Abstract
Status epilepticus (SE) describes persistent or recurring seizures without a return to baseline mental status, and is a common neurologic emergency. SE can occur in the context of epilepsy or may be symptomatic of a wide range of underlying etiologies. The clinician’s aim is to rapidly institute care that simultaneously stabilizes the patient medically, identifies and manages any precipitant conditions, and terminates seizures. Seizure management involves “emergent” treatment with benzodiazepines followed by “urgent” therapy with other anti-seizure medications. If seizures persist then refractory SE is diagnosed and management options include additional anti-seizure medications or infusions of midazolam or pentobarbital. This paper reviews the management of pediatric SE and RSE.
Keywords: Status epilepticus, Seizure, Pediatric, Management, EEG
Introduction
Status epilepticus (SE) describes a prolonged seizure or recurrent seizures without a return to baseline. It is the most common pediatric neurological emergency with an incidence of 18–23 per 100,000 children per year.1 Care involves simultaneously identifying and managing systemic complications, identifying and managing precipitant etiologies, and administering anticonvulsants to terminate ongoing seizures(s).
Historically, SE was defined as a seizure lasting longer than 30 minutes or a series of seizures without return to baseline level of alertness between seizures.2 During the prodromal or incipient stage (<5 minutes) it is unknown whether the seizure will self-terminate or evolve into SE. Persisting SE has been divided into early SE (5–30 minutes), established SE (>30 minutes), or refractory SE (RSE) (seizures persist despite treatment with adequate doses of two or three anticonvulsants). Due to increasing recognition that most seizures are brief (3–4 minutes)3 and anticonvulsant administration delays are associated with more refractory seizures, the temporal definition of SE has gradually shortened and the related terminology has been modified to convey a greater sense of urgency. The Neurocritical Care Society guideline for SE management in children and adults defines SE as “5 minutes or more of (i) continuous clinical and/or electrographic seizure activity or (ii) recurrent seizure activity without recovery (returning to baseline) between seizures” and opines that “definitive control of SE should be established within 60 minutes of onset.”4 Rather than labeling medications as first, second, and third line agents which provide no sense of timing urgency, the guideline uses the terms “emergent”, “urgent”, and “refractory” to help convey that medications should be administered sequentially and rapidly. RSE is defined as clinical or electrographic seizures which persist after an adequate dose of an initial benzodiazepine and a second appropriate anti-seizure medication; in contrast to prior definitions no specific time must elapse before initiation of RSE management.
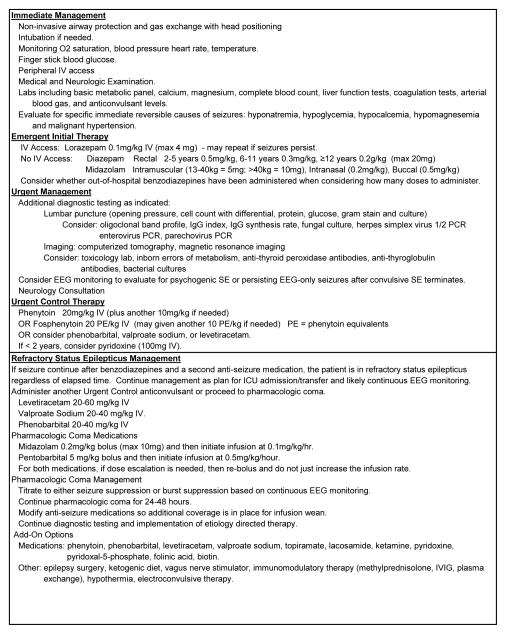
Variability in SE management and treatment delays are common. Studies of SE management in children in emergency departments have described that laboratory parameters were often not checked and some results were only available after long delays,5 the median time to administer a second-line anticonvulsant to a seizing child was 24 minutes,6 and that benzodiazepine dosing was outside usual dosing guidelines in 23% of children with SE.5 Excess benzodiazepine dosing (which often occurs when prehospital doses are administered) contributes to respiratory insufficiency and need for intensive care unit admission5,7,8 while inadequate dosing may reduce the likelihood of seizure termination. Several studies have described associations between SE management delays and more prolonged seizures9 as well as lower anticonvulsant responsiveness.10–13 To expedite therapeutic decisions, a consensus document recommended that all units have a written management pathway with a clear structured time frame.14 An example SE management pathway is provided in Figure 1 which is adapted from the Neurocritical Care Society guideline4 and other recent publications.15–17
Figure 1.
Status epilepticus evaluation and management pathway. Adapted from prior pathways.15–17
Medical Management and Precipitating Etiology Evaluation
Medical Stabilization of the acutely seizing patient should focus on airway, breathing and circulation with the goal maintain oxygenation, ventilation and adequate tissue perfusion while rapidly diagnosing and treating the source of the seizures. The Neurocritical Care Society guideline provides a timed treatment outline.4 Steps to be completed in the initial 2 minutes include non-invasive airway protection and gas exchange with head positioning and vital sign assessment. Steps to be included in the initial 5 minutes include neurologic examination and placement of peripheral intravenous access for administration of emergent anti-seizure medication therapy and fluid resuscitation. Steps to be completed in the initial 10 minutes include intubation if airway or gas exchange is compromised or intracranial pressure is elevated. Intubation may be necessary due to seizure associated hypoventilation, medication associated hypoventilation, inability to protect the airway or other causes of oxygenation or ventilation failure. Steps to be completed in the initial 15 minutes include vasopressor support if needed.4
Multiple studies have characterized the various potential etiologies for SE.1,18–20 The most common cause of pediatric SE is febrile SE, and SE may also occur in children with epilepsy.21 However, in most situations acute precipitating conditions must be considered. Acute symptomatic conditions are identified in 15–20% of children with SE.1,19,22 The American Academy of Neurology practice parameter addressing the diagnostic assessment of a child with convulsive SE reported that abnormal results among children who underwent testing included low anticonvulsant levels (32%), neuroimaging abnormalities (8%), electrolytes (6%), inborn errors of metabolism (4.%), ingestion (4%), central nervous system infections (3%), and positive blood cultures (3%).23 Rapidly reversible causes of seizures should be diagnosed and treated within minutes of hospital arrival, specifically evaluating for electrolyte disturbances such as hyponatremia, hypoglycemia, hypomagnesemia and hypocalcemia. The Neurocritical Care Society guideline provides suggestions regarding etiologic testing including: bedside finger stick blood glucose (0–2 minutes) and serum glucose, complete blood count, basic metabolic panel, calcium, magnesium, and anti-seizure medication levels (5 minutes). In some patients, other diagnostic testing may include neuroimaging or lumbar puncture (LP) (0–60 minutes), additional laboratory testing (including liver function tests, coagulation studies, arterial blood gas, toxicology screen, and inborn errors of metabolism screening), and continuous electroencephalographic (EEG) monitoring if the patient is not waking up after clinical seizures cease (15–60 minutes).4 These recommendations are similar to those of the prior American Academy of Neurology practice parameter.23 Rarer infectious, metabolic, autoimmune and paraneoplastic etiologies may be considered in specific situations.24
Neuroimaging abnormalities have been reported in 30% of children with SE and described to alter acute management in 24%.19 If no etiology is identified by computerized tomography (CT), magnetic resonance imaging (MRI) may still identify lesions. One study described that among 44 children who underwent head CT and MRI, 14 had a normal head CT but an abnormal MRI, leading to the conclusion that MRI had a superior yield and should be considered whenever available if head CT is non-diagnostic.19
There are two main urgent EEG indications. First, if the diagnosis of psychogenic SE is suspected, then rapid diagnosis using EEG monitoring may avoid continued exposure to anticonvulsants with potential adverse effects. Second, if there is concern that EEG-only (non-convulsive) seizures are ongoing despite cessation of clinically evident seizures, then EEG monitoring may be required for identification and to assess the impact of continued management.25,26 A multi-center study of children who underwent EEG monitoring while in the ICU reported that 33% of 98 children who presented with convulsive SE had electrographic seizures identified. The seizure burden was often high with electrographic SE in 47%. Further, 34% of children with seizures had exclusively EEG-only seizures which would not have been identified without EEG monitoring.27 Observational studies have reported that in multivariable analyses aiming to account for encephalopathy etiology and severity, high electrographic seizure burdens in critically ill children are associated with worse outcomes.26,28–31 The Neurocritical Care Society guideline states that in order to identify electrographic seizures, “continuous electroencephalographic monitoring should be initiated within one hour of SE onset if ongoing seizures are suspected” and that the management goal be termination of both convulsive and electrographic seizures.4 Further study is needed to determine whether efforts to identify and manage these electrographic seizures improve patient outcomes.
If no etiology is identified by the initial testing, then additional testing may be indicated. A targeted approach may be useful for some patients, but in some patients sending a full panel of tests initially may be optimal.
Central nervous system infections are a common cause of acute symptomatic SE19, accounting for 0.6%–40% of all SE in different series.32,33 In addition, SE occurs in 15% of encephalitis cases overall. Development of RSE in encephalitis has been associated with younger age, fever, the presence of a prodromal gastrointestinal illness, normal peripheral white blood cell count, and normal neuroimaging.34 The clinical presentation of encephalitis and other central nervous system infections is highly variable depending on the pathogen involved and specific host factors. In some cases, particularly in young children, individuals who are immunocompromised, or individuals who have received recent antibiotics, fever may be absent and clinical signs of infection very subtle. Therefore, LP and magnetic resonance imaging (MRI) should be performed in all cases of SE without an obvious non-infectious etiology. A diagnostic strategy to evaluate for possible infectious etiologies is outlined in Table 2 (adapted from prior publications35,36). Detailed consideration of potential infectious etiologies may lead to focused testing and in some etiologies, specific effective treatment (such as Bartonella in cat-scratch disease).
If an autoimmune etiology for RSE is suspected, a LP should be performed to evaluate for evidence of central nervous system inflammation. Routine studies from the cerebrospinal fluid (CSF) including a cell count and protein are useful as patients with underlying neuro-inflammatory processes will often have a pleocytosis and elevated CSF protein. Intrathecal immunoglobulin synthesis is a hallmark of central nervous system inflammation and should be evaluated with an oligoclonal band profile, IgG index, and IgG synthesis rate when a neuro-immune etiology is being considered. Oligoclonal bands are positive when two or more immunoglobulin bands are detected in CSF but not in accompanying serum.
Many causes of autoimmune encephalitis may be associated with neoplasm, although the frequency of tumor detection varies. Depending on the auto-antibody, distinct brain regions may be targeted, with seizures or SE resulting from autoimmunity to either the limbic system or cerebral cortex.37 Paraneoplastic antibodies may target intracellular antigens or antigens on the neuronal surface. Antibodies to intracellular neuronal antigens include Hu, Ma2, CV2/CRMP5, and amphiphysin; such antibodies have rates of tumor association (>90%).38 In general, for intracellular paraneoplastic antibodies, cytotoxic T-cell responses are believed to mediate neural inflammation and destruction, and there is poor response to immunotherapy.39 One exception is encephalitis associated with anti-GAD65 antibodies. Although GAD65 is an intracellular antigen, there less brain inflammation/destruction, a lower association with tumor (<5%), and greater response to immunotherapy.38,39 Of the intracellularly targeted antibodies, anti-Hu antibodies have the strongest association with isolated seizures and limbic encephalitis, but are rarely detected in children and most often they occur in association with neuroblastoma. Patients with anti-CRMP5 antibodies may have associated chorea, while anti-Ma2 antibodies often result in a diencephalic syndrome, and anti-GAD-65 and anti-amphiphysin both may associate with stiff person syndrome.40
In contrast to intracellularly targeted antibodies, disorders associated with antibodies to neuronal surface antigens have a distinct pathophysiology as these antibodies are thought to be directly pathogenic. Antibodies bind to the target antigen at synapses and result in altered synaptic function. There is less neuronal destruction and tissue inflammation, likely resulting in the more robust responses to immunotherapy, typically with agents targeting B-cells.39 Of these disorders, the most well established in the pediatric population is anti-NMDAR encephalitis, which is often associated with ovarian teratoma. This encephalitis usually begins with behavioral change or psychosis and then progresses to seizures in 70%, as well as decline of the level of consciousness, catatonia, dyskinesias, autonomic instability, and hypoventilation.37 Children comprise nearly half of identified patients, are more likely than adults to have seizure as the first presenting sign, and are less likely to have associated ovarian teratoma.41,42 Antibodies against the voltage gaited potassium channel (VGKC) complex, which includes leucine-rich glioma-inactivated protein 1 (LGI1) and contactin-associated protein-like 2 (CASPR2), are established as a cause of autoimmune epilepsy and limbic encephalitis in adults and case series of pediatric patients with likely autoimmune encephalitis have detected these antibodies in 4–15% of subjects. In these case series, a few patients with anti-CASPR2 encephalitis have been reported but no anti-LGI1 cases have yet been found. Similarly, although antibodies to the AMPA subtype of glutamate receptor have been reported as a cause of limbic encephalitis in adults, but these have not yet been found in children.43,44 Recently described autoantibodies to other synaptic proteins such as mGluR5, glycine receptors, GABA-A receptors and GABA-B receptors have been associated with seizures and refractory SE in children.40,45,46 Reports of these antibodies remain rare, but this is an emerging field, and both the spectrum of associated diseases and number of cases are expected to grow.
As discussed above, LP should be performed if autoimmune or paraneoplastic encephalitis is suspected. Elevated oligoclonal bands and CSF pleiocytosis support an autoimmune process with intrathecal synthesis of anti-neuronal antibodies, but these findings are not necessary for the diagnosis. Specific autoantibody testing for some of these disorders is available and should be pursued. In general, testing of CSF has superior sensitivity and specificity as compared to serum. Anti-NMDAR antibody testing can be send to a number of clinical laboratories. As the most commonly identified cause of encephalitis, testing for anti-NMDAR antibodies has higher diagnostic yield that other autoantibody testing or even testing for specific viral etiologies.47,48 If this test is negative or symptoms are atypical, additional autoantibody testing is commercially available with paraneoplastic autoantibody panels. In addition, any patient with known or suspected paraneoplastic disease should have appropriate tumor screening, which should include imaging of the chest and abdomen if ovarian teratoma is not detected in the pelvis.
Aside from paraneoplastic processes, Rasmussen’s encephalitis and Hashimoto’s encephalopathy are two of the most common autoimmune causes of SE. There are no specific diagnostic tests for Rasmussen’s encephalitis though patients will clinically present with focal seizures and unilateral cortical deficits and are found to have progressive unihemispheric cortical atrophy.49 If a biopsy is obtained, histopathology generally reveals T-cell dominated encephalitis with activated microglia.49 Hashimoto’s encephalopathy is an acute or subacute encephalopathy that occurs most commonly in women, and is associated with serum anti-thyroid peroxidase antibodies or anti-thyroglobulin antibodies. The majority of patients have seizures and cases of RSE have been reported.50 Anecdotal reports of patients with Hashimoto’s encephalopathy describe benefit with immunotherapy, and screening for anti-thyroid antibodies should be considered in patient with unexplained RSE.
Some genetic epilepsies may present with new-onset SE that do not produce obvious metabolic or imaging changes. While screening for genetic epilepsies in new-onset SE is usually not considered in the acute work-up, the question of an underlying genetic etiology is often considered in the subacute phase of the treatment, particularly when no other etiologies can be identified. So far, reports of patients with genetic epilepsies presenting with new-onset SE are anecdotal. Two genetic epilepsies commonly considered are epilepsies due to mutations in SCN1A51 or POLG1.52 Beyond the classical phenotypes of Dravet Syndrome and Alpers Syndrome, patients may present atypically with SE. However, in patients with severe epilepsies with new-onset SE such as febrile infection related epilepsy syndrome, these mutations seem to be rare.53
With increasing availability and decreasing costs of genetic testing, many genetic epilepsies are found to have a wider phenotypic spectrum than initially anticipated. Given that the genetic basis of a sizable fraction of genetic epilepsies may only be identified by parallel screening of a panel of candidate genes54 or through genome-wide approaches such as exome or genome sequencing,55 clinicians may consider adding either gene panel analysis or exome sequencing approaches into the systematic work flow of new onset SE, rather than only evaluating for them during a later stage of the workup. Improving the turnaround time for genetic tests will be crucial in establishing genetic testing as a routine part of the diagnostic workflow.
Status Epilepticus Management
The guideline states that “definitive control of SE should be established within 60 minutes of onset”4 with termination of both clinical and electrographic seizures. Benzodiazepines are the “emergent” medications of choice; lorazepam for intravenous administration, midazolam for intramuscular or intranasal administration, and diazepam for rectal administration.4 Repeat dosing may be provided in 5–10 minutes if needed. A double-blind randomized trial of 273 children with SE compared intravenous lorazepam (0.1mg/kg) and diazepam (0.2mg/kg) in the emergency department. A half-dose of either medication could be administered at 5 minutes if seizures persisted. The primary outcome was SE cessation by 10 minutes without recurrence in 30 minutes, and it was not significantly different in the two groups (72.1% with diazepam and 72.9% with lorazepam). Patients receiving lorazepam were more likely to be sedated (67% with lorazepam, 50% with diazepam) but there was no difference in requirement for assisted ventilation (18% with lorazepam, 16% with diazepam).56 If intravenous access cannot be obtained, then providers should administer rectal, intramuscular or buccal benzodiazepines. Providers can consider obtaining intraosseus access if prolonged intravenous access is unable to be obtained and the patient had respiratory or circulatory instability. Care should be taken to assess whether any pre-hospital benzodiazepines were administered since excess benzodiazepines may produce respiratory insufficiency.5,7,8 Unless the SE etiology has been identified and definitively corrected, all children should also receive an “urgent” category anticonvulsant in addition to a benzodiazepine.4
Nearly half of children will have persisting SE after receiving benzodiazepines,6,10 yet there are few comparative data evaluating the medication options available. Phenytoin is reported as the second-line agent by most respondents in surveys of pediatric emergency medicine physicians57 and neurologists.58 Fosphenytoin is a pro-drug of phenytoin, and while it may be administered more rapidly it must then be converted to phenytoin internally, so they likely reach therapeutic concentrations in the brain in about the same time. The Neurocritical Care Society guideline considers phenytoin/fosphenytoin to be an emergent treatment option, urgent treatment option, and refractory treatment option.4 Cardiac arrhythmias are rare, especially with fosphenytoin, but may occur with both. Fosphenytoin is associated with less tissue injury if infiltration occurs. Both are considered focal anticonvulsants, and they may be ineffective in treating SE related to generalized epilepsy. There are numerous drug interactions due to strong hepatic induction and high protein binding.
Phenobarbital is often considered a third or fourth line drug in most pediatric SE pathways. The Neurocritical Care Society guideline considers phenobarbital to be an emergent treatment option and an urgent control treatment option.4 Dosing is generally 20mg/kg followed by another 5–10mg/kg if needed. One study of 36 children with SE indicated that phenobarbital stopped seizures faster than a combination of diazepam and phenytoin and safety was similar,59 and several reports have described the use of high dose phenobarbital to control RSE and allow withdrawal of pharmacologic coma.60–62 Phenobarbital may cause sedation, respiratory depression and hypotension so cardiovascular and respiratory monitoring is generally required. It is a hepatic enzyme inducer leading to drug interactions.
Valproate sodium is a broad spectrum anticonvulsant and has been reported to be safe and highly effective in terminating SE and RSE. It is a broad spectrum anticonvulsant with multiple mechanisms of action including modulation of sodium and calcium channels and inhibitory GABA transmission.63 Because it has mechanisms independent of GABA receptors, valproate may be effective later in RSE once GABA receptors have been targeted by other agents. The Neurocritical Care Society guideline considers valproate sodium to be an emergent treatment option, an urgent control treatment option and a refractory treatment option.4 Several studies and reports have reported that valproate sodium at doses of 20–40 mg/kg is effective in terminating RSE in children without adverse effects.64–68 Black box warnings include hepatotoxicity (highest risk in children younger than two years, receiving anticonvulsant poly-therapy, and with suspected or known metabolic/mitochondrial disorders), pancreatitis, and teratogenicity. Other adverse effects include pancytopenia, thrombocytopenia, platelet dysfunction, hypersensitivity reactions (including Stevens-Johnson syndrome and toxic epidermal necrolysis), and encephalopathy (with or without elevated ammonia). There are numerous drug interactions due to strong hepatic inhibition.
Levetiracetam is a broad spectrum anticonvulsant and there is increasing evidence that levetiracetam may be safe and effective for treating SE. The Neurocritical Care Society guideline considers levetiracetam to be an urgent therapy option.4 Several observational studies in children have reported that levetiracetam may be safe and effective for managing SE and acute symptomatic seizures in children at doses of 20–60mg/kg.69–73 Levetiracetam has no hepatic metabolism, which may be beneficial in complex patients with liver dysfunction, metabolic disorders, or in those at risk for major drug interactions. In comparison to other intravenous anticonvulsants, levetiracetam has a low risk of sedation, cardio-respiratory depression, or coagulopathy. Since levetiracetam clearance is dependent on renal function, maintenance dosage reduction is required in patients with renal impairment.
Refractory Status Epilepticus
RSE is characterized by seizures that persist despite treatment with adequate doses of initial anticonvulsants. Definitions for RSE have varied in seizure durations (no time criteria, 30 minutes, one hour, or two hours) and/or lack of response to different numbers (two or three) and types of anticonvulsants. The Neurocritical Care Society guideline states that “patients who continue to experience either clinical or electrographic seizures after receiving adequate doses of an initial benzodiazepine followed by a second acceptable anticonvulsant will be considered refractory.”4 In contrast to prior definitions of RSE, there is no specific time that must elapse to define RSE, thereby emphasizing the importance of rapid sequential treatment. In the guideline, “refractory therapy” refers to anticonvulsants administered immediately if seizures persist after administration of an urgent control therapy medication, and the guideline discusses that “the main decision point at this step is to consider repeat bolus of the urgent control anticonvulsant or to immediately initiate additional agents.”4 Additional urgent control anticonvulsants may be reasonable if they have not yet been tried or if the patient needs to be transferred or stabilized prior to administration of continuous infusions. However, if an initial urgent control medication fails to terminate seizures then preparations should be initiated to achieve definitive seizure control with continuous infusions. Depending on RSE definitions and the cohorts described, RSE occurs in about 10–40% of children11,12,74 with SE. Studies in children have indicated that SE lasted more than one hour in 26–45% of patients,75,76 longer than two hours in 17–25% of patients,76,77 and longer than four hours in 10% of patients.76
In a subgroup of patients, RSE may last for weeks to months, despite treatment with multiple anticonvulsant medications. This lengthy course has been referred to as malignant RSE78 or super-refractory SE.79,80 Malignant RSE is associated with an infectious or inflammatory etiology, younger age, previous good health, and high morbidity and mortality.78,81,82 It has also been referred to as de-novo cryptogenic refractory multi-focal SE,82 new-onset refractory SE (NORSE),81,83,84 and febrile infection related epilepsy syndrome (FIRES).85–87 Some of these entities in which RSE occurs in a previously healthy person with no identified cause except a recent infection may represent overlapping terms describing similar or identical entities.88
The management of RSE has been reviewed previously in children89–92 and while there is variability in suggested pathways, all either administer additional anticonvulsants such as phenytoin/fosphenytoin, phenobarbital, valproate sodium, or levetiracetam, or they proceed to pharmacologic coma induction with intravenous or inhaled medications. A survey of 60 experts in SE management conveyed that there was substantial variability in the selected medications which included phenytoin, levetiracetam, valproate, and midazolam.93 Children who are treated for RSE should be done so in an intensive care unit under the supervision of a neurology and critical care team with experience in managing these patients. The Neurocritical Care Society guidelines recommend rapid advancement to pharmacologic coma induction rather than sequential trials of many urgent control anticonvulsants.4 Midazolam is a fast acting benzodiazepine that rapidly penetrates the blood brain barrier and has a short duration of action. Midazolam dosing usually involves an initial loading dose of 0.2 mg/kg followed by an infusion at 0.05–2 mg/kg/hour titrated as needed to achieve clinical or electrographic seizure suppression or EEG burst suppression. If seizures persist, escalating dosing through additional boluses is needed to rapidly increase levels and terminate seizures. Increasing the infusion rate without bolus dosing will lead to very slow increase in serum levels which is inconsistent with the goal of rapid seizure termination. A meta-analysis of 111 children indicated that midazolam was as effective as other coma inducing medications and had lower mortality94 and a multi-center, retrospective study suggested efficacy of both midazolam boluses and continuous infusion.13 An open label randomized study comparing midazolam and diazepam in 40 children indicated similar efficacy (86% and 89%), but midazolam was associated with higher recurrence (57% versus 16%) and higher mortality (38% versus 10.5%).95 Studies describe breakthrough seizures and seizures on weaning in 25–50% of children.
Pentobarbital is a barbiturate that may be used to to treat RSE. Dosing usually involves an initial loading dose of 5–15mg/kg (followed by another 5–10mg/kg if needed) followed by an infusion at 0.5–5 mg/kg/hour titrated as needed to achieve seizure suppression or EEG burst suppression. If seizures persist, escalating dosing through additional boluses is needed to rapidly increase levels and terminate seizures. Pentobarbital is long acting medication and therefore increasing the infusion rate without continue bolus dose administration will lead to very slow increases in pentobarbital levels which is inconsistent with the goal of rapid seizure termination. One case series of 26 children who received pentobarbital for RSE years provided a loading dose of 5 mg/kg followed by an infusion of 1–3 mg/kg/hour. Efficacy was 74% but 22% had relapse of seizures upon pentobarbital weaning.96 A case series of 30 patients who received pentobarbital for RSE described sustained burst suppression without relapse in 33%.97 Adverse effects include respiratory depression, hypotension, cardiac depression, paralytic ileus, infection, and suppression of brainstem reflexes. Anesthetics such as isoflurane are effective in inducing a burst suppression pattern and terminating seizures, but only case reports are available.98,99 Management of a RSE patient with volatile anesthestics should be performed the with support and guidance of an anesthesiologist. Propofol may also be used to terminate seizures, but is rarely used in children due to its FDA black box warning because of the risk of propofol infusion syndrome.
Patients treated with continuous infusions or inhaled anesthetics require intensive monitoring. All will require invasive continuous mechanical ventilation for both airway protection and to maintain appropriate oxygenation and ventilation as medications are titrated. Central venous access and arterial access should be considered as these patients require frequent laboratory sampling and are at high risk for developing hypotension requiring vasopressor or inotropic support. Because high dose sedatives and anesthetics can blunt the shivering response and endogenous thermoregulation, continuous core temperature monitoring should be employed and when needed external thermoregulation. Patients should undergo continued evaluation for the development lactic acidosis, anemia, thrombocytopenia, and end organ dysfunction such as acute liver or renal injury. Finally, these patients are at increased risk of secondary infections due to indwelling catheters (central catheters, endotracheal tubes, foley catheters) as well as some medications (pentobarbital). Clinicians should maintain a high index of suspicion for infection as hypotension and hypothermia can be perceived to be due to seizure treatment, when they may be due the onset of sepsis.
When coma inducing agents are employed, it remains unclear whether the treatment goal should be termination of seizures, burst suppression, or complete suppression of EEG activity. The Neurocritical Care Society guideline states that “dosing of continuous infusions anticonvulsants for RSE should be titrated to cessation of electrographic seizures or burst suppression.”4 Patients may have seizures, even when the inter-ictal background is primarily a burst suppression pattern or even complete suppression, so this level of suppression does not guarantee seizure suppression. Adult reports comparing treatment goals of burst suppression versus seizure termination are inconclusive100–102 and there are no data in children.
It remains unclear how long the patient should be maintained in pharmacologic coma. The Neurocritical Care Society guideline states that “a period of 24–48 hours of electrographic control is recommended prior to slow withdrawal of continuous infusion anticonvulsants for RSE”4 and a survey of experts in SE management across all age groups reported they would continue pharmacologic coma for 24 hours.93 Electrographic or electro-clinical seizures frequently recur during weaning of pharmacologic coma medications95,96,103,104 indicating that pharmacologic coma should be considered as a temporizing measure, and during this period other anticonvulsants should be initiated which may provide seizure control as coma inducing medications are weaned. Often coma inducing medications are weaned over 1–2 days, although this is not evidence based. If definite seizures occur, reinitiating pharmacologic coma may provide additional time to adjust other anticonvulsants. A survey of experts on SE management reported that if seizures recurred during weaning of the pharmacologic coma they would reintroduce pharmacologic coma for 24–48 hours.93 However, after several weaning attempts, reinitiating pharmacologic coma for recurrent seizures may not be optimal. First, continued pharmacologic coma use is associated with adverse effects. Second, in a report of prognosis of 22 children with RSE, all survivors had intractable epilepsy and many children had persisting seizures during or shortly after weaning of anti-seizure medications.105 Since future seizures are so likely, some seizures may be tolerated during weaning from pharmacologic coma.
Case reports and series have described several add-on medications and other techniques have been reported useful in reducing seizure recurrence as pharmacologic coma is weaned, but there are no large studies. These options include topiramate, ketamine, pyridoxine, the ketogenic diet, epilepsy surgery, immunomodulation, hypothermia, and electroconvulsive therapy.
Topiramate is a broad spectrum anticonvulsant with several mechanisms of action and may be logical to use in RSE once GABA receptors have been targeted by other medications. While not available in an intravenous form, tablets can be crushed for use with feeding tubes. Studies have not evaluated topiramate for early SE, but reports suggest it may a useful add-on medication for RSE.62,106–108 Dosing is varied in these reports, but often starts at 1–5mg/kg/day with escalation to 20–25mg/kg/day. Topiramate is a carbonic anhydrase inhibitor and may result in metabolic acidosis by preventing bicarbonate formation. Rare adverse reactions include nephrolithiasis, pancreatitis, acute angle closure glaucoma, and oligohydrosis with resulting hyperthermia.
Ketamine is a non-competitive N-methyl D-aspartate (NMDA)-type glutamate receptor antagonist that may be effective in later stages of RSE since it acts independently of GABA- related mechanisms. Only case reports and series are available reporting 0.5–2 mg/kg loading doses followed by continuous infusions. The largest case series described 9 children with RSE with ketamine administered at a median of 6 days of RSE at a median dose of 40 mcg/kg/min. RSE was controlled in 66% and no major adverse effects were reported.109 Ketamine’s sympathomimetic properties may produce hypertension and tachycardia.
Pyridoxine-dependent seizures are typically related to a rare autosomal recessive mutation in the ALDH7A1 gene which encodes antiquitin. While generally considered in neonates with seizures, there have been reports of older patients including infants and even adults with SE controlled by pyridoxine.110–114 The diagnosis of pyridoxine-responsive seizures is made when administration of intravenous pyridoxine (100mg given for one to five doses) terminates seizures, typically within hours of administration. Pyridoxine responsiveness may occur in children with ALDH7A1 mutations, pyridoxine 5′-phosphate oxidase (PNPO) deficiency, hypophosphatasia, nutritional pyridoxine deficiency, and some children with idiopathic epilepsy. Some children who do not respond to pyridoxine may respond to pyridoxal-5-phosphate (PLP, also known as P5P, 50–100 mg/kg/day) or to folinic acid (3–5 mg/kg/day).115,116 Diagnosis of pyridoxine dependent epilepsy may be established by evaluating for elevated urinary alpha-aminoadipic semialdehyde or a specific marker on neurotransmitter testing. For patients who have responded to therapy testing can be performed for mutations in known genes.117 Other vitamin response epilepsies will typically present during infancy, but late onset PNPO deficiency responding only to PLP as well as late onset biotinidase deficiency responding to biotin have both been described.118,119 Thus, a trial of PLP or biotin could be considered in refractory SE.
The ketogenic diet is a high fat, low carbohydrate diet which can be administered by parenteral nutrition of intravenously. It is considered the treatment of choice for GLUT-1 transporter deficiency and pyruvate dehydrogenase deficiency while it is contraindicated in patients with porphyria, pyruvate carboxylase deficiency, disorders of fatty acid oxidation and metabolism, and some other metabolic disorders. Screening labs include serum acylcarnitine profile, amino acids, lactate, ammonia, complete blood count, electrolytes, liver function tests, and urine organic acids.120,121 Implementation is complex and multiple adverse effects may occur, necessitating an experienced team.122 Adverse effects include hypoglycemia, metabolic acidosis, hypertriglyceridemia, gastroesophageal reflux, emesis, constipation, nephrolithiasis, esophagitis, renal tubular acidosis, hepatitis, lipoid pneumonia, pancreatitis, and metabolic abnormalities. Several case reports and series describe benefit with the ketogenic diet including benefit in 7 of 9 patients with febrile infection related epilepsy syndrome in a mean of 5 days after starting the diet.123 A literature review summarized 32 reported cases in which children and adults with SE were treated with dietary therapy and reported that 78% became seizure-free, with a response usually evident in 7–10 days.122
Immunomodulatory therapies may be useful in the context of cryptogenic RSE or when there is a confirmed autoimmune or inflammatory etiology, such as Rasmussen encephalitis, central nervous system vasculitis, NMDA receptor encephalitis, or Hashimoto’s encephalopathy. Though there have been no controlled studies of the use of corticosteroids, intravenous immunoglobulin (IVIG), or plasma exchange for RSE, small observational studies have described benefit. For example, in one case series of 5 adults with cryptogenic RSE, the 3 patients with good outcomes were all treated with early immunotherapy.124
When steroids are used for RSE, initial treatment consists of intravenous methylprednisolone at a dose of 30mg/kg/day (maximum of 1 gram) for 3 days. If infection is being considered as an etiology for RSE, infectious disease specialists should be involved before initiation of steroids, and patients should be covered with appropriate antiviral and antibiotic agents until infectious studies have resulted. IVIG has also been used in refractory epilepsy if an underlying autoimmune etiology is suspected.124,125 This therapy is typically employed if a patient has an inadequate response to steroids or if steroids are contraindicated. Standard dosage for IVIG is 2g/kg divided over 2–5 days. All diagnostic serum antibody testing should be obtained prior to initiation of IVIG. Though plasma exchange is more invasive than intravenous methylprednisolone and IVIG as it requires placement of an indwelling catheter, patients with RSE have had reported benefit from this treatment.126,127 While the mechanism of action of plasma exchange is likely multifactorial, it is thought to have an effect by removing pathogenic antibodies and immune complexes from blood, and by modulating pro-inflammatory cytokines. A typical course of plasma exchange consists of 5–7 exchanges over 14 days. A patient’s coagulation factors, electrolytes, and fluid balance must be closely monitored over this time period and levels of anti-epileptic medications should be checked regularly to ensure that they remain therapeutic. As with IVIG, all diagnostic serum antibody testing should be obtained before initiation of plasma exchange. If an autoimmune or paraneoplastic etiology is confirmed as the cause for RSE and a patient does not respond to initial treatment with methylprednisolone, IVIG, or plasma exchange, second-line treatment with rituximab or cyclophosphamide should be considered. As these second-line therapies are associated with potentially severe adverse effects, clinical response should closely monitored after initiation of these treatments to help determine whether subsequent maintenance immunosuppression is warranted. While response to immunotherapy can support a diagnosis of autoimmune epilepsy, direct benefit is often difficult to prove as patients are often concurrently being treated with conventional anti-epileptic agents. It is also important to note that steroids have also been found to be beneficial in epilepsies that are not autoimmune such as Landau-Kleffner syndrome, and in infantile spasms.
Case reports and series have reported efficacy of a variety of surgical procedures for many types of lesions when all seizures have onset in an identifiable location. One case series of 15 children with RSE who underwent surgical procedures reported that all had seizure control ranging from seizure freedom to substantial reductions, allowing transition out of the ICU.128 Vagus nerve stimulation has been reported effective in several case reports, but efficacy generally occurs over a prolonged period.
When used as a neuroprotective strategy for multiple types of brain injury, therapeutic hypothermia may reduce many destructive processes due to excitotoxicity, neuroinflammation, apoptosis, free radical production, seizures, and blood- brain barrier disruption. Only case reports and small case series have described the use of therapeutic hypothermia for SE and some indicate that 1–5 days of hypothermia to 32–36°C may terminate seizures and that some patients do not have seizure recurrence upon rewarming.129,130
RSE is considered an indication for electroconvulsive therapy by the American Psychiatric Association Task Force Report.131 Only case reports and small series are available but they indicate some patients have temporary improvement and rare patients have full functional recovery.132 Cardiovascular conditions are a relative contraindication, and in some patients electroconvulsive therapy may also provoke SE.
Conclusions
SE is a common neurologic emergency. Rapid efforts are needed to manage systemic complications, identify and manage precipitating conditions, and terminate seizures. A predetermined management plan that stresses urgent progression through appropriately dosed anticonvulsants may avoid delays. While data are limited regarding RSE management, a logical stepwise approach using available options is needed. Pharmacologic coma induction offers a window to identify and manage precipitant etiologies and to put in place anti-seizure strategies aiming to provide seizure control during pharmacologic coma weaning. While studies have compared benzodiazepines and administration strategies, there has been little study of medications used when seizures persist after administration benzodiazepines. However, research consortia are developing to identify and develop evidence-based interventions to improve the care of children with SE.133
Figure 2.
Suggested Infectious Disease Evaluation for Children with Refractory Status Epilepticus (RSE). Adapted from prior tables.35,36
Acknowledgments
Funding:
Nicholas Abend is funded by NIH K23-NS076550
Alexis Topjian is funded by NIH K23-NS075363
Jessica Panzer is funded by NIH K12-NS049453
Jennifer McGuire is funded by NIH K12NS049453
Dennis Dlugos is funded by NIH grants 1R01NS053998, 2U01NS045911, 1R01LM011124, and U01NS077276
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368:222–9. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- 2.Commission on Epidemiology and Prognosis. International League Against Epilepsy: Guidelines for epidemiologic studies on epilepsy. Epilepsia. 1993;34:592–6. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 3.Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol. 2001;49:659–64. [PubMed] [Google Scholar]
- 4.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 5.Tobias JD, Berkenbosch JW. Management of status epilepticus in infants and children prior to pediatric ICU admission: deviations from the current guidelines. South Med J. 2008;101:268–72. doi: 10.1097/SMJ.0b013e318164e3f0. [DOI] [PubMed] [Google Scholar]
- 6.Lewena S, Pennington V, Acworth J, et al. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care. 2009;25:83–7. doi: 10.1097/PEC.0b013e318196ea6e. [DOI] [PubMed] [Google Scholar]
- 7.Chin RF, Verhulst L, Neville BG, Peters MJ, Scott RC. Inappropriate emergency management of status epilepticus in children contributes to need for intensive care. J Neurol Neurosurg Psychiatry. 2004;75:1584–8. doi: 10.1136/jnnp.2003.032797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tirupathi S, McMenamin JB, Webb DW. Analysis of factors influencing admission to intensive care following convulsive status epilepticus in children. Seizure. 2009;18:630–3. doi: 10.1016/j.seizure.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. 2008;7:696–703. doi: 10.1016/S1474-4422(08)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia. 2008;49:615–25. doi: 10.1111/j.1528-1167.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewena S, Young S. When benzodiazepines fail: how effective is second line therapy for status epilepticus in children? Emerg Med Australas. 2006;18:45–50. doi: 10.1111/j.1742-6723.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson K, Metsaranta P, Huhtala H, Auvinen A, Kuusela AL, Koivikko M. Treatment delay and the risk of prolonged status epilepticus. Neurology. 2005;65:1316–8. doi: 10.1212/01.wnl.0000180959.31355.92. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K, Osawa M, Aihara M, et al. Efficacy of Intravenous Midazolam for Status Epilepticus in Childhood. Pediatr Neurol. 2007;36:366–72. doi: 10.1016/j.pediatrneurol.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Shorvon S, Baulac M, Cross H, Trinka E, Walker M. The drug treatment of status epilepticus in Europe: Consensus document from a workshop at the first London Colloquium on Status Epilepticus. Epilepsia. 2008;49:1277–86. doi: 10.1111/j.1528-1167.2008.01706_3.x. [DOI] [PubMed] [Google Scholar]
- 15.Abend NS, Loddenkemper T. Pediatric status epilepticus management. Curr Opin Pediatr. 2014 doi: 10.1097/MOP.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abend NS, Loddenkemper T. Management of pediatric status epilepticus. Curr Treat Options Neurol. 2014;16:301. doi: 10.1007/s11940-014-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abend NS, Gutierrez-Colina AM, Dlugos DJ. Medical Treatment of Pediatric Status Epilepticus. Semin Pediatr Neurol. 2010;17:169–75. doi: 10.1016/j.spen.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Hussain N, Appleton R, Thorburn K. Aetiology, course and outcome of children admitted to paediatric intensive care with convulsive status epilepticus: a retrospective 5-year review. Seizure. 2007;16:305–12. doi: 10.1016/j.seizure.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Singh RK, Stephens S, Berl MM, et al. Prospective study of new-onset seizures presenting as status epilepticus in childhood. Neurology. 2010;74:636–42. doi: 10.1212/WNL.0b013e3181d0cca2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishiyama I, Ohtsuka Y, Tsuda T, et al. An epidemiological study of children with status epilepticus in Okayama, Japan. Epilepsia. 2007;48:1133–7. doi: 10.1111/j.1528-1167.2007.01106.x. [DOI] [PubMed] [Google Scholar]
- 21.Berg AT, Shinnar S, Testa FM, et al. Status epilepticus after the initial diagnosis of epilepsy in children. Neurology. 2004;63:1027–34. doi: 10.1212/01.wnl.0000138425.54223.dc. [DOI] [PubMed] [Google Scholar]
- 22.Berg AT, Shinnar S, Levy SR, Testa FM. Status epilepticus in children with newly diagnosed epilepsy. Ann Neurol. 1999;45:618–23. doi: 10.1002/1531-8249(199905)45:5<618::aid-ana10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Riviello JJ, Ashwal S, Hirtz D, et al. Practice Parameter: Diagnostic assessment of the child with status epilepticus (an evidence-based review) Neurology. 2006;67:1542–50. doi: 10.1212/01.wnl.0000243197.05519.3d. [DOI] [PubMed] [Google Scholar]
- 24.Watemberg N, Segal G. A suggested approach to the etiologic evaluation of status epilepticus in children: what to seek after the usual causes have been ruled out. J Child Neurol. 2010;25:203–11. doi: 10.1177/0883073809337032. [DOI] [PubMed] [Google Scholar]
- 25.Abend NS, Gutierrez-Colina AM, Topjian AA, et al. Nonconvulsive seizures are common in critically ill children. Neurology. 2011;76:1071–7. doi: 10.1212/WNL.0b013e318211c19e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: Cohort study of risk factors and mortality. Neurology. 2013;81:383–91. doi: 10.1212/WNL.0b013e31829c5cfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez Fernandez I, Abend NS, Arndt DH, et al. Electrographic seizures after convulsive status epilepticus in children and young adults. A retrospective multicenter study. Journal of Pediatrics. 2014;164:339–46. doi: 10.1016/j.jpeds.2013.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkham FJ, Wade AM, McElduff F, et al. Seizures in 204 comatose children: incidence and outcome. Intensive Care Med. 2012;38:853–62. doi: 10.1007/s00134-012-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic Status Epilepticus is Associated with Mortality and Worse Short-Term Outcome in Critically Ill Children. Critical Care Medicine. 2013;31:215–23. doi: 10.1097/CCM.0b013e3182668035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagenman KL, Blake TP, Sanchez SM, et al. Electrographic status epilepticus and long-term outcome in critically ill children. Neurology. 2014;82:396–404. doi: 10.1212/WNL.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–38. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neligan A, Shorvon SD. Frequency and prognosis of convulsive status epilepticus of different causes: a systematic review. Arch Neurol. 2010;67:931–40. doi: 10.1001/archneurol.2010.169. [DOI] [PubMed] [Google Scholar]
- 33.Saz EU, Karapinar B, Ozcetin M, et al. Convulsive status epilepticus in children: etiology, treatment protocol and outcome. Seizure. 2011;20:115–8. doi: 10.1016/j.seizure.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 34.Glaser CA, Gilliam S, Honarmand S, et al. Refractory status epilepticus in suspect encephalitis. Neurocrit Care. 2008;9:74–82. doi: 10.1007/s12028-007-9042-y. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57:1114–28. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGuire JL, AMG . Central Nervous System Infections. In: Abend NS, Helfaer M, editors. Pediatric Neurocritical Care. New York, NY: Demos; 2013. pp. 267–336. [Google Scholar]
- 37.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis R, Dalmau J. Autoimmunity, seizures, and status epilepticus. Epilepsia. 2013;54 (Suppl 6):46–9. doi: 10.1111/epi.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bien CG, Vincent A, Barnett MH, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–38. doi: 10.1093/brain/aws082. [DOI] [PubMed] [Google Scholar]
- 40.Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698–701. doi: 10.1212/WNL.0b013e3182364a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–8. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haberlandt E, Bast T, Ebner A, et al. Limbic encephalitis in children and adolescents. Arch Dis Child. 2011;96:186–91. doi: 10.1136/adc.2010.183897. [DOI] [PubMed] [Google Scholar]
- 44.Suleiman J, Wright S, Gill D, et al. Autoantibodies to neuronal antigens in children with new-onset seizures classified according to the revised ILAE organization of seizures and epilepsies. Epilepsia. 2013;54:2091–100. doi: 10.1111/epi.12405. [DOI] [PubMed] [Google Scholar]
- 45.Kruer MC, Hoeftberger R, Lim KY, et al. Aggressive course in encephalitis with opsoclonus, ataxia, chorea, and seizures: the first pediatric case of gamma-aminobutyric acid type B receptor autoimmunity. JAMA Neurol. 2014;71:620–3. doi: 10.1001/jamaneurol.2013.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–86. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54:899–904. doi: 10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. The Lancet Infectious diseases. 2010;10:835–44. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 49.Varadkar S, Bien CG, Kruse CA, et al. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13:195–205. doi: 10.1016/S1474-4422(13)70260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai MH, Lee LH, Chen SD, Lu CH, Chen MT, Chuang YC. Complex partial status epilepticus as a manifestation of Hashimoto’s encephalopathy. Seizure. 2007;16:713–6. doi: 10.1016/j.seizure.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Dravet C, Oguni H. Dravet syndrome (severe myoclonic epilepsy in infancy) Handbook of clinical neurology. 2013;111:627–33. doi: 10.1016/B978-0-444-52891-9.00065-8. [DOI] [PubMed] [Google Scholar]
- 52.Wolf NI, Rahman S, Schmitt B, et al. Status epilepticus in children with Alpers’ disease caused by POLG1 mutations: EEG and MRI features. Epilepsia. 2009;50:1596–607. doi: 10.1111/j.1528-1167.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 53.Appenzeller S, Helbig I, Stephani U, et al. Febrile infection-related epilepsy syndrome (FIRES) is not caused by SCN1A, POLG, PCDH19 mutations or rare copy number variations. Dev Med Child Neurol. 2012;54:1144–8. doi: 10.1111/j.1469-8749.2012.04435.x. [DOI] [PubMed] [Google Scholar]
- 54.Lemke JR, Riesch E, Scheurenbrand T, et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia. 2012;53:1387–98. doi: 10.1111/j.1528-1167.2012.03516.x. [DOI] [PubMed] [Google Scholar]
- 55.Allen AS, Berkovic SF, Cossette P, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–21. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chamberlain JM, Okada P, Holsti M, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA. 2014;311:1652–60. doi: 10.1001/jama.2014.2625. [DOI] [PubMed] [Google Scholar]
- 57.Babl FE, Sheriff N, Borland M, et al. Emergency management of paediatric status epilepticus in Australia and New Zealand: practice patterns in the context of clinical practice guidelines. J Paediatr Child Health. 2009;45:541–6. doi: 10.1111/j.1440-1754.2009.01536.x. [DOI] [PubMed] [Google Scholar]
- 58.Claassen J, Hirsch LJ, Mayer SA. Treatment of status epilepticus: a survey of neurologists. J Neurol Sci. 2003;211:37–41. doi: 10.1016/s0022-510x(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 59.Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epileticus: a prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38:202–7. doi: 10.1212/wnl.38.2.202. [DOI] [PubMed] [Google Scholar]
- 60.Crawford TO, Mitchell WG, Fishman LS, Snodgrass SR. Very-high-dose phenobarbital for refractory status epilepticus in children. Neurology. 1988;38:1035–40. doi: 10.1212/wnl.38.7.1035. [DOI] [PubMed] [Google Scholar]
- 61.Wilmshurst JM, van der Walt JS, Ackermann S, Karlsson MO, Blockman M. Rescue therapy with high-dose oral phenobarbitone loading for refractory status epilepticus. J Paediatr Child Health. 2010;46:17–22. doi: 10.1111/j.1440-1754.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 62.Lin JJ, Lin KL, Wang HS, Hsia SH, Wu CT. Effect of topiramate, in combination with lidocaine, and phenobarbital, in acute encephalitis with refractory repetitive partial seizures. Brain Dev. 2009;31:605–11. doi: 10.1016/j.braindev.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–64. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 64.Yu KT, Mills S, Thompson N, Cunanan C. Safety and efficacy of intravenous valproate in pediatric status epilepticus and acute repetitive seizures. Epilepsia. 2003;44:724–6. doi: 10.1046/j.1528-1157.2003.41302.x. [DOI] [PubMed] [Google Scholar]
- 65.Uberall MA, Trollmann R, Wunsiedler U, Wenzel D. Intravenous valproate in pediatric epilepsy patients with refractory status epilepticus. Neurology. 2000;54:2188–9. doi: 10.1212/wnl.54.11.2188-a. [DOI] [PubMed] [Google Scholar]
- 66.Mehta V, Singhi P, Singhi S. Intravenous sodium valproate versus diazepam infusion for the control of refractory status epilepticus in children: a randomized controlled trial. J Child Neurol. 2007;22:1191–7. doi: 10.1177/0883073807306248. [DOI] [PubMed] [Google Scholar]
- 67.Campistol J, Fernandez A, Ortega J. Status epilepticus in children. Experience with intravenous valproate. Update of treatment guidelines. Rev Neurol. 1999;29:359–65. [PubMed] [Google Scholar]
- 68.Hovinga CA, Chicella MF, Rose DF, Eades SK, Dalton JT, Phelps SJ. Use of intravenous valproate in three pediatric patients with nonconvulsive or convulsive status epilepticus. Ann Pharmacother. 1999;33:579–84. doi: 10.1345/aph.18349. [DOI] [PubMed] [Google Scholar]
- 69.Abend NS, Chapman KE, Gallentine WB, et al. Electroencephalographic monitoring in the pediatric intensive care unit. Curr Neurol Neurosci Rep. 2013;13:330. doi: 10.1007/s11910-012-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reiter PD, Huff AD, Knupp KG, Valuck RJ. Intravenous levetiracetam in the management of acute seizures in children. Pediatric Neurology. 2010;43:117–21. doi: 10.1016/j.pediatrneurol.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Abend NS, Monk HM, Licht DJ, Dlugos DJ. Intravenous levetiracetam in critically ill children with status epilepticus or acute repetitive seizures. Pediatr Crit Care Med. 2009;10:505–10. doi: 10.1097/PCC.0b013e3181a0e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goraya JS, Khurana DS, Valencia I, et al. Intravenous Levetiracetam in Children With Epilepsy. Pediatr Neurol. 2008;38:177–80. doi: 10.1016/j.pediatrneurol.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Gallentine WB, Hunnicutt AS, Husain AM. Levetiracetam in children with refractory status epilepticus. Epilepsy Behav. 2009;14:215–8. doi: 10.1016/j.yebeh.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 74.Brevoord JC, Joosten KF, Arts WF, van Rooij RW, de Hoog M. Status epilepticus: clinical analysis of a treatment protocol based on midazolam and phenytoin. J Child Neurol. 2005;20:476–81. doi: 10.1177/08830738050200060201. [DOI] [PubMed] [Google Scholar]
- 75.Maytal J, Shinnar S, Moshe SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83:323–31. [PubMed] [Google Scholar]
- 76.Dunn DW. Status epilepticus in children: etiology, clinical features, and outcome. J Child Neurol. 1988;3:167–73. doi: 10.1177/088307388800300303. [DOI] [PubMed] [Google Scholar]
- 77.Eriksson KJ, Koivikko MJ. Status epilepticus in children: aetiology, treatment, and outcome. Dev Med Child Neurol. 1997;39:652–8. doi: 10.1111/j.1469-8749.1997.tb07358.x. [DOI] [PubMed] [Google Scholar]
- 78.Holtkamp M, Othman J, Buchheim K, Masuhr F, Schielke E, Meierkord H. A “malignant” variant of status epilepticus. Arch Neurol. 2005;62:1428–31. doi: 10.1001/archneur.62.9.1428. [DOI] [PubMed] [Google Scholar]
- 79.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134:2802–18. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 80.Shorvon S. Super-refractory status epilepticus: An approach to therapy in this difficult clinical situation. Epilepsia. 2011;52 (Suppl 8):53–6. doi: 10.1111/j.1528-1167.2011.03238.x. [DOI] [PubMed] [Google Scholar]
- 81.Wilder-Smith EP, Lim EC, Teoh HL, et al. The NORSE (new-onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singapore. 2005;34:417–20. [PubMed] [Google Scholar]
- 82.Van Lierde I, Van Paesschen W, Dupont P, Maes A, Sciot R. De novo cryptogenic refractory multifocal febrile status epilepticus in the young adult: a review of six cases. Acta Neurol Belg. 2003;103:88–94. [PubMed] [Google Scholar]
- 83.Rathakrishnan R, Wilder-Smith EP. New onset refractory status epilepticus (NORSE) J Neurol Sci. 2009;284:220. doi: 10.1016/j.jns.2009.03.023. author reply -1. [DOI] [PubMed] [Google Scholar]
- 84.Costello DJ, Kilbride RD, Cole AJ. Cryptogenic New Onset Refractory Status Epilepticus (NORSE) in adults-Infectious or not? J Neurol Sci. 2009;277:26–31. doi: 10.1016/j.jns.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Kramer U, Chi CS, Lin KL, et al. Febrile infection-related epilepsy syndrome (FIRES): Pathogenesis, treatment, and outcome: A multicenter study on 77 children. Epilepsia. 2011 doi: 10.1111/j.1528-1167.2011.03250.x. [DOI] [PubMed] [Google Scholar]
- 86.Kramer U, Shorer Z, Ben-Zeev B, Lerman-Sagie T, Goldberg-Stern H, Lahat E. Severe refractory status epilepticus owing to presumed encephalitis. J Child Neurol. 2005;20:184–7. doi: 10.1177/08830738050200030301. [DOI] [PubMed] [Google Scholar]
- 87.van Baalen A, Hausler M, Boor R, et al. Febrile infection-related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia. 2010;51:1323–8. doi: 10.1111/j.1528-1167.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 88.Ismail FY, Kossoff EH. AERRPS, DESC, NORSE, FIRES: Multi-labeling or distinct epileptic entities? Epilepsia. 2011 doi: 10.1111/j.1528-1167.2011.03293.x. [DOI] [PubMed] [Google Scholar]
- 89.Abend NS, Dlugos DJ. Treatment of refractory status epilepticus: literature review and a proposed protocol. Pediatr Neurol. 2008;38:377–90. doi: 10.1016/j.pediatrneurol.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 90.Owens J. Medical Management of Refractory Status Epilepticus. Semin Pediatr Neurol. 2010;17:176–81. doi: 10.1016/j.spen.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 91.Wheless JW. Treatment of Refractory Convulsive Status Epilepticus in Children: Other Therapies. Semin Pediatr Neurol. 2010;17:190–4. doi: 10.1016/j.spen.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 92.Wilkes R, Tasker RC. Pediatric intensive care treatment of uncontrolled status epilepticus. Crit Care Clin. 2013;29:239–57. doi: 10.1016/j.ccc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Riviello JJ, Jr, Claassen J, LaRoche SM, et al. Treatment of status epilepticus: an international survey of experts. Neurocrit Care. 2013;18:193–200. doi: 10.1007/s12028-012-9790-1. [DOI] [PubMed] [Google Scholar]
- 94.Gilbert DL, Gartside PS, Glauser TA. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children: a meta-analysis. J Child Neurol. 1999;14:602–9. doi: 10.1177/088307389901400909. [DOI] [PubMed] [Google Scholar]
- 95.Singhi S, Murthy A, Singhi P, Jayashree M. Continuous midazolam versus diazepam infusion for refractory convulsive status epilepticus. J Child Neurol. 2002;17:106–10. doi: 10.1177/088307380201700203. [DOI] [PubMed] [Google Scholar]
- 96.Kim SJ, Lee DY, Kim JS. Neurologic outcomes of pediatric epileptic patients with pentobarbital coma. Pediatr Neurol. 2001;25:217–20. doi: 10.1016/s0887-8994(01)00311-3. [DOI] [PubMed] [Google Scholar]
- 97.Barberio M, Reiter PD, Kaufman J, Knupp K, Dobyns EL. Continuous infusion pentobarbital for refractory status epilepticus in children. J Child Neurol. 2012;27:721–6. doi: 10.1177/0883073811424941. [DOI] [PubMed] [Google Scholar]
- 98.Kofke WA, Young RS, Davis P, et al. Isoflurane for refractory status epilepticus: a clinical series. Anesthesiology. 1989;71:653–9. doi: 10.1097/00000542-198911000-00005. [DOI] [PubMed] [Google Scholar]
- 99.Mirsattari SM, Sharpe MD, Young GB. Treatment of refractory status epilepticus with inhalational anesthetic agents isoflurane and desflurane. Arch Neurol. 2004;61:1254–9. doi: 10.1001/archneur.61.8.1254. [DOI] [PubMed] [Google Scholar]
- 100.Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol. 2005;62:1698–702. doi: 10.1001/archneur.62.11.1698. [DOI] [PubMed] [Google Scholar]
- 101.Krishnamurthy KB, Drislane FW. Depth of EEG suppression and outcome in barbiturate anesthetic treatment for refractory status epilepticus. Epilepsia. 1999;40:759–62. doi: 10.1111/j.1528-1157.1999.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 102.Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43:146–53. doi: 10.1046/j.1528-1157.2002.28501.x. [DOI] [PubMed] [Google Scholar]
- 103.Morrison G, Gibbons E, Whitehouse WP. High-dose midazolam therapy for refractory status epilepticus in children. Intensive Care Med. 2006;32:2070–6. doi: 10.1007/s00134-006-0362-8. [DOI] [PubMed] [Google Scholar]
- 104.Koul R, Chacko A, Javed H, Al Riyami K. Eight-year study of childhood status epilepticus: midazolam infusion in management and outcome. J Child Neurol. 2002;17:908–10. [PubMed] [Google Scholar]
- 105.Sahin M, Menache CC, Holmes GL, Riviello JJ. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42:1461–7. doi: 10.1046/j.1528-1157.2001.21301.x. [DOI] [PubMed] [Google Scholar]
- 106.Kahriman M, Minecan D, Kutluay E, Selwa L, Beydoun A. Efficacy of topiramate in children with refractory status epilepticus. Epilepsia. 2003;44:1353–6. doi: 10.1046/j.1528-1157.2003.11803.x. [DOI] [PubMed] [Google Scholar]
- 107.Perry MS, Holt PJ, Sladky JT. Topiramate loading for refractory status epilepticus in children. Epilepsia. 2006;47:1070–1. doi: 10.1111/j.1528-1167.2006.00564.x. [DOI] [PubMed] [Google Scholar]
- 108.Blumkin L, Lerman-Sagie T, Houri T, et al. Pediatric refractory partial status epilepticus responsive to topiramate. J Child Neurol. 2005;20:239–41. doi: 10.1177/08830738050200031701. [DOI] [PubMed] [Google Scholar]
- 109.Rosati A, L’Erario M, Ilvento L, et al. Efficacy and safety of ketamine in refractory status epilepticus in children. Neurology. 2012;79:2355–8. doi: 10.1212/WNL.0b013e318278b685. [DOI] [PubMed] [Google Scholar]
- 110.Yoshii A, Takeoka M, Kelly PJ, Krishnamoorthy KS. Focal status epilepticus as atypical presentation of pyridoxine-dependent epilepsy. J Child Neurol. 2005;20:696–8. doi: 10.1177/08830738050200081301. [DOI] [PubMed] [Google Scholar]
- 111.Goutieres F, Aicardi J. Atypical presentations of pyridoxine-dependent seizures: a treatable cause of intractable epilepsy in infants. Ann Neurol. 1985;17:117–20. doi: 10.1002/ana.410170203. [DOI] [PubMed] [Google Scholar]
- 112.Chou ML, Wang HS, Hung PC, Sun PC, Huang SC. Late-onset pyridoxine-dependent seizures: report of two cases. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1995;36:434–7. [PubMed] [Google Scholar]
- 113.Kluger G, Blank R, Paul K, et al. Pyridoxine-dependent epilepsy: normal outcome in a patient with late diagnosis after prolonged status epilepticus causing cortical blindness. Neuropediatrics. 2008;39:276–9. doi: 10.1055/s-0029-1202833. [DOI] [PubMed] [Google Scholar]
- 114.Russell KE, Mulligan SR, Mallory LA. Diagnosis of pyridoxine-dependent seizures in a nineteen-year-old patient. Pediatr Neurol. 2012;47:141–3. doi: 10.1016/j.pediatrneurol.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 115.Wang HS, Kuo MF, Chou ML, et al. Pyridoxal phosphate is better than pyridoxine for controlling idiopathic intractable epilepsy. Arch Dis Child. 2005;90:512–5. doi: 10.1136/adc.2003.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ficicioglu C, Bearden D. Isolated neonatal seizures: when to suspect inborn errors of metabolism. Pediatr Neurol. 2011;45:283–91. doi: 10.1016/j.pediatrneurol.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 117.Yeghiazaryan NS, Zara F, Capovilla G, Brigati G, Falsaperla R, Striano P. Pyridoxine-dependent epilepsy: an under-recognised cause of intractable seizures. J Paediatr Child Health. 2012;48:E113–5. doi: 10.1111/j.1440-1754.2010.01866.x. [DOI] [PubMed] [Google Scholar]
- 118.Joshi SN, Fathalla M, Koul R, Maney MA, Bayoumi R. Biotin responsive seizures and encephalopathy due to biotinidase deficiency. Neurol India. 2010;58:323–4. doi: 10.4103/0028-3886.63783. [DOI] [PubMed] [Google Scholar]
- 119.Agadi S, Quach MM, Haneef Z. Vitamin-responsive epileptic encephalopathies in children. Epilepsy research and treatment. 2013;2013:510529. doi: 10.1155/2013/510529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cervenka MC, Kossoff EH. Dietary treatment of intractable epilepsy. Continuum (Minneap Minn) 2013;19:756–66. doi: 10.1212/01.CON.0000431396.23852.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nordli DR, De Vivo DC. The Ketogenic Diet. In: Wyllie E, editor. Wyllie’s Treatment of Epilepsy. 5. Philadelphia, PA: Lippincott, Williams & Wilkins; 2011. pp. 790–6. [Google Scholar]
- 122.Kossoff EH, Nabbout R. Use of dietary therapy for status epilepticus. J Child Neurol. 2013;28:1049–51. doi: 10.1177/0883073813487601. [DOI] [PubMed] [Google Scholar]
- 123.Nabbout R, Mazzuca M, Hubert P, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51:2033–7. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 124.Gall CR, Jumma O, Mohanraj R. Five cases of new onset refractory status epilepticus (NORSE) syndrome: outcomes with early immunotherapy. Seizure. 2013;22:217–20. doi: 10.1016/j.seizure.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 125.Geva-Dayan K, Shorer Z, Menascu S, et al. Immunoglobulin treatment for severe childhood epilepsy. Pediatr Neurol. 2012;46:375–81. doi: 10.1016/j.pediatrneurol.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 126.Gedik AH, Demirkol D, Tatli B, et al. Therapeutic plasma exchange for malignant refractory status epilepticus: a case report. Pediatr Neurol. 2014;50:407–10. doi: 10.1016/j.pediatrneurol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 127.Bektas O, Yilmaz A, Kendirli T, Siklar Z, Deda G. Hashimoto encephalopathy causing drug-resistant status epilepticus treated with plasmapheresis. Pediatr Neurol. 2012;46:132–5. doi: 10.1016/j.pediatrneurol.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 128.Bhatia S, Ahmad F, Miller I, et al. Surgical treatment of refractory status epilepticus in children. J Neurosurg Pediatr. 2013;12:360–6. doi: 10.3171/2013.7.PEDS1388. [DOI] [PubMed] [Google Scholar]
- 129.Guilliams K, Rosen M, Buttram S, et al. Hypothermia for pediatric refractory status epilepticus. Epilepsia. 2013 doi: 10.1111/epi.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin JJ, Lin KL, Hsia SH, Wang HS. Therapeutic hypothermia for febrile infection-related epilepsy syndrome in two patients. Pediatr Neurol. 2012;47:448–50. doi: 10.1016/j.pediatrneurol.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 131.Weiner RD, Coffet CE, Fochtmann L. The Practice of ECT: Recommendations for Treatment, Training and Privileging. 2. Washington DC: American Psychiatric Press; 2001. [Google Scholar]
- 132.Lambrecq V, Villega F, Marchal C, et al. Refractory status epilepticus: electroconvulsive therapy as a possible therapeutic strategy. Seizure. 2012;21:661–4. doi: 10.1016/j.seizure.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 133.Sanchez Fernandez I, Abend NS, Agadi S, et al. Gaps and opportunities in refractory status epilepticus research in children: a multi-center approach by the Pediatric Status Epilepticus Research Group (pSERG) Seizure. 2014;23:87–97. doi: 10.1016/j.seizure.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]