Abstract
The health and economic burden of heart failure is significant, and continues to grow each year. Loop diuretics are an integral part of symptom management in heart failure. Furosemide is used disproportionately compared to other loop diuretics and there is currently no guidance for physicians regarding which agent to choose. However, there exist pharmacologic differences as well as other mechanistic differences that appear to favor torsemide use over furosemide. Compared to furosemide, torsemide improves surrogate markers of heart failure severity such as left ventricular function, plasma brain natriuretic peptide levels, and New York Heart Association functional class and may also reduce hospitalizations, readmissions, and mortality. Data suggest these benefits could be mediated through torsemide’s ability to positively affect the renin-angiotensin-aldosterone system. Specifically, torsemide has been shown to inhibit aldosterone secretion, synthesis, and receptor binding in vitro, as well as decrease transcardiac extraction of aldosterone, myocardial collagen production and cardiac fibrosis in patients with heart failure. We identified pertinent literature using keyword MEDLINE searches and cross-referencing prior bibliographies. We summarize the available data suggesting potential benefits with torsemide over furosemide, and call attention to the need for a reappraisal of diuretic use in heart failure patients and also for a well powered, randomized control trial assessing torsemide versus furosemide use.
Keywords: heart failure, torsemide, furosemide, diuretic
Introduction
Despite numerous advances in heart failure (HF) treatment in recent decades, the burden of HF remains significant for patients and the medical system. An estimated 5.1 million adult Americans have HF and with 825 000 new cases annually, by 2030 the prevalence is expected to increase by 46% resulting in more than 8 million affected adults. In 2012, the total cost for HF was $30.7 billion and by 2030 it is projected to increase by 127% to $69.7 billion [1]. Much of the cost is attributable to hospitalizations for acute decompensated heart failure (ADHF) and rates for hospitalization have steadily increased over the last several decades [2]. After hospitalization, the 30-day, 1-year, and 5-year fatality rates remain poor at 10.4%, 22%, and 42.3%, respectively [1]. Furthermore, 30-day readmission rates for patients with an initial diagnosis of HF are nearly 25%, with a median time to readmission of only 12 days, and over 1/3 of the readmissions are attributed to recurrent HF symptoms [3]. In the context of this growing burden of HF with high costs, morbidity, and mortality, loop diuretics are a cornerstone of therapy as indicated by published guidelines [4] and their optimization is an important component of clinical care.
Diuretics are a primary therapy to treat HF patients’ symptoms of dyspnea. Loop diuretics serve two main purposes, to maintain euvolemia for chronic HF patients and to achieve decongestion in ADHF patients [4, 5]. Among hospitalized acute HF patients, 70% were receiving diuretic therapy as an outpatient [6], and 90% of those admitted for ADHF received intravenous loop diuretics [7]. Challenges to achieving and maintaining decongestion include inadequate diuretic dosing, diuretic resistance, the “breaking phenomenon”, and post-diuretic sodium retention or the “rebound effect” as reviewed previously [8]. These challenges, as well as comorbidities such as underlying renal dysfunction make adequate decongestion difficult to accomplish. Even after aggressive diuretic administration during ADHF hospitalization, many patients have persistent congestive symptoms at discharge [9]. Patients with higher degrees of congestion are not only more likely to be rehospitalized for HF but also have higher rates of mortality [10]. Thus, decongestion is not just an important patient-reported outcome (i.e., dyspnea relief) but also serves as a clinical target for optimization of functional status. Improved decongestion may help to prevent ADHF and the need for hospitalizations. Current guidelines acknowledge that diuretic effects on morbidity and mortality are unknown, and no specific guidance is provided on loop diuretic choice [4]. There are three loop diuretics utilized in HF patients: furosemide, torsemide, and bumetanide.
In this review, we summarize pharmacological differences between loop diuretics and review the available data suggesting potential morbidity and mortality benefits with torsemide over furosemide in heart failure patients. In particular, we discuss the biochemical and molecular effects of torsemide on fibrosis via the renin-angiotensin-aldosterone system (RAAS). We also review previous data regarding cost considerations between torsemide and furosemide, update the data based on contemporary figures and provide a comparison of direct drug costs to Medicare patients throughout different geographic regions of the United States.
Methods
Publications were identified using MEDLINE searches with keywords that included torsemide, furosemide, bumetanide, loop diuretics, and/or heart failure, as well as cross-referencing prior bibliographies. Search results included animal and human trials and were filtered by English language. Data for our cost analysis was accessed using the online 2015 Medicare Plan Finder for Health, Prescription Drug and Medigap Plans. We assumed refills for 90 tablets (torsemide 20mg or furosemide 40mg daily) at 3-month intervals and selected the 3 prescription drug plans with the lowest estimated annual torsemide drug cost for the 10001, 27707, 34470, 95240, and 99201 ZIP codes. Dr. Mentz was supported by grant T32GM086330 from the National Institute of General Medical Sciences. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Pharmacology
Loop diuretics inhibit the Na+/2Cl−/K+ cotransporter in the thick ascending loop of Henle, resulting in increased excretion of urinary sodium and chloride and subsequent diuresis. Bumetanide and torsemide have consistent bioavailabilities of 80–100% compared with the wide range of 10–100% for furosemide [8] (Table 1).
Table 1.
Pharmacologic properties of loop diuretics
| Property | Furosemide | Torsemide | Bumetanide |
|---|---|---|---|
| Relative potency | 1x | 2x | 40x |
| Bioavailability (%) | 10 – 100 | 80 – 100 | 80 – 100 |
| Oral:Intravenous dosing | 2:1 | 1:1 | 1:1 |
| Time to onset (min) | 60 | 60 | 30 – 60 |
| Oral peak serum concentration (hr) | 1 | 1 | 1 – 2 |
| Absorption affected by food | Yes | No | Yes |
| Average half-life (hr) | 2 | 3.5 | 1 – 1.5 |
| Duration of effect (hr) | 6 – 8 | 6 – 16 | 4 – 6 |
| Decreased kaliuresis | No | Yes | No |
Abbreviations: hr: hour; min: minute
Torsemide’s bioavailability tends to be >90% in patients with renal insufficiency, liver cirrhosis, and heart failure [11–15]. Unlike furosemide and bumetanide, the bioavailability of torsemide remains unchanged with food intake [16, 17]. Oral administration of the three agents results in peak serum concentrations within 1–2 hours, but torsemide has the longest half-life of about 3.5 hours versus 1 and 2 hours with bumetanide and furosemide, respectively [15]. The pharmacologic properties of torsemide allow for a rapid onset and more predictable diuretic effect in HF patients, particularly when compared to the variable bioavailability of furosemide [15]. Pharmacologic comparisons of furosemide to bumetanide are generally lacking and most are least 15 years old [17–20]. Within the HF population furosemide and bumetanide have similarly prolonged absorption rates, but consistent with bumetanide’s greater bioavailabilty, more of the agent is absorbed overall compared to furosemide [18]. Increased gut edema, as a result of passive venous congestion in HF patients, has classically been considered to be a main contributor to the variable effect of diuretics such as furosemide [21]. Of note, while this concept is commonly referred to in the clinical setting, studies suggest that relatively substantial and sustained decreases in gut blood flow are required to exert a significant change in drug absorption rates [22]. This supports the hypothesis that pharmacologic factors inherent to furosemide may be the primary contributors to a variable diuretic response compared with other loop diuretics. For instance, the bioavailability of furosemide has been shown to be widely variable between different patients and within individual patients in a variety of different health states [17, 23]. In contrast, torsemide reliably has a bioavailability >80%, regardless of disease state [11–14, 24].
In addition to pharmacologic differences, genetic differences may also explain, in part, the variable responses to loop diuretics. Analysis of genetic polymorphisms seen in the sodium chloride contransporter, epithelial sodium channel, G nucleotide β-subunit 3, α-adducin, atrial natriuretic peptide precursor, and angiotensin I converting enzyme (ACE) may explain up to 1/6 of the inter-patient variability of loop diuretics on urinary electrolyte excretion, and a resulting 15% variation in urinary volume [25]. Interestingly, none of the polymorphisms affect the Na+/2Cl−/K+ cotransporter. The clinical relevance of these observations has yet to be determined.
The Renin-Angiotensin-Aldosterone System
The role of the RAAS in HF has been extensively studied and reviewed elsewhere [26]. In brief, the RAAS regulates intravascular volume and tissue repair through activation of inflammatory and proliferative mechanisms. Initially, decreased perfusion of the renal juxtaglomerular cells leads to a secretion of renin. Renin cleaves angiotensinogen to angiotensin I which is further cleaved into the active angiotensin II (Ang II) by ACE. In the final part of this pathway, Ang II promotes aldosterone synthesis and secretion from the adrenal cortex, as well as contributing to ventricular remodeling, myocardial hypertrophy, systemic vasoconstriction, and vascular smooth muscle cell (VSMC) growth via its actions on the Ang II type 1 receptor (AT1R) [27, 28]. Aldosterone has also been extensively studied and noted to have negative systemic and cardiac effects including promotion of inflammation, fibrosis, hypertrophy, and cell death [29–31] (Fig. 1). Loop diuretics have been shown to up regulate the RAAS [32, 33]. These potential adverse effects of loop diuretics have been cited as an explanation for the observation that higher dose diuretics are associated with worse outcomes [34]. However, these observations may be subject to residual confounding due to the fact that sicker patients require higher doses of diuretics.
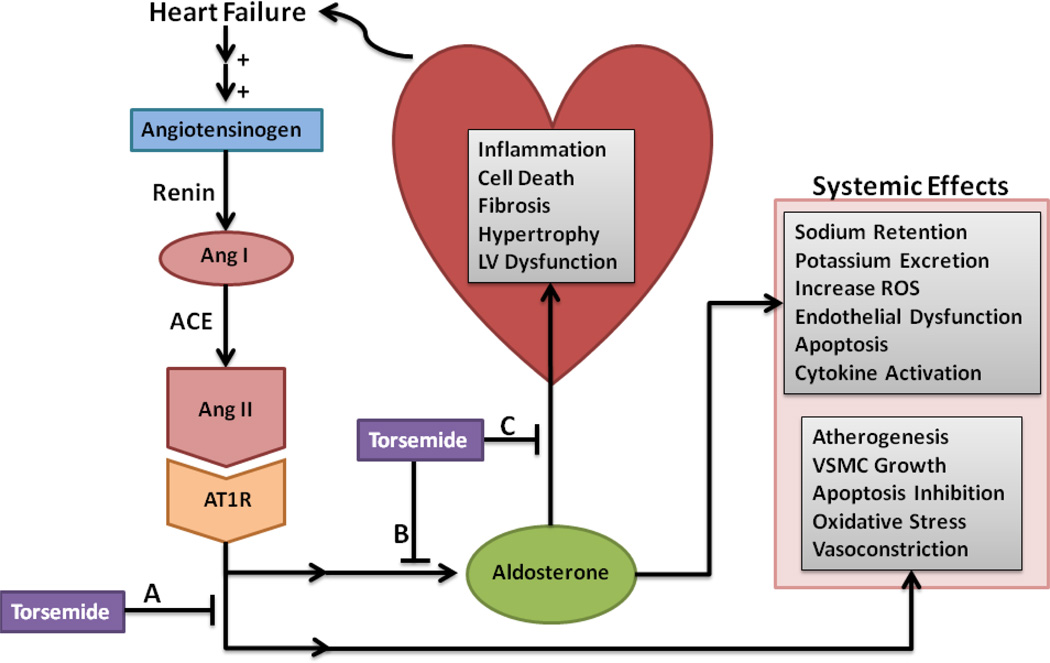
Figure 1. Potential effects of torsemide on the RAAS.
Heart failure leads to an up regulation of the RAAS. Renin converts angiotensinogen to Ang I, which is converted to Ang II by ACE. Ang II acts on AT1R leading to downstream effects including increasing aldosterone production and secretion, stimulating atherogenesis, VSMC growth, inhibition of apoptosis, increased oxidative stress, and promoting vasoconstriction. Circulating aldosterone acts on local myocardium receptors leading to myocardial inflammation, cell death, fibrosis, hypertrophy, and LV dysfunction leading to heart failure. Aldosterone stimulates sodium retention, potassium excretion, an increase in ROS, endothelial dysfunction, apoptosis, and increased cytokine activation. Torsemide may inhibit the downstream effects of Ang II (A), the secretion of aldosterone from adrenal cells (B), and aldosterone receptor binding (C).
Abbreviations: ACE: Angiotensin converting enzyme; Ang I: Angiotensin; Ang II: Angiotensin II; AT1R: Angiotensin II type 1 receptor; LV: left ventricular; RAAS: renin-angiotensin-aldosterone system; ROS: reactive oxygen species; VSMC: vascular smooth muscle cell
Chronic RAAS activation as seen in HF, leads to pathological remodeling and LV dysfunction. This process of pathologic remodeling serves as the underlying principle for current guideline recommendations of providing RAAS inhibition with an ACE inhibitor or angiotensin-receptor blockers (ARB), unless contraindicated, in all HF patients with a reduced ejection fraction [4]. Guidelines also recommend addition of an aldosterone receptor antagonist in patients with New York Heart Association (NYHA) class II-IV and LVEF ≤ 35% [4, 35, 36].
Similar to the guideline-recommended RAAS inhibitors above, studies have suggested potential RAAS benefits with torsemide. When compared to other diuretics, researchers initially noted in animal models that torsemide had longer lasting diuresis and less urinary potassium excretion [37], similar to effects seen with aldosterone blockade. These observations led to further studies to assess potential aldosterone antagonist-like activities with torsemide [38–42]. Subsequently, torsemide, but not furosemide, was found to inhibit aldosterone receptor binding in a dose-dependent manner in rat kidneys [38]. It has also been shown that torsemide can directly inhibit aldosterone secretion in animal models [39]. Recently, in vitro studies suggested that torsemide was unable to block aldosterone-mediated receptor translocation in monkey kidney cells [43]. In rat cardiomyocytes, torsemide had a weak inhibitory effect of aldosterone-mediated transactivation, but specific downstream gene expression was not inhibited [43]. Thus, in vitro data are conflicting and torsemide may exert some effects on both aldosterone production and receptor binding. In contrast, furosemide has been shown to increase circulating aldosterone levels as well as worsen underlying cardiac function in animal models [44].
One of the first human studies to assess RAAS activation with torsemide compared to furosemide, measured aldosterone levels and plasma active renin concentration (PARC) in 50 patients with NYHA class II or III symptoms and LVEF ≤45% who were taking 20 or 40 mg of furosemide daily, in a 6 month cross-over design [40]. Blockade of the RAAS through aldosterone receptor antagonism with spironolactone has reliably been shown to produce elevated aldosterone levels and PARC [41, 45], likely through the loss of feedback inhibition. Investigators found that similar to spironolactone, torsemide led to an increase in aldosterone and PARC [40]. Even though pre-clinical studies have suggested torsemide may inhibit aldosterone release, these findings may suggest that the more dominant mechanism in HF patients is likely its potential aldosterone receptor inhibition. Furthermore, torsemide improved clinical markers of HF severity in a dose-dependent manner as noted by a decrease in plasma brain natriuretic peptide (BNP) and improved echocardiographic measurements of LV function when compared to furosemide [40]. These observations with torsemide are similar to those seen in HF patients receiving spironolactone [45], which are thought to be mediated through direct myocardial aldosterone receptor inhibition. Several studies have provided evidence for torsemide’s ability to block myocardial aldosterone receptors. Early studies indicated that cardiac dysfunction results in greater uptake of circulating aldosterone by myocardial cells when compared to healthy controls [46], leading to further LV dysfunction and pathologic remodeling [46, 47]. Torsemide has been shown to reduce this myocardial aldosterone extraction compared to furosemide within HF patients [42], suggesting torsemide may directly antagonize the cardiac aldosterone receptors. Thus, in addition to torsemide’s potential effects on reducing aldosterone production and secretion, it may inhibit myocardial extraction of deleterious aldosterone from circulation. However, it is worth noting that despite the above studies, the clinical importance of any potential effect of torsemide on aldosterone or aldosterone receptors remains to be proven.
Although the majority of data regarding torsemide’s potential effect on RAAS inhibition have focused on aldosterone modulation, studies have also assessed potential benefits via effects on Ang II. Torsemide was found to inhibit the downstream effects of Ang II-induced protein synthesis during in vitro studies with rat VSMC, while furosemide had no such effect [48]. While this single, in vitro study is limited in extrapolating results to humans, it is suggestive that modulation of the systemic effect of Ang II may provide yet another mechanism by which torsemide provides benefit via RAAS inhibition. For instance, the blockade of the AT1R has proven mortality benefit within HF patients [49, 50].
Cardiac Fibrosis
The development of cardiac fibrosis in HF patients is mediated by myofibroblasts that respond to aldosterone, amongst other factors, by increasing the synthesis and secretion of fibrillar collagen precursors [51] (Fig. 2). Two of the more abundant precursors in the heart are collagen type I and III, which are preceded by triple-helix procollagen precursors that require cleavage of terminal propeptides before integration into the collagen molecule [51]. The carboxy-terminal propeptide of procollagen type I (PICP) can be quantified and used as a measurement of the amount of collagen, and thus myocardial fibrosis, produced [52].The correlation of increased PICP levels and myocardial fibrosis has been demonstrated in biopsy studies [53].
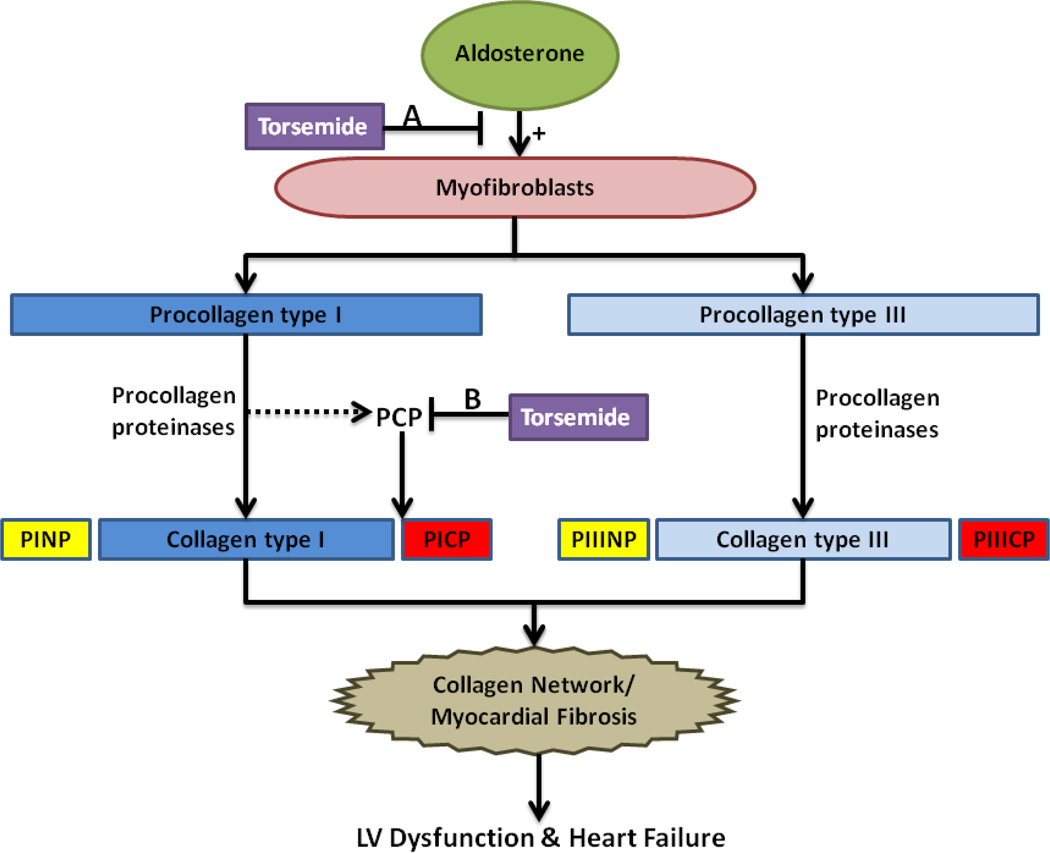
Figure 2. Potential effects of torsemide on myocardial fibrosis.
Aldosterone, as well as other cytokines, growth factors, and hormones, stimulate myofibroblasts to synthesize and secrete two main collagen precursors within the heart, procollagen type I and procollagen type III. Procollagen proteinases are enzymes that process the procollagen into collagen molecules by cleaving the terminal propeptides. The cleaved propeptides of procollagen type I (PINP and PICP) and procollagen type III (PIIINP and PIIICP) are released into circulation and can be quantified as an indirect measurement of collagen production. The mature collagen molecules are further processed and eventually form the collagen network responsible for myocardial fibrosis, subsequently leading to pathologic remodeling, LV dysfunction, and heart failure. Torsemide is thought to mainly inhibit downstream collagen synthesis through its inhibition at the level of the aldosterone receptor (A) but torsemide may also decrease the activity of the PCP enzyme, the procollagen proteinase responsible for cleavage of PICP (B).
Abbreviations: LV: left ventricular; PCP: procollagen type I carboxy-terminal proteinase; PICP: procollagen type I carboxy-terminal propeptide; PINP: procollagen type I amino-terminal propeptide; PIIICP: procollagen type III carboxy-terminal propeptide; PIIINP: procollagen type III amino-terminal propeptide
Prior studies have suggested potential benefits on fibrosis with torsemide. A randomized study of 39 patients with NYHA class II to IV symptoms and average LVEF ranging from 38 – 44% found that after 8 months those receiving torsemide (10 – 20 mg daily) had less myocardial collagen on septal biopsy and lower concentrations of PICP than those receiving furosemide (20 – 40 mg daily) [54]. Other investigators have used the aminoterminal propeptide of procollagen type III (PIIINP) as a surrogate marker of myocardial fibrosis. Unlike PICP, PIIINP does not have a 1:1 ratio of the number of molecules released to the number of collagen molecules produced [51]. Still, it has been suggested that in HF patients PIIINP levels positively correlate with cardiac aldosterone extraction [46], and PIIINP levels are reduced in patients receiving torsemide, along with the previously noted reduction in aldosterone extraction [42]. These data provide further support that torsemide has antifibrotic effects mediated through inhibition of aldosterone.
Another antifibrotic mechanism for torsemide has been described by Lopez and colleagues. The investigators found that activation of the enzyme responsible for cleavage of the PICP, procollagen type I carboxy-terminal proteinase (PCP), was decreased in a sample of 22 patients taking torsemide, and unchanged in furosemide-treated patients [55]. While inhibition of PCP may explain lower levels of PICP in torsemide-treated patients, there needs to be further investigation to elucidate whether this is a direct action or downstream effect of torsemide.
The largest study completed to-date investigating torsemide’s antifibrotic effects was TORAFIC [56], a multi-center study of 155 HF patients randomized to torsemide or furosemide. In contrast to prior data, the investigators found no significant differences between the two groups in changes of PICP levels [56]. Several potential confounding variables could explain the negative data. The TORAFIC study had a disproportionately large number of mild, or early-stage, HF patients, as evidenced by baseline characteristics revealing a low prevalence of baseline edema, and most patients had NYHA class II symptoms (96.1% and 89.7% in the torsemide and furosemide groups, respectively), no patients had NYHA class IV symptoms, and the average LVEF was 54.4% and 50.7% in the torsemide and furosemide groups, respectively. Comparatively, Lopez and colleagues [54] had a patient population with baseline NYHA class III-IV symptoms in ~58% of the torsemide group and ~70% of the furosemide group, with an associated average LVEF of 40% and 38% in the torsemide and furosemide groups, respectively. Thus, arguably the TORAFIC study’s patient population had less severe HF at baseline compared to prior study data, possibly explaining the low baseline serum levels of PICP noted and limiting the investigators ability to detect a significant change.
Preclinical Data and Surrogate Markers with Torsemide
Given the potential pharmacologic, RAAS, and antifibrotic benefits of torsemide over furosemide, several studies have directly compared these agents in an attempt to quantify effects on HF status and severity. Preclinical data is limited, but investigators demonstrated several differences between two groups of HF-induced rats that were randomized to receive varying doses of torsemide and furosemide [57]. After 4 weeks, 100% of the rats receiving torsemide remained alive compared to only 64.3% in the control group and 75% of the furosemide groups [57]. The torsemide group also had improved LV end diastolic pressures based on echocardiography and decreased myocardial fibrosis and collagen deposition compared to furosemide-treated rats [57].
Early clinical studies comparing torsemide with furosemide focused mainly on the diuretic effect and noted that patients receiving torsemide had more diuresis and subsequent weight loss compared to furosemide [58, 59]. In a prospective, multicenter trial of 237 HF patients randomized to torsemide or furosemide, investigators found that torsemide was superior for improvements in patient-reported quality of life and there was a trend for greater improvement in NYHA class [60]. Others studies have also demonstrated improvement in symptoms such as fatigue with torsemide over furosemide [61], but these studies were limited due to the lack of adequate blinding [60, 61]. Kasama and colleagues similarly found improvements in NYHA functional class in a small, randomized sample of 40 HF patients treated with torsemide compared to furosemide after 6 months of therapy [62]. Furthermore, the torsemide treated group had improvements in other surrogate measures such as decreased LV end diastolic and systolic volumes, decreased levels of BNP, and improved sympathetic nerve activity [62]. Several other studies have noted greater improvement in NYHA functional class with torsemide compared to furosemide [54, 55, 63], while only the aforementioned TORAFIC study found no significant difference in functional improvement. These data suggest relative concordance across multiple studies that torsemide improves surrogate markers of HF severity to a greater extent than furosemide.
Torsemide vs. Furosemide – Morbidity and Mortality
Trial data assessing morbidity and mortality of different loop diuretics are limited (Table 2). As such, a recent systematic review identified just 25 randomized trials comparing the two agents, and only two (total N=471) were included in the analysis because of limited data on clinical outcomes [64]. The landmark TORIC study, which was the largest study to-date comparing torsemide to furosemide, was not included in the above analysis as it was not a randomized controlled trial [63]. TORIC was principally designed for post-market surveillance of safety and tolerability. The original data analysis found a significant relative risk reduction in death among the torsemide group. This led to the post-hoc analysis that included adult patients with NYHA class II-IV symptoms, who had at least one follow-up visit, and had few exclusion criteria, even allowing for concomitant use of other diuretics. The main exclusion criteria were patients with torsemide hypersensitivity, significant electrolyte disturbances, severe ventricular arrhythmia, complete atrioventricular block, dyspnea due to lung disease, or protocol violations. The analysis included 1377 patients and found that after an average of 9 months there was a significant 51.5% reduction in the risk of overall mortality, 59.7% reduction in cardiac mortality, as well as significant improvement in functional status within the torsemide group [63]. However, the results remain limited by the study design and sample population, as the cohort of patients was rural, non-hospital based, and the use of other standard HF-pharmacotherapies such as beta-blockers and ACE inhibitors was low (~9.5% and ~30%, respectively). Nevertheless, the mortality benefit observed was significant and TORIC remains at the center of the discussion regarding torsemide’s mortality benefit.
Table 2.
Torsemide vs. Furosemide studies: Hospitalizations and mortality
| Study (Year) |
N | Patients | Design | Average Follow-up |
Comparison | Outcomes (Torsemide vs Furosemide) |
Limitations |
|---|---|---|---|---|---|---|---|
| TORIC (2002) | 1377 |
|
Open-label, non-randomized, post-marketing surveillance | 9.2 months (~276 days) |
Torsemide avg. dose 8.2 mg/day Vs. Furosemide avg. dose 35 mg/day + other diuretics |
|
|
| Müller et al. (2003) |
237 |
|
Open-label, randomized, prospective |
239 days in Torsemide arm 250 days in Furosemide arm |
Torsemide avg. dose 11.36 mg/day Vs. Furosemide avg. dose 40.04 mg/day |
|
|
| Murray et al. (2001) |
234 |
|
Open-label, randomized, prospective |
324 days in Torsemide arm 318 days in Furosemide arm |
Torsemide avg. dose 72 mg/day Vs. Furosemide avg. dose 136 mg/day |
|
|
Abbreviations: ACE: Angiotensin converting enzyme; avg: average; CV: cardiovascular; HF: heart failure; LV: left ventricular; mg: milligram; NYHA: New York Heart Association; RR: relative risk
One of the first randomized trials to address questions of morbidity and mortality was an open-label trial of 234 HF patients given torsemide or furosemide for up to one year [61]. The study was powered for a primary endpoint of HF-related readmissions. They found that patients given torsemide were less likely to be readmitted for HF or any cardiovascular event and had less HF-related hospital days (106 vs. 296 days) [61]. Analysis of secondary endpoints found that the torsemide group had reduced all-cause mortality (18 vs. 25 deaths) but it was not statistically significant [61]. One interesting difference between the baseline characteristics of the two groups was that patients randomized to torsemide had significantly more HF hospitalizations in the year preceding enrollment [61]. Thus, the observation of reduced HF readmissions and a trend toward reduced mortality with torsemide, even in the context of a potentially higher risk population [3], was another early observation supporting potential clinical benefits with torsemide. Furthermore, the study was not powered to detect differences in patient mortality, and the study population from two hospital centers within the same city may not be generalizable to the broad HF population.
Another study randomized 237 HF patients to torsemide or furosemide with a primary endpoint of overall hospitalizations [60]. After 9 months, the torsemide group had significantly fewer in-hospital days related to HF and cardiovascular disease combined (95 vs. 146 days). There was no difference in hospitalizations due to ADHF or cardiovascular disease alone, or all-cause mortality. The investigators indicated that the difference in hospitalization days was mainly due to a single patient in the furosemide group with a prolonged stay. Furthermore, the estimated sample size needed in each group for adequate power was 120, but only 237 patients were randomized and 194 completed the study. While this study and the previous study by Murray and colleagues [61] had similar sample sizes and endpoints, several key differences exist. Compared to the prior study the patient population in the Müller study [60] had lower baseline NYHA functional class and were required to be on an ACE inhibitor. They also had less total follow-up days and had only 14 deaths in the entire study, further limiting their ability to detect a mortality difference between treatment groups.
More recently, a meta-analysis of the existing studies [55, 56, 60–63] found a trend towards improvement in NYHA class functional status (Risk Ratio 0.93 [95% CI: 0.82 – 1.06]), and all-cause death (Risk Ratio 0.68 [95% CI: 0.39 – 1.18]) in torsemide-treated compared to furosemide-treated patients [65]. However, the meta-analysis was limited by the quality of the included studies, and each end-point had at least moderate degrees of heterogeneity (NYHA class I2=46%; Mortality I2=48%) across the studies. The limitations of the above-mentioned studies may have further limited the ability to detect a significant difference in either end-point. Nevertheless, the data are suggestive of a potential benefit with torsemide over furosemide in regards to morbidity and mortality.
Cost Analysis
Historically, the acquisition cost of furosemide has been less than torsemide, but since torsemide became generic in 2002 that difference has been minimized. Despite the higher acquisition cost, economic analyses while torsemide was on-patent found a reduction in total cost per patient compared to furosemide [66]. This difference was observed largely due to decreased hospital admissions in the torsemide group [66]. Another analysis of 240 randomized HF patients found no difference in average medical costs related to HF or cardiovascular-related diseases in torsemide treated patients [67]. The above studies, and several others have been reviewed previously [68], and suggest that torsemide compared to furosemide decreases, or at a minimum does not increase, total health care costs despite the higher acquisition costs [68].
Müller and colleagues present the more recent and conservative data [60] of the two trials reporting HF-related hospital days while comparing torsemide and furosemide [60, 61] (Table 2). Using Müller and colleagues’ unadjusted data, we estimated the 90-day inpatient cost difference between the torsemide and furosemide groups using the 2013 average reimbursement for Medicare’s diagnosis-related group (MS-DRG) weight unit of $5,774. We calculated a total daily cost of $1,252.27 using the most conservative MS-DRG HF-related weight unit from 2014. At 90-days, there were 0.05 less hospital days per patient in the torsemide group, accounting for $63 less total cost per patient. Based on local Wal-Mart pharmacy pricing, the difference in cost of obtaining a 90-day supply of torsemide 10 mg versus furosemide 20 mg daily is $52.28. Thus, when considering the difference in acquisition costs, and using conservative hospitalization and reimbursement rates, data suggest torsemide use does not increase total medical costs.
While total medical costs may be similar, a potential barrier to patient compliance with torsemide use may be higher direct costs to the patient. We estimated the average annual drug cost differences for Medicare patients taking torsemide 20 mg compared to furosemide 40 mg daily. The 2015 estimated Medicare costs were collected from five separate geographic regions across the U.S. and were adjusted based on delivery method (retail vs. mail order) and presence or absence of financial assistance with Medicaid dual eligibility (Table 3). The average annual drug costs differences to patients taking torsemide compared to furosemide without extra insurance was $33.70 ($0.094/day) for retail and $23.20 ($0.064/day) for mail order. While the average annual drug costs differences for patients with Medicaid dual eligibility was $1.00 ($0.003/day) for retail and $0.70 ($0.002/day) for mail order. There was minimal variation across geographic regions. Thus, the cost increment for torsemide versus furosemide use is less than $3 per month for Medicare patients not receiving financial assistance, and there is no difference for patients with dual Medicare and Medicaid eligibility. Limitations of our cost analysis include the lack of inclusivity of all geographic regions, furosemide availability on $4 formularies may be less than some copayments, and choices of prescription drug plans may be influenced by medications not included in our analysis.
Table 3.
Estimated 2015 Medicare patient average annual drug costs differences: Torsemide vs. Furosemide
| Location | Costs With No Extra Insurance | Costs With Medicaid Dual Eligible | |||
|---|---|---|---|---|---|
| ZIP Code | City, State | Retail Mean (Range) |
Mail Order Mean (Range) |
Retail Mean (Range) |
Mail Order Mean (Range) |
| 10001 | Manhattan, NY | $32.00($0, $63) | $24.30 ($0, $73) | $1.70 ($0, $5) | $1.70 ($0, $5) |
| 27707 | Durham, NC | $32.30 ($0, $64) | $24.70 ($0, $74) | $0.00 ($0, $0) | $0.00 ($0, $0) |
| 34470 | Ocala, FL | $21.70 ($0, $33) | $0.00 ($0, $0) | $1.70 ($0, $5) | $1.70 ($0, $5) |
| 95240 | Lodi, CA | $35.30 ($0, $73) | $28.30 ($0, $85) | $1.70 ($0, $5) | $0.00 ($0, $0) |
| 99201 | Spokane, WA | $47.00 ($38, $64) | $38.70 ($6, $74) | $0.00 ($0, $0) | $0.00 ($0, $0) |
| Total Average Costs Differences (Range) | $33.70 ($0, $73) | $23.20 ($0, $85) | $1.00 ($0, $5) | $0.70 ($0, $5) | |
Abbreviations: CA: California; FL: Florida; NC: North Carolina; NY: New York; WA: Washington
Conclusion
There is significant room for improvement in regards to preventing hospitalizations and reducing mortality within the HF patient population. The majority of studies that directly compared torsemide to furosemide have been small, underpowered, and with limited end-points. Nonetheless, most have suggested benefits with torsemide use, including improved NYHA functional class, improved LV function, decreased myocardial fibrosis, decreased rates of hospitalizations and potentially a reduction in mortality (Table 4). However, there remains a need for a prospective, large, randomized controlled trial directly comparing torsemide to furosemide within the HF population that is adequately powered for such endpoints.
Table 4.
Potential benefits of torsemide compared to furosemide
| Preclinical Data | Clinical Data |
|---|---|
|
|
Gravez et al. [43] found no receptor inhibition.
TORAFIC study [56] found no difference between variables.
Müller et al. [60] found no difference between variables.
Abbreviations: Ang II: Angiotensin II; BNP: brain natriuretic peptide; HF: heart failure; LV: left ventricular; NYHA: New York Heart Association; PCP: procollagen type I carboxy-terminal proteinase; PICP: procollagen type I carboxy-terminal propeptide; PIIINP: procollagen type III aminoterminal propeptide; RAAS: renin-angiotensin-aldosterone system
Current guidelines recommend diuretic use without providing guidance on therapy choice [4], and despite all of the preclinical and clinical differences noted, clinicians commonly use furosemide over other loop diuretics [65]. The preferential use of furosemide is likely due to furosemide being first to market in 1966 such that clinicians tend to have experience with its use and it generally has lower acquisition costs. In comparison, torsemide was not FDA approved until 1993, and tends to be reserved for patients refractory to furosemide or those with renal failure or suspected intestinal edema. The above data suggesting comparative clinical benefits with torsemide over furosemide and the availability of generic torsemide coupled with economic analyses finding similar total medical costs and minimal differences in direct patient drug costs, suggests clinicians should consider torsemide use first over furosemide, and at the very least emphasizes a need for a reappraisal of current diuretic use in HF patients.
Acknowledgments
Funding: Dr. Mentz was supported by grant T32GM086330 from the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no relevant conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. Journal of the American College of Cardiology. 2008;52:428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 3.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA : the journal of the American Medical Association. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Mentz RJ, Kjeldsen K, Rossi GP, Voors AA, Cleland JG, Anker SD, et al. Decongestion in acute heart failure. European journal of heart failure. 2014 doi: 10.1002/ejhf.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American heart journal. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Peacock WF, Costanzo MR, De Marco T, Lopatin M, Wynne J, Mills RM, et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–19. doi: 10.1159/000164149. [DOI] [PubMed] [Google Scholar]
- 8.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. Journal of the American College of Cardiology. 2012;59:2145–2153. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 9.Lala A, Vader J, Dunlay S, Ravichandran A, AbouEzzadine O, Zakeri R, et al. A Two-Symptom Congestion Score In Relation to Outcomes after Discharge with Acute Decompensated Heart Failure. Journal of cardiac failure. 2013;19:S39. [Google Scholar]
- 10.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. European heart journal. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 11.Bleske BE, Welage LS, Kramer WG, Nicklas JM. Pharmacokinetics of Torsemide in Patients with Decompensated and Compensated Congestive Heart Failure. The Journal of Clinical Pharmacology. 1998;38:708–714. doi: 10.1002/j.1552-4604.1998.tb04810.x. [DOI] [PubMed] [Google Scholar]
- 12.Gehr TW, Rudy DW, Matzke GR, Kramer WG, Sica DA, Brater DC. The pharmacokinetics of intravenous and oral torsemide in patients with chronic renal insufficiency. Clinical pharmacology and therapeutics. 1994;56:31–38. doi: 10.1038/clpt.1994.98. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb SS, Khatta M, Wentworth D, Roffman D, Fisher ML, Kramer WG. The effects of diuresis on the pharmacokinetics of the loop diuretics furosemide and torsemide in patients with heart failure. The American journal of medicine. 1998;104:533–538. doi: 10.1016/s0002-9343(98)00111-9. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz S, Brater DC, Pound D, Green PK, Kramer WG, Rudy D. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide in patients with cirrhosis. Clinical pharmacology and therapeutics. 1993;54:90–97. doi: 10.1038/clpt.1993.116. [DOI] [PubMed] [Google Scholar]
- 15.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clinical pharmacology and therapeutics. 1995;57:601–609. doi: 10.1016/0009-9236(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 16.Kramer WG. Effect of Food on the Pharmacokinetics and Pharmacodynamics of Torsemide. American journal of therapeutics. 1995;2:499–503. doi: 10.1097/00045391-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 17.McCrindle JL, Li Kam Wa TC, Barron W, Prescott LF. Effect of food on the absorption of frusemide and bumetanide in man. British journal of clinical pharmacology. 1996;42:743–746. doi: 10.1046/j.1365-2125.1996.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney international. 1984;26:183–189. doi: 10.1038/ki.1984.153. [DOI] [PubMed] [Google Scholar]
- 19.Sagar S, Sharma BK, Sharma PL, Wahi PL. A comparative randomized double-blind clinical trial of bumetanide and furosemide in congestive cardiac failure and other edema states. International journal of clinical pharmacology, therapy, and toxicology. 1984;22:473–478. [PubMed] [Google Scholar]
- 20.Ramsay F, Crawford RJ, Allman S, Bailey R, Martin A. An open comparative study of two diuretic combinations, frusemide/amiloride (‘Frumil’) and bumetanide/potassium chloride (‘Burinex’ K), in the treatment of congestive cardiac failure in hospital out-patients. Current medical research and opinion. 1988;10:682–689. doi: 10.1185/03007998809111119. [DOI] [PubMed] [Google Scholar]
- 21.Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC. Furosemide absorption altered in decompensated congestive heart failure. Annals of internal medicine. 1985;102:314–318. doi: 10.7326/0003-4819-102-3-314. [DOI] [PubMed] [Google Scholar]
- 22.Sica DA. Pharmacotherapy in congestive heart failure: drug absorption in the management of congestive heart failure: loop diuretics. Congestive heart failure. 2003;9:287–292. doi: 10.1111/j.1527-5299.2003.02399.x. [DOI] [PubMed] [Google Scholar]
- 23.Murray MD, Haag KM, Black PK, Hall SD, Brater DC. Variable furosemide absorption and poor predictability of response in elderly patients. Pharmacotherapy. 1997;17:98–106. [PubMed] [Google Scholar]
- 24.Vargo D, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. The pharmacodynamics of torsemide in patients with congestive heart failure. Clinical pharmacology and therapeutics. 1994;56:48–54. doi: 10.1038/clpt.1994.100. [DOI] [PubMed] [Google Scholar]
- 25.Vormfelde SV, Brockmoller J. The genetics of loop diuretic effects. The pharmacogenomics journal. 2012;12:45–53. doi: 10.1038/tpj.2010.68. [DOI] [PubMed] [Google Scholar]
- 26.Mentz RJ, Bakris GL, Waeber B, McMurray JJ, Gheorghiade M, Ruilope LM, et al. The past, present and future of renin-angiotensin aldosterone system inhibition. International journal of cardiology. 2013;167:1677–1687. doi: 10.1016/j.ijcard.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dell’Italia LJ. Translational success stories: angiotensin receptor 1 antagonists in heart failure. Circulation research. 2011;109:437–452. doi: 10.1161/CIRCRESAHA.110.238550. [DOI] [PubMed] [Google Scholar]
- 28.Touyz RM, Schiffrin EL. Angiotensin II regulates vascular smooth muscle cell pH, contraction, and growth via tyrosine kinase-dependent signaling pathways. Hypertension. 1997;30:222–229. doi: 10.1161/01.hyp.30.2.222. [DOI] [PubMed] [Google Scholar]
- 29.He BJ, Anderson ME. Aldosterone and cardiovascular disease: the heart of the matter. Trends in endocrinology and metabolism: TEM. 2013;24:21–30. doi: 10.1016/j.tem.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin W, Rudolph AE, Bond BR, Rocha R, Blomme EA, Goellner JJ, et al. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circulation research. 2003;93:69–76. doi: 10.1161/01.RES.0000080521.15238.E5. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi H, Kobara M, Abe M, Tanaka N, Gouda E, Toba H, et al. Aldosterone nongenomically produces NADPH oxidase-dependent reactive oxygen species and induces myocyte apoptosis. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31:363–375. doi: 10.1291/hypres.31.363. [DOI] [PubMed] [Google Scholar]
- 32.He XR, Greenberg SG, Briggs JP, Schnermann J. Effects of furosemide and verapamil on the NaCl dependency of macula densa-mediated renin secretion. Hypertension. 1995;26:137–142. doi: 10.1161/01.hyp.26.1.137. [DOI] [PubMed] [Google Scholar]
- 33.Bayliss J, Norell M, Canepa-Anson R, Sutton G, Poole-Wilson P. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. British heart journal. 1987;57:17–22. doi: 10.1136/hrt.57.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O’Connor CM, Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. European journal of heart failure. 2007;9:1064–1069. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. The New England journal of medicine. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 36.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. The New England journal of medicine. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 37.Ghys A, Denef J, de Suray JM, Gerin M, Georges A, Delarge J, et al. Pharmacological properties of the new potent diuretic torasemide in rats and dogs. Arzneimittel-Forschung. 1985;35:1520–1526. [PubMed] [Google Scholar]
- 38.Uchida T, Yamanaga K, Nishikawa M, Ohtaki Y, Kido H, Watanabe M. Anti-aldosteronergic effect of torasemide. European journal of pharmacology. 1991;205:145–150. doi: 10.1016/0014-2999(91)90812-5. [DOI] [PubMed] [Google Scholar]
- 39.Goodfriend TL, Ball DL, Oelkers W, Bahr V. Torsemide inhibits aldosterone secretion in vitro. Life sciences. 1998;63:PL45–PL50. doi: 10.1016/s0024-3205(98)00265-3. [DOI] [PubMed] [Google Scholar]
- 40.Yamato M, Sasaki T, Honda K, Fukuda M, Akutagawa O, Okamoto M, et al. Effects of torasemide on left ventricular function and neurohumoral factors in patients with chronic heart failure. Circulation journal : official journal of the Japanese Circulation Society. 2003;67:384–390. doi: 10.1253/circj.67.384. [DOI] [PubMed] [Google Scholar]
- 41.Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. The American journal of cardiology. 1995;76:1259–1265. doi: 10.1016/s0002-9149(99)80353-1. [DOI] [PubMed] [Google Scholar]
- 42.Tsutamoto T, Sakai H, Wada A, Ishikawa C, Ohno K, Fujii M, et al. Torasemide inhibits transcardiac extraction of aldosterone in patients with congestive heart failure. Journal of the American College of Cardiology. 2004;44:2252–2253. doi: 10.1016/j.jacc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Gravez B, Tarjus A, Jimenez-Canino R, El Moghrabi S, Messaoudi S, Alvarez de la Rosa D, et al. The diuretic torasemide does not prevent aldosterone-mediated mineralocorticoid receptor activation in cardiomyocytes. PloS one. 2013;8:e73737. doi: 10.1371/journal.pone.0073737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. Journal of the American College of Cardiology. 2004;44:1301–1307. doi: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 45.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. Journal of the American College of Cardiology. 2001;37:1228–1233. doi: 10.1016/s0735-1097(01)01116-0. [DOI] [PubMed] [Google Scholar]
- 46.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Spironolactone inhibits the transcardiac extraction of aldosterone in patients with congestive heart failure. Journal of the American College of Cardiology. 2000;36:838–844. doi: 10.1016/s0735-1097(00)00796-8. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi M, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Tsutsui T, et al. Relationship between transcardiac extraction of aldosterone and left ventricular remodeling in patients with first acute myocardial infarction: extracting aldosterone through the heart promotes ventricular remodeling after acute myocardial infarction. Journal of the American College of Cardiology. 2001;38:1375–1382. doi: 10.1016/s0735-1097(01)01539-x. [DOI] [PubMed] [Google Scholar]
- 48.Muniz P, Fortuno A, Zalba G, Fortuno MA, Diez J. Effects of loop diuretics on angiotensin II-stimulated vascular smooth muscle cell growth. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16(Suppl 1):14–17. doi: 10.1093/ndt/16.suppl_1.14. [DOI] [PubMed] [Google Scholar]
- 49.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 50.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 51.Lopez B, Gonzalez A, Diez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–1654. doi: 10.1161/CIRCULATIONAHA.109.912774. [DOI] [PubMed] [Google Scholar]
- 52.Querejeta R, Varo N, Lopez B, Larman M, Artinano E, Etayo JC, et al. Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation. 2000;101:1729–1735. doi: 10.1161/01.cir.101.14.1729. [DOI] [PubMed] [Google Scholar]
- 53.Querejeta R, Lopez B, Gonzalez A, Sanchez E, Larman M, Martinez Ubago JL, et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110:1263–1268. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 54.Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. Journal of the American College of Cardiology. 2004;43:2028–2035. doi: 10.1016/j.jacc.2003.12.052. [DOI] [PubMed] [Google Scholar]
- 55.Lopez B, Gonzalez A, Beaumont J, Querejeta R, Larman M, Diez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. Journal of the American College of Cardiology. 2007;50:859–867. doi: 10.1016/j.jacc.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 56.Group TI. Effects of prolonged-release torasemide versus furosemide on myocardial fibrosis in hypertensive patients with chronic heart failure: a randomized, blinded-end point, active-controlled study. Clinical therapeutics. 2011;33:1204–1213. doi: 10.1016/j.clinthera.2011.08.006. e3. [DOI] [PubMed] [Google Scholar]
- 57.Veeraveedu PT, Watanabe K, Ma M, Thandavarayan RA, Palaniyandi SS, Yamaguchi K, et al. Comparative effects of torasemide and furosemide in rats with heart failure. Biochemical pharmacology. 2008;75:649–659. doi: 10.1016/j.bcp.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 58.Broekhuysen J, Deger F, Douchamps J, Ducarne H, Herchuelz A. Torasemide, a new potent diuretic. Double-blind comparison with furosemide. European journal of clinical pharmacology. 1986;(31 Suppl):29–34. doi: 10.1007/BF00541464. [DOI] [PubMed] [Google Scholar]
- 59.Scheen AJ, Vancrombreucq JC, Delarge J, Luyckx AS. Diuretic activity of torasemide and furosemide in chronic heart failure: a comparative double blind cross-over study. European journal of clinical pharmacology. 1986;(31 Suppl):35–42. doi: 10.1007/BF00541465. [DOI] [PubMed] [Google Scholar]
- 60.Müller K, Gamba G, Jaquet F, Hess B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. European journal of heart failure. 2003;5:793–801. doi: 10.1016/s1388-9842(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 61.Murray MD, Deer MM, Ferguson JA, Dexter PR, Bennett SJ, Perkins SM, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. The American journal of medicine. 2001;111:513–520. doi: 10.1016/s0002-9343(01)00903-2. [DOI] [PubMed] [Google Scholar]
- 62.Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, et al. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart. 2006;92:1434–1440. doi: 10.1136/hrt.2005.079764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cosin J, Diez J, investigators T. Torasemide in chronic heart failure: results of the TORIC study. European journal of heart failure. 2002;4:507–513. doi: 10.1016/s1388-9842(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 64.DiNicolantonio JJ. Should torsemide be the loop diuretic of choice in systolic heart failure? Future cardiology. 2012;8:707–728. doi: 10.2217/fca.12.54. [DOI] [PubMed] [Google Scholar]
- 65.Bikdeli B, Strait KM, Dharmarajan K, Partovian C, Coca SG, Kim N, et al. Dominance of Furosemide for Loop Diuretic Therapy in Heart Failure: Time to Revisit the Alternatives? Journal of the American College of Cardiology. 2013;61:1549–1550. doi: 10.1016/j.jacc.2012.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stroupe KT. Healthcare Costs of Patients with Heart Failure Treated with Torasemide or Furosemide. PharmacoEconomics. 2000;17:429–440. doi: 10.2165/00019053-200017050-00002. [DOI] [PubMed] [Google Scholar]
- 67.Noe LL, Vreeland MG, Pezzella SM, Trotter JP. A pharmacoeconomic assessment of torsemide and furosemide in the treatment of patients with congestive heart failure. Clinical therapeutics. 1999;21:854–866. doi: 10.1016/s0149-2918(99)80007-1. [DOI] [PubMed] [Google Scholar]
- 68.Young M, Plosker GL. Torasemide: a pharmacoeconomic review of its use in chronic heart failure. Pharmacoeconomics. 2001;19:679–703. doi: 10.2165/00019053-200119060-00006. [DOI] [PubMed] [Google Scholar]