Abstract
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality and may frequently be complicated by sleep disorders. Insomnia and obstructive sleep apnea are commonly encountered in patients with COPD. Nocturnal hypoxemia is also prevalent in COPD may occur despite adequate awake oxygenation and can be especially severe in rapid eye movement sleep. Additionally, several factors—some of them unique to COPD—can contribute to sleep-related hypoventilation. Recognition of hypoventilation can be vital as supplemental oxygen therapy itself can acutely worsen hypoventilation and lead to disastrous consequences. Finally, accruing data establish an association between restless leg syndrome and COPD— an association that may be driven by hypoxemia and/or hypercapnia. Comorbid sleep disorders portend worse sleep quality, diminished quality of life, and multifarious other adverse consequences. The awareness and knowledge regarding sleep comorbidities in COPD has continued to evolve over past many years. There are still several lacunae, however, in our understanding of the etiologies, impact, and therapies of sleep disorders, specifically in patients with COPD. This review summarizes the latest concepts in prevalence, pathogenesis, diagnosis, and management of diverse sleep disorders in COPD.
Citation:
Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med 2015;11(3):259–270.
Keywords: COPD, insomnia, obstructive sleep apnea, restless legs syndrome, hypoventilation
That sleep is adversely affected in chronic obstructive pulmonary disease (COPD) has been long recognized.1 COPD affects 5% to 10% of the adult population in the United States and is a major contributor to global disease burden.2 The prevalence of insomnia symptoms, insomnia disorder, restless leg syndrome, and hypoxemia is increased in COPD.3–5 Furthermore, polysomnographic (PSG) evaluation generally reveals decreased sleep efficiency and lower mean overnight oxygen saturation in COPD patients compared to controls.6
In COPD, the pathogenesis of sleep disorders appears to be a complex and multifactorial process, likely consequent to one or more of the following: physiological changes associated with sleep, hypoxemia, hypercapnia, inflammation, COPD medications, and/or nicotine use. Comorbid disorders as well as primary sleep disturbances may also contribute to disrupted sleep in COPD patients. For example, nocturnal gastroesophageal reflux (GERD) is associated with both symptoms of sleep apnea and COPD, and may contribute to the pathogenesis, and concomitant occurrence of both disorders.7 GERD may also influence sleep quality which could potentially contribute to some of the sleep complaints reported by persons with COPD.8 The following sections describe the diverse sleep disorders and sleep-related abnormalities encountered in patients with COPD.
INSOMNIA
Epidemiology
Insomnia is defined as difficulty falling asleep, staying asleep, waking up too early, or having unrefreshing sleep. The prevalence of insomnia is increased in patients with COPD.6 One study found that DSM-IV insomnia was reported in 32.9% of those with COPD, compared with only 20.3% of those without COPD.3 A history of COPD was associated with significantly increased odds of insomnia 1.9 (1.5–2.5) after adjusting for age and gender (p < 0.001). PSG did not reveal a significant overall difference in sleep latency or sleep efficiency in those with or without COPD. However, a higher proportion of persons with COPD had a low sleep efficiency (< 82%) than those without COPD (44% vs. 31%, p = 0.04). A recent study found a high prevalence of insomnia disorder (27.3%), defined as presence of insomnia symptoms along with daytime manifestations, in patients with COPD.4
Whether COPD severity is related to worse sleep is unclear. Some studies suggest worse sleep in more severe COPD,9 while other studies have not shown an association between FEV1 and reported sleep quality.4,10 Associated respiratory symptoms such as cough and sputum production appear to be better predictors of sleep disturbances.1,10,11 However, in one study, severity of dyspnea using the Medical Research Council dyspnea scale did not correlate with prevalence of insomnia.4 The authors hypothesized that nocturnal dyspnea may have a different etiology than diurnal dyspnea. While the latter may be related to exertion and inability to do tasks due to shortness of breath, several other factors, such as nocturnal hypoxemia and associated increased pulmonary vascular pressures, may contribute to nocturnal dyspnea.
Etiology
An insight into the etiology of insomnia in COPD may be vital in devising therapeutic strategies. Several factors may plausibly contribute to these sleep disturbances (Table 1).4 COPD can be associated with disabling dyspnea. Dyspnea may be worse supine and while in bed attempting to sleep (vide supra). Hypoxemia may contribute to nocturnal dyspnea and sleep disturbances. Indeed, oxygen use was found in one study to be associated with lower odds of insomnia.4 Minimum oxygen saturation was an independent predictor for a high score on a psychiatric sleep symptom scale in another study.6 However, data on the role of oxygen in improving sleep in COPD have been conflicting. While some studies demonstrate a salutary effect of supplemental oxygen,4,12,13 others do not.9,14 Nocturnal dyspnea may also be attributable to the asthma/bronchitic phenotype of obstructive lung disease. Medications used for COPD, especially β-agonists, have also been suggested to contribute to insomnia. However, a recent study did not show an adverse influence of any inhalers on sleep.4 In fact, univariate analyses revealed lower insomnia prevalence in patients on β-agonist inhalers, although the effects were not statistically significant in multivariate analyses. Inhaled steroids are commonly used in COPD, but their effect on sleep has not been systematically assessed. Smoking has been associated with sleep disturbances in several studies,4 sympathetic activation from nicotine being one possible culprit. However, acute nicotine withdrawal when asleep may also be responsible for disturbed sleep. Furthermore, restless legs syndrome (RLS) may be encountered more frequently in obstructive lung disorders than in healthy controls (vide infra) and may contribute to insomnia. RLS symptoms worsen during COPD exacerbations, further affecting sleep.15
Table 1.
Possible etiologies of insomnia in patients with COPD.

Psychiatric disorders such as depression and anxiety frequently accompany chronic medical disorders. Prevalence rates of up to 80% for depression and 74% for anxiety have been reported in COPD patients.16,17 In one study, over 20% of patients with COPD reported using an antidepressant.18 Depression in COPD is independently associated with lower quality of life.19 Anxiety and depression can precipitate or worsen insomnia. Furthermore, the association between psychiatric disorders and insomnia is likely bidirectional.3 Insomniacs have a significantly higher likelihood of reporting one or more psychiatric disorders compared with those with no sleep complaints.20
Hyperarousal appears to be a feature of primary insomnia.21,22 Some studies have shown increased sleeping heart rate in insomniacs.23,24 Insomniacs have higher ACTH and cortisol secretion,25 metabolic rate,26 and global cerebral glucose metabolism during sleep and awake27 compared with normal controls. Several factors in COPD may alter the sympathovagal balance with a resultant increase in sympathetic activity. Chronic hypoxia may contribute to sympathetic activation.28–31 Hypercapnia has been shown to increase sympathetic activity in some studies.32–34 COPD is associated with systemic inflammation35,36 and oxidative stress,37,38 which in turn, also augment sympathetic outflow.39–41 Impaired baroreflex responses, hyperinflation, elevated pulmonary artery pressures, dyspnea, physical inactivity, pronounced swings in intrathoracic pressure, and medications can all contribute to autonomic dys-function.42–44 Indeed, COPD patients have increased muscle sympathetic nerve activity, which decreases with short term oxygen supplementation.45 Plasma norepinephrine levels are elevated in hypoxemic patients with COPD, and levels decrease with long-term oxygen therapy.46 Patients with COPD also demonstrate depressed heart rate variability in association with systemic inflammation and lung function impairment.47,48 Use of noninvasive positive-pressure ventilation improves heart rate variability in acute COPD exacerbation.49 Furthermore, six weeks of therapy with tiotropium suppresses the exercise-induced increase in sympathetic activity in COPD patients.50 It is possible that the synergistic effects of sympathetic activation in COPD and insomnia may contribute to some of the adverse outcomes seen in persons with COPD comorbid with insomnia.
Impact
Insomnia is associated with a decrement in quality of life. Presence of COPD augurs a further deterioration in health-related quality of life.4 Self-reported sleep quality is also worse in COPD patients with insomnia compared to COPD patients without insomnia.4 COPD patients with insomnia, compared to those without insomnia, are more likely to suffer from daytime sleepiness.4 This may potentially lead to decreased productivity at work, absenteeism, and traffic accidents.
Insomnia is associated with a gamut of adverse outcomes. As a caveat, information regarding several of these outcomes comes from studies in the general population or patients seen in sleep clinics rather than specifically from patients with COPD. Odds of prevalent51 and incident52 hypertension are increased in insomnia with objective short sleep duration. Insomnia with < 6 hours nightly sleep duration is associated with increased odds of diabetes.53 Insomnia symptoms alone are also associated with high hemoglobin A1c levels.54 Insomnia with short sleep duration is also associated with neuropsychological deficits including slower processing speed and increased visual memory errors and omissions.55 Both insomnia and short sleep duration (≤ 5 hours) were independently associated with atherosclerosis risk, as determined by ultrasonographic measurements of carotid intima-media thickness, in a study of 86 elderly volunteers (age ≥ 65 years).56 Risk of acute myocardial infarction is increased in a dose-dependent manner in persons with symptoms of insomnia.57 The Penn State Cohort data showed four-fold increased odds of mortality in insomniacs who slept less than 6 hours compared to the those with no insomnia and normal sleep duration.58 Analyses of data from the Finnish Twin Cohort showed a significant association between self-reported poor sleep and risk of mortality, especially in those with somatic disease (presumably including COPD).59 It is possible that insomnia may contribute to increased incidence of these adverse outcomes in patients with COPD. Indeed, one study followed 98 adults with spirometrically confirmed COPD for a median of 2.4 years and found that insomnia symptoms at baseline predicted increased COPD exacerbations and worse survival during follow-up.10
Evaluation and Management
Insomnia is primarily a clinical diagnosis. Patients should be asked about the duration, frequency, and severity of their sleep symptoms.60 The course and precipitants of the symptoms, and relationship to the symptoms of the lung disorder (cough, sputum production, dyspnea) should be assessed. Inquiries should be made regarding daytime habits that might contribute to insomnia (e.g., nicotine use, alcohol, and caffeine intake), sleep hygiene and possible daytime consequences of sleep problems, including fatigue, sleepiness, and quality of life.61 Patients should also be asked about any other disorders that could contribute to insomnia.3 Physical exam should be targeted towards assessing comorbidities. Sleep logs can help provide relatively objective evidence of presence and course of sleep disturbance. Scales such as the Insomnia Severity Index can help quantify the severity of insomnia at baseline as well as provide objective evidence of improvement with therapies. Actigraphy is largely limited to the research arena, but may be used clinically if history is not clearly indicative of type or severity of sleep problems. Several interventions improve sleep quality in COPD patients. Optimal treatment of COPD to minimize symptoms such as cough, secretions, and dyspnea will likely lead to better sleep quality. Smoking cessation should be strongly encouraged. Organic sleep disorders including RLS and sleep disordered breathing (SDB) should be optimally treated. Oxygen may theoretically have several salutary effects on sleep in COPD (Table 2). Larger studies are needed to assess effects of long-term oxygen supplementation on sleep in these patients.
Table 2.
Potential beneficial effects of oxygen on sleep and breathing in COPD.

Cognitive behavioral therapy for insomnia (CBT-I) is an effective therapy in primary insomnia and appears to be superior to sedatives in the long term.62,63 CBT-I also appears to be beneficial in insomnia comorbid with cancer, human immunodeficiency virus infection, chronic pain, psychiatric disorders such as depression.63,64 A small study suggests feasibility and efficacy of performing CBT-I in COPD patients.65 In view of the potential adverse effects of pharmacotherapy in COPD, larger trials need to be conducted assessing CBT in COPD. Other interventions, such as stimulus control therapy alone, may also be beneficial.63
Therapy of attendant anxiety and depression may help improve sleep. One randomized, controlled trial reported significant improvements not only in depressive symptoms after CBT, but also improved sleep efficiency at 8-month follow-up in patients with COPD and depression.66
Despite concerns regarding their respiratory depressant effects, benzodiazepines have been assessed for treatment of insomnia in COPD.67 Medications may be required to improve sleep when nonpharmacologic measures prove inadequate. One week of temazepam 10 mg therapy in 14 patients with stable, severe, normocapnic COPD did not cause a significant increase in carbon dioxide tension during sleep or worsen dyspnea or sleepiness.68 However, decrease in minute ventilation, worsening of diaphragmatic endurance and decrease in oxygen saturations have been reported with traditional benzodiazepines, suggesting need for caution.67 Furthermore, tolerance, dependence, cognitive impairment, and abnormal sleep-related behaviors are concerns with both benzodiazepines and nonbenzodiazepine benzodiazepine receptor agonists.61
Melatonin can also improve sleep quality in COPD.69 Use of the MT(1)/MT(2) melatonin receptor agonist ramelteon 8 mg for one night in 25 subjects (≥ 40 years) with moderate to severe COPD resulted in a significant increase in total sleep and sleep efficiency without causing respiratory depression or worse hypoxemia.70 The likelihood of cognitive impairment and abuse liability is also lower than that with benzodiazepine receptor agonists. However, more clinical trials need to be done to assess the effects of ramelteon on diverse physiological and polysomnographic parameters in persons with COPD.
Doxepin is a histamine-1 receptor antagonist that has been shown to alleviate psychophysiological insomnia.71 The sleep promoting effect is seen primarily at low doses (3 mg or 6 mg), in contrast to the higher doses (10 mg or more) required for antidepressant action. However, efficacy of other antihistaminic agents in insomnia has not been demonstrated consistently.72 Furthermore, these agents can be limited by their anticholinergic adverse effects which include precipitating narrow angle glaucoma or urinary retention. Trazodone is commonly used for insomnia. However, its efficacy, especially in the long-term is not clear. Mirtazapine binds to 5-HT2A and 5-HT2C in addition to the H1 receptor and may have a role in promoting weight gain apart from its effects on sleep. Thus, it may have a potential role in a subset of COPD patients where both these benefits would be desirable. Nevertheless, it needs to be reiterated that these agents have not been systematically evaluated in COPD. Finally, it is plausible that antioxidants73 and anti-inflammatory agents,36 if proven effective, may improve sleep by improving the symptoms of COPD as well as decreasing sympathetic activity.
SLEEP-RELATED HYPOXEMIA
Epidemiology
Isolated hypoxemia (desaturation in absence of primary sleep disorders such as obstructive sleep apnea) during sleep is a common occurrence in patients with advanced COPD, and may occur despite adequate awake oxygenation. Indeed, significant nocturnal hypoxemia has been reported in up to 70% of COPD patients with daytime saturations between 90% and 95%.74,75 Daytime oxygen saturation, however, is highly predictive of nocturnal desaturation. Owing to the mechanisms detailed below, desaturations are more frequent and more pronounced during REM sleep.
Medicare criteria to qualify for nocturnal oxygen include an arterial PO2 ≤ 55 mm Hg or an arterial oxygen saturation ≤ 88%, for at least 5 minutes taken during sleep. A decrease in arterial PO2 > 10 mm Hg or a decrease in arterial oxygen saturation > 5% for at least 5 minutes during sleep, associated with symptoms or signs reasonably attributable to hypoxemia (e.g., cor pulmonale, “P” pulmonale on EKG, documented pulmonary hypertension, and erythrocytosis), can qualify for nocturnal oxygen therapy per Medicare guidelines as well. It should be mentioned that there can be a significant variation among physicians in the interpretation of nocturnal oximetry.76
Etiology
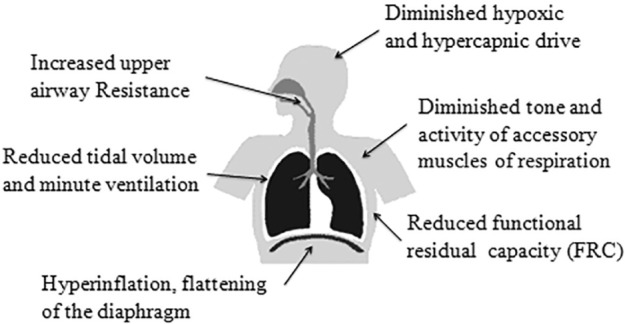
Several mechanisms contribute to a nocturnal decrease in oxygen levels in COPD (Figure 1). Alveolar hypoventilation leading to decreased minute ventilation may be the primary mechanism of nocturnal hypoxemia. Minute ventilation can drop approximately 16% during NREM sleep and 32% during REM sleep in patients with COPD.77 During wakefulness, respiration is not only under metabolic control, but also influenced by voluntary processes such as speaking and swallowing. During sleep, chemoreceptors and ventilatory centers become the sole controllers of respiration. Levels of PaO2, PaCO2, and pH influence the respiratory pattern. As a result, PaO2 can decrease by 3–10 mm Hg and PaCO2 can increase by 2–8 mm Hg. In persons with high oxygen reserve, this may portend only a slight drop in oxygen saturations. Limited oxygen reserves, however, as suggested by daytime saturations of 93% or below, may correspond to the steep portion of oxyhemoglobin dissociation curve (which describes the relationship between PO2 and oxygen saturations), whereby a slight drop in PaO2 culminates in pronounced oxygen desaturation. Hence, daytime oxygen saturation is among the strongest predictors of nocturnal desaturation in patients with COPD.78 In those with similar daytime oxygenation, COPD patients with daytime hypercapnia have worse nocturnal hypoxemia than those without daytime hypercapnia.79
Figure 1. Factors contributing to hypoxemia during sleep in patients with COPD.
Impact
Acute episodes of nocturnal desaturation can cause elevation in systemic systolic and mean pulmonary artery blood pressures.80 These repetitive and transient desaturations over time can lead to chronic pulmonary hypertension in patients with OSA.81 However, it is not clear if nocturnal hypoxemia in patients with COPD alone leads to development of right ventricular dysfunction or cor pulmonale. Cardiac arrhythmias have also been linked with nocturnal desaturations82 and may contribute to the higher than expected nocturnal death rate in COPD patients.83 Finally, nocturnal hypoxemia may be associated with arousals, and leads to sleep fragmentation.84 COPD patients with nocturnal hypoxemia have a lower survival rate than those without nocturnal hypoxemia, with oxygen therapy associated with a trend towards increased survival.85
Evaluation and Management
Patients with COPD with relatively low daytime saturation (< 93%) may be considered for overnight oximetry. However, PSG should be considered in those with symptoms suggestive of sleep disordered breathing (vide infra). A cyclical (sawtooth) pattern on overnight oximetry suggests sleep disordered breathing and may also merit a PSG.86
Supplemental oxygen is indicated in those who meet the Medicare criteria detailed above. In the landmark Nocturnal Oxygen Therapy Trial, continuous supplemental oxygen therapy was associated with lower mortality compared to only nocturnal therapy.87 Similarly, a Medical Research Council Trial from UK found improved mortality benefits of oxygen therapy used for 15 hours/day including sleep in comparison to no supplemental oxygen.88 However, the role of oxygen in symptomatic patients with COPD and moderate hypoxemia at rest and desaturation with activity is less clear. National Heart, Lung, and Blood Institute Long-term Oxygen Treatment Trial is expected to provide more information regarding the role of oxygen in this subset of COPD patients.
Oxygen therapy in COPD patients produces some decrease in mean pulmonary arterial pressure, even though it may not improve pulmonary hemodynamics significantly.89,90 Supplemental oxygen may help improve sleep quality in COPD patients with nocturnal hypoxemia.12 However, optimal treatment of obstructive lung disease with bronchodilators can also alleviate nocturnal hypoxemia and improve sleep quality.91–93 While oral steroids also improve total sleep time and oxygenation during sleep in stable COPD, numerous potential side effects including insomnia make this therapy undesirable.94 Lung volume reduction surgery decreases airflow obstruction, air-trapping, and hyperinflation, and improves sleep quality and nocturnal oxygenation.95
SLEEP HYPOVENTILATION
Epidemiology
As detailed above, some degree of hypoventilation and increase in PaCO2 from wake to sleep is physiologic. Sleep-related hypoventilation refers to a greater than normal increase in PaCO2 during sleep. It is defined as an increase in the PaCO2 to > 55 mm Hg for ≥ 10 minutes, or an increase in the PaCO2 by ≥ 10 mm Hg above the awake supine value to a value over 50 mm Hg for ≥ 10 minutes96 Due to difficulty in monitoring PaCO2 during sleep, data regarding sleep-related hypoventilation in COPD are limited. In one study of 54 stable hypercapnic COPD patients without concomitant sleep apnea or morbid obesity, 43% were found to have sleep hypoventilation.97 BMI, baseline PaCO2, and time spent in REM sleep were the strongest predictors of the severity of sleep hypoventilation. In contrast, another study of 23 COPD patients, most of whom did not have daytime hypercapnia, showed a mean increase in transcutaneous PCO2 during sleep of only 6 mmHg, similar to that in controls.98
AASM guidelines propose that end-tidal pCO2 (PETCO2) or transcutaneous PCO2 (tcPCO2) may be used as surrogates of arterial PaCO2 for diagnostic PSG and transcutaneous PCO2 for titration PSG. In a comparative study, however, neither PETCO2 nor tcPCO2 were a consistently accurate reflection of PaCO2.99 Another comparative study of anesthetized adult patients revealed that PETCO2 had a large negative bias and tcPCO2 has a small positive bias compared to PaCO2.100 Hence, there are limitations in using noninvasive monitoring of CO2 in diagnostic studies, especially in patients with COPD. Indeed, AASM guidelines advise using clinical judgment when assessing the accuracy of PETCO2 or tcPCO2 readings, especially when the values do not fit the clinical picture.99
Etiology
Sleep-related hypoventilation results from an exaggerated increase in PaCO2 from wake to sleep owing to mechanisms detailed above, including diminished ventilatory drive, increased upper airway resistance and mechanical disadvantages imposed by hyperinflation. Daytime hypercapnia, which can be seen in severe COPD as a result of significant decline in alveolar ventilation, is a strong predictor for sleep hypoventilation. A recurrent increase in nocturnal PaCO2 can plausibly lead to bicarbonate retention and blunting of the ventilatory responsiveness, which could in turn worsen daytime hypercapnia. Obesity causes loading of respiratory muscles and increased upper airway resistance, and is associated with blunted chemosensitivity. Hence, BMI is another predictor of sleep hypoventilation in several studies. Sleep apnea events, especially when frequent, can also contribute to the nocturnal increase in PaCO2.
Supplemental oxygen therapy, an integral therapy for patients with COPD and hypoxemia, itself can worsen hypoventilation. In one study of 80 clinically stable COPD patients with hypercapnic respiratory failure, 21% developed nocturnal hypoventilation after a night of supplemental oxygen therapy.101 BMI and daytime oxygenation were the best predictors for development of nocturnal hypoventilation. Similarly another study showed that use of an additional liter of oxygen over the daytime flow rate (as recommended by the American Thoracic Society/European Respiratory Society guidelines) in COPD patients with chronic hypercapnic respiratory failure improved nocturnal oxygenation, but was associated with greater hyper-capnia and acidosis the next morning.102
Impact
Hypercapnia in COPD patients is a poor prognostic indicator.103 It decreases myocardial and diaphragmatic contractility, increases pulmonary artery pressure and predisposes to arrhythmias. Sleep-related hypoventilation is associated with a reduced life expectancy.104 One study showed significantly greater improvement in sleep duration with oxygen plus nasal pressure support ventilation compared to oxygen alone, suggesting that hypoventilation may be a stronger determinant of sleep quality than nocturnal hypoxemia alone.105
Evaluation and Management
Morning headaches in patients with COPD may suggest nocturnal hypoventilation and CO2 retention. If nocturnal CO2 retention is suspected, CO2 monitoring should be considered along with the PSG. While serial PaCO2 determination, usually with an indwelling arterial catheter, is the gold standard for diagnosing sleep hypoventilation, it is invasive and is not practical outside of research studies. Surrogate measures of PaCO2, such as transcutaneous CO2 or end-tidal PCO2 monitoring can be used, but are not routinely performed during standard PSG. Moreover, the reliability and validity of these surrogates, especially in severe COPD, may be limited.
Medicare guidelines allow for nocturnal intermittent positive pressure ventilation (NIPPV) use in stable hypercapnic patients if daytime PaCO2 is ≥ 45 mm Hg and nocturnal oximetry reveals saturations ≤ 88% for at least 5 consecutive minutes not caused by obstructive upper airway events.106 Despite strong data favoring use of NIPPV in COPD patients with acute hypercapnia, studies assessing NIPPV use in chronic hypoventilation have shown conflicting results. A meta-analysis of 4 randomized controlled trials in hypercapnic patients with stable COPD did not find a consistent effect on sleep efficiency, lung function, gas exchange, respiratory muscle strength, or exercise tolerance. However, the small sample size of these studies precluded a definite conclusion regarding effect of NIPPV in COPD patients.107 In contrast, COPD patients treated with NIPPV along with oxygen compared to oxygen alone in one study demonstrated improved sleep quality and diminished sleep-related hypercarbia, albeit without significant effect on FEV1 or PaCO2.104 Additionally, NIPPV improved survival, but quality of life appeared to worsen in this study. More recently, controlled mode NIPPV was shown to improve diurnal PaCO2, vital capacity, and mean inspiratory pressure.108 Another study showed improvement in awake PaCO2 with use of nocturnal NIPPV in COPD.105
High intensity NIPPV (high pressure and high back-up rate) improves gas exchange and mortality in stable hypercapnic COPD patients.109 It has been proposed that the high pressure component of high intensity ventilation is actually responsible for these therapeutic improvements.110 If used, it should be ensured that respiratory support is sufficient to alleviate hypoventilation and hypercarbia. Unfortunately, tolerability and adherence may be an issue with higher pressures.
Average volume assured pressure support (AVAPS, Philips Respironics) and intelligent volume assured pressure support (iVAPS, ResMed) are newer hybrid modes that use proprietary algorithms to calculate the pressure support needed to achieve a target tidal volume or alveolar ventilation, respectively. iVAPS has been compared with high intensity NIPPV in a recent randomized crossover study.111 In stable chronic hypercapnic COPD patients, iVAPS showed a greater decrease in nocturnal hypercapnia and a trend towards more restful sleep at 6 weeks of treatment.
OBSTRUCTIVE SLEEP APNEA
Epidemiology
Obstructive sleep apnea (OSA) and chronic obstructive pulmonary (COPD) are both common pulmonary disorders. OSA may be present in ∼10% to 30% in persons with COPD, which is similar to its prevalence in the general population.112–114 Concurrence of OSA and COPD is termed “overlap syndrome” and occurs in approximately 1% of adults in the general population.115
Etiology
The coexistence of two common disorders, COPD and OSA, may likely just be a chance occurrence.115 However, several factors might actually predict a higher concurrence rate than merely by chance (Table 3). Some patients with severe COPD are on chronic oral steroids (or high dose of inhaled steroids), which may contribute to central obesity and fat deposition in neck, increasing the risk of OSA. Severe COPD may lead to elevated pulmonary pressures, right ventricular dysfunction and right heart failure (cor pulmonale). This may lead to edema in the pharyngeal soft tissues, predisposing to OSA. A decrease in exercise capacity may contribute to obesity, a prominent risk factor for OSA. COPD is also associated with generalized muscle weakness, which could portend higher upper airway collapsibility.
Table 3.
Plausible factors that may lead to a higher than chance concurrence of COPD and OSA.

Conversely, several mechanisms can be hypothesized whereby OSA could contribute to development and symptoms of COPD. OSA can lead to both local and systemic inflammation. Snoring related vibrations are postulated to cause soft tissue damage and local inflammation.116 OSA is associated with higher levels of inflammatory mediators such as interleukin 6 (IL-6), C reactive protein (CRP), intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin, and tumor necrosis factor alpha (TNF-α).117 Breath condensates of OSA patients have increased IL-6 and 8-isoprostane levels, suggesting bronchial airway inflammation.118 An overexpression of IL-8 in human bronchial epithelial cells has been demonstrated in response to a vibratory stimulus, similar to what may be seen in OSA.119 Inflammation can not only reduce the airway lumen, but it can potentially lead to alveolar wall destruction, which may be an important factor in development of COPD. Indeed, higher levels of IL-6, IL-8, CRP, and TNF-α are seen in patients with COPD.120 Furthermore, hypoxia from OSA can cause upregulation of xanthine oxidoreductase in pulmonary endothelial cells,121 similar to that produced by tobacco smoke,122 which could contribute to the pathogenesis of COPD. Additional factors mentioned in Table 3 may potentially lead to increased presence and/or severity of COPD in patients with OSA. OSA can worsen gastrointestinal reflux and PAP therapy can worsen nasal inflammation, both of which can worsen the asthmatic component of COPD and dyspnea.
Finally, several conditions contribute to both COPD and OSA and could lead to a higher than expected concurrence of the two. GERD may have a bidirectional relationship with OSA, and has also been shown to worsen the asthmatic component of COPD. Acid reflux into airways in turn can cause increased airway reactivity by either local inflammation or by enhancing vagal tone.123 It is plausible that nocturnal GERD may play a role in the development of obstructive lung disease and OSA symptoms. Indeed, a recent study showed worse respiratory and sleep apnea symptoms in those with GERD.7 Allergic rhinitis and nasal polyps may cause nasal obstruction and contribute to sleep-disordered breathing.124 Allergic rhinitis has been linked to asthma and chronic bronchitis as well,125 and could act as a potential link between COPD and OSA. Obesity is a major risk factor for OSA and has been associated with a higher incidence and severity of asthma. Asthma is more difficult to control with coexistent obesity and OSA.126 Weight reduction has been shown to improve severity of OSA and control of asthma.127 However, similar correlations between COPD and OSA are yet to be evaluated. Finally, smoking, the major risk factor for COPD, may also contribute to an increased prevalence and severity of OSA.128
Both OSA and COPD are associated with inflammatory cell activation and hypoxia.113 These may lead to endothelial dys-function, and consequently several adverse outcomes.113 However, data comparing endothelial dysfunction in COPD with comorbid OSA versus either alone are lacking.
Impact
COPD comorbid with OSA is associated with more pronounced hypoxemia and hypercapnia and adverse clinical outcomes compared with COPD or OSA alone.129 The concurrence of these two is associated with more cardiac dysrhythmias130 and portends more severe pulmonary hypertension and right heart failure.131 In one case series, pulmonary hypertension was observed in 86% of those with COPD comorbid with OSA (n = 17) compared to only 16% of patients with OSA but no COPD (n = 67).132 The incidence of right heart failure in comorbid COPD and OSA was 12% in another case series, and was associated with lower mean nocturnal oxygen saturation, lower awake PaO2, and higher PaCO2.133
COPD comorbid with OSA is associated with higher mortality compared to either disease alone.134 In a large study of 10,981 men, presence of COPD conferred a 7-fold increase in all-cause mortality in patients with OSA.135 A prospective study with median follow-up of 9.4 years also demonstrated higher mortality when untreated comorbid OSA was present than with COPD alone.136 The causes of death were primarily cardiovascular (28.1%), cancer (26%), and pulmonary (25.8%). The study also showed a higher prevalence of severe COPD exacerbation requiring hospitalization when OSA was present in COPD patients. However, in CPAP treated patients with COPD and OSA, the risk of mortality and severe exacerbations was similar to that observed in patients with COPD only. Furthermore, degree of positive airway pressure adherence appears to affect outcomes. In an observational large cohort of COPD comorbid with OSA patients, greater time on CPAP was associated with reduced mortality.137
Evaluation and Management
COPD patients with symptoms suggestive of OSA, such as snoring or witnessed apneas, should be evaluated by PSG. Additionally, in patients with COPD, presence of pulmonary hypertension out of proportion to the disease severity may indicate comorbid OSA, and PSG should be considered.138
While home sleep testing may be a cheaper and relatively more convenient way to diagnose sleep disordered breathing, it has not been validated in COPD and is not recommended by American Academy of Sleep Medicine.139 In-lab PSG is superior to in-home testing due to the ability to continuously monitor oxygen saturation and noninvasively monitor PCO2 either via PETCO2 or tcPCO2. For similar reasons, in-lab titration PSG may be better than auto-CPAP titration.
Continuous positive airway pressure (CPAP) therapy is the accepted standard for treatment of OSA. Apart from eliminating apneas, CPAP off-loads respiratory muscles and reduces work of breathing, which decreases hypoventilation and improves daytime oxygenation in patients with COPD.140 CPAP can also counteract auto-PEEP, and have a mild bronchodilator effect by decreasing chronic airway edema and hyperresponsiveness.140 Additionally, OSA may plausibly worsen COPD through diverse mechanisms (Table 3). Consequently, improvement in OSA with CPAP therapy may translate into a concomitant improvement in COPD.112 Indeed, apart from the expected improvement in sleep, spirometric parameters (FEV1, FVC) and gas exchange (PaO2, PaCO2) appear to improve with CPAP treatment of patients with OSA and COPD.141 CPAP treatment for OSA also reduces the number of COPD-related severe exacerbations and hospital admissions.136,142 Notably, oxygen alone is not an effective treatment for this condition. In a prospective cohort of 95 patients with moderate/ severe COPD (GOLD stage II-III) and moderate/severe OSA (AHI > 15), 5-year survival estimate was 71% in CPAP-treated patients compared to 26% in patients on long-term oxygen therapy alone.143 NIPPV has not been systematically evaluated in OSA comorbid with COPD. Bilevel PAP for nocturnal noninvasive ventilation is used for persistent hypoventilation leading to hypoxemia despite resolution of obstructive events with CPAP. Similarly, the specific role of oral devices is not clear in patients with coexistent COPD and OSA. Lifestyle modifications such as weight reduction and smoking cessation may have salutary effects on both these disorders, and should be strongly encouraged. Finally, treatment of underlying patho-physiology of obstructive lung disease may improve upper airway collapse. In a single-arm pilot study, upper airway collapsibility as measured by passive critical closing pressure (Pcrit) significantly improved after using orally inhaled fluticasone propionate for 16 weeks.144
CENTRAL SLEEP APNEA
There is a dearth of studies assessing prevalence of central sleep disordered breathing in patients with COPD. COPD is associated with several comorbidities or complications, which in turn can be associated with central sleep apnea or Cheyne-Stokes respiration. For example, patients with severe COPD can develop pulmonary hypertension and right ventricular dysfunction. One case series of 38 patients with pulmonary hypertension from different etiologies revealed Cheyne-Stokes respiration in 39% of the patients.145 Similarly, COPD is frequently associated with left ventricle diastolic dysfunction146 as well as systolic heart failure,147 both conditions known to be associated with Cheyne-Stokes respiration.
RESTLESS LEGS SYNDROME
Epidemiology
Restless legs syndrome (RLS) is a common sensorimotor disorder. It is characterized by the following four International Restless Legs Syndrome Study Group (IRLSSG) criteria: (1) An urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs, (2) The urge to move or unpleasant sensations that begin or worsen during periods of rest or inactivity such as lying or sitting, (3) The urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues, and (4) The urge to move or unpleasant sensations are worse in the evening or night than during the day or only occur in the evening or night.148 The urge to move is primarily reported in the legs, but arms and trunk may also be involved. Furthermore, in severe cases, symptoms may last all day, but a history of some worsening towards the end of the day may frequently be elicited.
RLS is present in 2% to 15% of the general population.149–151 It is more common in women and prevalence increases with age.149 A history of RLS in first-degree relatives is offered in majority of patients with RLS, and close to 80% of persons with RLS have periodic limb movements of sleep.152 However, whether these features are true for RLS comorbid with COPD is not known.
The prevalence of RLS is higher in persons with COPD than those without COPD.153 One study showed significantly higher odds of incidence of RLS in those with self-reported obstructive airway disease than those without obstructive airway disease (OR = 2.8).5
Etiology
While the etiology of RLS in COPD is yet to be clearly elucidated, hypoxemia and/or hypercapnia may contribute to the pathogenesis of RLS. Indeed, a higher prevalence of RLS has been reported in other pulmonary disorders including sarcoidosis and pulmonary hypertension. Hypoxia, through the hypoxia inducible factor-1 (HIF-1) pathway, may lead to an increase in tyrosine hydroxylase and vascular endothelial growth factor (VEGF). The former is a rate limiting enzyme in dopamine synthesis and is increased in RLS. VEGF expression is increased in the substantia nigra and in the anterior tibialis muscles of those suffering from RLS. Alterations in nigrostriatal and/or extrastriatal dopaminergic pathways may be seen in persons with RLS. Nicotine, the primary risk factor for COPD, exerts some effects through stimulation of dopaminergic pathways. Whether these are related, and could influence the association between COPD and RLS is unclear. Iron deficiency is likely causally related to RLS.154 Low ferritin in some COPD patients may be responsible for RLS. Similarly, comorbid renal failure may underlie RLS in some patients. Several medications including antidepressants and dopamine antagonists can worsen restless legs syndrome symptoms.155 Finally, some individuals may be genetically predisposed to develop RLS.156
Impact
RLS is associated with diminished quality of life.149,150,157 RLS patients usually have difficulty falling and staying asleep,158,159 and PSG demonstrates lower sleep efficiency and longer adjusted mean sleep latency and higher arousal index.160,161 The risk of depression, anxiety, and panic disorder is also increased in persons with RLS.162,163 RLS may also contribute to cardiovascular disease, although data assessing this association are conflicting.164,165
Evaluation and Management
Diagnosis of RLS is based on a typical history and does not need a PSG for confirmation. PSG may, however, be considered if history suggests sleep disordered breathing or another sleep disorder that would warrant this testing. It should be noted several conditions can mimic RLS and should be excluded prior to making this diagnosis.166 These include, but are not limited to, cramps, positional discomfort, arthritis, and neuropathy.
No specific studies have been conducted to assess the therapy of RLS specifically in COPD patients. Dopaminergic drugs should be the mainstay of RLS therapy in patients with COPD, as in those without this disorder.167 Levodopa is shorter acting (onset of action 10–15 minutes, half-life ∼ 1 hour) and has a higher risk of augmentation, especially at higher doses and with longer treatment duration. Hence it is not an optimal therapy for chronic use. It may, however, be used on as needed basis in case of infrequent symptoms. Pramipexole (half-life 8–12 hours) and ropinirole (half-life 5–6 hours) are effective for long-term treatment of RLS.168,169 Common side-effects of dopamine receptor agonists include nausea, dizziness, tiredness, headache, insomnia, and dry mouth.169 Excessive daytime sleepiness can occur at higher doses but is less common at the doses used for RLS. Pathological gambling and hypersexuality are other less commonly reported side effects.170 It has been suggested that dopamine agonists may potentially have salutary effects on symptoms such as cough and mucus secretion frequently seen in persons with advanced COPD.171 If borne out, this may be an additional benefit for persons with COPD and RLS. On the other hand, some studies suggest a suppressive effect of dopa-mine agonists on ventilatory responses to hypoxemia and hypercapnia via dopamine-mediated inhibition of carotid body chemoreceptors.172 This indicates a need for caution, and close follow-up after medication initiation, especially in persons with severe COPD with hypoxemia and/or hypercapnia.
A number of studies have shown that several other agents including alpha2-delta calcium channel ligands (e.g., gabapentin, pregabalin) are effective in the treatment of RLS.173 For example, gabapentin has been used in idiopathic RLS174 and RLS comorbid with renal failure,175 and may be a potentially useful drug in RLS comorbid with COPD. It can be especially useful when pain is the predominant manifestation of RLS. Opiates and benzodiazepines have been used to treat idiopathic RLS with varying efficacy.167 Opioids reduce dyspnea in patients with advanced COPD176 and may ameliorate comorbid RLS in such patients. However, possibility of respiratory depression may limit their use, especially in hypercarbic patients. Similarly, safety of benzodiazepines has not clearly been established in COPD.
A randomized, controlled trial showed efficacy of oral iron replacement in ameliorating symptoms in RLS patients with low-normal serum ferritin levels (15–75 ng/mL).177 Iron deficiency, if present, should be treated with an aim of keeping ferritin above 75 ng/mL.
Factors which may aggravate RLS symptoms should be avoided. These include use of nicotine, caffeine, and alcohol; and medications including selective serotonin reuptake inhibitors (SSRI) such as escitalopram and fluoxetine, other antidepressants including mianserin and mirtazapine, antipsychotics such as olanzapine, and L-thyroxine.155 Lifestyle modifications including exercise,178 sleep hygiene, massage, and warm water baths, may help ameliorate the symptoms.179 Other therapies including acupuncture,180 pneumatic compression devices,181 and near infrared light182 have been evaluated for RLS in small studies.
CONCLUSION
COPD is frequently associated with sleep-related abnormalities as well as primary sleep disorders. Presence of these comorbidities may worsen the already diminished quality of life in COPD patients and increase the odds of several other adverse health outcomes including higher mortality. A regular inquiry by health providers to COPD patients regarding sleep and potential sleep disorders, followed by management as warranted, may have the potential of ameliorating these risks and improving the quality of life and survival. While emerging data provide much needed information on the etiology, impact and management of sleep disorders in COPD, much still needs to be accomplished. Future studies should attempt to understand the specific role of diverse diagnostic techniques and pharmacologic and non-pharmacologic measures in diagnosis and treatment of insomnia, sleep disordered breathing and RLS in patients with COPD.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. Authors discuss potential treatments and therapies to be assessed in future, that may currently be considered off-label or investigational.
REFERENCES
- 1.Klink M, Quan SF. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest. 1987;91:540–6. doi: 10.1378/chest.91.4.540. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 3.Budhiraja R, Roth T, Hudgel DW, Budhiraja P, Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34:859–67. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budhiraja R, Parthasarathy S, Budhiraja P, Habib MP, Wendel C, Quan SF. Insomnia in patients with COPD. Sleep. 2012;35:369–75. doi: 10.5665/sleep.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budhiraja P, Budhiraja R, Goodwin JL, et al. Incidence of restless legs syndrome and its correlates. J Clin Sleep Med. 2012;8:119–24. doi: 10.5664/jcsm.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valipour A, Lavie P, Lothaller H, Mikulic I, Burghuber OC. Sleep profile and symptoms of sleep disorders in patients with stable mild to moderate chronic obstructive pulmonary disease. Sleep Med. 2011;12:367–72. doi: 10.1016/j.sleep.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Emilsson OI, Janson C, Benediktsdóttir B, Júlíusson S, Gíslason T. Nocturnal gastroesophageal reflux, lung function and symptoms of obstructive sleep apnea: results from an epidemiological survey. Respir Med. 2012;106:459–66. doi: 10.1016/j.rmed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Budhiraja R, Quan SF, Punjabi NM, Drake CL, Dickman R, Fass R. Power spectral analysis of the sleep electroencephalogram in heartburn patients with or without gastroesophageal reflux disease: a feasibility study. J Clin Gastroenterol. 2010;44:91–6. doi: 10.1097/MCG.0b013e3181a92a57. [DOI] [PubMed] [Google Scholar]
- 9.Fleetham J, West P, Mezon B, Conway W, Roth T, Kryger M. Sleep, arousals, and oxygen desaturation in chronic obstructive pulmonary disease. The effect of oxygen therapy. Am Rev Respir Dis. 1982;126:429–33. doi: 10.1164/arrd.1982.126.3.429. [DOI] [PubMed] [Google Scholar]
- 10.Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13:476–83. doi: 10.1016/j.sleep.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klink ME, Dodge R, Quan SF. The relation of sleep complaints to respiratory symptoms in a general population. Chest. 1994;105:151–4. doi: 10.1378/chest.105.1.151. [DOI] [PubMed] [Google Scholar]
- 12.Calverley PM, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis. 1982;126:206–10. doi: 10.1164/arrd.1982.126.2.206. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein RS, Ramcharan V, Bowes G, McNicholas WT, Bradley D, Phillipson EA. Effect of supplemental nocturnal oxygen on gas exchange in patients with severe obstructive lung disease. N Engl J Med. 1984;310:425–9. doi: 10.1056/NEJM198402163100704. [DOI] [PubMed] [Google Scholar]
- 14.McKeon JL, Murree-Allen K, Saunders NA. Supplemental oxygen and quality of sleep in patients with chronic obstructive lung disease. Thorax. 1989;44:184–8. doi: 10.1136/thx.44.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aras G, Kadakal F, Purisa S, Kanmaz D, Aynaci A, Isik E. Are we aware of restless legs syndrome in COPD patients who are in an exacerbation period? Frequency and probable factors related to underlying mechanism. COPD. 2011;8:437–43. doi: 10.3109/15412555.2011.623737. [DOI] [PubMed] [Google Scholar]
- 16.Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry. 2010;25:1209–21. doi: 10.1002/gps.2463. [DOI] [PubMed] [Google Scholar]
- 17.Fritzsche A, Clamor A, von Leupoldt A. Effects of medical and psychological treatment of depression in patients with COPD--a review. Respir Med. 2011;105:1422–33. doi: 10.1016/j.rmed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999-2008. BMC Pulm Med. 2012;12:26. doi: 10.1186/1471-2466-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundh J, Ställberg B, Lisspers K, Montgomery SM, Janson C. Co-morbidity, body mass index and quality of life in COPD using the Clinical COPD Questionnaire. COPD. 2011;8:173–81. doi: 10.3109/15412555.2011.560130. [DOI] [PubMed] [Google Scholar]
- 20.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 21.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60:610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Haynes SN, Adams A, Franzen M. The effects of presleep stress on sleep-onset insomnia. J Abnorm Psychol. 1981;90:601–6. doi: 10.1037//0021-843x.90.6.601. [DOI] [PubMed] [Google Scholar]
- 25.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet MH, Arand DL. 24-hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 27.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 28.Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol. 2000;121:173–84. doi: 10.1016/s0034-5687(00)00126-2. [DOI] [PubMed] [Google Scholar]
- 29.Calbet JA. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J Physiol. 2003;551(Pt 1):379–86. doi: 10.1113/jphysiol.2003.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy JC, Gray K, Whisler S, Leuenberger U. Sympathetic and blood pressure responses to voluntary apnea are augmented by hypoxemia. J Appl Physiol. 1994;77:2360–5. doi: 10.1152/jappl.1994.77.5.2360. [DOI] [PubMed] [Google Scholar]
- 31.Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2010;299:H925–31. doi: 10.1152/ajpheart.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y, Hayashida Y. Autonomic cardiovascular responses to hypercapnia in conscious rats: the roles of the chemo- and baroreceptors. Auton Neurosci. 2005;117:105–14. doi: 10.1016/j.autneu.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol. 1995;79:205–13. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- 34.Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–7. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holloway RA, Donnelly LE. Immunopathogenesis of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2013;19:95–102. doi: 10.1097/MCP.0b013e32835cfff5. [DOI] [PubMed] [Google Scholar]
- 36.Loukides S, Bartziokas K, Vestbo J, Singh D. Novel anti-inflammatory agents in COPD: targeting lung and systemic inflammation. Curr Drug Targets. 2013;14:235–45. doi: 10.2174/1389450111314020008. [DOI] [PubMed] [Google Scholar]
- 37.Sundar IK, Yao H, Rahman I. Oxidative stress and chromatin remodeling in chronic obstructive pulmonary disease and smoking-related diseases. Antioxid Redox Signal. 2013;18:1956–71. doi: 10.1089/ars.2012.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–55. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation. 2012;9:212. doi: 10.1186/1742-2094-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–70. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Jensen-Urstad M, Jensen-Urstad K, Ericson M, Johansson J. Heart rate variability is related to leucocyte count in men and to blood lipoproteins in women in a healthy population of 35-year-old subjects. J Intern Med. 1998;243:33–40. [PubMed] [Google Scholar]
- 42.van Gestel AJ, Steier J. Autonomic dysfunction in patients with chronic obstructive pulmonary disease (COPD) J Thorac Dis. 2010;2:215–22. doi: 10.3978/j.issn.2072-1439.2010.02.04.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gestel AJ, Kohler M, Clarenbach CF. Sympathetic overactivity and cardiovascular disease in patients with chronic obstructive pulmonary disease (COPD) Discov Med. 2012;14:359–68. [PubMed] [Google Scholar]
- 44.Andreas S, Anker SD, Scanlon PD, Somers VK. Neurohumoral activation as a link to systemic manifestations of chronic lung disease. Chest. 2005;128:3618–24. doi: 10.1378/chest.128.5.3618. [DOI] [PubMed] [Google Scholar]
- 45.Heindl S, Lehnert M, Criée CP, Hasenfuss G, Andreas S. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med. 2001;164:597–601. doi: 10.1164/ajrccm.164.4.2007085. [DOI] [PubMed] [Google Scholar]
- 46.Bratel T, Wennlund A, Carlström K. Impact of hypoxaemia on neuroendocrine function and catecholamine secretion in chronic obstructive pulmonary disease (COPD). Effects of long-term oxygen treatment. Respir Med. 2000;94:1221–8. doi: 10.1053/rmed.2000.0953. [DOI] [PubMed] [Google Scholar]
- 47.Corbo GM, Inchingolo R, Sgueglia GA, Lanza G, Valente S. C-reactive protein, lung hyperinflation and heart rate variability in chronic obstructive pulmonary disease: a pilot study. COPD. 2013;10:200–7. doi: 10.3109/15412555.2012.710667. [DOI] [PubMed] [Google Scholar]
- 48.van Gestel AJ, Kohler M, Steier J, et al. Cardiac autonomic function and cardiovascular response to exercise in patients with chronic obstructive pulmonary disease. COPD. 2012;9:160–5. doi: 10.3109/15412555.2011.647130. [DOI] [PubMed] [Google Scholar]
- 49.Skyba P, Joppa P, Orolín M, Tkácová R. Blood pressure and heart rate variability response to noninvasive ventilation in patients with exacerbations of chronic obstructive pulmonary disease. Physiol Res. 2007;56:527–33. doi: 10.33549/physiolres.931045. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura K, Maekura R, Hiraga T, et al. Effects of tiotropium on sympathetic activation during exercise in stable chronic obstructive pulmonary disease patients. Int J Chron Obstruct Pulmon Dis. 2012;7:109–17. doi: 10.2147/COPD.S28677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansson JK, Kronholm E, Jula AM. Variability in home-measured blood pressure and heart rate: associations with self-reported insomnia and sleep duration. J Hypertens. 2011;29:1897–905. doi: 10.1097/HJH.0b013e32834abccd. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–35. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kachi Y, Nakao M, Takeuchi T, Yano E. Association between insomnia symptoms and hemoglobin A1c level in Japanese men. PLoS One. 2011;6:e21420. doi: 10.1371/journal.pone.0021420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakazaki C, Noda A, Koike Y, Yamada S, Murohara T, Ozaki N. Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. Am J Hypertens. 2012;25:1149–55. doi: 10.1038/ajh.2012.107. [DOI] [PubMed] [Google Scholar]
- 57.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124:2073–81. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 58.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Heritability and mortality risk of insomnia-related symptoms: a genetic epidemiologic study in a population-based twin cohort. Sleep. 2011;34:957–64. doi: 10.5665/SLEEP.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 61.Doghramji K. The evaluation and management of insomnia. Clin Chest Med. 2010;31:327–39. doi: 10.1016/j.ccm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40. doi: 10.1186/1471-2296-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–9. [PubMed] [Google Scholar]
- 64.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–92. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Kapella MC, Herdegen JJ, Perlis ML, et al. Cognitive behavioral therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis. 2011;6:625–35. doi: 10.2147/COPD.S24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hynninen MJ, Bjerke N, Pallesen S, Bakke PS, Nordhus IH. A randomized controlled trial of cognitive behavioral therapy for anxiety and depression in COPD. Respir Med. 2010;104:986–94. doi: 10.1016/j.rmed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 67.Roth T. Hypnotic use for insomnia management in chronic obstructive pulmonary disease. Sleep Med. 2009;10:19–25. doi: 10.1016/j.sleep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Stege G, Heijdra YF, van den Elshout FJ, et al. Temazepam 10mg does not affect breathing and gas exchange in patients with severe normocapnic COPD. Respir Med. 2010;104:518–24. doi: 10.1016/j.rmed.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 69.Nunes DM, Mota RM, Machado MO, Pereira ED, Bruin VM, Bruin PF. Effect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary disease. Braz J Med Biol Res. 2008;41:926–31. doi: 10.1590/s0100-879x2008001000016. [DOI] [PubMed] [Google Scholar]
- 70.Kryger M, Roth T, Wang-Weigand S, Zhang J. The effects of ramelteon on respiration during sleep in subjects with moderate to severe chronic obstructive pulmonary disease. Sleep Breath. 2009;13:79–84. doi: 10.1007/s11325-008-0196-4. [DOI] [PubMed] [Google Scholar]
- 71.Roth T, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in adults with primary insomnia. Sleep. 2007;30:1555–61. doi: 10.1093/sleep/30.11.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vande Griend JP, Anderson SL. Histamine-1 receptor antagonism for treatment of insomnia. J Am Pharm Assoc (2003) 2012;52:e210–9. doi: 10.1331/JAPhA.2012.12051. [DOI] [PubMed] [Google Scholar]
- 73.Zheng JP, Wen FQ, Bai CX, et al. High-dose N-acetylcysteine in the prevention of COPD exacerbations: rationale and design of the PANTHEON study. COPD. 2013;10:164–71. doi: 10.3109/15412555.2012.732628. [DOI] [PubMed] [Google Scholar]
- 74.Lewis CA, Fergusson W, Eaton T, Zeng I, Kolbe J. Isolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleep. Thorax. 2009;64:133–8. doi: 10.1136/thx.2007.088930. [DOI] [PubMed] [Google Scholar]
- 75.Chaouat A, Weitzenblum E, Kessler R, et al. Sleep-related O2 desaturation and daytime pulmonary haemodynamics in COPD patients with mild hypoxaemia. Eur Respir J. 1997;10:1730–5. doi: 10.1183/09031936.97.10081730. [DOI] [PubMed] [Google Scholar]
- 76.Ramsey R, Mehra R, Strohl KP. Variations in physician interpretation of overnight pulse oximetry monitoring. Chest. 2007;132:852–9. doi: 10.1378/chest.07-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Becker HF, Piper AJ, Flynn WE, et al. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med. 1999;159:112–8. doi: 10.1164/ajrccm.159.1.9803037. [DOI] [PubMed] [Google Scholar]
- 78.Poongkunran C, Budhiraja R. Sleep board review question: nocturnal hypoxemia in COPD. Southwest J Pulm Crit Care. 2013:12–4. [Google Scholar]
- 79.Mulloy E, Fitzpatrick M, Bourke S, O'Regan A, McNicholas WT. Oxygen desaturation during sleep and exercise in patients with severe chronic obstructive pulmonary disease. Respir Med. 1995;89:193–8. doi: 10.1016/0954-6111(95)90247-3. [DOI] [PubMed] [Google Scholar]
- 80.Fletcher EC, Levin DC. Cardiopulmonary hemodynamics during sleep in subjects with chronic obstructive pulmonary disease. The effect of short- and long-term oxygen. Chest. 1984;85:6–14. doi: 10.1378/chest.85.1.6. [DOI] [PubMed] [Google Scholar]
- 81.Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis. 2009;51:363–70. doi: 10.1016/j.pcad.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Perin C, Fagondes SC, Casarotto FC, Pinotti AF, Menna Barreto SS, Dalcin PeT. Sleep findings and predictors of sleep desaturation in adult cystic fibrosis patients. Sleep Breath. 2012;16:1041–8. doi: 10.1007/s11325-011-0599-5. [DOI] [PubMed] [Google Scholar]
- 83.McNicholas WT, Fitzgerald MX. Nocturnal deaths among patients with chronic bronchitis and emphysema. BMJ (Clin Res Ed) 1984;289:878. doi: 10.1136/bmj.289.6449.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cormick W, Olson LG, Hensley MJ, Saunders NA. Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax. 1986;41:846–54. doi: 10.1136/thx.41.11.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fletcher EC, Donner CF, Midgren B, et al. Survival in COPD patients with a daytime PaO2 greater than 60 mm Hg with and without nocturnal oxyhemoglobin desaturation. Chest. 1992;101:649–55. doi: 10.1378/chest.101.3.649. [DOI] [PubMed] [Google Scholar]
- 86.Lacasse Y, Sériès F, Vujovic-Zotovic N, et al. Evaluating nocturnal oxygen desaturation in COPD--revised. Respir Med. 2011;105:1331–7. doi: 10.1016/j.rmed.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 88.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- 89.Nisbet M, Eaton T, Lewis C, Fergusson W, Kolbe J. Overnight prescription of oxygen in long term oxygen therapy: time to reconsider the guidelines? Thorax. 2006;61:779–82. doi: 10.1136/thx.2005.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fletcher EC, Luckett RA, Goodnight-White S, Miller CC, Qian W, Costarangos-Galarza C. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis. 1992;145:1070–6. doi: 10.1164/ajrccm/145.5.1070. [DOI] [PubMed] [Google Scholar]
- 91.Martin RJ, Bartelson BL, Smith P, et al. Effect of ipratropium bromide treatment on oxygen saturation and sleep quality in COPD. Chest. 1999;115:1338–45. doi: 10.1378/chest.115.5.1338. [DOI] [PubMed] [Google Scholar]
- 92.McNicholas WT, Calverley PM, Lee A, Edwards JC Investigators TSSiC. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J. 2004;23:825–31. doi: 10.1183/09031936.04.00085804. [DOI] [PubMed] [Google Scholar]
- 93.Ryan S, Doherty LS, Rock C, Nolan GM, McNicholas WT. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respiration. 2010;79:475–81. doi: 10.1159/000235619. [DOI] [PubMed] [Google Scholar]
- 94.Sposato B, Mariotta S, Palmiero G, Ricci A, Gencarelli G, Franco C. Oral corticosteroids can improve nocturnal isolated hypoxemia in stable COPD patients with diurnal PaO2 > 60 mmHg. Eur Rev Med Pharmacol Sci. 2007;11:365–72. [PubMed] [Google Scholar]
- 95.Krachman SL, Chatila W, Martin UJ, et al. Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysema. Chest. 2005;128:3221–8. doi: 10.1378/chest.128.5.3221. [DOI] [PubMed] [Google Scholar]
- 96.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Donoghue FJ, Catcheside PG, Ellis EE, et al. Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J. 2003;21:977–84. doi: 10.1183/09031936.03.00066802. [DOI] [PubMed] [Google Scholar]
- 98.Midgren B, Hansson L. Changes in transcutaneous PCO2 with sleep in normal subjects and in patients with chronic respiratory diseases. Eur J Respir Dis. 1987;71:388–94. [PubMed] [Google Scholar]
- 99.Sanders MH, Kern NB, Costantino JP, et al. Accuracy of end-tidal and transcutaneous PCO2 monitoring during sleep. Chest. 1994;106:472–83. doi: 10.1378/chest.106.2.472. [DOI] [PubMed] [Google Scholar]
- 100.Phan CQ, Tremper KK, Lee SE, Barker SJ. Noninvasive monitoring of carbon dioxide: a comparison of the partial pressure of transcutaneous and end-tidal carbon dioxide with the partial pressure of arterial carbon dioxide. J Clin Monit. 1987;3:149–54. doi: 10.1007/BF01695936. [DOI] [PubMed] [Google Scholar]
- 101.Tarrega J, Anton A, Guell R, et al. Predicting nocturnal hypoventilation in hypercapnic chronic obstructive pulmonary disease patients undergoing long-term oxygen therapy. Respiration. 2011;82:4–9. doi: 10.1159/000321372. [DOI] [PubMed] [Google Scholar]
- 102.Samolski D, Tárrega J, Antón A, et al. Sleep hypoventilation due to increased nocturnal oxygen flow in hypercapnic COPD patients. Respirology. 2010;15:283–8. doi: 10.1111/j.1440-1843.2009.01665.x. [DOI] [PubMed] [Google Scholar]
- 103.Cooper CB, Howard P. An analysis of sequential physiologic changes in hypoxic cor pulmonale during long-term oxygen therapy. Chest. 1991;100:76–80. doi: 10.1378/chest.100.1.76. [DOI] [PubMed] [Google Scholar]
- 104.McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64:561–6. doi: 10.1136/thx.2008.108274. [DOI] [PubMed] [Google Scholar]
- 105.Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med. 1995;152:538–44. doi: 10.1164/ajrccm.152.2.7633704. [DOI] [PubMed] [Google Scholar]
- 106.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation--a consensus conference report. Chest. 1999;116:521–34. doi: 10.1378/chest.116.2.521. [DOI] [PubMed] [Google Scholar]
- 107.Wijkstra PJ, Lacasse Y, Guyatt GH, Goldstein RS. Nocturnal non-invasive positive pressure ventilation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002:CD002878. doi: 10.1002/14651858.CD002878. [DOI] [PubMed] [Google Scholar]
- 108.Dellweg D, Schonhofer B, Haidl PM, et al. Short-term effect of controlled instead of assisted noninvasive ventilation in chronic respiratory failure due to chronic obstructive pulmonary disease. Respir Care. 2007;52:1734–40. [PubMed] [Google Scholar]
- 109.Windisch W, Haenel M, Storre JH, Dreher M. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int J Med Sci. 2009;6:72–6. doi: 10.7150/ijms.6.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murphy PB, Brignall K, Moxham J, Polkey MI, Davidson AC, Hart N. High pressure versus high intensity noninvasive ventilation in stable hypercapnic chronic obstructive pulmonary disease: a randomized crossover trial. Int J Chron Obstruct Pulmon Dis. 2012;7:811–8. doi: 10.2147/COPD.S36151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ekkernkamp E, Storre JH, Windisch W, Dreher M. Impact of intelligent volume-assured pressure support on sleep quality in stable hypercapnic chronic obstructive pulmonary disease patients: a randomized, crossover study. Respiration. 2014;88:270–6. doi: 10.1159/000364946. [DOI] [PubMed] [Google Scholar]
- 112.Zamarrón C, García Paz V, Morete E, del Campo Matías F. Association of chronic obstructive pulmonary disease and obstructive sleep apnea consequences. Int J Chron Obstruct Pulmon Dis. 2008;3:671–82. doi: 10.2147/copd.s4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med. 2009;180:692–700. doi: 10.1164/rccm.200903-0347PP. [DOI] [PubMed] [Google Scholar]
- 114.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration. 2005;72:142–9. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 115.Pronzato C. Chronic obstructive pulmonary disease and obstructive sleep apnea. Association, consequences and treatment. Monaldi Arch Chest Dis. 2010;73:155–61. doi: 10.4081/monaldi.2010.285. [DOI] [PubMed] [Google Scholar]
- 116.Teramoto S, Yamamoto H, Yamaguchi Y, Namba R, Ouchi Y. Obstructive sleep apnea causes systemic inflammation and metabolic syndrome. Chest. 2005;127:1074–5. doi: 10.1378/chest.127.3.1074. [DOI] [PubMed] [Google Scholar]
- 117.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3:409–15. [PMC free article] [PubMed] [Google Scholar]
- 118.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–7. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 119.Puig F, Rico F, Almendros I, Montserrat JM, Navajas D, Farre R. Vibration enhances interleukin-8 release in a cell model of snoring-induced airway inflammation. Sleep. 2005;28:1312–6. doi: 10.1093/sleep/28.10.1312. [DOI] [PubMed] [Google Scholar]
- 120.Kelly E, Owen CA, Pinto-Plata V, Celli BR. The role of systemic inflammatory biomarkers to predict mortality in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2013;7:57–64. doi: 10.1586/ers.12.82. [DOI] [PubMed] [Google Scholar]
- 121.Budhiraja R, Kayyali US, Karamsetty M, et al. Estrogen modulates xanthine dehydrogenase/xanthine oxidase activity by a receptor-independent mechanism. Antioxid Redox Signal. 2003;5:705–11. doi: 10.1089/152308603770380007. [DOI] [PubMed] [Google Scholar]
- 122.Kayyali US, Budhiraja R, Pennella CM, et al. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicol Appl Pharmacol. 2003;188:59–68. doi: 10.1016/s0041-008x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 123.Kasasbeh A, Kasasbeh E, Krishnaswamy G. Potential mechanisms connecting asthma, esophageal reflux, and obesity/sleep apnea complex--a hypothetical review. Sleep Med Rev. 2007;11:47–58. doi: 10.1016/j.smrv.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 124.Scharf MB, Cohen AP. Diagnostic and treatment implications of nasal obstruction in snoring and obstructive sleep apnea. Ann Allergy Asthma Immunol. 1998;81:279–87. doi: 10.1016/S1081-1206(10)63120-1. quiz 287–90. [DOI] [PubMed] [Google Scholar]
- 125.Larsson LG, Lindberg A, Franklin KA, Lundbäck B. Symptoms related to obstructive sleep apnoea are common in subjects with asthma, chronic bronchitis and rhinitis in a general population. Respir Med. 2001;95:423–9. doi: 10.1053/rmed.2001.1054. [DOI] [PubMed] [Google Scholar]
- 126.Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma. 2003;40:865–71. doi: 10.1081/jas-120023577. [DOI] [PubMed] [Google Scholar]
- 127.Simard B, Turcotte H, Marceau P, et al. Asthma and sleep apnea in patients with morbid obesity: outcome after bariatric surgery. Obes Surg. 2004;14:1381–8. doi: 10.1381/0960892042584021. [DOI] [PubMed] [Google Scholar]
- 128.Kim KS, Kim JH, Park SY, et al. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J Clin Sleep Med. 2012;8:367–74. doi: 10.5664/jcsm.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shteinberg M, Weiler-Ravel D, Adir Y. [The overlap syndrome: obstructive sleep apnea and chronic obstructive pulmonary disease] Harefuah. 2009;148:333–6. 348. [PubMed] [Google Scholar]
- 130.Shepard JW, Garrison MW, Grither DA, Evans R, Schweitzer PK. Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary disease. Am J Med. 1985;78:28–34. doi: 10.1016/0002-9343(85)90457-7. [DOI] [PubMed] [Google Scholar]
- 131.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151:82–6. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 132.Hawryłkiewicz I, Sliwiński P, Górecka D, Pływaczewski R, Zieliński J. Pulmonary haemodynamics in patients with OSAS or an overlap syndrome. Monaldi Arch Chest Dis. 2004;61:148–52. doi: 10.4081/monaldi.2004.693. [DOI] [PubMed] [Google Scholar]
- 133.Bradley TD, Rutherford R, Grossman RF, et al. Role of daytime hypoxemia in the pathogenesis of right heart failure in the obstructive sleep apnea syndrome. Am Rev Respir Dis. 1985;131:835–9. doi: 10.1164/arrd.1985.131.6.835. [DOI] [PubMed] [Google Scholar]
- 134.Chaouat A, Weitzenblum E, Krieger J, et al. Prognostic value of lung function and pulmonary haemodynamics in OSA patients treated with CPAP. Eur Respir J. 1999;13:1091–6. doi: 10.1034/j.1399-3003.1999.13e25.x. [DOI] [PubMed] [Google Scholar]
- 135.Lavie P, Herer P, Lavie L. Mortality risk factors in sleep apnoea: a matched case-control study. J Sleep Res. 2007;16:128–34. doi: 10.1111/j.1365-2869.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 136.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–31. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 137.Stanchina ML, Welicky LM, Donat W, Lee D, Corrao W, Malhotra A. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;9:767–72. doi: 10.5664/jcsm.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest. 1996;109:380–6. doi: 10.1378/chest.109.2.380. [DOI] [PubMed] [Google Scholar]
- 139.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 140.Mansfield D, Naughton MT. Effects of continuous positive airway pressure on lung function in patients with chronic obstructive pulmonary disease and sleep disordered breathing. Respirology. 1999;4:365–70. doi: 10.1046/j.1440-1843.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- 141.de Miguel J, Cabello J, Sánchez-Alarcos JM, Alvarez-Sala R, Espinós D, Alvarez-Sala JL. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath. 2002;6:3–10. doi: 10.1007/s11325-002-0003-6. [DOI] [PubMed] [Google Scholar]
- 142.Peker Y, Hedner J, Johansson A, Bende M. Reduced hospitalization with cardiovascular and pulmonary disease in obstructive sleep apnea patients on nasal CPAP treatment. Sleep. 1997;20:645–53. doi: 10.1093/sleep/20.8.645. [DOI] [PubMed] [Google Scholar]
- 143.Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. 2010;35:132–7. doi: 10.1183/09031936.00192008. [DOI] [PubMed] [Google Scholar]
- 144.Teodorescu M, Xie A, Sorkness CA, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med. 2014;10:183–93. doi: 10.5664/jcsm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ulrich S, Fischler M, Speich R, Bloch KE. Sleep-related breathing disorders in patients with pulmonary hypertension. Chest. 2008;133:1375–80. doi: 10.1378/chest.07-3035. [DOI] [PubMed] [Google Scholar]
- 146.López-Sánchez M, Muñoz-Esquerre M, Huertas D, et al. High prevalence of left ventricle diastolic dysfunction in severe COPD associated with a low exercise capacity: a cross-sectional study. PLoS One. 2013;8:e68034. doi: 10.1371/journal.pone.0068034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 148.Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 149.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 150.Allen RP, Stillman P, Myers AJ. Physician-diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med. 2010;11:31–7. doi: 10.1016/j.sleep.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 151.Nichols DA, Allen RP, Grauke JH, et al. Restless legs syndrome symptoms in primary care: a prevalence study. Arch Intern Med. 2003;163:2323–9. doi: 10.1001/archinte.163.19.2323. [DOI] [PubMed] [Google Scholar]
- 152.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lespérance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 153.Lo Coco D, Mattaliano A, Lo Coco A, Randisi B. Increased frequency of restless legs syndrome in chronic obstructive pulmonary disease patients. Sleep Med. 2009;10:572–6. doi: 10.1016/j.sleep.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 154.Sun ER, Chen CA, Ho G, Earley CJ, Allen RP. Iron and the restless legs syndrome. Sleep. 1998;21:371–7. [PubMed] [Google Scholar]
- 155.Hoque R, Chesson AL. Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6:79–83. [PMC free article] [PubMed] [Google Scholar]
- 156.Winkelmann J, Schormair B, Lichtner P, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–6. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 157.Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26:925–35. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 158.Ulfberg J, Bjorvatn B, Leissner L, et al. Comorbidity in restless legs syndrome among a sample of Swedish adults. Sleep Med. 2007;8:768–72. doi: 10.1016/j.sleep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 159.Phillips B, Hening W, Britz P, Mannino D. Prevalence and correlates of restless legs syndrome: results from the 2005 National Sleep Foundation Poll. Chest. 2006;129:76–80. doi: 10.1378/chest.129.1.76. [DOI] [PubMed] [Google Scholar]
- 160.Winkelman JW, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32:772–8. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Saletu B, Anderer P, Saletu M, Hauer C, Lindeck-Pozza L, Saletu-Zyhlarz G. EEG mapping, psychometric, and polysomnographic studies in restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) patients as compared with normal controls. Sleep Med. 2002;3(Suppl):S35–42. doi: 10.1016/s1389-9457(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 162.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7:545–52. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 163.Lee HB, Hening WA, Allen RP, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20:101–5. doi: 10.1176/jnp.2008.20.1.101. [DOI] [PubMed] [Google Scholar]
- 164.Koo BB, Blackwell T, Ancoli-Israel S, et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–31. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Budhiraja R, Quan SF. Is restless legs syndrome associated with cardiovascular disease? Am J Med. 2013;126:189–90. doi: 10.1016/j.amjmed.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 166.Hening WA, Allen RP, Washburn M, Lesage SR, Earley CJ. The four diagnostic criteria for Restless Legs Syndrome are unable to exclude confounding conditions (“mimics”) Sleep Med. 2009;10:976–81. doi: 10.1016/j.sleep.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23:2267–302. doi: 10.1002/mds.22254. [DOI] [PubMed] [Google Scholar]
- 168.Garcia-Borreguero D, Grunstein R, Sridhar G, et al. A 52-week open-label study of the long-term safety of ropinirole in patients with restless legs syndrome. Sleep Med. 2007;8:742–52. doi: 10.1016/j.sleep.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 169.Montplaisir J, Fantini ML, Desautels A, Michaud M, Petit D, Filipini D. Long-term treatment with pramipexole in restless legs syndrome. Eur J Neurol. 2006;13:1306–11. doi: 10.1111/j.1468-1331.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- 170.Driver-Dunckley ED, Noble BN, Hentz JG, et al. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol. 2007;30:249–55. doi: 10.1097/wnf.0b013e31804c780e. [DOI] [PubMed] [Google Scholar]
- 171.Birrell MA, Crispino N, Hele DJ, et al. Effect of dopamine receptor agonists on sensory nerve activity: possible therapeutic targets for the treatment of asthma and COPD. Br J Pharmacol. 2002;136:620–8. doi: 10.1038/sj.bjp.0704758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Olson LG, Hensley MJ, Saunders NA. Ventilatory responsiveness to hypercapnic hypoxia during dopamine infusion in humans. Am Rev Respir Dis. 1982;126:783–7. doi: 10.1164/arrd.1982.126.5.783. [DOI] [PubMed] [Google Scholar]
- 173.Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14:675–84. doi: 10.1016/j.sleep.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 174.Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59:1573–9. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 175.Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am J Kidney Dis. 2001;38:104–8. doi: 10.1053/ajkd.2001.25202. [DOI] [PubMed] [Google Scholar]
- 176.Marciniuk DD, Goodridge D, Hernandez P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18:69–78. doi: 10.1155/2011/745047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang J, O'Reilly B, Venkataraman R, Mysliwiec V, Mysliwiec A. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: a randomized, double-blind, placebo-controlled study. Sleep Med. 2009;10:973–5. doi: 10.1016/j.sleep.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 178.Aukerman MM, Aukerman D, Bayard M, Tudiver F, Thorp L, Bailey B. Exercise and restless legs syndrome: a randomized controlled trial. J Am Board Fam Med. 2006;19:487–93. doi: 10.3122/jabfm.19.5.487. [DOI] [PubMed] [Google Scholar]
- 179.Mitchell UH. Nondrug-related aspect of treating Ekbom disease, formerly known as restless legs syndrome. Neuropsychiatr Dis Treat. 2011;7:251–7. doi: 10.2147/NDT.S19177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cui Y, Wang Y, Liu Z. Acupuncture for restless legs syndrome. Cochrane Database Syst Rev. 2008:CD006457. doi: 10.1002/14651858.CD006457.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lettieri CJ, Eliasson AH. Pneumatic compression devices are an effective therapy for restless legs syndrome: a prospective, randomized, double-blinded, sham-controlled trial. Chest. 2009;135:74–80. doi: 10.1378/chest.08-1665. [DOI] [PubMed] [Google Scholar]
- 182.Mitchell UH, Myrer JW, Johnson AW, Hilton SC. Restless legs syndrome and near-infrared light: an alternative treatment option. Physiother Theory Pract. 2011;27:345–51. doi: 10.3109/09593985.2010.511440. [DOI] [PubMed] [Google Scholar]


