Abstract
The short-term mortality of cirrhotic patients who develop renal dysfunction remains unacceptably high, and as such the treatment of this condition is an unmet need. Although features of kidney injury are well recognized in these patients, the pathophysiology is complex and not completely understood. Improved understanding of the pathophysiological mechanisms involved in renal dysfunction occurring on a background of cirrhosis is key to developing effective treatment strategies to improve survival. Renal dysfunction due to hepatorenal syndrome (HRS) is characteristic of cirrhosis. Our current understanding is that HRS is functional in nature and occurs as a consequence of hemodynamic changes associated with portal hypertension. However, there is evidence in the literature suggesting that, histologically, the kidneys are not always normal in the vast majority of patients who present with renal dysfunction on the background of cirrhosis. Furthermore, there is emerging data implicating nonvasomotor mechanisms in the pathophysiology of renal dysfunction in cirrhosis. This mini-review aims to present the evidence suggesting that factors other than hemodynamic dysregulation have an important role in the development of this major complication for patients with progressive cirrhosis.
Keywords: cirrhosis, hepatorenal syndrome, renal failure
Renal dysfunction is a common manifestation of advanced cirrhosis that is associated with significant mortality and morbidity. Although acute renal dysfunction in cirrhosis can be due to a number of causes such as hypovolemia and nephrotoxins, hepatorenal syndrome (HRS) is the most characteristic. However, it is becoming increasingly evident that renal dysfunction in cirrhosis is a heterogeneous condition, and some patients who were previously diagnosed with HRS actually have renal dysfunction associated with infection/inflammation, which is likely to have a different pathophysiological basis.
An estimated 11% of patients with advanced cirrhosis and refractory ascites develop HRS.1 This condition is traditionally ascribed to functional renal failure in patients with chronic liver disease associated with no significant morphologic changes in renal histology and with largely preserved tubular function.2, 3 This is because kidneys from patients with HRS have been reported to recover function post liver transplantation,4 and they have also been successfully used as renal allografts for kidney transplantation.5 However, only a small proportion of patients who develop renal dysfunction in association with cirrhosis suffer from HRS.
Two types of HRS are recognized. Type 1 HRS occurs in an acute setting, with a rapidly progressive decline in renal function, which is characterized by a doubling of the initial creatinine to a level >226 μmol/l (2.5 mg/dl) in <2 weeks.6 Untreated, type 1 HRS is associated with a mortality rate of 80% at 2 weeks.7 Type 2 HRS follows a more progressive course with a moderate rise in serum creatinine levels to a level >133 μmol/l (1.5 mg/dl).6 Type 2 HRS has a median survival of 4–6 months.7 In addition to the above definitions for HRS 1 and 2, in 2007, the International Ascites Club proposed a revised version of the original criteria, and this is shown in the table below (Table 1).
Table 1. Diagnostic criteria for hepatorenal syndrome in cirrhosis (Adapted from Salerno et al. 6).
| Cirrhosis with ascites |
| Serum creatinine >133 μmol/l (1.5 mg/dl) |
| No improvement of serum creatinine (decrease to a level of ⩽133 μmol/l) after at least 2 days with diuretic withdrawal and volume expansion with albumin |
| Absence of shock |
| No current or recent treatment with nephrotoxic drugs |
| Absence of parenchymal kidney disease, as indicated by proteinuria >500 mg/day, microhematuria (>50 red blood cells per high power field), and/or abnormal renal ultrasonography |
As is evident from the above criteria, the diagnosis of HRS requires a set of stringent criteria that rely on serum creatinine levels. Evidence suggests that a smaller increase in serum creatinine, insufficient to make a diagnosis of HRS, is also associated with a poor prognosis in patients with cirrhosis.8 It is possible that the severity of renal dysfunction is underestimated by the measurement of serum creatinine levels, as it is most commonly measured using a modified colorimetric Jaffe assay, which is prone to interference from bilirubin and other compounds.9, 10 In addition, patients with cirrhosis often have muscle wasting, reduced hepatic creatine synthesis, and increased renal tubular creatinine secretion.11 As such, smaller increases in serum creatinine reflect much larger changes in renal function than would be anticipated from the rises in serum creatinine. For this reason, there has been a move to redefine HRS to fall in line with the Acute Kidney Injury Network (AKIN) criteria for acute renal failure,12 which is more sensitive for the early detection of smaller increases in serum creatinine.13 Several studies have been carried out using the AKIN criteria in a cirrhotic population, but there is a lack of consensus as to whether the AKIN or classical HRS criteria best predict prognosis in cirrhotic patients with acute renal dysfunction.14, 15, 16
Although the classical diagnostic criteria for HRS now includes patients with HRS secondary to infection (but not septic shock), it is likely that patients with renal dysfunction or HRS associated with infection are distinct from patients with ‘classical HRS' (HRS not associated with infection).17 A recent study by Barreto et al.17 describing outcomes in patients diagnosed with HRS associated with infection showed that in approximately two-thirds of patients this condition is not reversible with standard of care for HRS using terlipressin and albumin, indicating a different pathophysiological underlying mechanism of disease.12 In addition, patients with renal dysfunction associated with infection have been shown to have higher levels of urinary biomarkers of tubular damage compared with patients with classical HRS.13 It therefore stands to reason that different pathophysiological mechanisms may be responsible for the development of renal dysfunction in the patients with classical HRS compared with patients with renal dysfunction associated with infection.18
‘TRADITIONAL' VIEW OF HRS
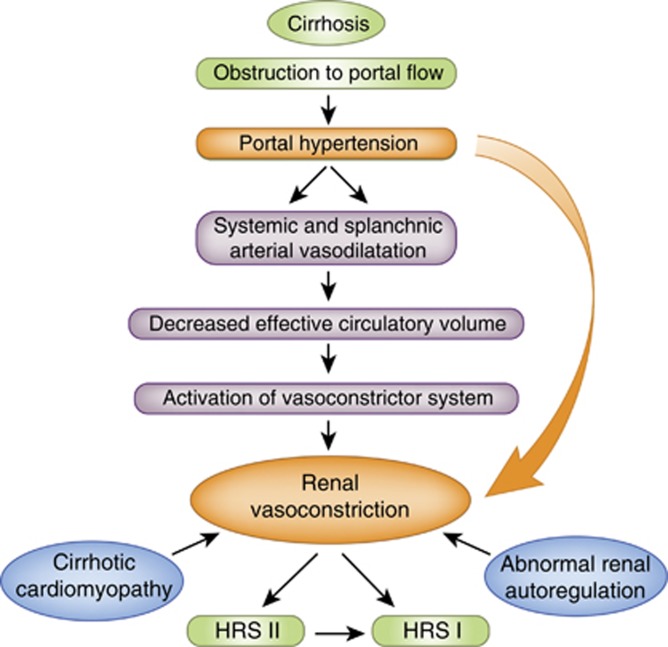
In 1970, Epstein et al.19 demonstrated using renal angiography that in cirrhotic patients with renal failure, the main pathophysiological feature is marked vasoconstriction of the renal vasculature associated with a redistribution of blood flow away from the renal cortex. The hypothesis is that vasoconstriction of the renal circulation, which occurs in HRS, develops as a result of the hemodynamic dysregulation associated with portal hypertension. In this setting, the increase in shear stress in the splanchnic vascular bed leads to overproduction of nitric oxide and other potent vasodilators, thus resulting in splanchnic vasodilatation.20 The consequence of this is a decrease in effective arterial volume, which leads to severe renal vasoconstriction via activation of the renin–angiotension–aldosterone system, causing renal hypoperfusion.13, 21 Impaired cardiac function in patients with decompensated cirrhosis leads to further arterial underfilling, decreased mean arterial pressure, and further impairment of renal blood flow and function.22 In addition, activation of the sympathetic nervous system through a hepatorenal reflex arc also contributes to the pathophysiology of HRS.23 There is an altered autoregulation of renal blood flow in patients with HRS (Figure 1).24
Figure 1.
The vasodilatation hypothesis of hepatorenal syndrome. (Adapted from Wong et al.13). In cirrhosis, portal hypertension leads to splanchnic and systemic vasodilatation, which results in a decrease in effective arterial volume. This in turn leads to the activation of vasoconstrictor systems leading to a reduction in renal blood flow. An impairment of cardiac function (cirrhotic cardiomyopathy) and abnormal renal blood flow autoregulation further contributes to renal hypoperfusion, resulting in HRS.
The evidence in the literature implicating the above factors in the pathophysiology of HRS is strong, as increasing the mean arterial pressure in patients with HRS by using splanchnic vasoconstrictors and albumin improves renal function.25, 26 Furthermore, a recent pilot study showed that patients with diuretic-refractory ascites, who have the highest risk of developing HRS, have lower renal plasma flow and higher right main kidney and arcuate artery resistive indices compared with patients without ascites.27 However, this study only involved 10 patients and was therefore underpowered for any statistical inferences to be made. Clearly, there is a need to investigate whether these findings are reproducible in a larger cohort.
Despite the evidence summarized above, attempts to restore the circulatory dysfunction associated with HRS using splanchnic vasoconstrictors and volume expanders do not reverse the syndrome in up to 40% of patients.28, 29 Therefore, it is likely that other pathophysiological mechanisms have a role in the pathogenesis of renal dysfunction in cirrhosis.
NEW CONCEPTS ON THE PATHOPHYSIOLOGY OF RENAL DYSFUNCTION IN CIRRHOSIS
Evidence that renal dysfunction is associated with infection/inflammation and is not just a vasomotor nephropathy
Renal dysfunction is a defining feature of acute on chronic liver failure (ACLF), and it occurs in ∼35% of patients with ACLF.30 ACLF is defined as ‘an acute deterioration of preexisting, chronic liver disease, usually relating to a precipitating event and associated with increased mortality at 3 months due to multisystem organ failure'.31 Dysregulated inflammation and infection, considered a hallmark of ACLF, has also been implicated in renal dysfunction.32, 33, 34 At present, it is not clear as to which of these patients have the classical HRS and which of these patients have renal dysfunction secondary to inflammation.
Thabut et al.32 found the presence of a systemic inflammatory response syndrome (SIRS) in ∼40% of cirrhotic patients with functional renal failure (including HRS with or without infection). In these patients, the in-hospital mortality rate was 68%, which was significantly higher than in patients without SIRS. They concluded that the presence of SIRS is an independent prognostic factor in patients with cirrhosis and acute functional renal failure, and treating SIRS could potentially lead to a reduction in mortality.32 Further evidence that SIRS has an important role in renal dysfunction is derived from studies that have shown that the use of anti-inflammatory agents such as pentoxifylline improves renal function or significantly decreases the risk of developing renal failure in patients with alcoholic hepatitis.35, 36 The therapeutic effect of pentoxifylline in this setting may be partly owing to its hemorrheologic and beneficial effects on the microcirculation, thus leading to an improvement in renal blood flow.37 It seems plausible that the mechanism responsible for the development of renal dysfunction in these patients with alcoholic hepatitis differs from the ‘classical' HRS, as the presence of alcoholic hepatitis has a negative effect on survival in patients with renal dysfunction treated with terlipressin.38
Renal dysfunction may also occur following a gastrointestinal bleed.39 Infection/inflammation may also have a role in this. It is well recognized that most cases of acute kidney injury following a gastrointestinal bleed occur as a consequence of acute tubular necrosis following hypovolemia. However, there is evidence that following a gastrointestinal bleed there is a transient increase in plasma endotoxin levels,40 and endotoxemia has a critical role in the development of acute renal dysfunction in cirrhosis.41
The mechanism of the inflammatory basis of renal dysfunction in cirrhotic patients is currently unknown, but one hypothesis is that in cirrhosis gut bacterial translocation is increased, which primes the kidneys to the effect of a superimposed inflammatory insult such as an infection. This hypothesis is supported by studies of selective gut decontamination using prophylactic administration of norfloxacin, which reported both reduced incidence of renal dysfunction and improved survival.42 Reducing the direct effects of bacterial products on the renal tubules may modulate this effect of gut decontamination.
The kidneys of some cirrhotic patients with presumed HRS show histologic evidence of acute kidney injury. However, it is likely that the patients described may have renal dysfunction that is associated with infection and/inflammation. A study by Mandal et al.43 reported light and electron microscopy changes in five patients diagnosed with HRS. They observed evidence of acute tubular necrosis on light microscopy, whereas electron microscopy demonstrated necrosis of the proximal tubules. It is important to state that, in this study, the samples were obtained postmortem, and thus the changes observed may reflect terminal changes. Kanel et al.39 further described the presence of an unusual renal lesion consisting of the reflux of the proximal convoluted tubular epithelium into Bowman's space in ∼70% of patients diagnosed with renal dysfunction on the background of cirrhosis. However, the authors felt that this lesion was unlikely to be responsible for renal failure observed in these patients.44 More recently, Shah et al.45 reported evidence of tubular injury on periodic methenamine and silver staining of five renal biopsy specimens derived from patients with ACLF and renal failure, i.e., renal dysfunction associated with inflammation. In addition to the human studies, histological examination of the kidneys in an animal model of ACLF revealed glomerular mesangial hypercellularity in the early stages of the syndrome. Furthermore, in the later stages, there was evidence of hydropic degeneration of the proximal and distal tubules.46 Similarly, another animal study, involving a rat model of cirrhosis treated with lipopolysaccharide (which is clinically similar to a patient with renal dysfunction associated with infection), showed evidence of tubular vacuolar degeneration in the proximal tubules, and this was associated with sloughing of the tubular cells. There was also an increased expression of caspase-3 expression signifying tubular cell apoptosis.47 It is recognized that apoptosis of tubular cells by inflammatory cytokines occurs in renal dysfunction associated with endotoxemia.48 A similar pathophysiology may underlie infection/inflammation-associated renal dysfunction in cirrhotic patients. These observations suggest that immunologic mechanisms are important in mediating the renal injury and that hemodynamic factors do not operate in isolation.
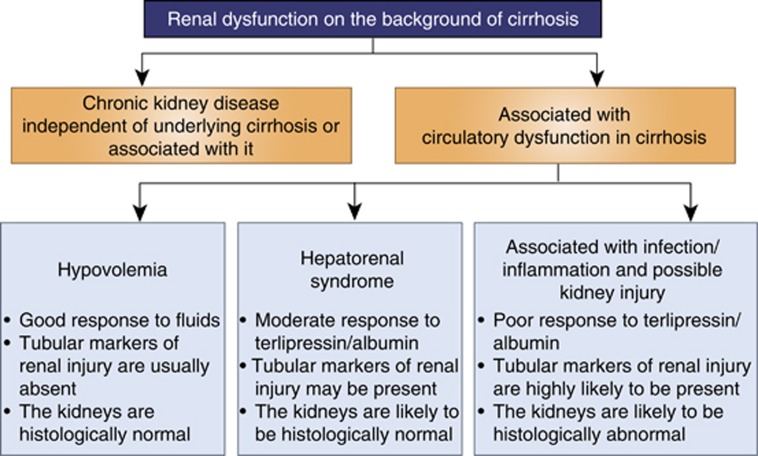
Further evidence supporting the hypothesis that renal dysfunction in cirrhotic patients is more than just functional renal failure and that tubular injury occurs is derived from studies that have shown that markers of tubular injury are elevated in some patients with renal dysfunction in cirrhosis. Rector et al.49 observed an increase in urinary beta-2-microglobulin, which is an index of tubular function in cirrhotic patients with presumed HRS compared with controls. Neutrophil gelatinase–associated lipocalin is a protein expressed by the renal tubules, which is upregulated after renal tubular injury. Patients diagnosed with HRS were shown to have significantly higher plasma and urinary neutrophil gelatinase–associated lipocalin levels compared with stable cirrhotic patients.50 In addition, neutrophil gelatinase–associated lipocalin was identified as a predictor of mortality in patients with HRS. It is likely that these investigators were indeed describing patients with renal dysfunction that is associated with infection/inflammation rather than patients with HRS. Figure 2 highlights the main features distinguishing between ‘classical HRS' and renal dysfunction associated with infection/inflammation.
Figure 2.
Renal dysfunction in cirrhosis. A Figure depicting our current understanding on the main features distinguishing between the hypovolemia, hepatorenal syndrome, and renal dysfunction associated with inflammation and infection.
A common misconception is that the kidneys are morphologically normal in HRS; thus, they function normally when transplanted into patients without liver disease and recover post liver transplantation. However, up to 21% of patients with presumed HRS have ongoing renal dysfunction at 30 days post liver transplantation.51 Similarly, up to 42% of patients have persistent renal failure following liver transplantation, suggesting that there might be underlying intrinsic renal parenchymal damage.52 Interestingly, patients with renal dysfunction with liver disease secondary to excess alcohol consumption are less likely to recover renal function. This ties in with our current thinking that there is an inflammatory basis to the tubular damage in some patients with renal dysfunction, as patients with alcoholic hepatitis and renal dysfunction have been shown to have increased serum TNF-α levels.53 In further support of the notion that tubular damage does occur in renal dysfunction observed in some cirrhotic patients, Gonwa et al.54 also demonstrated that this group of patients has a significantly higher incidence of progressing to end-stage renal failure after liver transplantation compared with cirrhotic patients without renal dysfunction (10% vs. 0.8% P<0.005).
On the basis of the emerging new concepts on the pathophysiology of HRS, a recent report from the International Ascites Club suggests that many other factors can impair the glomerular filtration rate (GFR) in cirrhotic patients with renal dysfunction beyond the control of renal blood flow, such as endothelial dysfunction following an infectious stimuli.55
Possible nonvasomotor mechanisms of renal dysfunction in cirrhotic patients
There is a paucity of data on the possible mechanisms by which renal tubular damage occurs in patients with renal dysfunction in cirrhosis, in particular that associated with superimposed infection/inflammation. A brief overview of possible mechanisms is described below:
Upregulation of inflammatory mediators potentiating renal injury
The presence of the SIRS significantly correlates with the development of renal dysfunction in cirrhotic patients.32, 56 The proinflammatory cytokines generated as a consequence of SIRS may contribute by causing direct renal injury. Although a range of proinflammatory cytokines, chemokines, circulating immune complexes, and adhesion molecules have a role in mediating renal dysfunction,57 to our knowledge, only the two mediators described below have been directly implicated. It should be noted that these two mediators have mainly been described in animal models. Therefore, there is a need to further validate their importance in humans.
Toll-like receptor 4
The first, recently described inflammatory mediator is the toll-like receptor 4 (TLR4). Receptors from this family have an important role in the innate immune system recognizing molecules derived from microbes. Activation of TLR4 leads to the production of proinflammatory mediators. Renal TLR4 (mRNA and protein) expression is regulated in an animal model of HRS.58 More recently, using an animal model of cirrhosis, Shah et al.47 demonstrated increased TLR4 expression in the proximal renal tubules. The upregulation of this receptor is most likely because of increased gut bacterial translocation. A subsequent inflammatory insult in this animal model led to a further increase in proximal tubule TLR4 expression, which corresponded with evidence of tubular injury on histology, as well as deterioration in renal function. Treatment with norfloxacin, a selective gut decontaminant, resulted in an attenuation of renal TLR4 expression and improvement in renal histology and renal function tests, suggesting that renal dysfunction in this model is partly mediated by proinflammatory cytokines consequent on increased bacterial translocation. A similar upregulation of tubular TLR4 expression was also observed in cirrhotic patients with renal dysfunction associated with infection/inflammation and, to a lesser degree, in those with HRS, supporting the hypothesis that renal dysfunction in cirrhosis is more than just a vasomotor disorder.45
Interleukin 17A
IL-17A is a proinflammatory cytokine released by T cells that have a role in host immune defense and inflammation. Intestinal Paneth cells have been shown to synthesize IL-17A and respond to inflammatory stimuli by overproducing IL-17A. This mechanism has recently been implicated in liver-related acute kidney injury.59 Takahashi and colleagues, using a mouse model of hepatic ischemia/reperfusion injury (which also develops significant renal dysfunction), demonstrated increased intestinal Paneth cell degranulation and increased IL-17A levels in the portal vein and small intestine.60 Both an IL-17A–neutralizing antibody and genetic deletion of IL-17A were found to be protective against hepatic ischemia/reperfusion and kidney injuries. In addition, depletion of Paneth cells attenuated not only hepatic ischemia/reperfusion injury but also renal dysfunction in this model,59 suggesting that Paneth cell–derived IL-17A may also have a role in the pathophysiology of renal dysfunction in cirrhotic patients.
Other important nonvasomotor mechanisms in renal dysfunction in cirrhosis
Bile cast nephropathy
Patients with liver dysfunction who develop renal dysfunction have increased serum concentrations of bilirubin and bile acids. On the basis of animal and human studies, it has been proposed that elevated levels of bilirubin and bile acids may have a direct toxic effect on the tubules61, 62, 63 and lead to renal impairment. In a recently published study, the authors looked at the prevalence and characteristics of renal bile casts in a cohort of jaundiced patients. They found that ∼85% of patients in this study who were diagnosed with HRS had evidence of renal tubular bile casts. Furthermore, patients with bile casts were found to have higher serum creatinine levels, although this did not reach statistical significance. The authors concluded that bile cast nephropathy is an important pathologic entity that may account for renal impairment in many patients with liver dysfunction. In addition, they suggested that the current paradigm of renal dysfunction in cirrhotic patients is incomplete without incorporating the contribution from renal bile casts.64 These findings may explain why bilirubin has been highlighted as an independent predictive factor of response to terlipressin therapy in cirrhotic patients with renal dysfunction. Terlipressin was effective in only 13% of patients with serum bilirubin levels >10 mg/dl (171 μmol/l) compared with 67% of patients with lower serum bilirubin levels. It may well be that the predominant mechanism for the development of renal dysfunction in patients with high serum bilirubin values is bilirubin-induced tubular epithelial injury rather than vasomotor nephropathy.28
To further corroborate the above findings, another study involving a bile duct–ligated mouse model (a model of liver injury, as well as cholestasis) demonstrated that a significantly higher serum bile acid level in this model was associated with biochemical evidence of renal failure, as well as histological evidence of tubular epithelial injury.65 Clearly, there is a need for additional studies looking at the renal effects of elevated bile acids and bilirubin levels in a cohort of patients diagnosed with renal dysfunction in cirrhosis. However, it is likely that studies of this nature in humans will be limited by a lack of histological samples, as renal biopsies are not often performed in cirrhotic patients with renal dysfunction.
Elevated intra-abdominal pressure
Intra-abdominal hypertension is defined as an intra-abdominal pressure (IAP) of >12 mm Hg. As up to 11% of patients with refractory ascites develop HRS, an association between increased IAP and the development of HRS has been postulated. In 1987, Cade et al.66 studied the effect of increased intra-abdominal pressure in 11 patients with HRS. They showed that a reduction in IAP to below 17 cm H2O was associated with an improvement in GFR, renal blood flow, and urine flow. Furthermore, insertion of a peritoneovenous shunt that maintains a low IAP also led to an improvement in GFR and renal blood flow. In another study carried out on 19 patients with HRS, a reduction of mean IAP from 22 mm Hg to 9 mm Hg was associated with a significant increase in creatinine clearance in the context of fluid substitution guided by assessment of the global end-diastolic volume.67 Chang et al.68 further described the mechanism by which an elevated IAP contributes to the development of HRS using a mouse model of cirrhosis and increased IAP. In their study, they observed that an increase in IAP above 10 mm Hg in this model was associated with a significant increase in serum creatinine. In addition, the renal biopsy specimen of the animals with IAP measurements above 10 mm Hg showed evidence of constrictive renal tubular lumen, inflammatory infiltrates in the interstitium, as well as formed casts and hyperemia in the renal interstitium. The interesting findings from this study need to be further validated in other models. Although the results from these studies suggest an association between raised IAP and HRS, one cannot automatically equate this with causality, especially in view of the fact that there is evidence in the literature suggesting that an abrupt drop in IAP following large volume paracentesis, without the use of plasma expanders, may actually precipitate HRS via hemodynamic changes.69, 70 More recently, data in cirrhotic patients showed that the post-paracentesis circulatory dysfunction, which sometimes results in renal dysfunction, is associated with activation of the circulating monocytes, arguing strongly that the renal dysfunction in this scenario is more than just a vasomotor dysfunction.71
CONCLUSION
During the past century, important progress has been made in the pathogenesis and treatment of renal dysfunction in cirrhosis, but it is clear that the journey into understanding the pathophysiology of this condition is far from complete. First, there is an urgent need to clarify the controversies surrounding the definition of renal dysfunction in cirrhosis.
In this review, we have summarized new and evolving concepts on the pathophysiology of renal dysfunction in cirrhosis separating HRS from renal dysfunction that is consequent upon infection/inflammation. In our opinion, the available evidence highlights the fact that the condition is complex, and its pathophysiology is likely to involve both vasomotor and nonvasomotor mechanisms. It is also likely that the predominant mechanism may differ depending on the clinical scenario.
All the authors declared no competing interests.
References
- Planas R, Montoliu S, Balleste B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–1394. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Papper S, Belsky JL, Bleifer KH. Renal failure in Laennec's cirrhosis of the liver. I. Description of clinical and laboratory features. Ann Inter Med. 1959;51:759–773. doi: 10.7326/0003-4819-51-4-759. [DOI] [PubMed] [Google Scholar]
- Goresky CA, Kumar G. Renal failure in cirrhosis of the liver. Can Med Assoc J. 1964;90:353–356. [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki S, Popovtzer MM, Corman JL, et al. Recovery from "hepatorenal syndrome" after orthotopic liver transplantation. N Engl J Med. 1973;289:1155–1159. doi: 10.1056/NEJM197311292892201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel MH, Coburn JW, Mims MM, et al. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence for the functionalnature of renal failure in advanced liver disease. N Engl J Med. 1969;280:1367–1371. doi: 10.1056/NEJM196906192802501. [DOI] [PubMed] [Google Scholar]
- Salerno F, Gerbes A, Gines P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gines P, Guevara M, Arroyo V, et al. Hepatorenal syndrome. Lancet. 2003;362:1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62:131–137. doi: 10.1136/gutjnl-2011-301255. [DOI] [PubMed] [Google Scholar]
- Daugherty NA, Hammond KB, Osberg IM. Bilirubin interference with the kinetic Jaffe method for serum creatinine. Clin Chem. 1978;24:392–393. [PubMed] [Google Scholar]
- Lolekha PH, Jaruthunyaluck S, Srisawasdi P. Deproteinization of serum: another best approach to eliminate all forms of bilirubin interference on serum creatinine by the kinetic Jaffe reaction. J Clin Lab Anal. 2001;15:116–121. doi: 10.1002/jcla.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269–278. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Nadim MK, Kellum JA, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702–709. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- Fagundes C, Barreto R, Guevara M, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–481. doi: 10.1016/j.jhep.2013.04.036. [DOI] [PubMed] [Google Scholar]
- Wong F, O'Leary JG, Reddy KR, et al. New consensus definition of acute kidney injury accurately predicts 30-day mortality in patients with cirrhosis and infection. Gastroenterology. 2013;145:e1281. doi: 10.1053/j.gastro.2013.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto R, Fagundes C, Guevara M, et al. Type-1 hepatorenal syndrome associated with infections in cirrhosis. Natural history, outcome of kidney function and survival. Hepatology. 2013;59:1505–1513. doi: 10.1002/hep.26687. [DOI] [PubMed] [Google Scholar]
- Fagundes C, Pepin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267–273. doi: 10.1016/j.jhep.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Epstein M, Berk DP, Hollenberg NK, et al. Renal failure in the patient with cirrhosis. The role of active vasoconstriction. Am J Med. 1970;49:175–185. doi: 10.1016/s0002-9343(70)80073-0. [DOI] [PubMed] [Google Scholar]
- Blendis L, Wong F. The hyperdynamic circulation in cirrhosis: an overview. Pharmacol Ther. 2001;89:221–231. doi: 10.1016/s0163-7258(01)00124-3. [DOI] [PubMed] [Google Scholar]
- Ring-Larsen H. Renal blood flow in cirrhosis: relation to systemic and portal haemodynamics and liver function. Scand J Clin Lab Invest. 1977;37:635–642. doi: 10.3109/00365517709100657. [DOI] [PubMed] [Google Scholar]
- Krag A, Bendtsen F, Henriksen JH, et al. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105–110. doi: 10.1136/gut.2009.180570. [DOI] [PubMed] [Google Scholar]
- Solis-Herruzo JA, Duran A, Favela V, et al. Effects of lumbar sympathetic block on kidney function in cirrhotic patients with hepatorenal syndrome. J Hepatol. 1987;5:167–173. doi: 10.1016/s0168-8278(87)80569-x. [DOI] [PubMed] [Google Scholar]
- Stadlbauer V, Wright GA, Banaji M, et al. Relationship between activation of the sympathetic nervous system and renal blood flow autoregulation in cirrhosis. Gastroenterology. 2008;134:111–119. doi: 10.1053/j.gastro.2007.10.055. [DOI] [PubMed] [Google Scholar]
- Angeli P, Volpin R, Gerunda G, et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690–1697. doi: 10.1002/hep.510290629. [DOI] [PubMed] [Google Scholar]
- Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: a pooled analysis of clinical trials. Am J Kidney Dis. 2011;58:928–938. doi: 10.1053/j.ajkd.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindikoglu AL, Dowling TC, Wong-You-Cheong JJ, et al. A pilot study to evaluate renal hemodynamics in cirrhosis by simultaneous glomerular filtration rate, renal plasma flow, renal resistive indices and biomarkers measurements. Am J Nephrol. 2014;39:543–552. doi: 10.1159/000363584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar A, Pereira GH, Guevara M, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219–226. doi: 10.1002/hep.23283. [DOI] [PubMed] [Google Scholar]
- Fabrizi F, Dixit V, Martin P. Meta-analysis: terlipressin therapy for the hepatorenal syndrome. Aliment Pharmacol Ther. 2006;24:935–944. doi: 10.1111/j.1365-2036.2006.03086.x. [DOI] [PubMed] [Google Scholar]
- Garg H, Kumar A, Garg V, et al. Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis. 2012;44:166–171. doi: 10.1016/j.dld.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Olson JC, Wendon JA, Kramer DJ, et al. Intensive care of the patient with cirrhosis. Hepatology. 2011;54:1864–1872. doi: 10.1002/hep.24622. [DOI] [PubMed] [Google Scholar]
- Thabut D, Massard J, Gangloff A, et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872–1882. doi: 10.1002/hep.21920. [DOI] [PubMed] [Google Scholar]
- Follo A, Llovet JM, Navasa M, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495–1501. doi: 10.1002/hep.1840200619. [DOI] [PubMed] [Google Scholar]
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- Mookerjee RP, Sen S, Davies NA, et al. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut. 2003;52:1182–1187. doi: 10.1136/gut.52.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E. Pentoxifylline for intermittent claudication. A critical review. Angiology. 1994;45:339–345. doi: 10.1177/000331979404500502. [DOI] [PubMed] [Google Scholar]
- Boyer TD, Sanyal AJ, Garcia-Tsao G, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315–321. doi: 10.1016/j.jhep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadei HM, Mai ML, Ahsan N, et al. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066–1079. doi: 10.2215/CJN.01340406. [DOI] [PubMed] [Google Scholar]
- Fukui H, Matsumoto M, Bode C, et al. Endotoxaemia in patients with liver cirrhosis and upper gastrointestinal bleeding: detection by the chromogenic assay with plasma Tween 80 pretreatment. J Gastroenterol Hepatol. 1993;8:577–581. doi: 10.1111/j.1440-1746.1993.tb01656.x. [DOI] [PubMed] [Google Scholar]
- Clemente C, Bosch J, Rodes J, et al. Functional renal failure and haemorrhagic gastritis associated with endotoxaemia in cirrhosis. Gut. 1977;18:556–560. doi: 10.1136/gut.18.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Navasa M, Planas R, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- Mandal AK, Lansing M, Fahmy A. Acute tubular necrosis in hepatorenal syndrome: an electron microscopy study. Am J Kidney Dis. 1982;2:363–374. doi: 10.1016/s0272-6386(82)80096-6. [DOI] [PubMed] [Google Scholar]
- Kanel GC, Peters RL. Glomerular tubular reflux—a morphologic renal lesion associated with the hepatorenal syndrome. Hepatology. 1984;4:242–246. doi: 10.1002/hep.1840040212. [DOI] [PubMed] [Google Scholar]
- Shah N, Mohamed FE, Jover-Cobos M, et al. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int. 2013;33:398–409. doi: 10.1111/liv.12047. [DOI] [PubMed] [Google Scholar]
- Rivera-Huizar S, Rincon-Sanchez AR, Covarrubias-Pinedo A, et al. Renal dysfunction as a consequence of acute liver damage by bile duct ligation in cirrhotic rats. Exp Toxicol Pathol. 2006;58:185–195. doi: 10.1016/j.etp.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Shah N, Dhar D, El Zahraa Mohammed F, et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J Hepatol. 2012;56:1047–1053. doi: 10.1016/j.jhep.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Jo SK, Cha DR, Cho WY, et al. Inflammatory cytokines and lipopolysaccharide induce Fas-mediated apoptosis in renal tubular cells. Nephron. 2002;91:406–415. doi: 10.1159/000064280. [DOI] [PubMed] [Google Scholar]
- Rector WG, Jr., Kanel GC, Rakela J, et al. Tubular dysfunction in the deeply jaundiced patient with hepatorenal syndrome. Hepatology. 1985;5:321–326. doi: 10.1002/hep.1840050229. [DOI] [PubMed] [Google Scholar]
- Gungor G, Ataseven H, Demir A, et al. Neutrophil gelatinase-associated lipocalin in prediction of mortality in patients with hepatorenal syndrome: a prospective observational study. Liver Int. 2013;34:49–57. doi: 10.1111/liv.12232. [DOI] [PubMed] [Google Scholar]
- Nadim MK, Genyk YS, Tokin C, et al. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transplant. 2012;18:539–548. doi: 10.1002/lt.23384. [DOI] [PubMed] [Google Scholar]
- Marik PE, Wood K, Starzl TE. The course of type 1 hepato-renal syndrome post liver transplantation. Nephrol, Dial, Transplant. 2006;21:478–482. doi: 10.1093/ndt/gfi212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GL, Sheron N, Goka AK, et al. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917–920. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- Gonwa TA, Morris CA, Goldstein RM, et al. Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome—experience in 300 patients. Transplantation. 1991;51:428–430. doi: 10.1097/00007890-199102000-00030. [DOI] [PubMed] [Google Scholar]
- Angeli P, Sanyal A, Moller S, et al. Current limits and future challenges in the management of renal dysfunction in patients with cirrhosis: report from the International Club of Ascites. Liver Int. 2013;33:16–23. doi: 10.1111/j.1478-3231.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- Cazzaniga M, Dionigi E, Gobbo G, et al. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475–482. doi: 10.1016/j.jhep.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Davis CL, Gonwa TA, Wilkinson AH. Pathophysiology of renal disease associated with liver disorders: implications for liver transplantation. Part I. Liver Transplant. 2002;8:91–109. doi: 10.1053/jlts.2002.31516. [DOI] [PubMed] [Google Scholar]
- Yan CG, Zhu DF, Wang F. [Study on the expressions and roles of renal heat shock protein 72 and Toll-like receptor 4 in hepatorenal syndrome in rat] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19:731–734. [PubMed] [Google Scholar]
- Park SW, Kim M, Brown KM, et al. Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology. 2011;53:1662–1675. doi: 10.1002/hep.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Vanlaere I, de Rycke R, et al. IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med. 2008;205:1755–1761. doi: 10.1084/jem.20080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betjes MG, Bajema I. The pathology of jaundice-related renal insufficiency: cholemic nephrosis revisited. J Nephrol. 2006;19:229–233. [PubMed] [Google Scholar]
- Gollan JL, Billing BH, Huang SN. Ultrastructural changes in the isolated rat kidney induced by conjugated bilirubin and bile acids. Br J Exp Pathol. 1976;57:571–581. [PMC free article] [PubMed] [Google Scholar]
- Song J, Chang A. Jaundice-associated acute kidney injury. NDT Plus. 2009;2:82–83. doi: 10.1093/ndtplus/sfn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Slambrouck CM, Salem F, Meehan SM, et al. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192–197. doi: 10.1038/ki.2013.78. [DOI] [PubMed] [Google Scholar]
- Fickert P, Krones E, Pollheimer MJ, et al. Bile acids trigger cholemic nephropathy in common bile-duct-ligated mice. Hepatology. 2013;58:2056–2069. doi: 10.1002/hep.26599. [DOI] [PubMed] [Google Scholar]
- Cade R, Wagemaker H, Vogel S, et al. Hepatorenal syndrome. Studies of the effect of vascular volume and intraperitoneal pressure on renal and hepatic function. Am J Med. 1987;82:427–438. doi: 10.1016/0002-9343(87)90442-6. [DOI] [PubMed] [Google Scholar]
- Umgelter A, Reindl W, Wagner KS, et al. Effects of plasma expansion with albumin and paracentesis on haemodynamics and kidney function in critically ill cirrhotic patients with tense ascites and hepatorenal syndrome: a prospective uncontrolled trial. Crit Care. 2008;12:R4. doi: 10.1186/cc6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Qi X, Li Z, et al. Hepatorenal syndrome: insights into the mechansims of intra-abdominal hypertension. Int J Clin Exp Pathol. 2013;6:2523–2528. [PMC free article] [PubMed] [Google Scholar]
- Cabrera J, Falcon L, Gorriz E, et al. Abdominal decompression plays a major role in early postparacentesis haemodynamic changes in cirrhotic patients with tense ascites. Gut. 2001;48:384–389. doi: 10.1136/gut.48.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno F, Guevara M, Bernardi M, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937–947. doi: 10.1111/j.1478-3231.2010.02272.x. [DOI] [PubMed] [Google Scholar]
- Carl DE, Ghosh S, Cheng J, et al. Post-paracentesis circulatory derangements are related to monocyte activation. Liver Int. 2013;34:1001–1007. doi: 10.1111/liv.12450. [DOI] [PubMed] [Google Scholar]