Abstract
Background
The American Heart Association (AHA) established recommendations based on 7 ideal health behaviors and factors with the goal of improving cardiovascular health (CVH) and reducing both morbidity and mortality from cardiovascular disease (CVD) by 20% by 2020. Few studies have investigated their association with subclinical coronary heart disease (CHD). We sought to examine whether the 7 AHA CVH metrics were associated with calcified atherosclerotic plaque in the coronary arteries.
Methods and Results
In a cross-sectional design, we studied 1731 predominantly Caucasian men and women from the National Heart, Lung, and Blood Institute Family Heart Study without prevalent CHD. Diet was assessed by a semi-quantitative food frequency questionnaire. Coronary artery calcium (CAC) was measured by cardiac CT. We defined prevalent CAC using an Agatston score of 100+ and fitted generalized estimating equations to calculate prevalence odds ratios of CAC. Mean age was 56.8 years and 41% were male. The median number of ideal CVH metrics was 3, and no participants met all 7. There was a strong inverse relationship between number of ideal CVH metrics and prevalent CAC. Odds ratios (95% CI) for CAC of 100+ were 1.0 (reference), 0.37 (0.29–0.45), 0.35 (0.26–0.44), and 0.27 (0.20–0.36) among subjects with 0–1, 2, 3, and 4+ ideal CVH metrics, respectively (p for trend: 0.0001), adjusting for sex, age, field center, alcohol, income, education, and calorie consumption.
Conclusions
These data demonstrate a strong and graded inverse relationship between AHA ideal CVH metrics and prevalent CAC in adult men and women.
Keywords: cardiovascular health, epidemiology, subclinical disease, coronary calcium
Introduction
Cardiovascular disease (CVD) remains a major public health burden in the United States with total costs in excess of $315 billion.1 Epidemiologic studies have demonstrated inverse relation between number of cardiovascular health behaviors or factors met and CVD events.2–4
The American Heart Association (AHA) published recommendations for the general population aimed at reducing CVD incidence and mortality by achieving seven specific cardiovascular health (CVH) metrics.5 These include 3 health factors (blood pressure, total cholesterol, and blood glucose) and 4 health behaviors (physical activity, not smoking, body mass index [BMI], and healthy diet), categorized as ideal, intermediate, or poor.
Although several studies have examined the relationship between these CVH metrics and CVD events6–8, few have investigated the joint-effects of all 7 AHA metrics with subclinical CHD.9–10 Such quantification could be used by clinicians to convey potential benefits of modifiable behaviors and factors. The prognosis of patients with CHD is closely related to the burden of atherosclerotic disease and its stability11, and coronary artery calcification (CAC) is a well-described marker for subclinical atherosclerotic disease that can help stratify CHD risk and predict future events.12–14 Conversion of no CAC to any measurable CAC confers a continuously increased risk of CVD and underscores the importance of identifying and targeting modifiable risk factors for the development of coronary atherosclerosis.15 We sought to investigate this relationship in men and women sampled from four regions of the US.
Methods
Study population
The National Heart, Lung, and Blood Institute Family Heart Study (NHLBI FHS) is a multi-center, population-based study designed to identify and evaluate genetic and non-genetic determinants of CHD, preclinical atherosclerosis, and cardiovascular risk factors.16–17 Briefly, families in the study had been chosen randomly (random group) or based on a higher than expected risk of CHD (high-risk group) from previously established population-based cohort studies. A total of 588 families were chosen at random (with 2673 subjects) and 566 families were selected based on higher than expected risk of CHD (3037 subjects). Of the 5710 subjects, 265 were African-American. The high-risk group was defined based on a family risk score, which compares the family’s age and sex-specific incidence of CHD to that expected in the general population.18 All family members were invited for a clinical evaluation (between 1993–1995) which included a detailed lifestyle and medical history and laboratory measurements including blood glucose and cholesterol levels. Between 2002–2003, about one-third of the NHLBI FHS participants were invited to participate in a clinical examination that included measurement of CAC. In addition to the initial study centers, an African-American center - University of Alabama at Birmingham - was recruited from the Hypertension Genetic Epidemiology Network Study, where subjects underwent cardiac CT but did not have dietary assessments.
Of the 3360 subjects who had data on cardiac CT, we excluded 389 subjects with prevalent CHD and 62 subjects with extreme caloric intake (> 4200 and 3500 calories or <800 and 600 calories for men and women, respectively). A total of 993 participants were missing diet information; 185 additional participants had incomplete or missing data on total cholesterol (n=122), blood pressure (n=37), glucose (n=19), education (n=3), alcohol consumption (n=2) and physical activity (n=2). Thus, current analyses are based on the remaining 1731 subjects. Each participant gave informed consent and the study protocol was reviewed and approved by all participating institutions.
Assessment of Baseline Characteristics
Dietary Assessment
Dietary information was collected through a staff-administered semi-quantitative food frequency questionnaire (FFQ) developed by Willett et al.19 The reproducibility and validity of the FFQ have been documented elsewhere.20 Nutrients (including sodium) were computed using the food-composition database from the Harvard School of Public Health and manufacturer information, and this technique has been described previously.21 Subjects were asked how often on average they consumed a typical portion size of each respective food in the questionnaire, with answers ranging from 0 to >6 per day.
Laboratory assays
Fasting triglyceride concentrations were measured using triglyceride GB reagent on the Roche COBAS FARA centrifugal analyzer (Boehringer Mannheim Diagnostics, Indianapolis). Serum total cholesterol was measured using a commercial cholesterol oxidase method on a Roche COBAS FARA centrifugal analyzer (Boehringer Mannheim Diagnostics, Indianapolis). For samples with triglyceride concentrations less than 4.5 mmol/L (400 mg/dL), LDL-cholesterol was calculated using the Friedewald formula,22 otherwise LDL was measured by ultracentrifugation.
Other Variables
Resting blood pressure was measured three times on seated participants after a 5-minute rest and we used the average systolic and diastolic blood pressures from the second and third measurements for analyses. Information on alcohol consumption, education, exercise, and cigarette smoking was obtained by interview during the initial clinic visit. Total physical activity was assessed using a questionnaire that captured average minutes per day of strenuous, moderate, and light leisure-time activities; frequency of sweating with exercise; miles walked and steps climbed; and level of occupational activity.23 Anthropometric data were collected with subjects wearing scrub suits. Comorbidities including diabetes mellitus, CHD, and hypertension were assessed via questionnaires and current medications. All variables used in these analyses were ascertained during the initial examination (1993–1995) except for CAC scores, which were obtained during a follow-up examination (2002–2003).
Definition of Cardiovascular Health Metrics
Cardiovascular health factors and behaviors were classified according to AHA definitions5 as follows: (a) smoking: ideal (never), intermediate (not currently smoking but smoked ≥100 cigarettes), and poor (current) due to a lack of information about time since quitting; (b) BMI: ideal (<25 kg/m2), intermediate (25 to <30 kg/m2), and poor (≥30 kg/m2); (c) physical activity: ideal (≥150 min/wk moderate intensity, ≥75 min/wk vigorous intensity, or equivalent combination), intermediate (1–149 min/wk moderate intensity, 1–74 min/wk vigorous intensity, or equivalent combination), and poor (no moderate and vigorous activity); (d) diet: ideal (4–5 healthy components), intermediate (2–3 healthy components), and poor (0–1 healthy component) based on 5 health dietary metrics (≥4.5 cups of fruits and vegetables a day, 2 or more 3.5-oz servings of fish a week, 3 or more 1-oz equivalent servings fiber-rich whole grains per day, <1500 mg sodium/d, and ≤450 kcal [or 36 ounces] of sugar-sweetened beverages a week); (e) total cholesterol: ideal (untreated and <200 mg/dL), intermediate (treated to <200 mg/dL or 200–239 mg/dL), and poor (≥240 mg/dL); (f) blood pressure: ideal (untreated and <120/<80 mm Hg), intermediate (treated to <120/<80 mm Hg or 120–139/80–89 mm Hg), and poor (≥140/90 mm Hg); and (g) fasting plasma glucose: ideal (untreated and <100 mg/dL), intermediate (treated to <100 mg/dL or 100–125/mg/dL), and poor (≥126 mg/dL).
Measurement of calcified atherosclerotic plaque in the coronary arteries
Cardiac CT examinations were obtained using General Electric Health Systems LightSpeed Plus and LightSpeed Ultra, Siemens Volume Zoom, or Philips MX 8000 machines as described previously.24,25 Briefly, Agatston scores, which are based on the area and density of calcified plaque, were modified to account for slice thickness and calculated using a 130 CT number threshold and a minimum lesion size of 0.9 mm. The sum of the vessel plaque was reported as the total CAC score, and CAC scores from two sequential CT scans during the same examination were averaged to generate a final score.
Statistical analysis
CAC was dichotomized using a cutoff point of 100 as described previously.17 Baseline characteristics are presented according to number of ideal CVH metrics met (0–1, 2, 3, and 4+). To account for familial clustering, we used generalized estimating equations to calculate prevalence odds ratios with corresponding 95% confidence interval. Model 1 adjusted for age (continuous) and gender. Model 2 also controlled for field center, alcohol intake (none, >0–≤7 drinks/week, >7–≤14 drinks/week, >14 drinks/week), income (<$25,000, $25,000–<$75,000, ≥$75,000), education (high school or less, bachelor’s or some college, advanced degree), and caloric intake (tertiles).
In secondary analysis, we calculated ORs using different CAC cut points (CAC >0 and CAC≥50) as well as sex-specific analyses. We explored the relationship between individual CVH metrics and CAC severity by calculating regression coefficients for individual CVH metrics and repeated main analyses using only four metrics that were inversely related with CAC. All analyses were completed using SAS, version 9.3 (SAS institute Inc., Cary, NC). P –values were 2-tailed and alpha level was set at 0.05.
Results
Of the total 1731 subjects, 41% were men; mean age was 56.8 years; and the median number of ideal CVH metrics was three. Table 1 shows the baseline characteristics by number of ideal CVH metrics met. Increasing number of ideal CVH metrics was associated with younger age, lower BMI, smaller waist circumference, female sex, higher caloric consumption and HDL, and lower LDL. Table 2 shows the distribution of each CVH metric in the total cohort and stratified by sex. Overall, no participants met all 7 ideal CVH metrics, few (3.0%) subjects met 6 ideal CVH metrics, 4.9% had 0 ideal CVH metrics, and the majority (64.4%) met between 1 to 3 ideal CVH metrics.
Table 1.
Baseline Characteristics of Study Population in the NHLBI FHS by number of Ideal Cardiovascular Health Metrics met
| Characteristics | Number of ideal Cardiovascular Health Metrics met | p for linear trend |
|||
|---|---|---|---|---|---|
| 0–1 N=379 |
2 N=415 |
3 N=405 |
4–7 N=532 |
- - |
|
| Age, mean (SD) | 62.4 ± 10.9 | 59.4 ± 12.3 | 56.6 ± 13.1 | 50.9 ± 12.2 | <0.0001 |
| BMI, kg/m2 (SD) | 31.4 ± 5.4 | 30.5 ± 5.9 | 28.6 ± 5.2 | 25.6 ± 4.4 | <0.0001 |
| Male, % | 48.0 | 40.5 | 42.0 | 36.1 | 0.0011 |
| White, % | 98.9 | 99.0 | 99.5 | 99.6 | 0.1587 |
| Cigarette pack years, % | - | - | - | - | |
| 0 | 28.2 | 56.6 | 71.4 | 82.5 | <0.0001 |
| 1–15 | 24.3 | 14.9 | 13.1 | 10.0 | <0.0001 |
| 16–30 | 16.9 | 12.3 | 8.4 | 3.2 | <0.0001 |
| 30+ | 25.3 | 12.1 | 4.4 | 2.1 | <0.0001 |
| Drinking status, % | - | - | - | - | |
| Never | 61.5 | 62.7 | 68.6 | 67.3 | 0.0183 |
| 1–7 drinks/wk | 16.4 | 20.2 | 19.8 | 22.0 | 0.0603 |
| 8–14 drinks/wk | 10.6 | 11.8 | 7.2 | 8.5 | 0.0931 |
| >14 drinks/wk | 11.6 | 5.3 | 4.4 | 2.3 | <0.0001 |
| Income, % | |||||
| <$25,000 | 16.6 | 13.7 | 11.9 | 7.0 | <0.0001 |
| $25,000–$75,000 | 58.1 | 52.3 | 54.1 | 48.7 | 0.0111 |
| ≥$75,000 | 22.4 | 31.0 | 31.6 | 41.5 | <0.0001 |
| Education, % | |||||
| High school or less | 49.3 | 34.0 | 30.9 | 21.1 | <0.0001 |
| Bachelor's or some college | 14.5 | 13.7 | 12.6 | 11.5 | 0.1578 |
| Advanced degree | 36.2 | 52.3 | 56.4 | 67.5 | <0.0001 |
| Hypertension, % | 66.0 | 45.6 | 28.6 | 8.8 | <0.0001 |
| Diabetes mellitus, % | 18.2 | 9.6 | 4.7 | 1.7 | <0.0001 |
| Statin use, % | 38.3 | 21.4 | 12.1 | 4.1 | <0.0001 |
| Calorie Consumption, median (IQR) | 1563 (1211–2055) | 1660 (1312–2088) | 1717 (1265–2112) | 1710 (1329–2157) | 0.0086 |
| Waist circumference, cm (SD) | 106.9 ± 14.4 | 102.5 ± 14.7 | 97.6 ± 14.5 | 88.5 ± 13.7 | <0.0001 |
| LDL, mg/dL (SD) | 116.4 ± 34.8 | 120.0 ± 34.4 | 116.0 ± 33.4 | 106.3 ± 32.3 | <0.0001 |
| HDL, mg/dL (SD) | 46.8 ± 12.8 | 48.9 ± 14.6 | 50.8 ± 15.3 | 52.4 ± 15.0 | <0.0001 |
Abbreviations: CVH, cardiovascular health; SD, standard deviation; BMI, body mass index; IQR, interquartile range
Table 2.
Prevalence of Meeting Cardiovascular Health Metrics in Adults-NLHBI FHS
| Cardiovascular Health Metric | Total Cohort | Female (n = 1019) | Male (n = 712) | ||||
|---|---|---|---|---|---|---|---|
| Smoking | No. | Prevalence, % | No. | Prevalence, % | No. | Prevalence, % | |
| Poor | 162 | 9.4% | 85 | 8.3% | 77 | 10.8% | |
| Intermediate | 521 | 30.1% | 289 | 28.4% | 232 | 32.6% | |
| Ideal | 1048 | 60.5% | 645 | 63.3% | 403 | 56.6% | |
| BMI | |||||||
| Poor | 601 | 34.7% | 342 | 33.6% | 259 | 36.4% | |
| Intermediate | 674 | 38.9% | 344 | 33.8% | 330 | 46.4% | |
| Ideal | 456 | 26.3% | 333 | 32.7% | 123 | 17.3% | |
| Cholesterol | |||||||
| Poor | 162 | 9.4% | 118 | 11.6% | 44 | 6.2% | |
| Intermediate | 789 | 45.6% | 491 | 48.2% | 298 | 41.9% | |
| Ideal | 780 | 45.1% | 410 | 40.2% | 370 | 52.0% | |
| Blood Pressure | |||||||
| Poor | 206 | 11.9% | 138 | 13.5% | 68 | 9.6% | |
| Intermediate | 780 | 45.1% | 418 | 41.0% | 362 | 50.8% | |
| Ideal | 745 | 43.0% | 463 | 45.4% | 282 | 39.6% | |
| Plasma Glucose | |||||||
| Poor | 113 | 6.5% | 67 | 6.6% | 46 | 6.5% | |
| Intermediate | 463 | 26.8% | 205 | 20.1% | 258 | 36.2% | |
| Ideal | 1155 | 66.7% | 747 | 73.3% | 408 | 57.3% | |
| Physical Activity | |||||||
| Poor | 545 | 31.5% | 335 | 32.9% | 210 | 29.5% | |
| Intermediate | 629 | 36.3% | 385 | 37.8% | 244 | 34.3% | |
| Ideal | 557 | 32.2% | 299 | 29.3% | 258 | 36.2% | |
| Diet | |||||||
| Poor | 1203 | 69.4% | 638 | 62.6% | 565 | 79.4% | |
| Intermediate | 523 | 30.2% | 376 | 36.9% | 147 | 20.7% | |
| Ideal | 5 | 0.3% | 5 | 0.5% | 0 | 0.0% | |
| Individual components met | |||||||
| Fruit/Veg | 285 | 16.5% | 201 | 19.7% | 84 | 11.8% | |
| Fish | 326 | 18.8% | 204 | 20.0% | 122 | 17.1% | |
| Sodium | 853 | 49.3% | 548 | 53.8% | 305 | 42.8% | |
| Whole grain | 87 | 5.0% | 44 | 4.3% | 43 | 6.0% | |
| Sugar-sweetened beverages | 495 | 28.6% | 349 | 34.3% | 146 | 20.5% | |
| Number of cardiovascular health metrics | |||||||
| 0 | 85 | 4.9% | 33 | 3.2% | 52 | 7.3% | |
| 1 | 294 | 17.0% | 164 | 16.1% | 130 | 18.3% | |
| 2 | 415 | 24.0% | 247 | 24.2% | 168 | 23.6% | |
| 3 | 405 | 23.4% | 235 | 23.1% | 170 | 23.9% | |
| 4 | 305 | 17.6% | 196 | 19.2% | 109 | 15.3% | |
| 5 | 175 | 10.1% | 109 | 10.7% | 66 | 9.3% | |
| 6 | 52 | 3.0% | 35 | 3.4% | 17 | 2.4% | |
| 7 | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |
Among health behaviors, the prevalence of nonsmoking was 60.5%, whereas ideal diet had the lowest prevalence (0.3%). Ideal sodium intake was present in 49.3%, followed by ideal consumption of sugar-sweetened beverage (28.6%), fish (18.8%), fruits and vegetables (16.5%), and whole grains (5.0%). The prevalence of ideal BMI, physical activity, fasting glucose, blood pressure, and cholesterol was 26.3%, 32.2%, 66.7%, 43.0%, and 45.1%, respectively.
There was a strong inverse relationship between the number of ideal CVH metrics and prevalent CAC. Compared to subjects meeting 0–1 ideal CVH metrics, multivariable adjusted odds ratios (95% CI) for CAC of ≥100 were 0.37 (0.29–0.45), 0.35 (0.26–0.44), and 0.27 (0.20–0.36) among subjects with 2, 3, and 4+ ideal CVH metrics, respectively (p for linear trend: 0.0001, Table 3), adjusting for age, sex, field center, alcohol, income, education, and energy intake. Inverse association between ideal CVH metrics and prevalent CAC was also observed when stratified by age. Adjusted odds ratios (95% CI) for CAC of ≥100 were 1 (reference), 0.39 (0.26–0.55), 0.22 (0.09–0.41), and 0.24 (0.12–0.43) among subjects < 60 years with 0–1, 2, 3, and 4+ ideal CVH metrics, respectively (p for linear trend: 0.031); and 1 (reference), 0.36 (0.27–0.46), 0.40 (0.30–0.52), and 0.30 (0.22–0.41), respectively in subjects 60 years and older (p for linear trend: 0.0029).
Table 3.
Prevalence odds ratios (95% confidence intervals) of CAC according to cardiovascular health metrics in 1731 participants in the NHLBI Family Heart Study
| Odds Ratios (95% CI) | |||||
|---|---|---|---|---|---|
| CVH metrics | n | Cases | Crude* | Age and Sex adjusted | Full Adjustment† |
| 0–1 | 379 | 169 | 1 | 1 | 1 |
| 2 | 415 | 110 | 0.31 (0.25–0.37) | 0.34 (0.27–0.42) | 0.37 (0.29–0.45) |
| 3 | 405 | 84 | 0.25 (0.19–0.31) | 0.30 (0.23–0.39) | 0.35 (0.26–0.44) |
| ≥4 | 532 | 54 | 0.12 (0.09–0.16) | 0.22 (0.16–0.30) | 0.27 (0.20–0.36) |
| p for trend | <0.0001 | <0.0001 | 0.0001 | ||
Coronary calcification was defined as total coronary calcium score of ≥ 100
Adjusted for: Sex, age, field center, alcohol, income, education, and calorie consumption
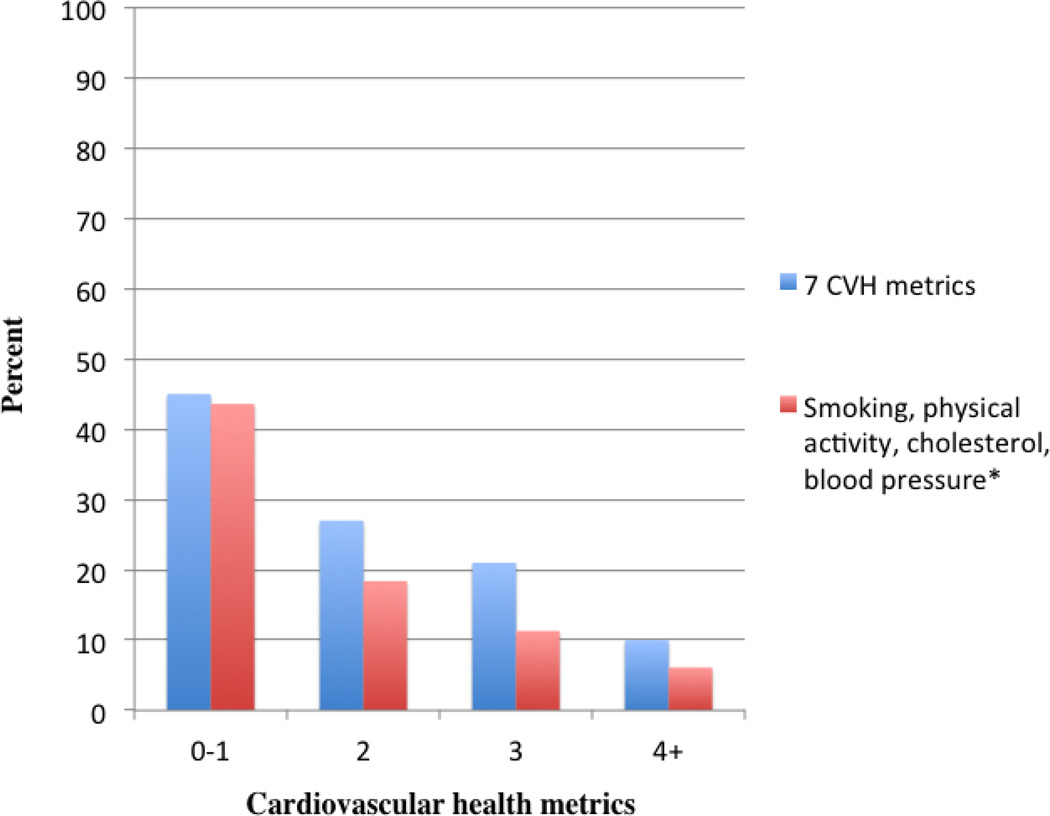
In a secondary analysis of individual metrics, not smoking, regular exercise, normal cholesterol and blood pressure were inversely associated with CAC (supplemental Table S1). Using only these metrics in the fully adjusted model, odds ratios (95% CI) for CAC > 100 were 1 (reference), 0.35 (0.26–0.46), 0.23 (0.15–0.32), and 0.19 (0.12–0.29) among subjects with 0–1, 2, 3, and 4 ideal CVH metrics, respectively (p for linear trend < 0.0001). Figure 1 shows the prevalence of subjects with CAC > 100 according to number of all 7 ideal CVH metrics met, and when restricting the analysis to only those metrics inversely associated with CAC (e.g. physical activity, smoking, cholesterol, and blood pressure).
Fig 1.
Prevalence of subjects with CAC > 100 according to number of ideal CVH metrics
Smoking and lack of physical activity were associated with CAC severity. Regression coefficients (95% CI) among ideal, intermediate, and poor smoking categories were: 1 (reference), 0.7615 (0.4366, 1.0865), and 1.1257 (0.6047, 1.6467). Corresponding coefficients (95% CI) for ideal, intermediate, and poor physical activity were 1 (reference), 0.1573 (−0.1827, 0.4973), and 0.5682 (0.2407, 0.8958) categories. We could not provide a stable estimate for diet because of limited number of ideal events (n=5). Blood pressure, cholesterol, glucose, and BMI were not associated with CAC severity (all P>0.05).
In a sensitivity analysis, we observed an inverse relationship between the number of ideal CVH metrics and prevalent CAC using CAC cut points of >0 and ≥50.
Discussion
In this cohort of individuals without known CHD, the number of ideal CVH metrics was inversely related to prevalent CAC. This strong and graded relationship was present using different CAC cutpoints and in participants younger and older than 60 years. Among individual metrics, not smoking, regular exercise, normal cholesterol and blood pressure were inversely associated with CAC whereas diet, glucose, and BMI were not. To our knowledge, our study is the first to investigate the relationship between the AHA 7 CVH metrics and prevalent CAC in a large cohort of US residents. Kulshreshtha et al. published data suggesting a strong inverse relationship between AHA CVH metrics and carotid intima-media thickness9 while Alman et al. found a similar relationship between ideal cardiovascular health and prevalent CAC in a smaller cohort of mostly Caucasian male and female adults with a high prevalence of type 1 diabetes (46%).26 Our study adds to the limited body of research investigating the AHA CVH factors and behaviors and subclinical disease, and further supports the application of ideal CVH metrics in its primary prevention.
Data from the Atherosclerosis Risk in Communities (ARIC) study showed a graded incidence of CVD in relation to the number of ideal CVH metrics.6 Similarly, a strong inverse relationship between ideal CVH and incident CVD was found among a more racially and ethnically heterogeneous cohort in the Northern Manhattan Study (NOMAS).7 This association was reproduced in a larger, nationally representative sample conducted over multiple periods spanning more than two decades.8
In comparison to the above-mentioned studies and to nationally representative data, the prevalence of ideal CVH in our study showed similar trends.27 We found that no participants met all 7 ideal CVH metrics, and few met 6. Similar to National Health and Nutrition Examination Surveys (NHANES) data, ideal smoking behavior was found in over half of our study’s cohort, and ideal cholesterol and blood pressure were present in over 40% of the cohort.8, 27
Ideal BMI and physical activity were less prevalent in our cohort (26.5% and 32.1%, respectively) in comparison to NHANES data from 2005–2010 (32.5% and 45.2%, respectively). Conversely, prevalence of ideal plasma glucose was slightly higher in the NHLBI FHS participants (66.7%) than in the NHANES III (59.5%).27
Ideal diet was very low in our cohort (1.3%) and in line with NHANES data (0.6% and 1.4% in men and women, respectively) and NOMAS data (0.4%). It is noteworthy that the prevalence of meeting ideal sodium intake in our cohort (49.5%) was much higher than the corresponding prevalence reported in a 24-hour dietary recall within the NHANES (<1%),28 suggesting that sodium intake might have been poorly captured by the FFQ.
CAC is a well-established predictor of CHD events even after adjustment for traditional risk factors.29 Given its strong predictive value, there has been significant interest in identifying those modifiable risk factors most closely associated with its incidence and progression.
Epidemiologic studies have demonstrated that current smoking, higher BMI, hypertension, and diabetes mellitus are associated with the development and progression of CAC.30,31 LDL cholesterol and HDL cholesterol were associated with risk of incident CAC but not progression of preexisting CAC in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort.32 Similarly, a recent study found that sedentary behavior was associated with progression of preexisting CAC, and that vigorous physical activity was only weakly protective against incident CAC.32 Numerous studies have investigated the role of diet in CVD events but limited data exist on its relationship to subclinical disease.33
Limitations of our study include its cross-sectional design and unmeasured confounding that may be present. The sample is restricted to middle-aged, Caucasian individuals and our results may not be generalizable to younger subjects or persons with other race-ethnic backgrounds. CVH metrics were assessed at baseline and may have changed over time before CAC measurement. Cardiac CT data was limited to calcified atherosclerotic plaque, and information regarding non-calcified plaque, a strong predictor of cardiovascular events, was unavailable to us. Misclassification due to self-reported data is possible. Nonetheless, the current study has several strengths including a detailed dietary questionnaire; large sample size; availability of data on key covariates to control for confounding; and robustness of findings in sensitivity analyses using various cut-points to define prevalent CAC.
In conclusion, our study shows that the AHA ideal CVH metrics, especially not smoking, regular exercise, normal cholesterol and blood pressure, are strongly and inversely related to prevalent CAC in men and women.
Supplementary Material
Acknowledgements
JMR and LD designed research; ABP and LD analyzed data and performed statistical analyses; JMR and LD drafted the paper. Supervision (LD). Funding (RCE, DKA, JSP, GH, SH). CAC measurement (JC). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. Appreciation is expressed to the staff of the study and especially to the study participants who volunteered for the project.
Funding:
This study was supported by grants from the National Heart, Lung, & Blood Institute (U01 HL56563, U01 HL56564, U01 HL56565, U01 HL56566, U01 HL56567, U01 HL56568, U01 HL56569, and K01-HL70444).
Abbreviations
- CVD
cardiovascular disease
- CHD
coronary heart disease
- AHA
American Heart Association
- CVH
cardiovascular health
- CAC
coronary artery calcification
- NHLBI FHS
National Heart, Lung, and Blood Institute Family Heart Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Executive Summary: Heart Disease and Stroke Statistics – A Report From the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 3.Daviglus ML, Stamler J, Pirzada A, et al. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292:1588–1592. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 4.Stampfer MJ, Hu FB, Manson JE, et al. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343(1):16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong C, Rundek T, Wright CB, et al. Ideal Cardiovascular Health Predicts Lower Risks of Myocardial Infarction, Stroke, and Vascular Death Across Whites, Blacks, and Hispanics: The Northern Manhattan Study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Q, Cogswell ME, Flanders WD, et al. Trends in Cardiovascular Health Metrics and Associations With All-Cause and CVD Mortality Among US Adults. JAMA. 2012;307(12):1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulshreshtha A, Goyal A, Veledar E. Association Between Ideal Cardiovascular Health and Carotid Intima Media Thickness: A Twin Study. J Am Heart Assoc. 2014;3:e000282. doi: 10.1161/JAHA.113.000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laitinen TT, Pahkala K, Magnussen CG, et al. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2012;125:1971–1978. doi: 10.1161/CIRCULATIONAHA.111.073585. [DOI] [PubMed] [Google Scholar]
- 11.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92(5):1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 12.Haberl R, Becker A, Leber A, et al. Correlation of coronary calcification and angiographically documented stenoses in patients with suspected coronary artery disease: results of 1,764 patients. J Am Coll Cardiol. 2001;37:451–457. doi: 10.1016/s0735-1097(00)01119-0. [DOI] [PubMed] [Google Scholar]
- 13.Arad Y, Spadaro LA, Goodman K, et al. Predictive value of electron beam computed tomography of the coronary arteries. 19-month follow-up of 1173 asymptomatic subjects. Circulation. 1996;93:1951–1953. doi: 10.1161/01.cir.93.11.1951. [DOI] [PubMed] [Google Scholar]
- 14.Greenland P, LaBree L, Azen SP, et al. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 15.Erbel R, Budoff M. Improvement of cardiovascular risk prediction using coronary imaging: subclinical atherosclerosis: the memory of lifetime risk factor exposure. Eur Heart J. 2012;33(10):1201–1213. doi: 10.1093/eurheartj/ehs076. [DOI] [PubMed] [Google Scholar]
- 16.Higgins M, Province M, Heiss G, et al. NHLBI Family Heart Study: objectives and design. Am J Epidemiol. 1996;143:1219–1228. doi: 10.1093/oxfordjournals.aje.a008709. [DOI] [PubMed] [Google Scholar]
- 17.Djoussé L, Arnett DK, Carr JJ, et al. Dietary linolenic acid is inversely associated with calcified atherosclerotic plaque in the coronary arteries: the NHLBI Family Heart Study. Circulation. 2005;111:2921–2926. doi: 10.1161/CIRCULATIONAHA.104.489534. [DOI] [PubMed] [Google Scholar]
- 18.Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chronic Dis. 1986;39:809–821. doi: 10.1016/0021-9681(86)90083-4. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Agriculture. Agriculture handbook no. 8. Washington, DC: US Government Printing Office; 1989. Composition of foods: raw, processed, and prepared, 1963–1988. [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Ellison RC, Zhang Y, Qureshi MM, et al. Lifestyle determinants of high-density lipoprotein cholesterol: The national heart, lung, and blood institute family heart study. Am Heart J. 2004;147:529–535. doi: 10.1016/j.ahj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 25.Djoussé L, Hopkins PN, Arnett DK, et al. Chocolate consumption is inversely associated with calcified atherosclerotic plaque in the coronary arteries: The NHLBI Family Heart Study. Clinical Nutrition. 2011;30:38–43. doi: 10.1016/j.clnu.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alman A, Maahs DM, Rewers MJ, et al. Ideal cardiovascular health and the prevalence and progression of coronary artery calcification in adults with and without type I diabetes. Diabetes Care. 2014;37:521–528. doi: 10.2337/dc13-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shay CM, Ning H, Allen NB, et al. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelton PK, Appel LJ, Sacco RL, et al. Sodium, Blood Pressure, and Cardiovascular Disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–2889. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- 29.Pletcher MJ, Tice JA, Pignone M, et al. Using the coronary artery calcium score to predict coronary heart disease events. Arch Intern Med. 2004;164(12):1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 30.Kronmal RA, McClelland RL, Detrano R, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the multiethnic study of atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann N, Möhlenkamp S, Mahabadi AA, et al. Effect of smoking and other traditional risk factors on the onset of coronary artery calcification: Results of the Heinz Nixdorf recall study. Atherosclerosis. 2014;232:339–345. doi: 10.1016/j.atherosclerosis.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 32.Delaney JA, Jensky NE, Criqui MH, et al. The association between physical activity and both incident coronary artery calcification and ankle brachial index progression: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;230:278–283. doi: 10.1016/j.atherosclerosis.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mente A, Koning LD, Shannon HS, et al. A Systematic Review of the Evidence Supporting a Causal Link Between Dietary Factors and Coronary Heart Disease. Arch Intern Med. 2009;169(7):659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.