Abstract
Bar-headed geese cross the Himalayas on one of the most iconic high-altitude migrations in the world. Heart rates and metabolic costs of flight increase with elevation and can be near maximal during steep climbs. Their ability to sustain the high oxygen demands of flight in air that is exceedingly oxygen-thin depends on the unique cardiorespiratory physiology of birds in general along with several evolved specializations across the O2 transport cascade.
“On one cold and still night in early April, I stood beside the Barun glacier [near Mount Makalu, the fifth highest mountain in the world at 8,463 m above sea level] . . . Coming from the south, the distant hum became a call. Then, as if from the stars above me, I heard the honking of bar-headed geese.”–Lawrence Swan (46)
Since early mountaineers and naturalists first sighted bar-headed geese migrating amidst the Himalayan mountains, the migration of this species has been a fascination to both scientists and the general public. Bar-headed geese can be found anywhere from Mongolia to the Tibetan plateau in the summer, where they raise young before the majority take long flights south to the Indian subcontinent in the autumn, and return again the following spring (24, 47). As the most metabolically intense form of vertebrate locomotion, flight demands an extremely high rate of oxygen consumption (51), yet the air at high altitudes in the Himalayas contains only one-third to one-half of the oxygen that is available in air at sea level. Therein lies the apparent paradox that has intrigued so many scientists: bar-headed geese must be capable of sustaining the high oxygen demands of flight in air that is exceedingly oxygen-thin. What is the evidence that bar-headed geese can in fact accomplish this paradoxical feat? What physiological mechanisms underlie high-altitude flight? Recent efforts to address these questions, from characterizing the physiological ecology of the natural migration to elucidating the unique respiratory and metabolic physiology that underlies it, are shedding new insight into the paradox of high-altitude flight in this species.
The Challenges of High-Altitude Flight
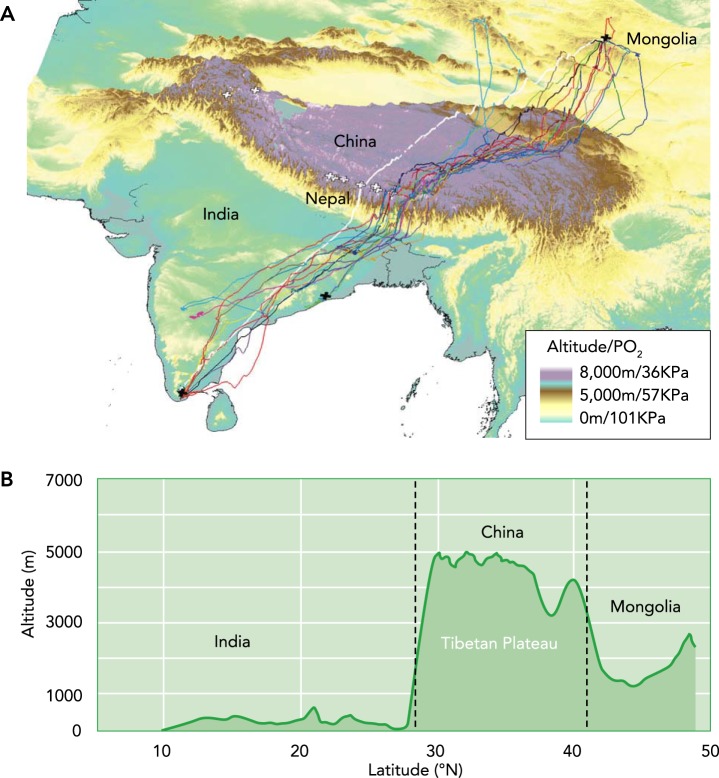
Bar-headed geese fly at altitudes that are extremely challenging to lowland humans and animals. Geese migrating between India and Mongolia have been tracked by satellite telemetry crossing the Himalayan mountains across a broad front (47) (FIGURE 1). Most birds reach altitudes of 5,000–6,000 m during the migration, where the Po2 is roughly half of that at sea level, and they occasionally fly even higher (e.g., one recorded bird reached 7,290 m) (16, 24, 47). Although the accuracy of auditory and visual observations are questionable (16), there are anecdotal reports of bar-headed geese flying even higher, above the highest peaks in the Himalayas (of which there are 14 above 8,000 m), where the Po2 is only one-third of the sea-level value (46). The level of hypoxia at these elevations, even the lowest at which bar-headed geese cross the mountains, is sufficient to reduce maximal O2 uptake rates in humans substantially (53). In fact, the atmosphere atop the highest peaks in the Himalayas is believed to have scarcely enough oxygen to support basal metabolism in humans (53). Therefore, bar-headed geese face the challenge of sustaining the high rates of O2 consumption needed for flapping flight, which ranges from 10- to 15-fold above resting levels during steady flight in a wind tunnel at sea level (51), in air that can severely limit aerobic metabolism in many lowland animals (49). At the same time, the temperatures at high altitudes can be very low, well below freezing year round in the high Himalayas (56), which could require additional metabolic energy for thermogenesis if the heat production from exercise is not sufficient to maintain body temperature. Maintaining water balance during flight should also be a major challenge in the dry air at high altitudes, given that water loss can constrain flight duration at sea level in some species (11).
FIGURE 1.
The high-altitude migration of bar-headed geese
A: satellite tracking of migrating bar-headed geese (colored lines are individual geese) shows where individuals from a Mongolian population cross the Himalayas on their southward migration to India. The colored background shading indicates elevation, and the white crosses are the world's highest mountains (all over 8,000-m elevation). B: on their northward migration out of India, the ascent over the mountains is very steep (minimum climb rates of 0.8–2.2 km/h) and occurs very quickly (<1 day) (17). Image was modified and reproduced from Ref. 16 with permission.
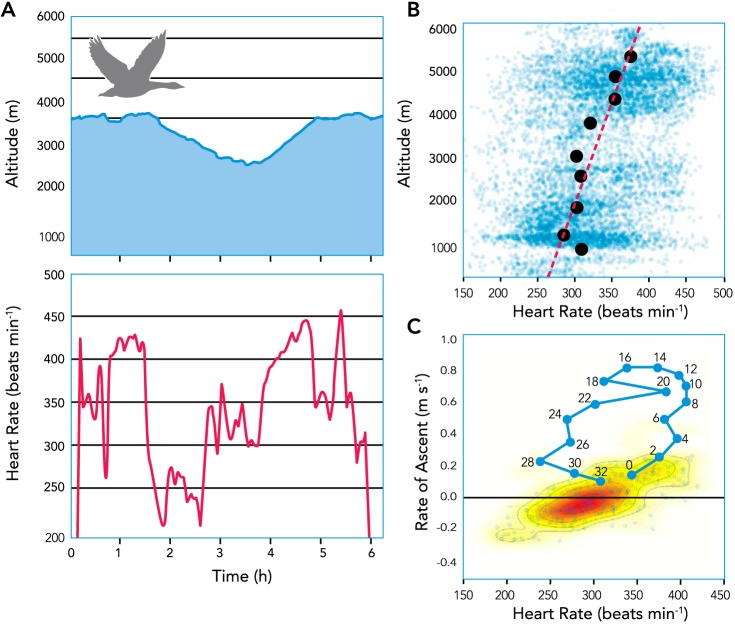
As bar-headed geese fly to higher elevations, it becomes progressively more difficult to generate lift in the decreasing air density. Logging of physiological variables during migration has shown that average heart rates during flight increase with rising elevation (FIGURE 2B), and geese spend a greater proportion of time flying with near maximal heart rates when altitude exceeds 4,800 m (4). When possible, geese will offset the metabolic power requirements of flight at high altitude (as estimated by logged heart rates) by taking lower altitude routes, such as through riverine valleys, or by taking advantage of the orographic lift or katabatic winds near mountains (4, 16) (FIGURE 2C). Nevertheless, bar-headed geese are flapping fliers that very rarely glide, even during steep descents (4).
FIGURE 2.
The metabolic cost of high-altitude flight can be substantial, as reflected by heart rates logged during the natural migration
A: altitudes and heart rates of an individual bar-headed goose flying across the Tibetan plateau, showing the changes in heart rate during descent and ascent. B: the average heart rates exhibited during flight increase with elevation. C: environmental assistance (e.g., uplifting winds) can lessen the heart rates and presumably the metabolic costs of climbing flight when available. An individual goose is shown as an example, with blue lines indicating sequential data points (numbered in minutes) for an event of assisted lift that lies outside the typical relationship between ascent rate and heart rate (red color intensity reflects the overall density of observations). Image modified and reproduced from Ref. 4 with permission.
Crossing the Himalayas from India onto the Tibetan plateau also requires bar-headed geese to ascend for many hours, sustaining the longest sustained rates of climbing flight recorded to date, and possibly even into headwinds (17). Climbing flight presents a much greater metabolic challenge than level flight and generally requires higher average heart rates and wing-beat frequencies (4). Indeed, heart rates of individual geese have been shown to increase as they ascend and to decrease as they descend (FIGURE 2A), and there is a positive relationship between rate of ascent and heart rate (FIGURE 2C) (4). Therefore, it would be advantageous for bar-headed geese to make use of upslope tailwinds during ascent (7, 9). However, upslope tailwinds predominate only during the day in mountainous regions, and bar-headed geese often migrate at night and in the early morning when the predominant winds travel downslope (17). Although these nighttime flights likely entail a greater metabolic cost than flying later in the day when updrafts predominate, the darkness should lessen predation risk (e.g., from predatory birds), the wind currents are more stable and less turbulent, and the air is cooler and will have a slightly higher density and Po2. These benefits may outweigh the metabolic costs of having to flap harder to climb to high altitudes.
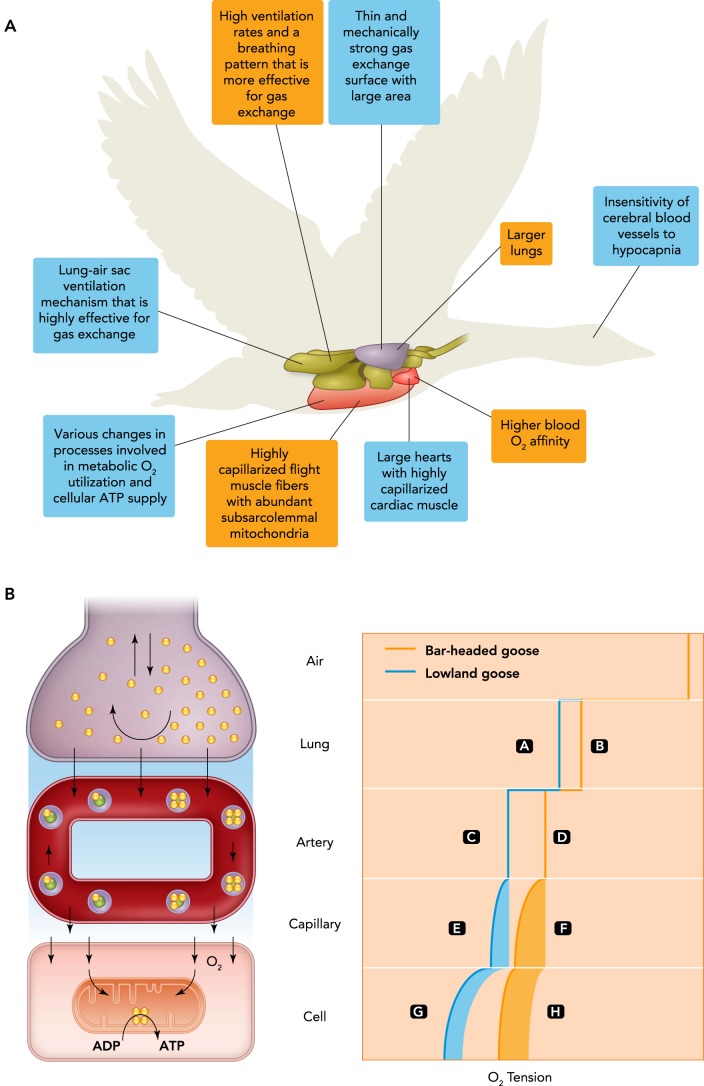
The ecophysiological studies of the natural migration of bar-headed geese emphasize the challenges of high-altitude flight. Although bar-headed geese may take help from wind assistance to lessen the metabolic requirements of flight when possible, much like lowland geese (9), they also experience prolonged periods of relatively high heart rates and intense metabolic activity (4). That these periods of intense activity occur in hypoxic air (Po2 of ≤50% of that at sea level) makes this physiological feat all the more impressive. The ability of bar-headed geese to transport enough O2 to the flight muscle and other tissues to sustain the high metabolic requirements of flight appears to require a variety of physiological traits, some of which are general traits of all birds (i.e., high-altitude exaptations, evolved traits that are important at high altitudes but are not considered to be adaptations) and some additional traits that likely evolved during the process of evolutionary adaptation to high altitude (FIGURE 3).
FIGURE 3.
High-altitude flight is facilitated by several general avian traits
A: high-altitude flight is facilitated by several general avian traits (blue) as well as many evolved specializations in bar-headed geese (orange). B: the qualitative effects of the evolved specializations increase oxygen tensions (Po2) across the oxygen transport cascade (shown at left) compared with lowland geese (with letters added for comparison between bar-headed geese and lowland geese). The capillary Po2 driving diffusion decreases along the length of capillaries as the blood loses O2 to the tissues. The decline in capillary Po2 and decrease in Po2 with distance from capillaries lead to a range of potential cellular Po2. The oxygen transport cascade is adapted from Refs. 21, 48.
Avian Physiology as High-Altitude Exaptations
The exceptional hypoxia tolerance of birds in general, which may have arisen with the evolution of increased cardiorespiratory performance to support flight, is likely an important contributor to the ability of bar-headed geese to fly at high altitudes (12, 38). Early work showed that even lowland sparrows can fly in a wind tunnel while breathing air that simulated the Po2 at 6,100 m, which was sufficiently hypoxic to render domestic mice comatose (50). Birds are generally more tolerant of hypoxia than mammals, and bar-headed geese and some other hypoxia-tolerant birds can survive lower Po2 (lowest survivable Po2, ∼2.7 kPa) than the most hypoxia-tolerant euthermic mammals (e.g., mole rats, ∼4.7 kPa) (49). Several unique features of avian respiratory and cardiovascular physiology, distributed across the oxygen cascade (21, 48) (shown in FIGURE 3), are likely responsible for this heightened hypoxia tolerance of birds. These unique traits probably act as important exaptations that facilitate high-altitude flight.
Birds are thought to be capable of higher ventilation rates than mammals in hypoxia. The decline in arterial Po2 (“hypoxemia”) drives the increase in breathing in response to environmental hypoxia, but CO2 excretion increases as a secondary consequence (42). Low Pco2 in the blood (“hypocapnia”) ensues, which restrains the hypoxic ventilatory response and can lead to an alkalosis of the blood. Based on the extremely low arterial Pco2 (<1 kPa) recorded in birds breathing heavily in severe hypoxia, it has been suggested that birds can ventilate more than mammals in severe hypoxia because they can tolerate a greater depletion of blood CO2 before normal cellular function is impaired (35). This may result from an enhanced capacity to restore blood pH rapidly when blood Pco2 changes (10) or because the brain vasculature is insensitive to hypocapnia (as discussed in more detail below). As a result, O2 transport to the gas-exchange surface in hypoxia may be enhanced in birds compared with mammals.
The structure and function of the avian lung imparts an inherently greater gas-exchange capacity than that of the mammalian lung. Birds possess a system of unidirectional airflow in the lungs, which arose in reptiles (14) and later evolved into a highly effective gas exchanger (36). Blood in the pulmonary capillaries flows perpendicular to the air flowing through the parabronchi, so the bird lung functions as a cross-current gas exchanger (28). Cross-current gas exchange is inherently more effective than the alveolar exchange mechanism of mammalian lungs, such that birds in hypoxia can have an arterial Po2 that exceeds that of the expired gas (33, 35). The capacity for O2 diffusion in the lungs is also very high in birds because the gas-exchange tissue is exceptionally thin (0.1–0.2 μm compared with 0.4–0.8 μm or more in mammals) and generally has a larger surface area (40–100 cm2/g compared with 15–40 cm2/g in nonflying mammals) (52, 54).
The unique physiology of the pulmonary vessels in birds may also impart resistance to high-altitude pulmonary edema, a major contributor to acute mountain sickness in mammals (37). The pulmonary vessels of mammals constrict in response to hypoxia, which can result in pulmonary hypertension, impairment of gas exchange, and pulmonary edema (26, 37). In contrast, the pulmonary vasculature does not constrict in response to hypoxia in birds, and pulmonary arterial pressures increase in hypoxia only when cardiac output rises (6, 13, 55). The avian blood-gas barrier is also thought to be mechanically stronger and more resistant to stress failure than that of mammals (54).
There are several differences in the hearts of birds compared with mammals that should help support higher cardiac outputs and greater convective O2 delivery during hypoxia. Birds have ∼50% larger hearts and cardiac stroke volumes than mammals of similar body size, and birds can sustain heart rates during free flight that are similar to or greater than those of mammals during maximal exercise (3, 15). Capillary density in the cardiac muscle also appears to be higher in birds compared with mammals (12), which would be associated with higher oxygen diffusion capacity and should presumably make bird hearts more resistant to cardiac oxygen limitation in hypoxia.
The capacity for O2 diffusion into the peripheral tissues appears to be higher in birds than in mammals and other vertebrates. The capillary exchange capacity is higher in the flight muscle of birds (capillary length per fiber volume of 6,015 and ∼13,910 mm−2 in pigeons and hummingbirds) compared with the locomotory muscles of nonflying mammals (5,700 and 1,890 mm−2 in the hindlimb of deer mice and dogs) (29). This distinction exists largely because there is a mesh of branching capillaries that surrounds avian muscle fibers, which are themselves smaller in size compared with nonflying mammals of a similar body size (fiber diameters of 20 and 14 μm in pigeons and hummingbirds, and 29 and 45 μm in the hindlimb of deer mice and dogs) (29).
Several differences in the brain physiology of birds compared with mammals may protect against cerebral dysfunction in hypoxia. In birds, unlike in mammals (1), cerebral blood flow is not inhibited by respiratory hypocapnia (12). This should improve brain oxygenation during environmental hypoxia, although this has not yet been confirmed by direct measurement. Avian neurons also have an inherently higher tolerance of low cellular O2 levels (as reflected by the greater survival of cerebellar slices from ducks and chickens than that from rats in 60 min of anoxia) (25) and therefore appear to be well protected from cellular damage induced by O2 limitation. An intriguing question that has yet to be addressed is whether birds suffer hypoxic cerebral edema, one of the most dangerous consequences of high-altitude exposure in humans (19).
The unique respiratory and cardiovascular physiology of birds enhances hypoxia tolerance and exercise capacity by improving the overall capacity for O2 transport, but most birds probably cannot fly at extremely high altitudes. Many species cannot tolerate the levels of hypoxia that exist atop the world's highest mountains (5), and some birds fly exceptionally long distances during their migration to avoid mountain barriers (20). Why bar-headed geese do not do the same is unclear, but it is suggested that the species (or its ancestor) may have begun migrating between South and Central Asia in the late Pliocene or early Pleistocene at a time in geological history when the Himalayas were not nearly as high (46). Because migration routes can be genetically programmed, the species may have slowly evolved to fly higher and higher as they continued along the same migration route over thousands of years. Whatever the evolutionary path that has led to modern day bar-headed geese, it has resulted in the evolution of several specialized traits that set this species apart from most other birds and that allow them to sustain the high O2 requirements of flight in the oxygen-thin air at high altitudes.
The Specialized Physiology of Bar-Headed Geese
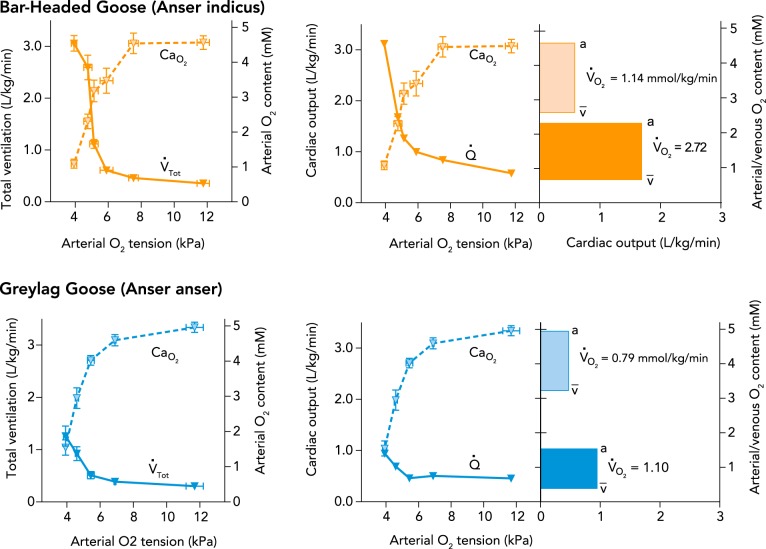
The capacity of bar-headed geese to transport and consume oxygen at high rates in hypoxia distinguishes this species from similar lowland waterfowl. Bar-headed geese can tolerate extreme hypoxia at rest (inspired Po2 tensions as low as ∼2.7 kPa, approximating ∼12,000 m), which far exceeds the tolerance of many lowland waterfowl (5, 41). Bar-headed geese also maintain body temperature in hypoxia to a lower inspired Po2 (∼9 kPa) than lowland waterfowl (∼12 kPa), and they depress body temperature less (39). In fact, they elevate metabolic rate two- to threefold in hypoxia (inspired Po2 between ∼4 and 9 kPa) at rest, presumably to support the O2 demands of the respiratory and cardiovascular responses to hypoxia (FIGURE 4) (5, 41). Bar-headed geese are also capable of achieving the high metabolic rates needed for flight in a normobaric wind-tunnel and maximal running on a treadmill at comparable levels of hypoxia to those on the summit of Mount Everest (∼7 kPa) (18, 31a). As discussed below, this impressive ability to sustain high metabolic rates in hypoxia appears to arise from increases in the capacity of several steps in the O2 transport pathway that augment cellular Po2 (FIGURE 3).
FIGURE 4.
The cardiorespiratory and metabolic responses to hypoxia at rest are enhanced in bar-headed geese compared with lowland waterfowl
Total ventilation (V̇Tot) and cardiac output (Q̇) increase in hypoxia below the arterial O2 tension at which the blood begins to desaturate, but this increase is much greater in bar-headed geese than in greylag geese. Associated with this heightened cardiorespiratory response to hypoxia in bar-headed geese is a much larger increase in O2 consumption rate (V̇o2), which is reflected by the area of the bars in the right panels for each species in normoxia and in 5% inspired O2 fraction. Data are from Ref. 41, with the exception of cardiac output, which was calculated by the Fick equation (V̇o2= Q̇ [CaO2 − CvV̄o2]) from data in Ref. 41. CaO2, arterial O2 content; CvV̄o2, mixed venous O2 content; a, arterial; V̄, mixed venous.
The control of breathing has evolved in bar-headed geese to improve O2 uptake into the respiratory system in hypoxia. Bar-headed geese have been shown to exhibit larger increases in total ventilation in response to severe hypoxia (inspired Po2 of ≤6 kPa) than any other bird species studied to date (5, 41, 42). For example, total ventilation in bar-headed geese during severe hypoxia is roughly twice that in the greylag goose, a closely related species that does not generally fly at high altitudes (FIGURE 4). Furthermore, bar-headed geese breathe more deeply (with higher tidal volumes) and less frequently than low-altitude birds at a given level of total ventilation (39, 41), a breathing pattern that is more effective for gas exchange because it reduces dead-space ventilation and produces a higher Po2 at the gas-exchange surface in the lungs (compare FIGURE 3, A AND B). Bar-headed geese also have ∼25% larger lungs than lowland waterfowl of comparable body mass (45), which should enhance the area and diffusion capacity of the pulmonary gas-exchange surface. These specializations allow bar-headed geese to maintain higher Po2 in the arterial blood than their lowland counterparts during hypoxia (compare FIGURE 3 C AND D; FIGURE 4).
Circulatory O2 delivery in hypoxia is improved in bar-headed geese by evolved changes in blood physiology. The hemoglobin of bar-headed geese has a higher affinity for O2 (whole-blood P50 of 4.0 kPa at pH 7.4 and CO2 tension of ∼5 kPa) than that of closely related lowland geese (5.3 kPa in greylag goose under the same conditions) (32), which increases pulmonary O2 loading and peripheral O2 delivery in hypoxia by increasing hemoglobin saturation at a given blood Po2 (43). The genetic basis for this increase in affinity could involve several amino-acid substitutions in the α-subunit of the hemoglobin protein. Birds possess major (HbA) and minor (HbD) forms of hemoglobin, and in bar-headed geese the α-subunits of these forms contain four (αA) and two (αD) derived substitutions, respectively (30). Site-directed mutagenesis has shown that one of the substitutions in αA (proline-119 → alanine) can account for much of the increase in O2 affinity (23), likely by altering the interaction between α- and β-subunits and destabilizing the deoxygenated state of the protein (57). Hemoglobin-O2 binding is also more sensitive to temperature in bar-headed geese than in other birds and mammals (31). This could have significant implications for O2 transport if there is thermal heterogeneity between the lungs and flight muscle during flight (27, 43). For example, warming of blood in the active flight muscle (8) would transiently decrease hemoglobin O2 affinity and favor O2 unloading. The benefit of this mechanism for O2 transport depends on the magnitude of thermal heterogeneity, which is currently unknown. Theoretical analyses suggest that a 10°C temperature difference between the lungs and flight muscle would increase O2 transport in hypoxia by ∼40–60%, and this potential effect will be magnified by the enhanced thermal sensitivity of bar-headed goose hemoglobin (43).
Circulatory O2 delivery in hypoxia may also be improved in bar-headed geese by evolved changes in heart function. Bar-headed geese have a 30–40% higher capillary density in the left ventricle of the heart than closely related lowland geese but similar myoglobin concentration and maximal activity of several metabolic enzymes (e.g., citrate synthase, hydroxyacyl-coA dehydrogenase, lactate dehydrogenase, pyruvate kinase) (34, 45). This should increase the Po2 in cardiac myocytes, improve the hypoxemia tolerance of the heart, and allow bar-headed geese to increase cardiac output during hypoxia (FIGURE 4).
The capillarity of the flight muscle is also higher in bar-headed geese than in lowland waterfowl (40), which increases the capacity for O2 diffusion from the blood in hypoxia. Furthermore, a greater proportion of the mitochondria in oxidative muscle fibers are in a subsarcolemmal location (located next to the cell membrane) in bar-headed geese (∼50%) compared with lowland geese (∼35%) (40), which reduces intracellular O2 diffusion distances. Each of these evolved specializations will increase the capacity for extracting O2 from the blood (compare FIGURE 3, E AND F) and maintain a high Po2 at the mitochondria (compare FIGURE 3, G AND H) during flight at high altitudes.
The increases in the capacity of bar-headed geese to transport O2 during hypoxia (FIGURE 3) are accompanied by various evolved changes in the properties of metabolic O2 utilization in the flight muscle and heart. The proportional abundance of oxidative fibers in the flight muscle is higher in bar-headed geese than in lowland waterfowl (∼70% vs. ∼60% by area in the superficial pectoralis) (40). In contrast, the mitochondrial respiratory capacity and O2 kinetics (i.e., sensitivity to low O2 tension) as well as the abundance of mitochondria in oxidative fibers are similar in bar-headed geese and lowland geese (40). The affinity of cytochrome c oxidase (COX; the enzyme that consumes O2 in oxidative phosphorylation) for cytochrome c is also higher in bar-headed geese than in lowland waterfowl (45). This change may have arisen from a single mutation in COX subunit 3 at a site that is otherwise conserved across vertebrates (tryptophan-116 → arginine) and appears to alter inter-subunit interactions (45). The physiological importance of this unique trait is not completely understood, but it may act to reduce the propensity of mitochondria to produce reactive oxygen species (ROS) by allowing the electron transport chain to operate in a less reduced state. If so, this trait could reduce the propensity of bar-headed geese to experience oxidative stress, an underappreciated stressor associated with prolonged migration (22).
There also appear to be mechanisms in place that better match cellular ATP supply and demand in the flight muscle of bar-headed geese. Mitochondrial respiration in situ in permeabilized muscle fibers is more strongly regulated by creatine in bar-headed geese than in low-altitude waterfowl (44). These results suggest that ATP supply and demand is better coupled via the creatine kinase shuttle, a system important for moving ATP-equivalents around the cell (2). This unique trait could even be related to the subsarcolemmal localization of mitochondria in the flight muscle (see above), compensating for the greater distance between these organelles and the contractile elements.
Conclusions
High-altitude flight is an extremely challenging feat of performance that is underpinned by a number of specialized physiological traits. Bar-headed geese can reach high altitudes during their migration across the Himalayas and Tibetan plateau because they can continue supporting the metabolic costs of flight as the air becomes extremely hypoxic. Like other migrating birds, they may occasionally make use of updraft wind assistance to help offset flight costs. However, they also experience periods of intense flapping flight that require extremely high heart rates, wing-beat frequencies, and metabolic power, such as during level flight at high elevation or during climbs that are not assisted by wind. Physiological specializations have evolved at all steps in the O2 cascade of bar-headed geese that help them accomplish this feat by improving O2 transport in hypoxia. However, most of what is known about the physiology of bar-headed geese comes from comparing this species to lowland birds in a common environment at sea level. It is likely that the evolved specializations that have already been discovered do not entirely explain high-altitude flight. For example, we know much more about how bar-headed geese cope with hypoxia than how they deal with low barometric pressure, cold, and dry air at high altitudes. We also know relatively little about the influence of phenotypic plasticity (i.e., acclimatization) and developmental plasticity on the physiology of this species. We therefore have much yet to learn about the migration of this fascinating species, which will undoubtedly continue to shed light on nature's impressive solutions to oxygen deprivation.
Footnotes
We are grateful to all field team members and for the additional support for field work from the Mongolian Academy of Sciences, Wildlife Science and Conservation Centre, Max Planck Institute for Ornithology, U.S. Geological Survey, Western Ecological and Patuxent Wildlife Research Centres and Avian Influenza Programme, United Nations Food and Agriculture Organization, and Beaumaris Instruments.
Research is supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants to G. R. Scott and W. K. Milsom, and a UK Biotechnology and Biological Sciences Research Council (BBSRC) award to C. M. Bishop and P. J. Butler (grant no. BB/FO15615/1).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: G.R.S., L.A.H., and C.M.B. prepared figures; G.R.S. drafted manuscript; G.R.S., L.A.H., P.B.F., P.J.B., C.M.B., and W.K.M. edited and revised manuscript; G.R.S., L.A.H., P.B.F., P.J.B., C.M.B., and W.K.M. approved final version of manuscript.
References
- 1.Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Exp Physiol 95: 251–262, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Andrienko T, Kuznetsov AV, Kaambre T, Usson Y, Orosco A, Appaix F, Tiivel T, Sikk P, Vendelin M, Margreiter R, Saks VA. Metabolic consequences of functional complexes of mitochondria, myofibrils and sarcoplasmic reticulum in muscle cells. J Exp Biol 206: 2059–2072, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bishop CM. Heart mass and the maximum cardiac output of birds and mammals: implications for estimating the maximum aerobic power input of flying animals. Philos Trans R Soc Lond B Biol Sci 352: 447–456, 1997. [Google Scholar]
- 4.Bishop CM, Spivey RJ, Hawkes LA, Batbayar N, Chua B, Frappell PB, Milsom WK, Natsagdorj T, Newman SH, Scott GR, Takekawa JY, Wikelski M, Butler PJ. The roller coaster flight strategy of bar-headed geese conserves energy during Himalayan migrations. Science 347: 250–254, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Black CP, Tenney SM. Oxygen transport during progressive hypoxia in high altitude and sea level waterfowl. Respir Physiol 39: 217–239, 1980. [DOI] [PubMed] [Google Scholar]
- 6.Black CP, Tenney SM. Pulmonary hemodynamic responses to acute and chronic hypoxia in 2 waterfowl species. Comp Biochem Physiol A 67: 291–293, 1980. [Google Scholar]
- 7.Butler PJ. High fliers: the physiology of bar-headed geese. Comp Biochem Physiol A Mol Integr Physiol 156: 325–329, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Butler PJ, West NH, Jones DR. Respiratory and cardiovascular responses of the pigeon to sustained, level flight in a wind-tunnel. J Exp Biol 71: 7–26, 1977. [Google Scholar]
- 9.Butler PJ, Woakes AJ. Heart rate, respiratory frequency and wing beat frequency of free flying barnacle geese Branta leucopsis. J Exp Biol 85: 213–226, 1980. [Google Scholar]
- 10.Dodd GAA, Scott GR, Milsom WK. Ventilatory roll off during sustained hypercapnia is gender specific in pekin ducks. Respir Physiol Neurobiol 156: 47–60, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Engel S, Biebach H, Visser GH. Water and heat balance during flight in the rose-colored starling (Sturnus roseus). Physiol Biochem Zool 79: 763–774, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Faraci FM. Adaptations to hypoxia in birds: how to fly high. Annu Rev Physiol 53: 59–70, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Faraci FM, Kilgore DL, Fedde MR. Attenuated pulmonary pressor response to hypoxia in bar-headed geese. Am J Physiol Regul Integr Comp Physiol 247: R402–R403, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Farmer CG, Sanders K. Unidirectional airflow in the lungs of alligators. Science 327: 338–340, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Grubb BR. Allometric relations of cardiovascular function in birds. Am J Physiol Heart Circ Physiol 245: H567–H572, 1983. [DOI] [PubMed] [Google Scholar]
- 16.Hawkes LA, Balachandran S, Batbayar N, Butler PJ, Chua B, Douglas DC, Frappell PB, Hou Y, Milsom WK, Newman SH, Prosser DJ, Sathiyaselvam P, Scott GR, Takekawa JY, Natsagdorj T, Wikelski M, Witt MJ, Yan B, Bishop CM. The paradox of extreme high-altitude migration in bar-headed geese Anser indicus. Proc R Soc B 280: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkes LA, Balachandran S, Batbayar N, Butler PJ, Frappell PB, Milsom WK, Tseveenmyadag N, Newman SH, Scott GR, Sathiyaselvam P, Takekawa JY, Wikelski M, Bishop CM. The trans-Himalayan flights of bar-headed geese (Anser indicus). Proc Natl Acad Sci USA 108: 9516–9519, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkes LA, Butler PJ, Frappell PB, Meir JU, Milsom WK, Scott GR, Bishop CM. Maximum running speed of captive bar-headed geese is unaffected by severe hypoxia. PLos One 9: e94015, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imray C, Wright A, Subudhi A, Roach R. Acute mountain sickness: pathophysiology, prevention, and treatment. Prog Cardiovasc Dis 52: 467–484, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Irwin DE, Irwin JH. Siberian migratory divides: the role of seasonal migration in speciation. In: Birds of Two Worlds: The Ecology and Evolution of Migration, edited by Greenberg R, Marra PP. Baltimore, MD: Johns Hopkins Univ. Press, 2005. p. 27–40. [Google Scholar]
- 21.Ivy CM, Scott GR. Control of breathing and the circulation in high-altitude mammals and birds. Comp Biochem Physiol A Mol Integr Physiol. In press. [DOI] [PubMed] [Google Scholar]
- 22.Jenni-Eiermann S, Jenni L, Smith S, Costantini D. Oxidative stress in endurance flight: an unconsidered factor in bird migration. PLos One 9: e97650, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jessen TH, Weber RE, Fermi G, Tame J, Braunitzer G. Adaptation of bird hemoglobins to high altitudes: demonstration of molecular mechanism by protein engineering. Proc Natl Acad Sci USA 88: 6519–6522, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köppen U, Yakovlev A, Barth R, Kaatz M, Berthold P. Seasonal migrations of four individual bar-headed geese Anser indicus from Kyrgyzstan followed by satellite telemetry. J Ornithol 151: 703–712, 2010. [Google Scholar]
- 25.Ludvigsen S, Folkow LP. Differences in in vitro cerebellar neuronal responses to hypoxia in eider ducks, chicken and rats. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 195: 1021–1030, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Maggiorini M, Melot C, Pierre S, Pfeiffer F, Greve I, Sartori C, Lepori M, Hauser M, Scherrer U, Naeije R. High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 103: 2078–2083, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Maginniss LA, Bernstein MH, Deitch MA, Pinshow B. Effects of chronic hypobaric hypoxia on blood oxygen binding in pigeons. J Exp Zool 277: 293–300, 1997. [Google Scholar]
- 28.Maina JN. Development, structure, and function of a novel respiratory organ, the lung-air sac system of birds: to go where no other vertebrate has gone. Biol Rev 81: 545–579, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Mathieu-Costello O. Histology of flight: tissue and muscle gas exchange. In: Hypoxia: The Adaptations, edited by Sutton JR, Coates G, Remmers JE. Toronto, Canada: B. C. Decker, 1990, p. 13–19. [Google Scholar]
- 30.McCracken KG, Barger CP, Sorenson MD. Phylogenetic and structural analysis of the HbA (αA/βA) and HbD (αD/βA) hemoglobin genes in two high-altitude waterfowl from the Himalayas and the Andes: bar-headed goose (Anser indicus) and Andean goose (Chloephaga melanoptera). Mol Phylogenet Evol 56: 649–658, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Meir JU, Milsom WK. High thermal sensitivity of blood enhances oxygen delivery in the high-flying bar-headed goose. J Exp Biol 216: 2172–2175, 2013. [DOI] [PubMed] [Google Scholar]
- 31a.Meir JU, Jardine W, York J, Chua B, Milsom WK. Heart rate and metabolic rate of bar-headed geese flying in hypoxia (Abstract). FASEB J 27: 1149.16, 2013. [Google Scholar]
- 32.Petschow D, Würdinger I, Baumann R, Duhm J, Braunitzer G, Bauer C. Causes of high blood O2 affinity of animals living at high altitude. J Appl Physiol 42: 139–143, 1977. [DOI] [PubMed] [Google Scholar]
- 33.Piiper J, Scheid P. Maximum gas transfer efficacy of models for fish gills, avian lungs and mammalian lungs. Respir Physiol 14: 115–124, 1972. [DOI] [PubMed] [Google Scholar]
- 34.Saunders DK, Fedde MR. Physical conditioning: effect on the myoglobin concentration in skeletal and cardiac muscle of bar-headed geese. Comp Biochem Physiol A 100: 349–352, 1991. [Google Scholar]
- 35.Scheid P. Avian respiratory system and gas exchange. In: Hypoxia: The Adaptations, edited by Sutton JR, Coates G, Remmers JE. Toronto, Canada: B. C. Decker, 1990, p. 4–7. [Google Scholar]
- 36.Scheid P, Slama H, Piiper J. Mechanisms of unidirectional flow in parabronchi of avian lungs: measurements in duck lung preparations. Respir Physiol 14: 83–95, 1972. [DOI] [PubMed] [Google Scholar]
- 37.Scherrer U, Rexhaj E, Jayet PY, Allemann Y, Sartori C. New insights in the pathogenesis of high-altitude pulmonary edema. Prog Cardiovasc Dis 52: 485–492, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Scott GR. Elevated performance: the unique physiology of birds that fly at high altitudes. J Exp Biol 214: 2455–2462, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Scott GR, Cadena V, Tattersall GJ, Milsom WK. Body temperature depression and peripheral heat loss accompany the metabolic and ventilatory responses to hypoxia in low and high altitude birds. J Exp Biol 211: 1326–1335, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Scott GR, Egginton S, Richards JG, Milsom WK. Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc R Soc Lond B Biol Sci 276: 3645–3653, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott GR, Milsom WK. Control of breathing and adaptation to high altitude in the bar-headed goose. Am J Physiol Regul Integr Comp Physiol 293: R379–R391, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Scott GR, Milsom WK. Control of breathing in birds: implications for high altitude flight. In: Cardio-Respiratory Control in Vertebrates: Comparative and Evolutionary Aspects, edited by Glass ML, Wood SC. Berlin: Springer-Verlag, 2009, p. 429–448. [Google Scholar]
- 43.Scott GR, Milsom WK. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir Physiol Neurobiol 154: 284–301, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Scott GR, Richards JG, Milsom WK. Control of respiration in flight muscle from the high-altitude bar-headed goose and low-altitude birds. Am J Physiol Regul Integr Comp Physiol 297: R1066–R1074, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Scott GR, Schulte PM, Egginton S, Scott ALM, Richards JG, Milsom WK. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol Biol Evol 28: 351–363, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Swan LW. Goose of the Himalayas. Nat Hist 70: 68–75, 1970. [Google Scholar]
- 47.Takekawa JY, Heath SR, Douglas DC, Perry WM, Javed S, Newman SH, Suwal RN, Rahmani AR, Choudhury BC, Prosser DJ, Yan B, Hou Y, Batbayar N, Natsagdorj T, Bishop CM, Butler PJ, Frappell PB, Milsom WK, Scott GR, Hawkes LA, Wikelski M. Geographic variation in bar-headed geese Anser indicus: connectivity of wintering areas and breeding grounds across a broad front. Wildfowl 59: 100–123, 2009. [Google Scholar]
- 48.Taylor CR, Weibel ER. Design of the mammalian respiratory system. I. Problem and strategy. Respir Physiol 44: 1–10, 1981. [PubMed] [Google Scholar]
- 49.Thomas SP, Follette DB, Thomas GS. Metabolic and ventilatory adjustments and tolerance of the bat Pteropus poliocephalus to acute hypoxic stress. Comp Biochem Physiol A 112: 43–54, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Tucker VA. Respiratory physiology of house sparrows in relation to high-altitude flight. J Exp Biol 48: 55–66, 1968. [DOI] [PubMed] [Google Scholar]
- 51.Ward S, Bishop CM, Woakes AJ, Butler PJ. Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). J Exp Biol 205: 3347–3356, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Watson RR, Fu Z, West JB. Morphometry of the extremely thin pulmonary blood-gas barrier in the chicken lung. Am J Physiol Lung Cell Mol Physiol 292: L769–L777, 2007. [DOI] [PubMed] [Google Scholar]
- 53.West JB. American medical research expedition to Everest. High Alt Med Biol 11: 103–110, 2010. [DOI] [PubMed] [Google Scholar]
- 54.West JB. Comparative physiology of the pulmonary blood-gas barrier: the unique avian solution. Am J Physiol Regul Integr Comp Physiol 297: R1625–R1634, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West JB, Watson RR, Fu Z. Major differences in the pulmonary circulation between birds and mammals. Respir Physiol Neurobiol 157: 382–390, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X, Zhang T, Qin D, Kang S, Qin X. Characteristics and changes in air temperature and glacier's response on the north slope of Mt. Qomolangma (Mt. Everest). Arct Antarct Alp Res 43: 147–160, 2011. [Google Scholar]
- 57.Zhang J, Hua ZQ, Tame JRH, Lu GY, Zhang RJ, Gu XC. The crystal structure of a high oxygen affinity species of haemoglobin (bar-headed goose haemoglobin in the oxy form). J Mol Biol 255: 484–493, 1996. [DOI] [PubMed] [Google Scholar]