Abstract
Introduction:
Obstructive sleep apnea (OSA) is common among bariatric surgery candidates. After surgical weight loss, OSA frequently persists and untreated OSA can lead to weight gain. Long-term continuous positive airway pressure (CPAP) adherence is unclear and poor adherence may worsen weight loss outcomes. We sought to determine the impact of CPAP use on long-term weight-loss outcomes in a cohort of bariatric patients.
Methods:
Long-term observational study of bariatric surgery patients with OSA. Patients were evaluated with polysomnography preoperatively and one-year postoperatively. The cohort was again evaluated a mean of 7.2 years later to determine the relationship between long-term CPAP use and subsequent regain of weight.
Results:
Twenty-four consecutive patients (aged 48.5 ± 9.4 years at time of surgery; 73% female) were included in the initial assessment, and long-term outcome data were available on 22 subjects. Persistent OSA was documented in 21 of 22 subjects (95%) one year postoperatively. Final evaluation occurred 7.2 ± 2.3 years following surgery. Weight (213.3 ± 39.1 to 235.3 ± 47.1 lb, p = 0.10) and BMI (32.5 ± 5.4 to 37.3 ± 8.2 kg/m2, p = 0.03) increased in most (n = 19, 86.4%) from postoperative to final evaluation. CPAP use declined from 83.3% (preoperatively) to 38.1% (one year) and to 23.8% (final evaluation). BMI increased among those not using CPAP at long-term follow-up compared to those with continued CPAP use (6.8% v −1.8%, p = 0.05).
Conclusions:
In our cohort of bariatric patients with OSA, long-term adherence to CPAP therapy was poor, and non-adherence was associated with weight gain. Ongoing follow-up of OSA in this population may help to preserve initial achievements after surgical weight loss.
Commentary:
A commentary on this article appears in this issue on page 195.
Citation:
Collen J, Lettieri CJ, Eliasson A. Postoperative CPAP use impacts long-term weight loss following bariatric surgery. J Clin Sleep Med 2015;11(3):213–217.
Keywords: obesity, bariatric surgery, obstructive sleep apnea, sleep disorder, CPAP adherence
The prevalence of obesity in the United States has increased dramatically in recent decades. Current literature estimates that upwards of 66% of the US population is overweight (body mass index [BMI] ≥ 25 kg/m2), half of whom are obese (≥ 30 kg/m2). The fastest growing subpopulations among the obese are those with a BMI ≥ 40 kg/m2 (5-fold increase in prevalence from 1986 to 2005), and those with a BMI ≥ 50 kg/m2 (10-fold increase).1 Comorbidities of obesity, including diabetes mellitus, cardiovascular disease, and obstructive sleep apnea have risen in concert. Life expectancy in those with morbid obesity, particularly those with very severe morbid obesity (BMI ≥ 50 kg/m2), may be reduced by 5 to 20 years.1 Diet and intensive lifestyle interventions have limited efficacy for durable weight loss. The National Institutes of Health Consensus Statement specifies gastrointestinal surgery as a treatment option for patients with severe obesity (≥ 40 kg/m2 or ≥ 35 kg/m2 and significant comorbid disease). Currently, bariatric surgery is the most effective therapy for sustained weight loss, decreased morbidity and mortality, and improved quality of life measures.1,2
The evaluation and management of sleep disorders in this population is critical, as poor quality and fragmented sleep can undermine efforts to lose weight and sustain weight loss. Notably, insufficient sleep quantity and poor sleep quality cause alterations in the secretion and response to mediators involved in appetite stimulation and satiety (ghrelin and leptin, respectively). These hormonal alterations increase appetite and specifically increase cravings for high-calorie foods that lead to weight gain.3
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is common among bariatric surgical candidates and often persists following weight loss. Adherence with continuous positive airway pressure (CPAP) therapy at follow-up is poor and may impact long-term clinical outcomes.
Study Impact: Long-term use of CPAP is dismal among bariatric surgery patients. Weight-loss outcomes are significantly worsened in patients who do not use CPAP postoperatively.
The prevalence of obstructive sleep apnea (OSA) in obesity ranges from 39% to 98%.4–7 The highest rates are seen in those with a BMI ≥ 40 kg/m2.7 Prevalence rates and measures of disease severity (nocturnal saturation nadir and apnea-hypopnea index) may increase several-fold with increasing weight and BMI. Among patients being considered for bariatric surgery, the prevalence of OSA ranges between 64% and 100%.8–14 Increasing BMI, age, and male gender were able to predict the likelihood of OSA in patients evaluated for bariatric surgery in one recent study.8 Although other markers of pretest probability for OSA, such as increased daytime sleepiness (Epworth Sleepiness Scale) and the Functional Outcomes of Sleep Questionnaire (FOSQ), have demonstrated efficacy in predicting the likelihood of OSA, these markers have not been reliable in predicting OSA severity in bariatric surgery candidates.14 Therefore universal screening for OSA is typically performed in the preoperative evaluation for bariatric surgery.
The literature assessing the impact of surgical weight loss on OSA has often been limited by lack of follow-up polysomnogram (PSG) data to document disease resolution objectively instead relying on surrogate markers such as improvement in snoring and daytime somnolence. Use of these subjective surrogate markers may lead patients to feel they no longer require CPAP.15,16 Previous research from our institution found that although OSA improved following surgical weight loss, moderate-to-severe residual disease persisted (mean AHI 24.5 ± 18.1/h) in the majority of patients (71%), which by any measure necessitates therapy with CPAP.10 A subsequent meta-analysis evaluating twelve studies in bariatric patients confirmed this finding.17 It has been consistently demonstrated that bariatric surgery significantly reduces AHI, but in many cases does not completely resolve OSA, and clinical variables such as age, gender, and preoperative OSA severity may predict residual disease after peak weight loss.1 In addition, postoperative CPAP adherence is poor in this population. In our initial report of a bariatric cohort, only 26% of patients continued use of CPAP after surgery, similar to other studies.18,19 Given that untreated OSA can contribute to weight gain, it is likely that non-adherence with CPAP may worsen long-term weight-related outcomes.
We sought to evaluate long-term CPAP use and associations with weight change in a cohort of bariatric surgery patients who had undergone gastric banding at our institution.
METHODS
Subjects
Twenty-four patients who underwent preoperative bariatric surgery evaluations between 2003 and 2005 at our sleep disorders center were initially included.10 These patients were evaluated prior to and one year after bariatric surgery. The results of this study were previously reported.10 This patient population was derived from 145 consecutive patients who presented for bariatric surgery during the study period. Bariatric procedures for weight loss were performed on 118 patients; of these, 25 patients were referred to our sleep center for preoperative evaluation. One patient was excluded from this analysis due to postoperative death from pulmonary embolus. Otherwise, all records were included. The study cohort (n = 24) was evaluated with PSG preoperatively and at one year postoperatively to assess for weight change and for the impact of surgical weight loss on sleep disordered breathing. Five years after the publication of this initial study, long-term outcome data were available for 22 patients (91.7%) of the original study cohort. We assessed weight changes and CPAP use to determine pertinent associations. The study protocol was approved by our hospital's institutional review board.
Measurements
Subjects were clinically evaluated by a board certified sleep medicine physician preoperatively and one year postoperatively. All patients underwent level 1 in-laboratory, attended overnight polysomnography preoperatively and at one year postoperatively using a standard 12-channel montage (Sensor-medics Alpha Somnostar System, Sensormedics, Yorba Linda, CA), and studies were scored in 30-s epochs in accordance with standard criteria for sleep staging.20,21 OSA was diagnosed in accordance with the American Academy of Sleep Medicine recommendations during the study period.22,23 At long-term follow-up, the investigators reviewed a closed electronic medical record to abstract clinical data from the primary care manager, sleep medicine provider, and durable medical equipment vendor reports (CPAP use). Data collection included age, gender, weight (kg), body mass index (BMI, kg/m2), preoperative and postoperative apnea-hypopnea index (AHI, events/h), prescribed CPAP settings, and the continued use of CPAP (based on documented use in the sleep medicine physician clinical encounter, self-report, and continued mask replacements based on durable medical equipment vendor reports).
Endpoints
Our primary endpoint was the absolute change in BMI at long-term follow-up. The secondary endpoint was the percentage of patients with continued CPAP use at long-term follow-up. Changes in BMI were correlated with CPAP usage.
Statistical Analysis
We compared continuous variables with the Student t-test and analyzed categorical variables with the Fisher exact test. All tests were 2-tailed, and p values ≤ 0.05 were assumed to represent statistical significance. When applicable, data are presented as mean ± standard deviation. All analyses were completed using Stata ver. 9.2 (StataCorp, College Station, TX).
RESULTS
At the initial evaluation, the mean age of the cohort was 48.5 ± 9.4 years, and 73% were female. Over the duration of our evaluation, the subgroups (those using CPAP versus not using CPAP) were similar with regard to age and gender. The mean preoperative BMI was 51.1 ± 10.9 kg/m2 (range of 37–73 kg/m2). At the one-year postoperative evaluation, the mean decrease in weight was 121.1 ± 50.2 lb (36% of baseline weight), and BMI decreased by 18.6 kg/m2. Preoperative polysomno-grams demonstrated severe sleep apnea among most of the patients, with a mean AHI of 48.2 ± 32.8 events/hour. At one year postoperatively, 21 of 22 subjects (95%) had persistent obstructive sleep apnea based on repeat postoperative PSG, with a mean AHI of 24.5 ± 18.8 events/h, and 36% (n = 8) were using CPAP. At that time, one subject had an AHI of 2/h, demonstrating reversal of the subject's sleep apnea, and CPAP therapy was no longer indicated. This subject was not further considered for analysis of long-term outcome data.
Mean follow-up duration from time of surgery was 7.2 ± 2.3 years (range 2.1–10.0 years). At long-term follow-up the mean age of the cohort was 56.9 ± 9.0 years. Compared to the one-year postoperative visit, there were substantial increases in the mean absolute weight (213.3 ± 39.1 to 235.3 ± 47.1 lb, p = 0.10) and BMI (32.5 ± 5.4 to 37.3 ± 8.2 kg/m2, p = 0.03). The mean increase in weight from the one-year postoperative visit to the final evaluation was 22.0 lb, or a 10% increase in weight.
CPAP use declined dramatically, from 83% preoperatively, to 36% at one year postoperatively, and 23% (n = 5 patients) at long-term follow-up (Table 1). We evaluated weight loss outcomes based on CPAP use at long-term follow-up (Figure 1). BMI increased significantly more among those not using CPAP at their long-term follow-up compared to those who had been adherent to CPAP therapy (6.8 v −1.8 kg/m2, p = 0.05). Patients using CPAP lost an average of 1.4 lb per year, compared to an average gain of 4.3 lb per year among those not using CPAP.
Table 1.
Comparison of clinical variables and outcome measures over long-term follow-up.
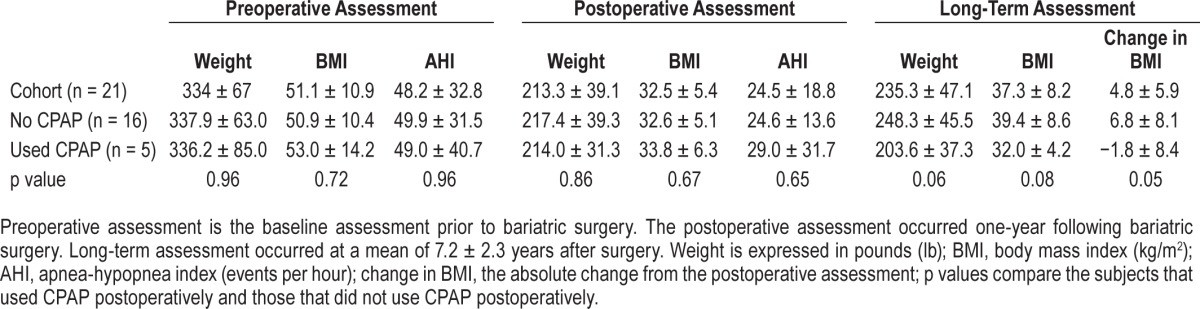
Figure 1. Comparison of change in BMI over long-term follow-up.
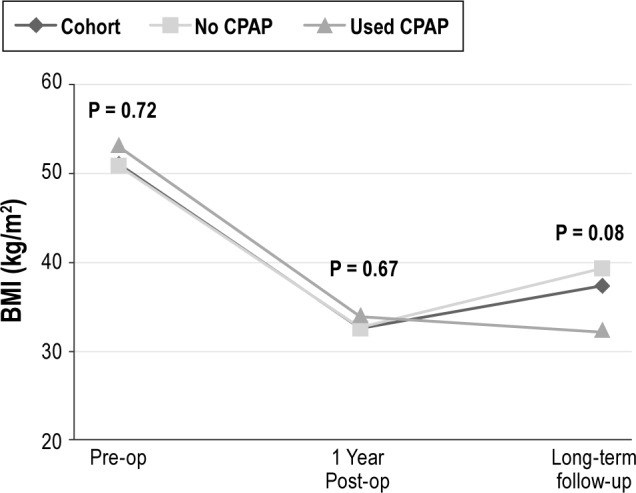
The change in BMI is displayed over time between three groups (the overall cohort, subjects using CPAP postoperatively, and subjects not using CPAP postoperatively). p values are displayed for the three time-points evaluated (baseline or preoperative, one-year postoperative visit, and long-term follow-up), and compare subjects using CPAP versus those not using CPAP.
DISCUSSION
Among our cohort of bariatric surgery patients, the majority had severe OSA preoperatively. Despite substantial weight loss, OSA persisted in nearly all individuals. Unfortunately, CPAP was discontinued in most. After 7.2 years, the majority of patients had regained weight over time. Weight gain was significantly greater among those who did not use CPAP postoperatively (nearly 6 lb differential per year between patients who did not use CPAP and those who did use CPAP in the intervening years).
Although several studies have assessed long-term weight loss outcomes after bariatric surgery, polysomnographic documentation of residual OSA in these studies is limited. Many prior studies reported resolution of OSA based upon surrogate markers such as subjective reports by bed partners of resolved snoring and patient self-reports of improved excessive daytime somnolence or the “feeling” that they can stop using CPAP therapy.1 This is unreliable as substantial weight loss can reduce fatigue and sleep disruptions, potentially leading to a misconception that OSA had resolved. As such, an objective assessment of the AHI is needed. Unfortunately, few studies have presented follow-up data on residual apnea severity verified by PSG.12
Studies that objectively document OSA severity based on follow-up PSG demonstrate that while patients do experience significant decreases in the AHI, moderate severity OSA remains for many. In our population, 71% of patients had moderate-to-severe OSA at one-year follow-up. A meta-analysis of 12 trials also reflected that the majority (62%) of patients have moderate-to-severe OSA postoperatively with a mean AHI > 15 events/hour.10,17 A recent study by Ravesloot et al. evaluated 110 bariatric surgery patients with OSA with PSG at ≥ 6- and ≥ 12-month follow-up. Only 50 patients completed the second follow-up PSG, and the mean AHI declined from 49.5/h preoperatively to 22.7/h at a mean of 7.1 months and 17.4/h at a mean of 16.9 months.12 Peak improvements in the AHI occurred in the first 6 months, in parallel with weight loss. Thereafter, reductions in AHI and weight continued, but appeared to plateau. Similar to our results, moderate OSA persisted in most. Overall however, reductions in AHI were significant, with patients tending to decrease in severity by one class (from severe to moderate, moderate to mild, and mild to none). Disease severity matters, and patients with mild disease preoperatively were more likely to be cured than those with severe disease (53.6% v 17.9%, respectively). Although “cure” is typically based on reducing the AHI to less than five events per hour, alternate criteria may be more realistic, such as a 50% reduction in the AHI, or an AHI ≤ 20/h, which occurred in over half of the cohort. Although CPAP use was not delineated, their results also indicate the need for ongoing evaluation and management. The majority of publications evaluating residual OSA based upon PSG have found that while the initial reduction in AHI is profound after surgical weight loss, a significant number of patients may have moderate-to-severe residual disease, which still necessitates CPAP therapy.19,24,25
Unfortunately, literature on CPAP adherence in this population is sparse. Although several studies comment on the decreased need for CPAP as a marker for improvement in OSA, only three studies have published data on rates of CPAP use after surgical weight loss, including our prior report on this cohort. The other studies demonstrated similarly disappointing rates of adherence. Haines et al.19 evaluated 349 patients referred for PSG prior to bariatric surgery, and 101 patients followed-up for postoperative PSG. CPAP use decreased from 83 patients preoperatively to 31 patients (37.3%) approximately one year postoperatively. Similarly, Dixon et al.18 prospectively evaluated 25 patients and found that CPAP use decreased from 14 patients preoperatively to 4 patients (28.6%) at the final annual follow-up. Overall, CPAP use appears to decline dramatically in the months following bariatric surgery as weight loss goals are achieved.
Because untreated OSA can lead to weight gain, strategies that improve CPAP use and promote better adherence are indicated. CPAP use has been found to improve weight loss outcomes in overweight and obese patients,26 and untreated OSA likely facilitates weight gain3,4 and hinders ongoing weight loss. OSA severity has been shown to decrease significantly with surgical weight loss, and CPAP requirements do decrease in concert.10,19 This is notable, as prior CPAP settings may be higher than required to ablate residual sleep disordered breathing following weight loss. Intolerance to an inappropriately high pressure may promote abandonment of therapy. Ultimately, a multi-pronged approach to optimizing sleep in this population, focusing on sleep quantity, quality, and CPAP adherence may improve clinical outcomes.
Limitations
Our study may be limited by selection bias given the observational study design, and may be underpowered to detect significant differences in clinical outcomes and response to therapy due to the small size of our cohort. It is notable that the mean BMI in our cohort was 51.1 kg/m2, and nearly all patients had severe OSA based on an AHI > 30 events/h preoperatively. Because preoperative BMI and OSA severity can affect outcomes with regards to weight loss and resolution of sleep disordered breathing, our results may not be applicable to all patients. CPAP use at long-term follow-up was low and limited our ability to perform subgroup analyses. A final potential limitation is that all patients in our cohort underwent gastric banding. In the present study, it is not possible to discern how the type of surgery or patient variables that lead to their candidacy for this procedure over other surgical options (such as gastric bypass, gastroplasty, or biliopancreatic diversion among others), may have influenced long-term weight loss outcomes.
CONCLUSIONS
Among our cohort of bariatric surgery patients with OSA, most experienced substantial regain in weight over time. CPAP use correlated with improved long-term weight loss outcomes. This finding suggests that untreated OSA undermines long-term weight loss outcomes in bariatric surgery patients. Future prospective studies that track weight loss longitudinally in bariatric patients, coupled with PSG assessment of residual OSA severity and objective measures of CPAP adherence, may help to delineate more precisely the effect of OSA therapy on bariatric outcomes.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. The views expressed in this paper are those of the authors and do not reflect the official policy of the Department of the Army, Department of Defense, or the US Government.
ACKNOWLEDGMENTS
All of the authors have participated in the production of this manuscript and have approved its final version. Dr. Collen participated in collection of data, interpretation of results, and the majority of manuscript writing. Dr. Lettieri participated in study design, data analysis and interpretation, and manuscript editing. Dr. Eliasson participated in data analysis and interpretation, and edited the final version of the manuscript.
REFERENCES
- 1.Pannain S, Mokhlesi B. Bariatric surgery and its impact on sleep architecture, sleep-disordered breathing, and metabolism. Best Pract Res Clin Endocrinol Metab. 2010;24:745–61. doi: 10.1016/j.beem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Julia C, Ciangura C, Capuron L, et al. Quality of life after Roux-en-Y gastric bypass and changes in body mass index and obesity-related comorbidities. Diabetes Metab. 2013;39:148–54. doi: 10.1016/j.diabet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Leinum CJ, Dopp JM, Morgan BJ. Sleep-disordered breathing and obesity: pathophysiology, complications, and treatment. Nutr Clin Pract. 2009;24:675–87. doi: 10.1177/0884533609351532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowan DC, Livingston E. Obstructive sleep apnoea syndrome and weight loss: review. Sleep Disord. 2012;2012:163296. doi: 10.1155/2012/163296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25:669–75. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 6.Sergi M, Rizzi M, Comi AL, et al. Sleep apnea in moderate-severe obese patients. Sleep Breath. 1999;3:47–52. doi: 10.1007/s11325-999-0047-y. [DOI] [PubMed] [Google Scholar]
- 7.Valencia-Flores M, Orea A, Castano VA, et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res. 2000;8:262–9. doi: 10.1038/oby.2000.31. [DOI] [PubMed] [Google Scholar]
- 8.Carneiro G, Florio RT, Zanella MT, et al. Is mandatory screening for obstructive sleep apnea with polysomnography in all severely obese patients indicated? Sleep Breath. 2012;16:163–8. doi: 10.1007/s11325-010-0468-7. [DOI] [PubMed] [Google Scholar]
- 9.Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13:676–83. doi: 10.1381/096089203322509228. [DOI] [PubMed] [Google Scholar]
- 10.Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med. 2008;4:333–8. [PMC free article] [PubMed] [Google Scholar]
- 11.O'Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–6. doi: 10.1381/096089204772787248. [DOI] [PubMed] [Google Scholar]
- 12.Ravesloot MJ, Hilgevoord AA, van Wagensveld BA, deVries N. Assessment of the effect of bariatric surgery on obstructive sleep apnea at two postoperative intervals. Obes Surg. 2014;24:22–31. doi: 10.1007/s11695-013-1023-y. [DOI] [PubMed] [Google Scholar]
- 13.Sareli AE, Cantor CR, Williams NN, et al. Obstructive sleep apnea in patients undergoing bariatric surgery--a tertiary center experience. Obes Surg. 2011;21:316–27. doi: 10.1007/s11695-009-9928-1. [DOI] [PubMed] [Google Scholar]
- 14.Sharkey KM, Orff HJ, Tosi C, Harrington D, Roye GD, Millman RP. Subjective sleepiness and daytime functioning in bariatric patients with obstructive sleep apnea. Sleep Breath. 2013;17:267–74. doi: 10.1007/s11325-012-0685-3. [DOI] [PubMed] [Google Scholar]
- 15.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 16.Catheline JM, Fysekidis M, Bachner I, et al. Five-year results of sleeve gastrectomy. J Visc Surg. 2013;150:307–12. doi: 10.1016/j.jviscsurg.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–42. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond) 2005;29:1048–54. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 19.Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141:354–8. doi: 10.1016/j.surg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A, editors. Los Angeles, CA: University of California Los Angeles, Brain Information Service/Brain Research Institute; 1968. A manual of standardized techniques and scoring system for sleep stages of human sleep. [Google Scholar]
- 21.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 22.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406–22. [PubMed] [Google Scholar]
- 23.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 24.Rao A, Tey BH, Ramalingam G, et al. Obstructive sleep apnoea (OSA) patterns in bariatric surgical practice and response of OSA to weight loss after laparoscopic adjustable gastric banding (LAGB) Ann Acad Med Singapore. 2009;38:587. [PubMed] [Google Scholar]
- 25.Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg. 2003;13:58–61. doi: 10.1381/096089203321136593. [DOI] [PubMed] [Google Scholar]
- 26.Loube DI, Loube AA, Erman MK. Continuous positive airway pressure treatment results in weight less in obese and overweight patients with obstructive sleep apnea. J Am Diet Assoc. 1997;97:896–97. doi: 10.1016/s0002-8223(97)00220-4. [DOI] [PubMed] [Google Scholar]


