Abstract
The Board of Directors of the American Academy of Sleep Medicine (AASM) commissioned a Task Force to develop quality measures as part of its strategic plan to promote high quality patient-centered care. Among many potential dimensions of quality, the AASM requested Workgroups to develop outcome and process measures to aid in evaluating the quality of care of five common sleep disorders: insomnia, obstructive sleep apnea in adults, obstructive sleep apnea in children, restless legs syndrome, and narcolepsy. This paper describes the rationale, background, general methods development, and considerations in implementation of these quality measures in obstructive sleep apnea (OSA) in children. This document describes measurement methods for five desirable process measures: assessment of symptoms and risk factors of OSA, initiation of an evidence-based action plan, objective evaluation of high-risk children with OSA by obtaining a polysomnogram (PSG), reassessment of signs and symptoms of OSA within 12 months, and documentation of objective assessment of positive airway pressure adherence. When these five process measures are met, clinicians should be able to achieve the two defined outcomes: improve detection of childhood OSA and reduce signs and symptoms of OSA after initiation of a management plan. The AASM recommends the use of these measures as part of quality improvement programs that will enhance the ability to improve care for patients with childhood OSA.
Citation:
Kothare SV, Rosen CL, Lloyd RM, Paruthi S, Thomas SM, Troester MM, Carden KA. Quality measures for the care of pediatric patients with obstructive sleep apnea. J Clin Sleep Med 2015;11(3):385–404.
Obstructive sleep apnea (OSA) is a disorder of breathing during sleep in which episodic upper airway collapse disrupts ventilation and leads to oxyhemoglobin desaturation and poor sleep quality. It is a common condition in childhood, with a prevalence rate of 1% to 5%, which can result in a range of adverse health outcomes if left untreated.1,2 Childhood OSA is associated with neurocognitive impairment, behavioral problems, failure to thrive, hypertension, cardiac dysfunction, systemic inflammation, and increased health care costs. Risk factors include adenotonsillar hypertrophy, obesity, craniofacial anomalies, and neuromuscular disorders. Increases in pediatric obesity rates have markedly increased the risk of OSA in children. Symptoms include habitual snoring (often with intermittent pauses, snorts, or gasps), disturbed sleep, and daytime neurobehavioral problems. In contrast to OSA in adults, daytime sleepiness is not usually obvious in young children. Although there are many similarities, distinctions must be made between adult and pediatric (defined as birth to 18 years of age) OSA, especially in terms of key risk factors and treatment. In children, adenotonsillar hypertrophy is the most common cause for OSA and adenotonsillectomy (AT) is recommended as the first-line treatment.1,2
Since the pathophysiology and treatment of pediatric OSA is quite different from adult OSA, the AASM thought it would be important to develop separate quality measures to assess the processes and outcomes unique to children. This Pediatric OSA Quality Measures Workgroup was one of five Workgroups that the AASM commissioned to develop quality care measures aimed at optimizing care for patients suffering from the most common sleep-related disorders (the others being adult OSA, restless legs syndrome, narcolepsy, and insomnia).3 These quality care measures focus on both outcomes, that is, what happens to a patient as a result of the care received, and processes, or the steps taken by a healthcare provider in the care of an individual patient. Both outcomes and processes are important in the care of the patient. Outcomes are often more directly relevant to the patient, whereas processes tend to be less influenced by factors outside an individual provider's control. All of the pediatric OSA outcomes and processes detailed in this report were developed by the Pediatric OSA Quality Measures Workgroup and received final approval from the AASM Quality Measures Task Force and the AASM Board of Directors.
METHODS
Literature Review
As described in the parent paper,3 a comprehensive search was conducted to identify any publications which addressed sleep apnea in terms of quality care or measures. All searches were limited to articles published between 2002–2013, pertaining to humans, and in the English language. Publication types such as news, letters, editorials, and case reports were excluded. A total of 795 articles were retrieved for review using this search.
An additional search was conducted to identify clinical practice guidelines, measures, systematic reviews, meta-analyses, and consensus recommendations published by the AASM or other organizations or groups in the National Guidelines Clearinghouse, the National Quality Measures Clearinghouse, PubMed, EMBASE, PsycInfo, and the Cochrane Library pertaining to obstructive sleep apnea (and all associated MeSH terms). All searches were limited to articles published between 2002–2013, pertaining to humans and pediatric populations, and in the English language. Publication types other than the ones listed above were excluded. A total of 165 articles resulted from this additional search. The results of our literature search revealed articles that provided an empirical basis for the selection of outcome measures or that linked processes to outcomes to assure quality pediatric OSA care. The most pertinent available literature comprised the Childhood Adenotonsillectomy (CHAT) study, as well as statements and guidelines from the American Academy of Pediatrics (AAP), AASM, and American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS).1,2,4–7
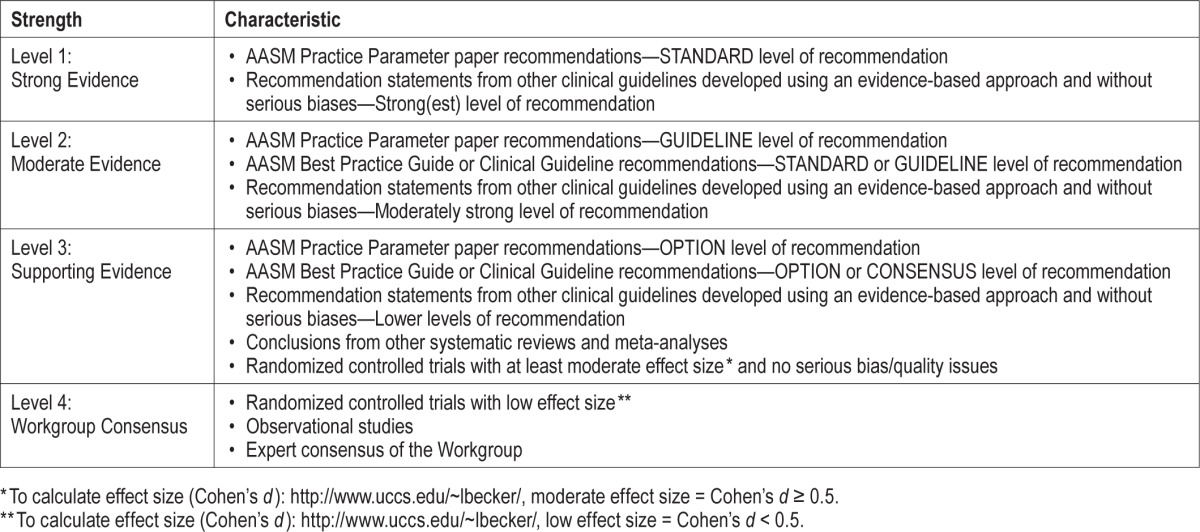
The titles and abstracts of all articles were reviewed by two Workgroup members for any relevant literature on childhood OSA. Any disagreements about whether to include a particular reference were resolved by a third Workgroup member. Full articles of publications thought to be relevant were obtained and reviewed in full to identify and provide support for the drafted quality measures. Two Workgroup members graded every article selected from the literature searches for the strength of association between the proposed process and the desired outcome. Differences were resolved by a third Workgroup member. For this, they used the grading scheme shown in Table 1.
Table 1.
Strength of association between process measure and desired outcome.
Measure Selection
Initial identification of candidate quality measures was guided by the published practice parameters and clinical guidelines identified in our literature search and the collective expertise and clinical experiences of the Workgroup members.1,2,4,5,7 Three groups of health care providers (pediatricians, otolaryngologists, and sleep medicine specialists) are closely involved in the evaluation and management of children with OSA. Each group has recently published evidence-based clinical practice guidelines1,2,5,7–9 that differ somewhat in their recommendations regarding the role of polysomnography (PSG) in the diagnostic evaluation of childhood OSA. Given these clinical practice variations, the Workgroup developed a harmonized range of quality measures to assess whether practitioners from various disciplines apply a consistent and evidence-based set of assessment and management strategies.
Initially, the Workgroup identified 4 candidate outcomes and 7 process measures that were considered pertinent to quality care of children with OSA. In attempts to develop these outcomes and delineate the process measures required to assure their achievement, the Workgroup realized that the initial outcomes selected were overly ambitious. Whereas the Workgroup believed it important that pediatric OSA patients first receive a comprehensive diagnostic assessment, there was difficulty agreeing upon how this would be defined. The Workgroup also realized that the various processes would be used differently depending on the clinical settings—for example, sleep specialty care versus primary care versus otolaryngology practice. Furthermore, the Workgroup recognized that many of the patient outcome measures had been operationalized only in clinical research settings. Thus, the Workgroup reduced the idealized outcomes down to two outcomes: (1) improve detection of childhood OSA, and (2) reduce signs and symptoms of OSA. Once the Workgroup settled on this decision, candidate process measures were identified that could be practically measured across multiple clinical venues and that would likely result in improved detection and reduced disease burden. These candidate process measures include: (1) Assessment of OSA symptoms and risk factors, (2) Initiation of an evidence-based action plan, (3) Objective assessment of OSA signs and symptoms in children with complex medical condition(s), (4) Reassessment of OSA signs and symptoms and (5) Objective assessment of positive airway pressure (PAP) therapy adherence.
As discussed earlier, children with OSA may present to various types of providers (e.g., primary care physicians; sleep specialists; and ear, nose and throat (ENT) surgeons), some of whom may or may not be specialists in sleep medicine. The Workgroup was challenged to identify process measures that were applicable to the different clinical venues where children with OSA are likely to be seen and to operationalize them so that they could be easily abstracted from patient health records as documented by key providers involved in pediatric OSA care. Symptom inventories, physical examinations, and management strategies for childhood OSA are not currently standardized across clinical practices. An evidence-based action plan may vary widely across disciplines from referral to a specialist by the primary care physician, to ordering a PSG by the sleep specialist, or performing surgery by the otolaryngologist, all of which are currently endorsed in the AAP clinical practice guideline for diagnosis and management of OSA in childhood.1 In some cases, the only “contact” with a sleep medicine specialist is the interpretation of the PSG ordered by the primary care physician and subsequently reviewed by the otolaryngologist. Many children with OSA may never have contact with a sleep medicine specialist if they are directly referred to an otolaryngologist for adenotonsillectomy. For otolaryngologists, a diagnostic PSG for OSA is recommended for cases involving children with complex medical conditions or for children with OSA symptoms where there is a discrepancy in the signs and symptoms.7
Given the variety of settings in which childhood OSA patients may be seen and the diverse set of professional disciplines that are involved in their management, we decided that the quality measures for childhood OSA needed to be more inclusive and widely applicable than those that might be required for some of the other sleep disorders. Hence, the childhood OSA measures offer choices and flexibility to clinicians so that they are acceptable and practical across diverse settings, but they still ensure that certain minimal practice standards are maintained.
As a result of our deliberations, the Workgroup developed the driver diagram shown in Figure 1. This diagram shows the two primary outcomes: (1) improve detection of childhood OSA and (2) reduce signs and symptoms of OSA. It also details how these outcomes are related to the process measures. The technical specifications associated with each of these quality measures can be found in the Appendix. These specifications outline how to calculate an individual provider's performance in meeting these measures using a combination of diagnostic and CPT codes and chart review.
Figure 1. Pediatric OSA quality measures driver diagram.
QUALITY MEASURES
Outcome 1 – Improve Detection of Childhood OSA
Description
Outcome 1, which is not a measured outcome, but rather a broad goal of care, is to improve detection of childhood OSA.
Supporting Evidence and Rationale
There are over 70 million children between the ages of 0 and 17 years in the United States.10 Between 10% and 30% (7.4 to 22 million children) habitually snore,11 and between 1% and 5% (743,000–3,715,000 children) have obstructive sleep apnea syndrome.1 Both habitual snoring and obstructive sleep apnea syndrome have been linked to neurodevelopmental and behavioral difficulties.1,12,13 Data suggest that sleep disorders, including habitual snoring and obstructive sleep apnea, are under-diagnosed.14 Because these sleep disorders are treatable when recognized, it is imperative to improve their detection and ultimately reduce the associated neurodevelopmental consequences.
Issues Addressed During Development
There was no debate that this outcome was of paramount importance to the AASM, the expert panel, and the involved stakeholders. The main discussion points centered on how the outcome might be measured in a practical sense. It became clear that in this particular instance, this outcome was more of a guiding principle toward which the other quality measures drive the provider. This outcome addresses the importance of screening for OSA in otherwise healthy children as well as children with complex medical problems who are at high risk for childhood OSA.
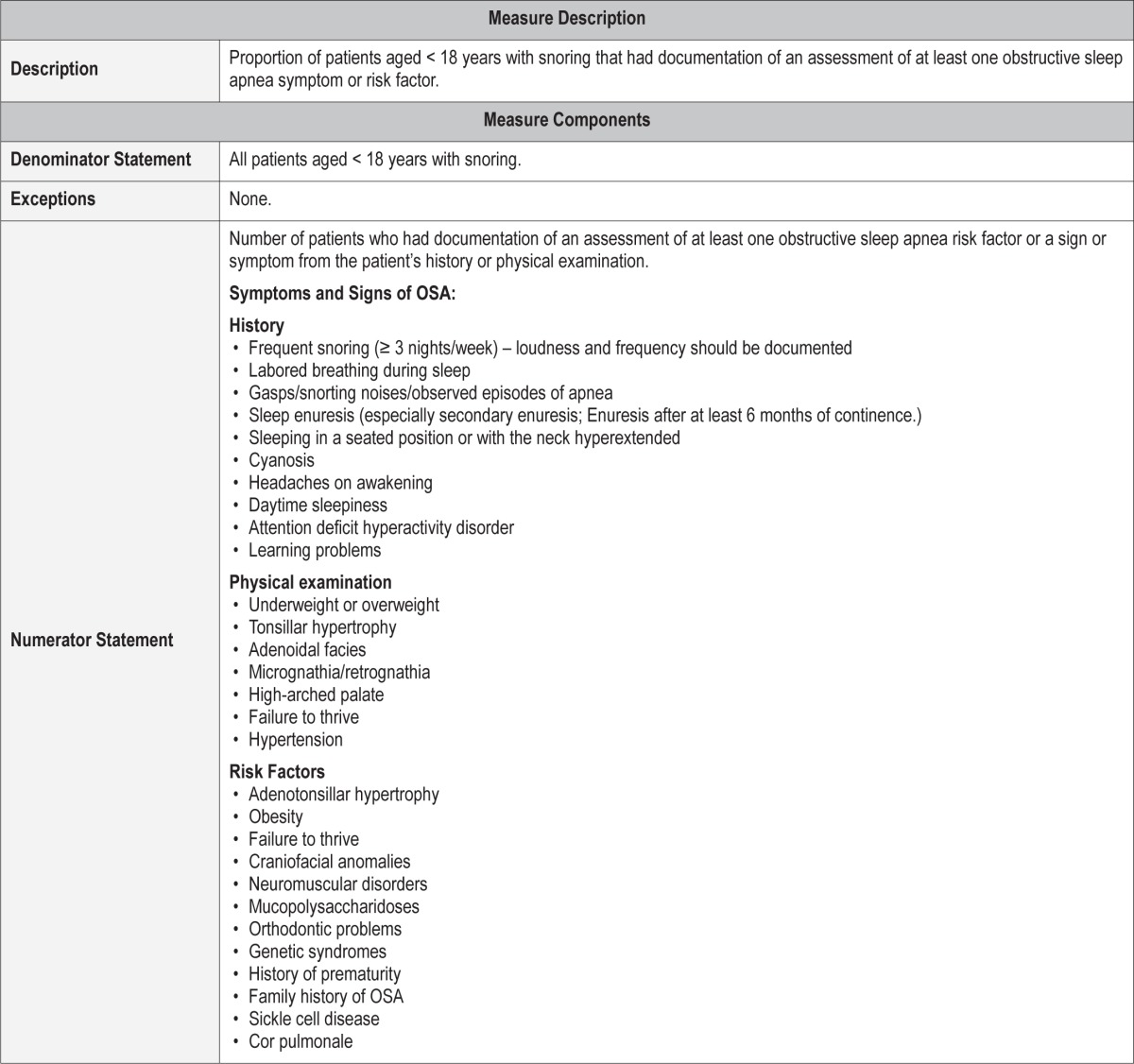
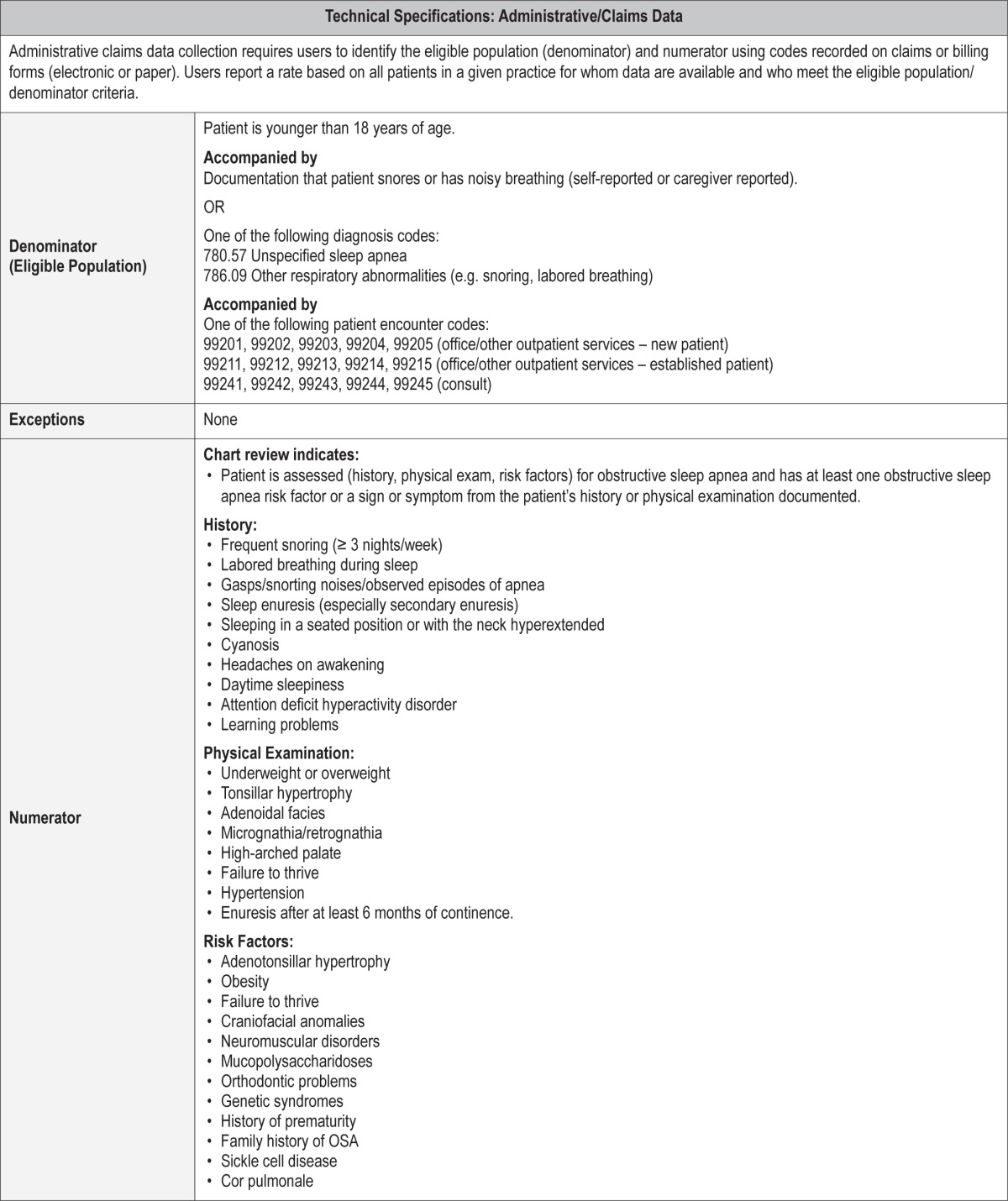
Process Measure 1 – Assessment of Obstructive Sleep Apnea Symptoms and Risk Factors
Description
Proportion of patients aged < 18 years with snoring who had documentation of an assessment of at least one obstructive sleep apnea symptom or risk factor.
Exceptions and Exception Justifications
There were no exceptions for this measure.
Supporting Evidence and Rationale
There are well-described signs and symptoms of pediatric OSA which, when assessed, will improve diagnosis and management of childhood obstructive sleep apnea (see Table 2).1,2 These clinical parameters focus on detection of uncomplicated OSA associated with adenotonsillar hypertrophy and/or obesity. Appropriate assessment should include history, physical exam, and screening for risk factors associated with pediatric OSA.1
Table 2.
Symptoms and signs of OSA.1,2

The AASM identified certain conditions associated with an elevated prevalence of pediatric OSA in the Practice Parameters for the Respiratory Indications for Polysomnography in Children.4 These include obesity, Down syndrome, Prader-Willi syndrome, neuromuscular disorders, Chiari malformations and myelomeningocele, and craniofacial anomalies that obstruct the upper airway (including achondroplasia and Pierre Robin sequence), history of prematurity, allergic rhinitis, African American race, and family history of OSA. Furthermore, OSA was identified as an independent risk factor for hypertension.4
Relationship to Desired Outcome
Multiple studies cite risk factors for pediatric OSA including anatomic crowding from adenotonsillar hypertrophy, craniofacial features, obesity, genetic syndromes, neuromuscular weakness, prematurity, and ethnic variations. Well-documented neurobehavioral consequences and a growing body of cardiometabolic consequences are associated with pediatric OSA. Screening for symptoms, signs, and risk factors of OSA may help improve the accuracy of a comprehensive diagnosis.
Opportunities for Improvement/Gaps
Pediatric OSA is underdiagnosed. It is associated with a wide range of airway anomalies namely related to crowding of the oropharynx. Assessing for symptoms, signs, and risk factors of OSA may lead to improved identification and outcomes of OSA.
Issues Addressed During Development
There was no disagreement about the need to assess basic symptoms and risk factors when assessing a child for suspected OSA.
Stakeholders reviewing the process measure were generally in agreement as well, though some expressed concerns about collecting and measuring data. Most recognized that this is part of a basic assessment of pediatric OSA and should be included as part of the health record.
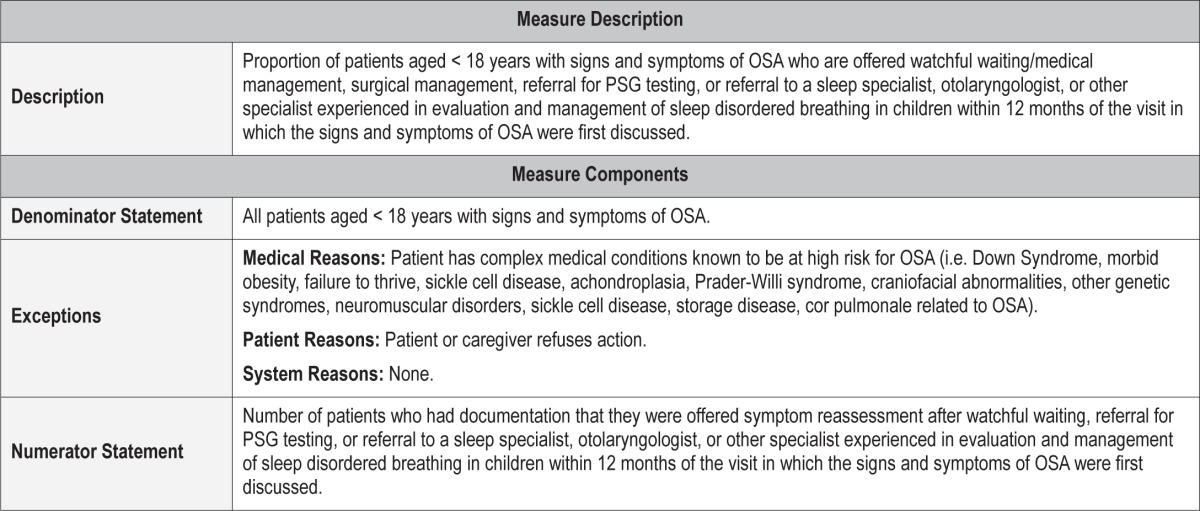
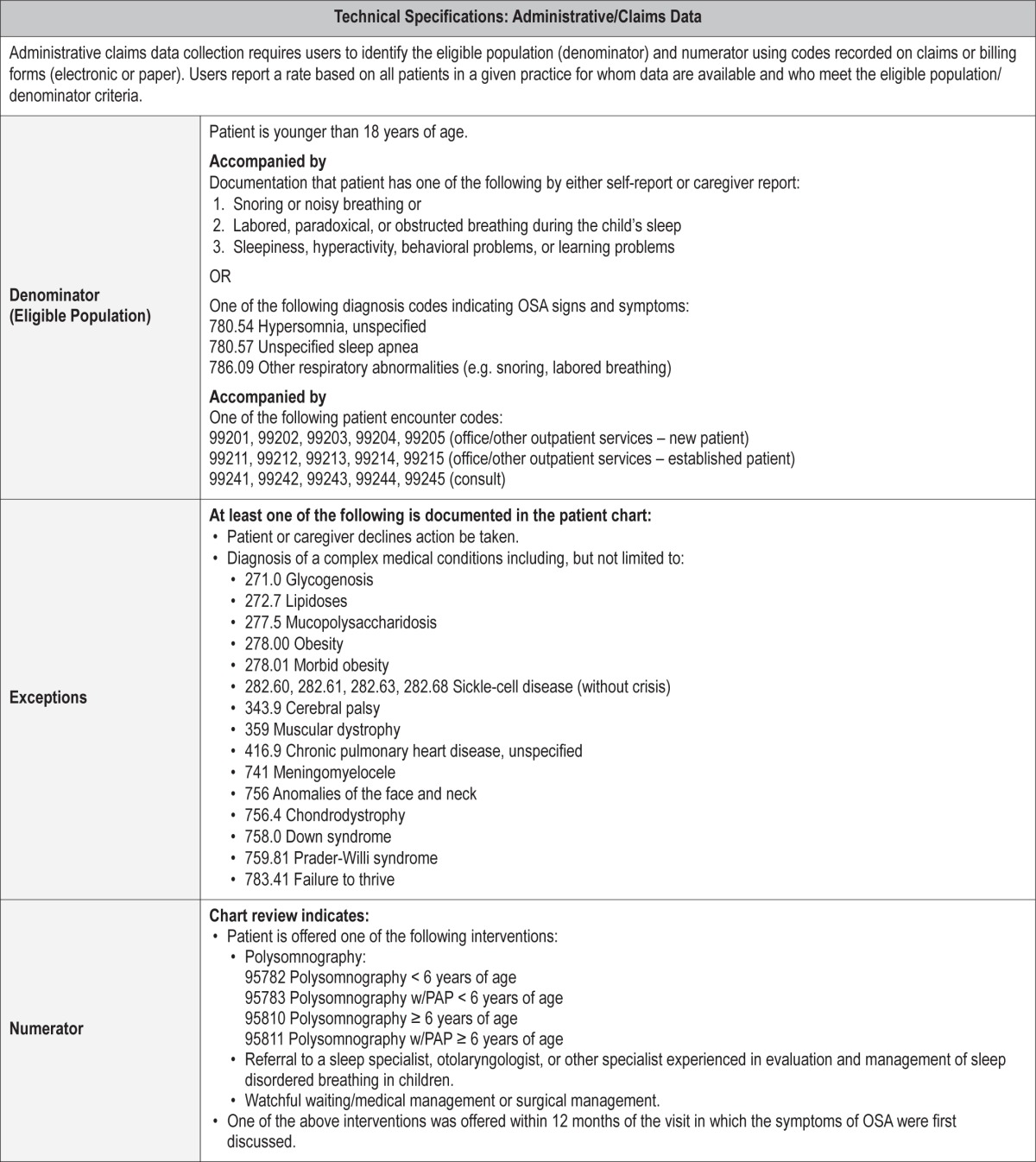
Process Measure 2 – Initiation of an Evidence-Based Action Plan
Description
Proportion of patients aged < 18 years with signs and symptoms of OSA that are offered watchful waiting/medical management, surgical management, referral for PSG testing, or referral to a sleep specialist, otolaryngologist, or other specialist experienced in evaluation and management of sleep disordered breathing in children within 12 months of the visit in which the signs and symptoms of OSA were first discussed.
Exceptions and Exception Justifications
Medical Reasons: Patient has a complex medical condition known to be at high-risk for OSA (i.e., Down Syndrome, morbid obesity, failure to thrive, sickle cell disease, achondroplasia, Prader-Willi syndrome, craniofacial abnormalities, other genetic syndromes, neuromuscular disorders, sickle cell disease, storage diseases, cor pulmonale related to OSA).
These complex patients with OSA would require a sleep study first (see process measure 3), followed by implementation of an action plan.
Patient Reasons: Patient or caregiver declines action be taken.
System Reasons: None.
Supporting Evidence and Rationale
If a child or adolescent snores on a regular basis and has any of the signs, symptoms or physical findings of OSA, clinicians should either (1) obtain a polysomnogram [Level 1] or (2) refer the patient to a sleep specialist or otolaryngologist for a more extensive evaluation.1 [Level 3]
If a child is determined to have OSA, has a clinical examination consistent with adenotonsillar hypertrophy, and does not have a contraindication to surgery, the clinician should recommend an evaluation by an otolaryngologist for surgical management, and adenotonsillectomy should be considered as a treatment option. If the child has OSA but does not have adenotonsillar hypertrophy, other treatments should be considered. Clinical judgment is required to determine the benefits of adenotonsillectomy compared with other treatment in obese children with varying degrees of adenotonsillar hypertrophy.1 [Level 2]
Clinicians should recommend weight loss in addition to other therapy if a child/adolescent with OSA is overweight or obese.1 [Level 3]
If the child is referred for testing, the preferred test is overnight in-lab polysomnography, which typically includes sensors for nasal and oral airflow, abdominal and chest wall movements, capnography, oximetry, continuous electroencephalography (six or more leads), electrooculography, electromyography, electrocardiography, and snore microphone. Esophageal pressure monitoring may be offered in some sleep centers.
There also exist a few alternative sleep tests such as ambulatory PSG, nap PSG, oximetry, and audio/video recording from home, which have been most extensively studied in the research setting with some limited clinical testing. Most of these alternative tests have a low negative predictive value, indicating that a negative result is insufficient to exclude OSA. These tests are not currently used in routine practice and are not the standard of care in the evaluation of pediatric sleep disordered breathing.
Relationship to Desired Outcome
Snoring and other signs and symptoms of OSA may be abnormal. It is critical for all children who snore to have an evidence-based action plan developed by the clinician during the visit in which snoring was discussed. This evaluation plan may include testing, referral to a specialist for additional evaluation/treatment, initiation of treatment, or watchful waiting with planned symptom reassessment within 12 months. Additionally, if a child with snoring presents directly to a specialist, the specialist may proceed to testing or offer surgical or nonsurgical treatment options utilizing an evidence-based action plan.
Opportunities for Improvement/Gaps
Not all primary care or specialty clinicians receive adequate training in sleep medicine, leading to varying detection, evaluation and treatment for the signs and symptoms of OSA. This process measure provides the opportunity for the provider to discuss the multiple treatment options available with the child and family. This measure helps to make the provider more aware of various surgical and noninvasive evaluations and treatment options, so the next step can be initiated (after assessment of obstructive sleep apnea symptoms and risk factors) during the visit in which signs and symptoms of obstructive sleep apnea were discussed.
Issues Addressed During Development
Several issues were discussed during development. Initial drafts of the process measures included separate measures for implementation of an evaluation plan and for education on the management and treatment options. The measures were initially separate, as implementation of an evaluation plan would lead to an increase in outcome #1 to improve the detection of childhood OSA, and education on the management and treatment options would lead to an increase in outcome #2 to improve the signs and symptoms of OSA. However, after review of stakeholder feedback and extensive Workgroup discussion, it was determined that the focus should be shifted to assess whether the clinician evaluated and initiated an evidence-based action plan. This allows each individual clinician to provide care that is compatible with the major guidelines regarding sleep disordered breathing. For example, a pediatrician or family physician who identifies a child with snoring may develop a plan with the parent or caregiver that includes watchful waiting, testing, or referral to a specialist. On the other hand, if a child with snoring presents to an otolaryngologist, then the otolaryngologist may develop a plan to proceed to adenotonsillectomy, in line with the AAO-HNS guidelines. Additionally, a sleep specialist's plan may focus on medical management options including PAP therapy. With the broadening of Process #2 to include initiation of an evidence-based action plan, both outcome measures will improve.
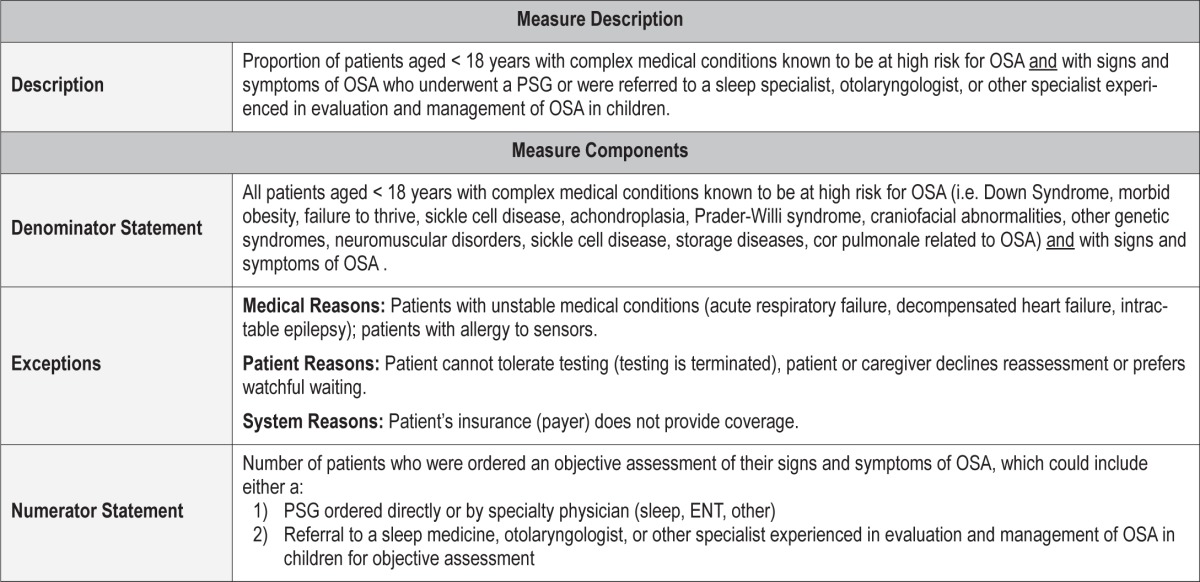
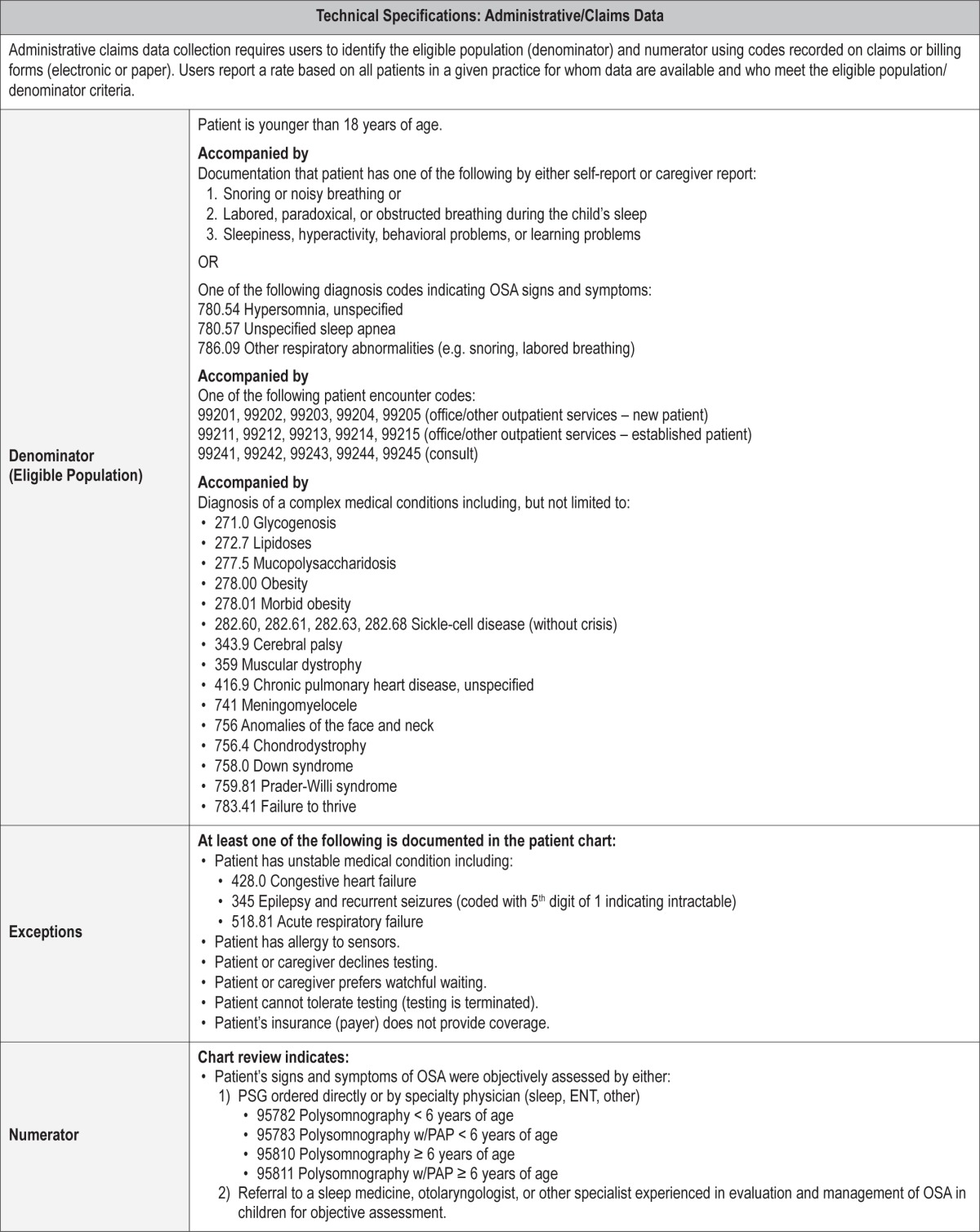
Process Measure 3 – Objective Assessment of OSA Signs and Symptoms in Children with Complex Medical Conditions
Description
Proportion of patients aged < 18 years with complex medical conditions known to be at high risk for OSA AND with signs and symptoms of OSA who underwent a PSG or were referred to a sleep specialist, otolaryngologist, or other specialist experienced in the evaluation and management of OSA in children.
Exceptions and Exception Justifications
Medical Reasons: Patient has an unstable medical condition (acute respiratory failure, decompensated heart failure, intractable epilepsy), patient has an allergy to sensors or materials used during PSG.
Patient Reasons: Patient cannot tolerate or cooperate with testing (testing is terminated), patient or caregiver declines or prefers watchful waiting.
System Reasons: Patient's insurance (payer) does not provide coverage.
Polysomnography (PSG) may not be performed if the patient is medically unstable and cannot safely undergo the PSG. PSG may not be successful in patients with limited ability to cooperate or when caregiver support/cooperation is not available. In these cases, more limited channel studies may provide sufficient estimates of the presence and severity of suspected OSA. Alternative evaluation can include assessment by a specialist experienced in evaluation and management of sleep disordered breathing in children.
Supporting Evidence and Rationale
While there is incomplete consensus among various medical societies as to whether to recommend PSG as part of the diagnostic process for OSA in otherwise healthy children, there is agreement about its role in the evaluation of children with complex medical conditions known to be at high risk for OSA. Familiar examples include obesity, Down syndrome, Prader-Willi syndrome, craniofacial abnormalities, achondroplasia, neuromuscular disorders, sickle cell disease, and mucopolysaccharidoses.1–5 Considered together, the evidence bases for these various medical society practice parameters and guidelines, in addition to several Level 4 studies that support that children with these specific conditions are at high risk for OSA or SDB15–18 provide a strong rationale for using PSG to assess signs and symptoms of OSA in high-risk children.
Relationship to Desired Outcome
The purpose of this measure is to improve quality of care and assist with the development of clinical treatment plans in children who (1) have complex medical conditions associated with a higher prevalence of OSA, (2) have signs and symptoms of OSA, and (3) are at increased risk for surgical or anesthetic complications because of their complex medical conditions and increased risk for residual OSA needing additional therapies.
Opportunities for Improvement/Gaps
In children who are at high risk of peri/postoperative respiratory compromise due to complex medical conditions, pre-operative PSG helps determine level of care and the need for peri/postoperative monitoring. Postoperative reassessment of residual OSA in patients at high risk will identify children in need of additional therapy for OSA.
Issues Addressed During Development
The pediatric OSA Workgroup concluded that this process measure was universally supported in the clinical practice guidelines of three major professional organizations. A potential limitation in implementation was that some children with complex medical conditions may have challenging behaviors or limited ability to cooperate with the PSG procedure. This problem was resolved by identifying specific exclusions and exceptions for this measure and by offering alternatives to PSG including evaluation by a medical specialist.
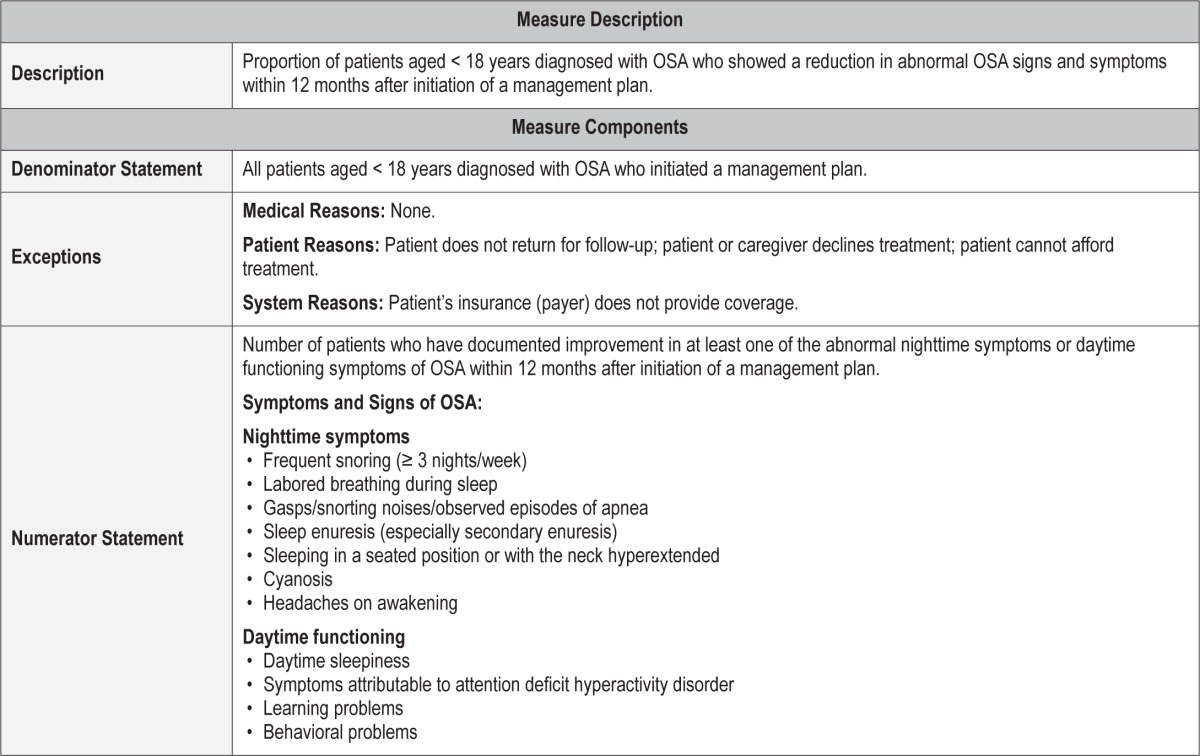
Outcome Measure 2 – Reduce Signs or Symptoms of OSA
Description
Proportion of patients aged < 18 years diagnosed with OSA who showed a reduction in OSA signs or symptoms within 12 months after initiation of a management plan
Exceptions and Exception Justifications
Medical Reasons: None.
Patient Reasons: Patient does not return for follow-up; patient or caregiver declines treatment; patient cannot afford treatment.
System Reasons: Patient's insurance (payer) does not provide coverage.
Supporting Evidence and Rationale
The proportion of patients who had residual OSA after tonsillectomy and adenoidectomy (AT) ranged from 13% to 29% in low risk populations to 73% when obese children were included and stricter PSG criteria were used.1,2 [Level 1] As compared with a strategy of watchful waiting, surgical treatment for the obstructive sleep apnea in school-age children did not significantly improve attention or executive function as measured by neuropsychological testing but did reduce OSA symptoms and improve secondary outcomes of behavior, quality of life, and PSG findings, thus providing evidence of beneficial effects of early adenotonsillectomy.19 [Level 3] CPAP is effective in the treatment of OSA, but adherence is a major barrier.1,2 [Level 1] Intranasal corticosteroids are an option for children with mild OSA in whom AT is contraindicated or for mild residual OSA after AT.1,2 [Level 1] Weight loss is recommended in addition to other therapies in patients who are overweight or obese.1,2 [Level 1] Follow-up sleep studies are recommended in high-risk patients and patients with comorbidities to assess residual OSA.1,2,4,8 [Level 1]
Hence, it is important to assess outcomes after initiating various types of treatment options for OSA, so as to implement alternate treatment plans in case of partial or incomplete response to treatment. This is important because of the effect of inadequate treatment of OSA on neurocognitive performance, daytime functioning and behavior, and overall quality of life.19
Opportunities for Improvement/Gaps
Every effort must be made by the treating physician, whether it is the primary care, ENT or sleep physician, to assess for improvement in signs and symptoms of OSA after treatment. In the future, better documentation of improvement in daytime functioning and school performance could also be imported into the electronic medical record (EMR) for meaningful assessment of outcomes due to treatment of the OSA.
Issues Addressed During Development
The outcome measures needed to be clinically relevant, meaningful to the patient, easily assessed by the clinician, and easily extracted from chart review. Additionally, several outcome measurement tools are only validated in research form. Lastly, given the many specialty physicians who provide care to children with sleep disordered breathing, each may have limited knowledge of or time to implement several sleep-specific research tools into a busy practice. Thus, this outcome measure is straightforward and part of the reassessment of any evidence-based action plan.
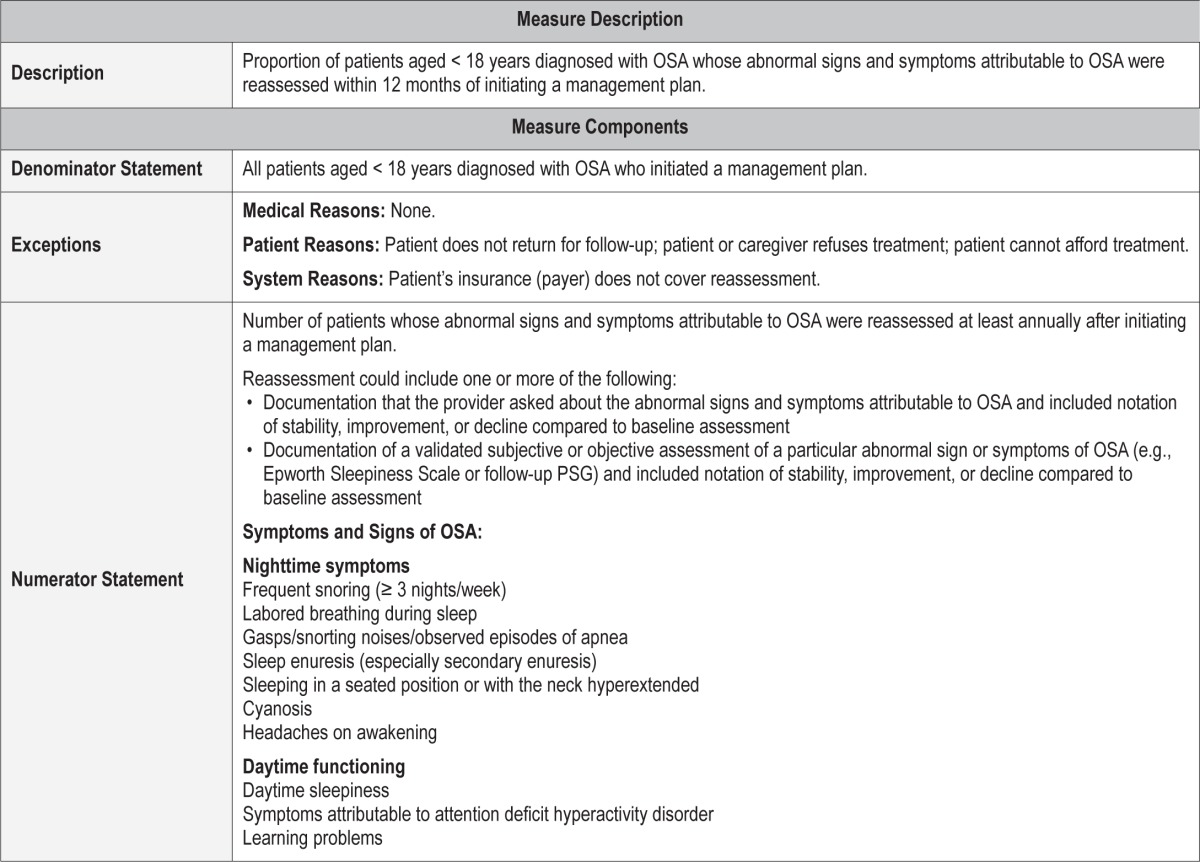
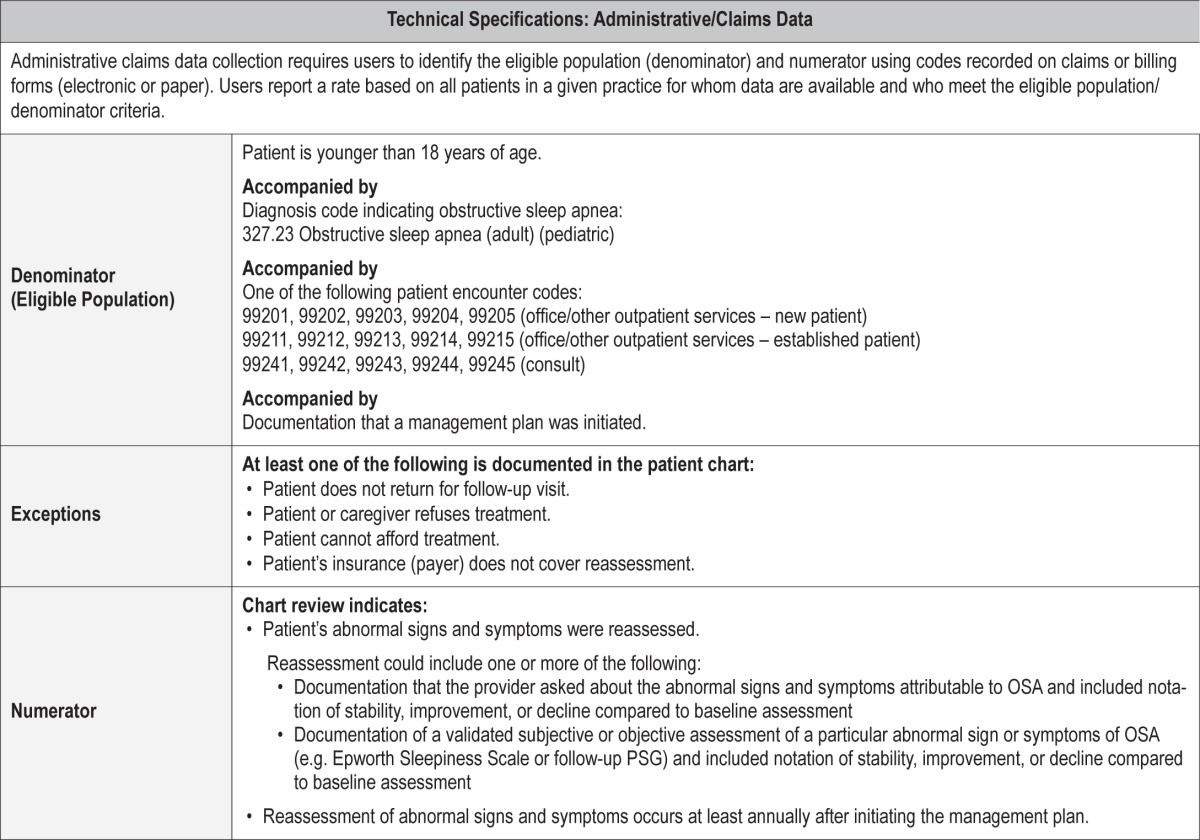
Process Measure 4 – Reassessment of OSA Signs and Symptoms
Description
Proportion of patients aged < 18 years diagnosed with OSA whose abnormal signs and symptoms attributable to OSA were reassessed within 12 months of initiating a management plan.
Exceptions and Exception Justifications
Medical Reasons: None.
Patient Reasons: Patient does not return for follow-up; patient or caregiver declines treatment; patient cannot afford treatment.
System Reasons: Patient's insurance (payer) does not cover reassessment.
Supporting Evidence and Rationale
The value of reassessment of OSA symptoms after implementation of a management plan is supported by the American Academy of Sleep Medicine (AASM) Practice Parameters,4 an American Academy of Pediatrics Practice Guideline,1 and a randomized controlled clinical trial regarding AT in children.19
Relationship to Desired Outcome
Reassessment of children diagnosed with OSA after starting treatment to monitor response to therapy will identify children in need of additional or alternative therapy/intervention.
Opportunities for Improvement/Gaps
Patients identified as having significant residual symptoms can be offered additional effective therapies or have a new/alternative treatment plan discussed and implemented.
Issues Addressed During Development
There was no disagreement about needing reassessment after an intervention for pediatric OSA. Most of the discussion issues regarding development of this particular process measure centered around timing and terminology. There was debate about what time zero was for assessment and what the final time was for reassessment. We settled on 12 months for reassessment, in spite of some stakeholder feedback asking that all reassessment be complete within 6 months, in part because of the highly specialized nature of the testing and limited pediatric resources for the testing. We were concerned that not all testing, analysis, intervention, and reassessment could reliably be completed in a majority of practice settings by 6 months.
Some believed we needed to specifically state the sequelae of inadequate reassessment of signs and symptoms. Our psychology colleagues felt strongly that we needed to address behavioral problems that might impact the willingness to initiate PAP therapy, maintain PAP adherence, or have a complete response to the treatment.
There was deliberation as to the need to separate assessment of PAP therapy adherence from general “reassessment.” Given the complex nature of PAP management, it soon became clear that would require a separate process measure.
This was a relatively straightforward process measure with limited level 1 supportive evidence (a single article), but considerable level 2 and 3 supportive data.
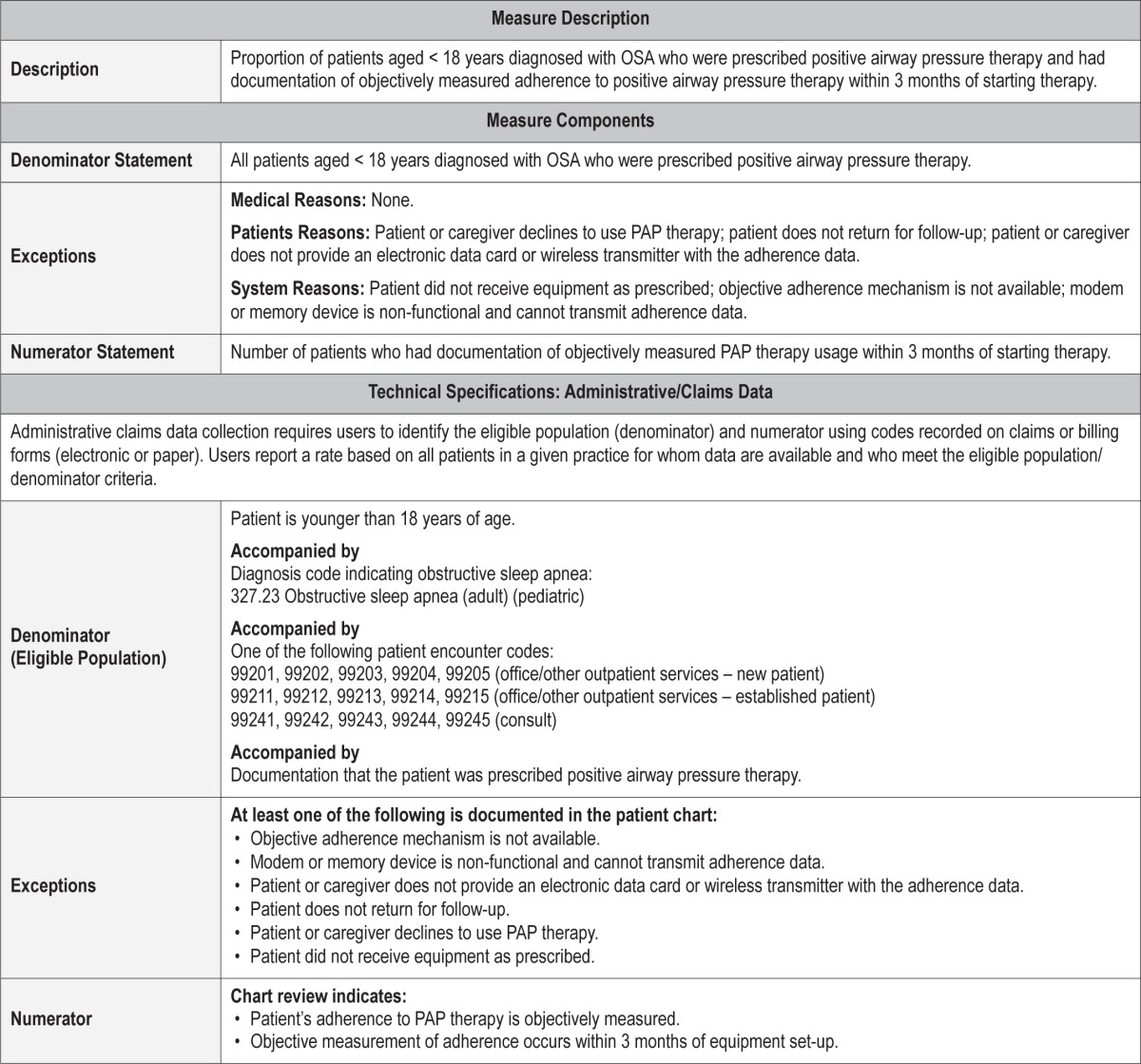
Process Measure 5 – Objective Assessment of Positive Airway Pressure Therapy Adherence
Description
Proportion of patients aged < 18 years diagnosed with OSA who were prescribed positive airway pressure (PAP) therapy and had documentation of objectively measured adherence to PAP therapy within 3 months of starting therapy.
Exceptions and Exception Justifications
Medical Reasons: None.
Patient Reasons: Patient or caregiver declines to use PAP therapy at all; patient does not return for follow-up; patient or caregiver does not provide an electronic data card or wireless transmitter with the adherence data.
System Reasons: Patient did not receive equipment as prescribed; objective adherence mechanism is not available; modem or memory device is nonfunctional and cannot transmit adherence data.
PAP usage cannot be objectively assessed if the device does not have recording capability or if its recording system is faulty. Furthermore, usage cannot be assessed if the patient/ caregiver does not implement therapy or return for follow-up.
Supporting Evidence and Rationale
In adults, this recommendation was based on overwhelming evidence at all levels that patients with OSA overestimate their PAP utilization.20 [Level 1] There is similar evidence that parents of children also overestimate their PAP utilization.2 [Level 2, Level 4] Greater usage early in treatment predicts better longer term usage, so intervention with families who are struggling to implement PAP therapy may improve long-term PAP use.21
Relationship to Desired Outcome
Neurobehavioral deficits such as daytime sleepiness, behavioral problems, inattentiveness, and decreased quality of life are common in children with OSA. Children treated with PAP therapy exhibit significant improvements in multiple behavioral domains.22
Opportunities for Improvement/Gaps
If adherence is suboptimal, then the clinician can institute measures to improve adherence (such as behavioral modification or treating side effects of CPAP) and institute alternative treatments if these measures are ineffective.
Issues Addressed During Development
There was strong agreement within the Workgroup supporting objective documentation of PAP adherence in children using this therapy. In practice, the pediatric clinical population who use PAP therapy for OSA treatment are often children with more severe OSA, comorbidities, and vulnerability to the adverse consequences of untreated OSA.23,24
Typical documentation should include the average number of hours used per night that the PAP device is used (all nights and/or nights used), the percentage of nights per month that the device is used, and the percentage of nights used for at least 4 hours per night. [Level 4] At this time, there are no universally agreed upon measures to determine a threshold for optimum use of PAP therapy in children.
In terms of the frequency at which adherence should be documented, the Workgroup selected 3 months after starting therapy at a minimum [Level 4]. Ideally, adherence should be reassessed at each encounter with the managing physician. Especially in children, the effect of growth on PAP needs has not been established. Reassessment of need for CPAP and the ideal pressure prescription may be needed every one to two years. Future data about the role of auto-titrating PAP devices in management of OSA in children may change those empiric recommendations.
IMPLEMENTATION STRATEGIES
These measures were developed taking into account the various types of providers who may be evaluating, treating, and following pediatric patients with OSA. These providers may include primary care physicians (pediatricians and family medicine physicians), surgeons (primarily otolarynologists), sleep medicine physicians (who may or may not have had formal training in pediatrics), and other providers within these fields including nurse practitioners and physician assistants. The measures were written with the intent that any of these providers will be able to provide acceptable documentation of their assessments, treatments or follow-up evaluations within the context of their individual practices. The measures were left intentionally broad to allow flexibility within the individual practices.
Given the broad spectrum of providers that may care for children with OSA, and the recognition that there may be limited pediatric-specific training, these measures are designed to help the provider implement a basic level of care required for diagnosing and managing pediatric OSA. One of the biggest challenges in diagnosing pediatric OSA is the recognition that it is not just dependent on an apnea-hypopnea index, but rather integration of the constellation of symptoms, physical findings, and descriptive nature of sleep (including positional preferences, airway protective maneuvers, and paradoxical breathing). Other challenges include the technical considerations in performing polysomnography on children with intolerance of monitoring. Also, there are differences in scoring rules over the course of childhood. Currently, there is a lack of consensus about utilizing the pediatric versus adult criterion in the adolescent population (ages 13 to 18 years).25 It is recognized that using the 4% hypopnea rule results in fewer adolescents meeting the criteria for OSA than the 3% rule.26
Practitioners will want to remain current with regard to the diagnostic criteria for pediatric OSA, as cited in the most recent International Classification of Sleep Disorders (ICSD) manual.27 Likewise, it will be necessary to remain updated on pediatric polysomnography scoring as provided by the most recent AASM Manual for the Scoring of Sleep and Associated Events.
FUTURE DIRECTIONS
Ideally, an electronic medical record ensuring truly meaningful use will have built in prompts/reminders or “smart phrases” initiated by diagnosis codes to help guide providers who do not deal with particular diagnoses regularly on how to proceed and to make sure those who do are properly documenting their evidence-based intervention's success or failure. Development of these measures are a primitive, but important first step on that journey.
This manuscript, along with relevant clinical practice guidelines, should be used to assist physicians involved in the care of children with OSA in improved assessment, treatment, and follow-up, as well as inform them which relevant clinical information should be documented for future revision and improvement of outcome measures.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Rosen has received research support from Jazz Pharmaceuticals, and has consulted for Jazz Pharmaceuticals, Advance-Medical, and Natus Medical. Dr. Thomas is an employee of the American Academy of Sleep Medicine. Dr. Carden has received royalties from UpToDate and is a current member of the American Academy of Sleep Medicine Board of Directors. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The American Academy of Sleep Medicine would like to thank the following parties for their review of these measures, and for providing feedback and suggestions to improve the relevancy and utility of these measures in their field of practice: Ron Mitchell, MD; Bonnie Norris, RN; Ruth Boland, RN; Richard Bogan, MD; Madhu Kaloji, MD; American Academy of Otolaryngology-Head and Neck Surgery (AAOHNS); American Sleep Apnea Association (ASAA). The AASM did not seek or receive endorsement of these measures from any of the reviewers who provided feedback. The authors carefully considered all feedback provided, and implemented as many suggestions as were feasible in the refining of these measures. We would also like to thank Carolyn Winter-Rosenberg, AASM Director of Coding and Compliance, for constructing the technical specifications associated with these quality measures.
APPENDIX
The following are the technical specifications for the pediatric OSA quality measures, which can be used to calculate an individual provider's performance in meeting these measures. Tracking and periodically reviewing this performance data will help providers identify opportunities for improvement within their own practices.
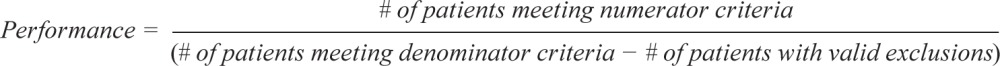 |
Outcome Measure #2: Reduce signs or symptoms of OSA
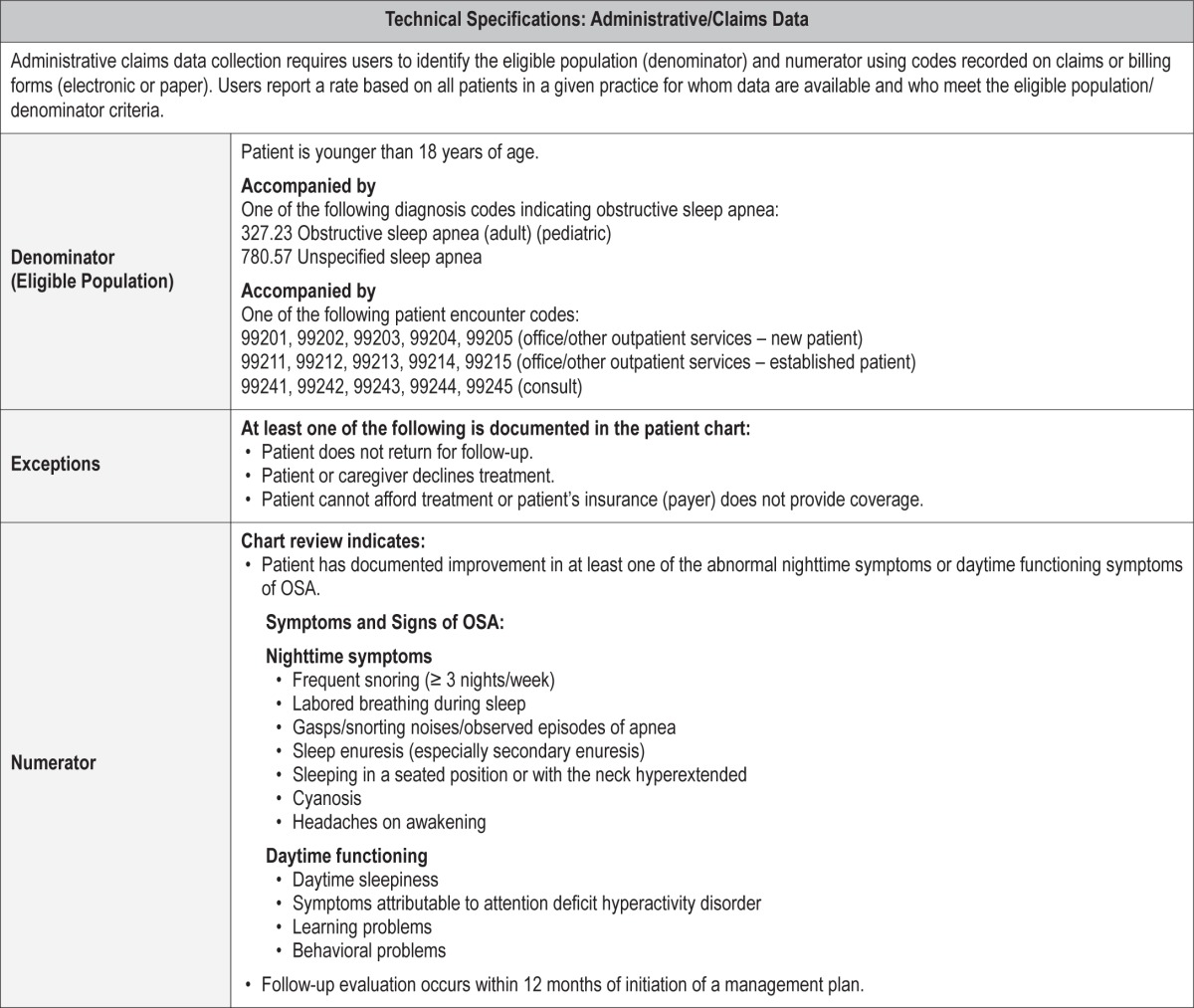
Process Measure #1: Assessment of OSA symptoms and risk factors
Process Measure #2: Initiation of an evidence-based action plan
Process Measure #3: Objective assessment of OSA signs and symptoms in children with complex medical conditions
Process Measure #4: Reassessment of OSA signs and symptoms
Process Measure #5: Objective assessment of positive airway pressure therapy adherence
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 3.Morgenthaler TI, Aronsky AJ, Carden KA, Chervin RD, Thomas SM, Watson NW. Measurement of quality to improve care in sleep medicine. J Clin Sleep Med. 2015;11:279–91. doi: 10.5664/jcsm.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34:379–88. doi: 10.1093/sleep/34.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baugh RF, Archer SM, Mitchell RB, et al. Clinical practice guideline: tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;144(1 Suppl):S1–30. doi: 10.1177/0194599810389949. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: survey of current practice. Laryngoscope. 2006;116:956–8. doi: 10.1097/01.MLG.0000216413.22408.FD. [DOI] [PubMed] [Google Scholar]
- 7.Roland PS, Rosenfeld RM, Brooks LJ, et al. Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 Suppl):S1–15. doi: 10.1177/0194599811409837. [DOI] [PubMed] [Google Scholar]
- 8.Wise MS, Nichols CD, Grigg-Damberger MM, et al. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34:389–98AW. doi: 10.1093/sleep/34.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wise MS, Nichols CD, Grigg-Damberger MM, et al. Respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34:389–398AR. doi: 10.1093/sleep/34.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ChildStats.gov - Forum on Child and Family Statistics [19 September 2014]; Available from: www.childstats.gov.
- 11.Erichsen D, Godoy C, Granse F, Axelsson J, Rubin D, Gozal D. Screening for sleep disorders in pediatric primary care: are we there yet? Clin Pediatr (Phila) 2012;51:1125–9. doi: 10.1177/0009922812464548. [DOI] [PubMed] [Google Scholar]
- 12.Beebe DW, Rausch J, Byars KC, Lanphear B, Yolton K. Persistent snoring in preschool children: predictors and behavioral and developmental correlates. Pediatrics. 2012;130:382–9. doi: 10.1542/peds.2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonuck K, Freeman K, Chervin RD, Xu L. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. Pediatrics. 2012;129:e857–65. doi: 10.1542/peds.2011-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meltzer LJ, Johnson C, Crosette J, Ramos M, Mindell JA. Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics. 2010;125:e1410–8. doi: 10.1542/peds.2009-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bull MJ Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- 16.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S193–228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 17.McCandless SE Committee on Genetics. Clinical report-health supervision for children with Prader-Willi syndrome. Pediatrics. 2011;127:195–204. doi: 10.1542/peds.2010-2820. [DOI] [PubMed] [Google Scholar]
- 18.Trotter TL, Hall JG American Academy of Pediatrics Committee on Genetics. Health supervision for children with achondroplasia. Pediatrics. 2005;116:771–83. doi: 10.1542/peds.2005-1440. [DOI] [PubMed] [Google Scholar]
- 19.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 21.Nixon GM, Mihai R, Verginis N, Davey MJ. Patterns of continuous positive airway pressure adherence during the first 3 months of treatment in children. J Pediatr. 2011;159:802–7. doi: 10.1016/j.jpeds.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Marcus CL, Radcliffe J, Konstantinopoulou S, et al. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:998–1003. doi: 10.1164/rccm.201112-2167OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiFeo N, Meltzer LJ, Beck SE, et al. Predictors of positive airway pressure therapy adherence in children: a prospective study. J Clin Sleep Med. 2012;8:279–86. doi: 10.5664/jcsm.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prashad PS, Marcus CL, Maggs J, et al. Investigating reasons for CPAP adherence in adolescents: a qualitative approach. J Clin Sleep Med. 2013;9:1303–13. doi: 10.5664/jcsm.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Accardo JA, Shults J, Leonard MB, Traylor J, Marcus CL. Differences in overnight polysomnography scores using the adult and pediatric criteria for respiratory events in adolescents. Sleep. 2010;33:1333–9. doi: 10.1093/sleep/33.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry RB, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2014. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.1. [Google Scholar]
- 27.American Academy of Sleep Medicine. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. International classification of sleep disorders. [Google Scholar]



