Abstract
During voluntary contraction, firing rates of individual motor units (MUs) increase modestly over a narrow force range beyond which little additional increase in firing rate is seen. Such saturation of MU discharge may be a consequence of extrinsic factors that limit net synaptic excitation acting on motor neurons (MNs) or may be due to intrinsic properties of the MNs. Two sets of experiments involving recording of human biceps brachii MUs were carried out to evaluate saturation. In the first set, the extent of saturation was quantified for 136 low-threshold MUs during isometric ramp contractions. Firing rate-force data were best fit by a saturating function for 90% of MUs recorded with a maximum rate of 14.8 ± 2.0 impulses/s. In the second set of experiments, to distinguish extrinsic from intrinsic factors underlying saturation, we artificially augmented descending excitatory drive to biceps MNs by activation of muscle spindle afferents through tendon vibration. We examined the change in firing rate caused by tendon vibration in 96 MUs that were voluntarily activated at rates below and at saturation. Vibration had little effect on the discharge of MUs that were firing at saturation frequencies but strongly increased firing rates of the same units when active at lower frequencies. These results indicate that saturation is likely caused by intrinsic mechanisms that prevent further increases in firing rate in the presence of increasing synaptic excitation. Possible intrinsic cellular mechanisms that limit firing rates of motor units during voluntary effort are discussed.
Keywords: firing rate, force, motor neuron, motor unit, saturation
motor neurons (MNs) represent the final processing stage within the mammalian central nervous system, the site at which synaptic signals are ultimately transformed into overt motor behaviors. MNs receive a complex array of synaptic inputs and have long been thought to generate action potentials at firing rates proportional to the net excitatory synaptic drive (Granit et al. 1966; Heckman and Binder 1991a; Kernell 1969; Kernell and Sjöholm 1973; Powers et al. 1992; Schwindt and Calvin 1973). Each action potential generated by a MN is replicated with near perfect fidelity in the hundreds of skeletal muscle fibers innervated by each MN, causing these muscle fibers to contract as a group. The MN and its innervated muscle fibers therefore comprise a functional entity called a motor unit (MU). The contractile force exerted by a MU, in turn, varies precisely as a function of the firing rate of the MN. In this way, the intensity of neural activity delivered synaptically to MNs is transduced into the constituent mechanical signals that operate on tendon and bone.
Because it is relatively easy to record the discharge of MUs (and hence, MNs) in awake human subjects, the relation between naturally occurring synaptic input and firing rate responses in MNs can be indirectly assessed. Furthermore, detailed computer models of MU populations have shown isometric muscle force to be a monotonically increasing function of the net excitatory synaptic drive (Fuglevand et al. 1993; Heckman and Binder 1991a). This means that isometric muscle force can serve as a coarse indicator of synaptic excitation during voluntary muscle contraction. This in turn enables, at least to a first approximation, assessment of the relation between natural synaptic input and spike-frequency output in an unanesthetized, fully intact subject.
Interestingly, when MU activity is recorded during experiments in which human subjects progressively increase the strength of an isometric contraction, a marked nonlinearity in the relation between firing rate and synaptic drive (as inferred from muscle force) is often exhibited. Specifically, firing rate increases only modestly over a relatively narrow range of isometric force beyond which little further increase in firing rate is seen (Bailey et al. 2007; Bracchi et al. 1966; De Luca and Contessa 2012; De Luca et al. 1982; Kiehn and Eken 1997; McGill et al. 2005; Monster and Chan 1977; Moritz et al. 2005; Mottram et al. 2009, 2014; Person and Kudina 1972). Such saturation of firing rate with maximum rates often <20 impulses/s (imp/s) in response to presumed increases in synaptic drive is in contrast to that observed with intracellular current injection during which, for example, cat hindlimb MNs increase firing rates linearly up to high levels (usually >50 imp/s, occasionally >100 imp/s) with increased current (Granit et al. 1966; Kernell 1965; Schwindt 1973; Schwindt and Calvin 1972; Schwindt and Crill 1982).
From the broadest perspective, there are two categories of factors that could account for such saturation associated with natural activation of MNs. Extrinsic factors represent those processes that might limit the net synaptic excitation acting on MNs during voluntary contraction. As such, during voluntary effort, the depolarizing current delivered synaptically may simply be less than that needed to drive the MNs to high firing rates. This might come about, in part, because of progressive increases in concurrent inhibition tending to curb increases in firing rate (Denny-Brown 1929; Granit 1958; Granit et al. 1960; Heckman and Binder 1993; Powers et al. 2012; Tansey and Botterman 1996).
Alternatively, MNs themselves might be intrinsically less able to respond with increases in firing rate as synaptic drive increases. One candidate for such an intrinsic limitation in firing rate is a progressive loss in driving potential at excitatory synapses as the dendritic membrane depolarizes (Barrett and Crill 1974; Cushing et al. 2005; Powers and Binder 2000). Such a loss in driving potential has been shown to markedly reduce synaptic currents and thereby lessen the net current transferred to the soma and spike-initiating zone (Cushing et al. 2005).
Another intrinsic factor is that associated with diminution of cell input resistance as the quantity of open transmitter-gated (and other type) channels increases (Binder et al. 1996; Burke 1967; Coombs et al. 1955; Destexhe and Paré 1999; Fuortes 1959; Kuhn et al. 2004; Llinas and Terzuolo 1964; Powers and Binder 2000). Such a reduction in membrane input resistance should lessen the effective change in membrane potential caused by a given increment in synaptic current (due to Ohm's law) and also lead to an abbreviation in the time course of postsynaptic potentials (due to the decrease in membrane time constant). Theoretically, such decreases in membrane input resistance and time constant should progressively erode the efficacy by which excitatory synaptic inputs are transformed into spiking output as synaptic activity increases.
Therefore, to help distinguish extrinsic from intrinsic factors underlying firing rate saturation, we artificially augmented descending excitatory drive to MNs by activation of muscle spindle afferents through tendon vibration. We measured the effects of tendon vibration on the change in firing rates of MUs voluntarily activated at firing rates below saturation and when saturated. We hypothesized that if MUs discharging at saturation increase their rate of firing with additional synaptic excitation provided by tendon vibration, then this would imply that extrinsic limitations in voluntary synaptic drive underlie saturation. On the other hand, if tendon vibration does not increase firing rate in otherwise saturated MUs, then this would imply that intrinsic cellular mechanisms limit increases in firing rate.
METHODS
Two sets of experiments were carried out, both involving the recording of MU activity in the lateral (long) head of the biceps brachii in healthy adult human subjects. In the first set of experiments (13 experimental sessions involving 4 female and 6 male subjects) we quantitatively characterized firing rate saturation in biceps MUs during voluntary contractions involving slow ramp increases in isometric elbow flexion force. In the second set of experiments (12 experimental sessions on 5 female and 5 male subjects) we applied vibration to the distal biceps tendon while subjects held MU firing rates at different steady levels. We selected biceps brachii as the test muscle because it has been widely used in previous studies of human MU activity and, based on preliminary studies, it was a muscle that could be readily excited with tendon vibration, a prerequisite for the present experiments. The Institutional Human Investigation Committee approved the procedures, and all subjects gave informed consent.
Experimental Setup
For both sets of experiments, subjects were seated comfortably in a dental chair with their right arm supported on a horizontal platform. The medial surface of the elbow rested on a padded, thermoplastic support that held the shoulder abducted ∼70° and flexed ∼45°. A thermoplastic cuff encircled the distal forearm just proximal to the wrist joint and was molded to hold the forearm midway between fully supinated and fully pronated positions. The cuff was connected to a force transducer (Grass FT-10) by a small metal chain. The length of the chain was adjusted so as to hold the elbow joint in a relatively extended position (∼20° from full extension). This extended elbow position was used to maintain some degree of resting tension on the force transducer. A vertically directed cord, supported by an overhead pulley, was attached to a Velcro strap encircling the hand to help support the weight of the hand and forearm. As a consequence of this overall arrangement, elbow flexion force was largely exerted in the horizontal plane. Force signals were amplified (gain 1,000) using a World Precision Instruments Transbridge amplifier.
Motor Unit Recording
Motor unit action potentials were recorded using sterilized, lacquer-coated tungsten microelectrodes (diameter 250, impedance ∼10 MΩ before insertion; Frederick Haer) inserted through alcohol-cleansed skin and into the lateral head of biceps brachii. A small surface electrode (diameter 4 mm) was fixed to the skin over the lateral epicondyle of the elbow to provide a reference signal for the differentially amplified intramuscular electromyographic (IEMG) signal (gain 1,000, bandpass filter 0.3–3 kHz). The IEMG signal was displayed on an oscilloscope and broadcast audibly to provide feedback of MU activity. Force and IEMG signals were digitally sampled (1 and 20 kHz, respectively; Power 1401 Cambridge Electronics Design), displayed on a computer monitor, and stored for subsequent analysis.
Tendon Vibration
Mechanical oscillatory inputs (80 Hz) were applied to the distal tendon of biceps brachii on the ventral aspect of the elbow using a mechanical vibrator (Panasonic EV 297). The vibrator was stabilized within a custom-built frame that allowed positioning of a 1-cm-diameter, 20-cm-long probe on and perpendicular to the distal portion of the biceps tendon. A noncalibrated accelerometer was mounted on the head of the vibrator and was simply used to monitor when vibration was applied. The accelerometer signal was digitally sampled (1 kHz) concurrently with force and EMG signals.
Protocol
Experiment 1.
In the first set of experiments (carried out to characterize firing rate saturation in biceps MUs), subjects first performed three brief (duration ∼2 s) maximum voluntary contractions (MVCs) of elbow flexion. MVCs were performed before insertion of microelectrodes into biceps. To record the large forces associated with this task, custom-built heavy-duty springs were inserted into the housing of the Grass FT-10 force transducer, providing an overall sensitivity of 0.072 mV/kg. Subjects were provided with about 1 min of rest between MVCs. The greatest peak force recorded from any of the three trials was taken as the MVC force.
After the MVCs were performed, the heavy-duty springs in the FT-10 transducer were replaced with springs allowing more sensitive measures of force (0.65 mV/kg). The microelectrode was inserted into the lateral head of biceps, and the position of the electrode was manually adjusted while the subject exerted weak isometric contractions until clear MU action potentials could be discerned. The subject was then instructed to perform slow, smooth ramp increases in elbow flexion force while MU activity was recorded. Subjects had visual feedback of force and MU activity. Auditory feedback of MU activity was also available to the subject. Subjects were instructed to increase force in a very gradual ramp, but no other constraints were put on the rate of rise of force. Force increased up to a level where recruitment of other units made detection of target-unit activity difficult (as judged by the experimenter), at which time the subject was instructed to relax. A rest of 10–20 s was provided between trials. The microelectrode position was then adjusted (which sometimes involved removal of the electrode and reinsertion at a new location) to sample from presumed different MUs, and the procedures were repeated.
To relate MU firing rates to the mechanical properties of the lateral biceps, additional experimental sessions were carried out in five of the subjects who participated in experiment 1 during which we electrically stimulated the lateral biceps at different frequencies using the intramuscular microelectrodes. The tip of the microelectrode was inserted to a depth that was judged to be near the central core of the lateral biceps. A small surface electrode attached over the lateral epicondyle of the elbow served as the return pathway for stimulation. Trains of monophasic, cathodic constant-current pulses (duration 0.5 ms) were delivered through the microelectrode using a Grass S88 stimulator and optical isolation unit. Initially, during stimulation at 1 Hz, the intensity of the pulses was increased up to a level that evoked moderately strong twitches but without discomfort to the subject. The stimulus intensity was then maintained at this level while trains of stimuli (∼2 s in duration and with ∼5 s of rest between trains) were delivered at frequencies of 5, 10, 15, 20, 30, 40, and 50 imp/s during which the evoked isometric flexion force was recorded. Subjects were instructed to relax the arm during stimulation.
Experiment 2.
The procedures for the second set of experiments involving tendon vibration were similar to those described above for MU recording, with the following differences. Initially, the probe of the tendon vibrator was applied to the biceps tendon with its position and applied pressure optimized to elicit a consistent reflex contraction in response to brief periods of vibration as judged by increased isometric force. The microelectrode was then inserted into the lateral biceps, and a target MU was identified during weak voluntary contractions. Subjects were then instructed to perform stepwise ramp-and-hold contractions beginning with a holding-force level just sufficient to activate the MU to fire tonically. Each hold phase consisted of an initial ∼4-s period of maintained isometric force followed by an ∼3- to 5-s period during which tendon vibration was superimposed on the voluntary contraction. Subjects were instructed to maintain the same level of voluntary effort during the vibration as during the initial holding period (Hagbarth et al. 1976). Subjects were then instructed to increase force slightly to a new holding-force level and the procedures were repeated. Such increasing force steps were continued until activity of newly recruited units made identification of the target unit difficult. The subject then relaxed for a period of 30–60 s, after which the microelectrode position was adjusted to search for a different MU. This process was repeated several times during an ∼2-h experimental session.
Data Analysis
In off-line analysis, MU action potentials detected in IEMG signals were discriminated on the basis of waveform shape and amplitude using a template-matching algorithm (Spike2; Cambridge Electronics Design). From the discriminated spike trains, the firing rates of each MU were calculated. Sporadic doublet discharges (interspike intervals <15 ms) occurring at the outset of a contraction were excluded from the analysis.
Experiment 1.
For the first set of experiments to characterize firing rate saturation, instantaneous firing rate was plotted as a function of isometric force (% MVC). This was done only for the rising force phase of the contractions. Based on previous work (e.g., De Luca and Contessa 2012; De Luca et al. 1982; McGill et al. 2005; Monster and Chan 1977), the profiles of these types of plots indicate that such data can be reasonably fit by rising exponentials of the form (see Fig. 1)
| (1) |
where R(F) represents the rate (R) of spiking as a function of muscle force (F), Rmax is the maximum rate of spiking, e is the base of the natural logarithms, F is normalized isometric force, Fth is the threshold force at which a unit begins to discharge, and Rmin is the minimum rate of spiking at recruitment threshold. The parameter Φ can be thought of as a force constant (akin to a time constant) and represents the amount of force increase associated with a 63% increase in firing rate. For each MU, we tested the hypothesis that such an exponential (i.e., saturating) function provided a significantly better fit of firing rate data than a simple linear function. As such, firing rate-force data for each unit were also fit with linear regression. The sum of squared errors (SSE) associated with linear regression was compared with that of the exponential fit using the F statistic. The firing rate profile of a unit was then designated as exponential only if the F statistic indicated a significant (P < 0.01) improvement in fit over linear regression. Otherwise, the firing rate relation was deemed to be linear. If neither linear nor exponential regression provided a significant fit of the data, then no designation was applied to the unit's firing rate profile.
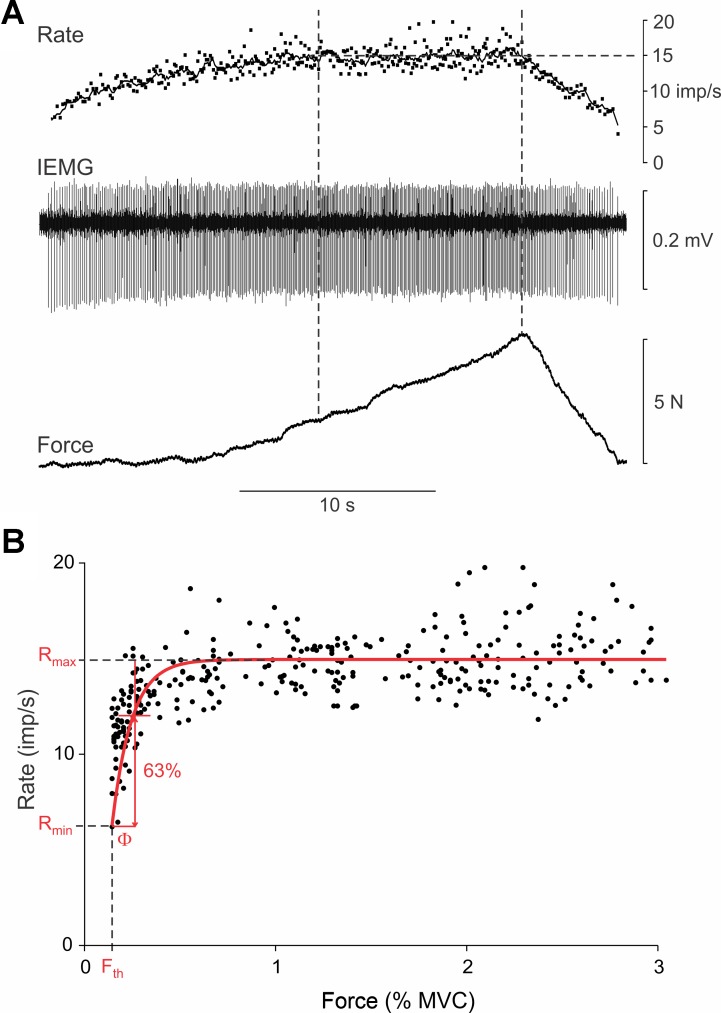
Fig. 1.
Example recording from biceps motor unit (MU). A: isometric force exerted by biceps muscle (bottom trace), intramuscular electromyography (IEMG) signal recorded in biceps muscle depicting discharge of a single MU (middle trace), and instantaneous (dots) and moving average (1-s window; line) firing rate of recorded unit (top trace). Vertical dashed lines indicate period during which force was increasing yet MU firing rate had leveled off at a firing rate of ∼15 impulses/s (imp/s). B: plot of instantaneous firing rate vs. muscle force for the unit shown in A. Force was normalized to a percentage of the maximum voluntary contraction (% MVC) force. Data were fit with a rising exponential (red line) of the form represented by Eq. 1. Parameters derived from this fit were recruitment threshold force (Fth), firing rate at recruitment threshold (Rmin), maximum rate (Rmax), and force constant (Φ).
Regression analyses were also carried out to determine if there existed significant associations between recruitment threshold (Fth) and the maximum rate of spiking (Rmax), between recruitment threshold and the rate of spiking at threshold (Rmin), and between recruitment threshold and the initial firing rate gain (calculated as the ratio of the change in firing rate from the minimum rate to that at the force constant, divided by the force constant).
Experiment 2.
For the second experiment involving tendon vibration, the firing rate of each unit was determined over a 2-s period immediately preceding tendon vibration and for a 2-s period 0.5 s after the onset of vibration. This was done for each of the holding-force levels. For each holding-force level, the difference in firing rate (Δrate) between that measured during the superimposed vibration (vibration rate) and that prior to vibration (initial rate) represented the efficacy with which the supplemental excitation arising from peripheral receptors increased firing rate in the MU. Likewise, to obtain a coarse index of the overall excitatory effect of vibration on the entire MU pool, we measured the change in isometric muscle force averaged over a 2-s window before vibration and over a 0.5-s window 2-s after the onset of vibration. This delayed window for measuring vibration-evoked force was selected because force was relatively stable and nearly fully developed during this period (e.g., see Fig. 6).
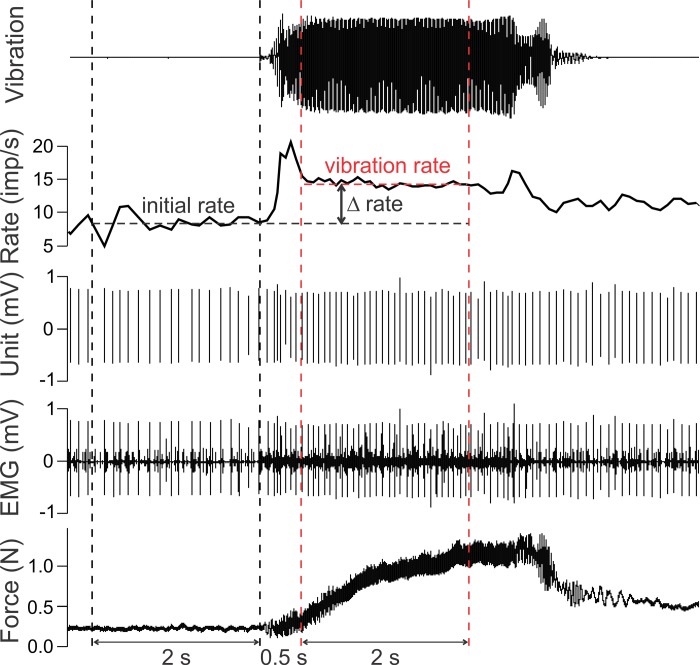
Fig. 6.
Quantification of vibration-mediated changes in firing rate. Example recording of biceps MU responding to tendon vibration illustrates quantification. The initial rate was determined as the average spike rate in a 2-s window (black vertical dashed lines) immediately before vibration. The vibration rate was calculated as the average spike rate in a 2-s window (red vertical dashed lines) 0.5 s after the onset of vibration. The difference in firing rate (Δrate) was calculated as the difference between the vibration rate and initial rate. Traces from bottom to top show elbow flexion force, intramuscular EMG signal, discriminated MU spikes, moving average (0.5-s window) MU firing rate, and noncalibrated acceleration signal indicating application of vibration.
Regression analysis was performed on Δrate as a function of the initial rate for all units and for all target levels to establish whether there was a systematic change in firing rate responsiveness of MUs to peripheral excitatory input as initial firing rates increased. Data are reported as means ± SD, and effects were considered statistically significant when P < 0.01.
RESULTS
Experiment 1: Firing Rate Saturation
The discharge patterns of 136 MUs were recorded from the biceps brachii during slow, weak ramp contractions in 10 subjects. The average trial duration was 14.2 ± 6.5 s with a slow rate of rise of force (0.77 ± 0.71 N/s or 0.40 ± 0.36% MVC/s). In 75 recordings, only 1 unit was analyzed; in 26 recordings, 2 units were identified and analyzed; and in 3 recordings, 3 units were identified and analyzed. Figure 1A shows an example recording in which one unit was followed throughout the ramp contraction. In this low-threshold unit, firing rate initially increased with subtle increases in force but then leveled off to an average rate of ∼15 imp/s even though force (and presumably, synaptic drive) continued to increase (period between dashed vertical lines).
In Fig. 1B, the instantaneous firing rate of this unit is replotted as a function of rising isometric force, normalized to the MVC of the subject. It is evident in this representation that firing rate was modulated over only a small increase in force beyond which firing rate showed little further systematic increases. Nevertheless, linear regression performed on these data was significant (P < 0.001, R2 = 0.35, SSE = 1,094). As expected, the exponential fit (Eq. 1, methods) was also significant (P < 0.001, R2 = 0.58, SSE = 714). Using the F statistic and accounting for differences in the number of parameters used to fit the data, the reduction in SSE from linear to exponential fits was highly significant (F ratio = 84.4, P < 0.0001). As such, this unit was designated as having an exponential (saturating) firing rate function, which is displayed as a red trace in Fig. 1B. From this function, the following parameters were identified (see Eq. 1): initial minimum spiking rate (Rmin = 6.2 imp/s), maximum spiking rate (Rmax = 14.9 imp/s), threshold force (Fth = 0.20% MVC), and force constant (Φ = 0.17% MVC).
Despite the narrow force range (<1% MVC) over which this unit modulated firing rate, the subject was still able to precisely regulate firing of the unit within this range. As shown in Fig. 1A, the increase in firing rate played out over a relatively long duration (>10 s). This prolonged period of rate modulation presumably occurred because the subject was capable of finely grading the net synaptic excitation driving the MN during this time. As such, the unit did not exhibit an uncontrollable jump from low to high firing rate; instead, firing rate was purposefully modulated despite the initial high gain. Indeed, the subject was able to extend the initial period of rate modulation of this unit over a period of ∼5 min (data not shown) involving a very gradual increase in force. Because we did not examine this issue systematically in the present study, it is not possible to say how prevalent this capability of controlled rate modulation is for biceps MUs.
The profile of firing rate vs. force for the unit depicted in Fig. 1A appeared different for the rising phase compared with the falling phase of the contraction. We did not analyze the falling phase in these experiments because subjects typically abruptly ceased the contraction when instructed to relax (unlike the trial shown in Fig. 1A). Nevertheless, such intriguing asymmetry in firing rate modulation has been noted previously (e.g., Mottram et al. 2014) and warrants dedicated further investigation.
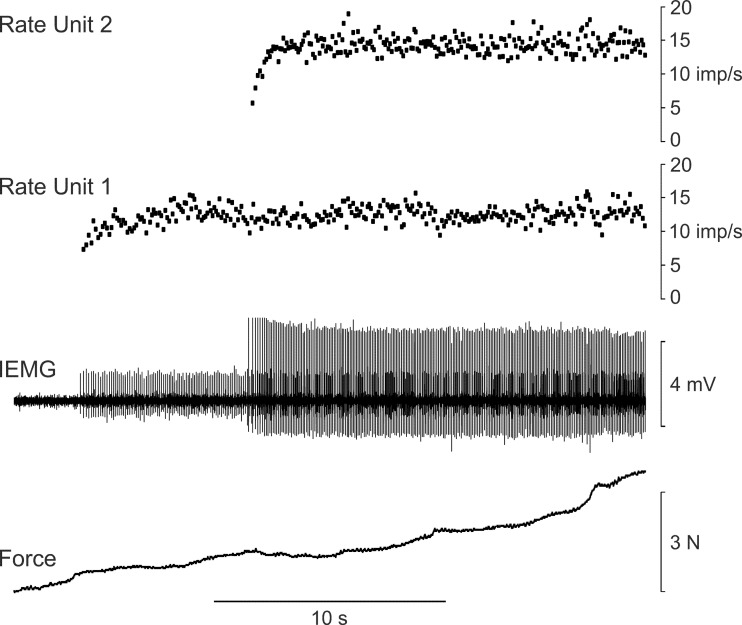
Figure 2 shows an example recording in which the activities of two units were clearly discernable during the ramp contraction. The first unit increased its firing rate over a period of about 5 s up to a level of ∼13 imp/s, after which no further systematic increases in firing rate occurred. The second unit was recruited at a time when the firing rate of the first unit had leveled off. The firing rate of the second unit then increased rapidly and with very modest changes in force up to a rate of ∼14.5 imp/s, beyond which no further increases in firing rate were observed. Such saturation in firing rate suggests that these units were relatively impervious to further increases in synaptic excitation delivered to the motor nucleus as a whole as evidenced by increased muscle force and recruitment of other units. Furthermore, this example demonstrates that the period over which a unit increased firing rate occurred relatively independently of that occurring in other units within the same muscle.
Fig. 2.
Example recording depicting firing rate saturation in 2 MUs. Isometric force (bottom trace), IEMG signal (middle trace), and instantaneous firing rates of the 2 units detected in the IEMG signal (top traces). The firing rates of both units leveled off despite continued increase in muscle force. In addition, unit 2 was recruited at a time when the firing rate of unit 1 had saturated.
Of the 136 MUs recorded, 123 (90%) were best fit by the exponential function, 10 were best fit with a linear function, and 3 were not significantly fit by either exponential or linear regression. For those units designated as linear, oftentimes they too exhibited the initial steep increase in firing rate characteristic of saturating firing but with insufficient data points in the initial high-gain region for the exponential fit to represent a significant improvement over the linear fit.
Figure 3A shows a plot of the exponential and linear firing-rate vs. force functions for all 133 MUs whose data were significantly fit by one of those two relations. Cases that were best fit by a linear function are shown in red. Virtually all the MUs that we could record and reliably discriminate had recruitment thresholds <10% MVC, and the majority of these were recruited at forces <5% MVC. For improved clarity at the low force range where many units are represented, we have replotted the firing-rate force relations over the initial 2.5% MVC in Fig. 3B.
Fig. 3.
Firing rate-force relation for 133 biceps MUs. A: each trace indicates the firing rate-force relation for an individual MU. Force is normalized as % MVC. Black traces indicate MUs (n = 123) whose firing rate-force data were significantly better fit by a rising exponential function (Eq. 1) than a linear function. Red traces indicate MUs (n = 10) whose firing rate data were best fit by a linear function. B: for improved clarity, firing rate-force relations shown in A are redrawn for a force range up to 2.5% MVC.
Most units depicted in Fig. 3 exhibited an initial steep rise in firing rate and then leveled out at modest firing rates, usually <20 imp/s. The average (SD) minimum firing rate across the entire population of MUs depicted in Fig. 3A was 6.7 ± 2.3 imp/s. The average recruitment threshold force of all units sampled was 2.1 ± 2.2% MVC. For this population of low-threshold MUs, there was no significant (P = 0.89) relation between recruitment threshold force and minimum firing rate.
For the 123 units that were best fit by an exponential function (and for which we could estimate the upper limit of firing rate), the average maximum firing rate was 14.8 ± 2.0 imp/s. This value is nearly identical to that (15.2 imp/s) reported for biceps MUs in healthy control subjects by Mottram et al. (2014). There was no significant (P = 0.53) relation between maximum firing rate and recruitment threshold force. The average extent of firing rate modulation (maximum firing rate minus minimum firing rate) was 8.0 ± 2.9 imp/s.
Force constant Φ values were highly skewed to the right (skewness = 3.6) with values ranging from 0.02 to 3.3% MVC, an average value of 0.35 ± 0.48% MVC, and a median value of 0.19% MVC. Likewise, initial gain values were also broadly distributed (range = 1–251 imp/s/% MVC) and rightward skewed (skewness = 2.4), with a mean value of 37 ± 38 imp/s/% MVC and a median value of 24 imp/s/% MVC. To ascertain whether initial gain varied systematically with recruitment threshold, the data were first log transformed (due to their lack of normality), and then linear regression was performed. Whereas the regression was significant (P < 0.01) and negative (i.e., lower gains for higher threshold MUs), the overall variance accounted for by the regression was small (R2 = 0.08). As such, some degree of caution is warranted in interpreting this result.
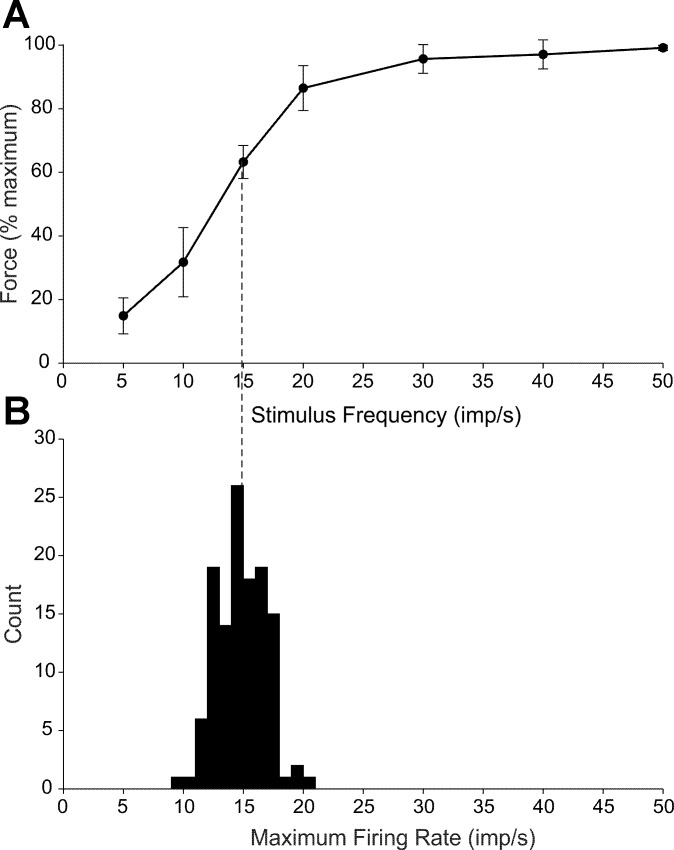
To understand whether firing rate saturation exhibited by most biceps MUs might limit force output, it is important to know the relation between MU firing rate and MU force. The human biceps is not experimentally amenable to intraneural microstimulation of single motor axons as are some distal muscles. Therefore, we instead electrically stimulated the lateral head of the biceps with intramuscular electrodes by using submaximal constant current pulses delivered at different stimulus frequencies in five of the subjects who participated in the first experiment. Figure 4A shows the average (SD) peak force exerted for each stimulus frequency normalized to the maximum evoked force. Maximum force was achieved at 50 imp/s in 3 of the 5 subjects and at 40 imp/s in the other 2 subjects. The average maximum evoked force for these experiments was 5.6 ± 2.8% MVC. Isometric force increased most steeply in the stimulus frequency range from 10 to 20 imp/s.
Fig. 4.
Relation between stimulus frequency and isometric force. A: mean (SD) steady-state elbow flexion force exerted during submaximal stimulation of biceps at different pulse frequencies using intramuscular electrodes. Force increased as a sigmoid function of stimulus frequency with maximum force attained at a stimulus frequency of ∼30 imp/s. B: histogram depicting maximum firing rates recorded during ramp contractions in 123 MUs that exhibited saturating firing rate responses (black traces in Fig. 3). Average maximum rate (14.8 imp/s) is depicted as a vertical dashed line projecting to A. Based on the force-stimulus frequency curve in A, MUs discharging at this average maximum rate would likely generate ∼63% of their force capacity.
Figure 4B shows the distribution of the maximum firing rates for the 123 exponentially designated MUs (depicted in Fig. 2) plotted on an abscissa with the same scaling as for Fig. 4A. The average maximum firing rate (14.8 imp/s) in Fig. 4B is indicated with a dashed vertical line projecting to the average force-stimulus frequency relation shown in Fig. 4A. The isometric force that would be evoked with electrical stimuli delivered at this frequency would only be about 63% of the maximal evoked force. These results suggest that biceps MUs discharging at saturation rates are unlikely to elicit their maximum force capacity. This issue will be more fully explored in the discussion.
Experiment 2: Tendon Vibration
As described in the Introduction, saturation of MU discharge may result from limitations in the net excitatory drive acting on the MNs (an extrinsic factor), from intrinsic properties of the MNs, or both. Excitatory drive to a MN pool can be augmented artificially by activation of muscle spindle Ia afferents through tendon vibration. Therefore, we examined the effects of tendon vibration on the change in firing rate of individual biceps brachii MUs that were voluntarily activated at various rates below saturation and at saturation.
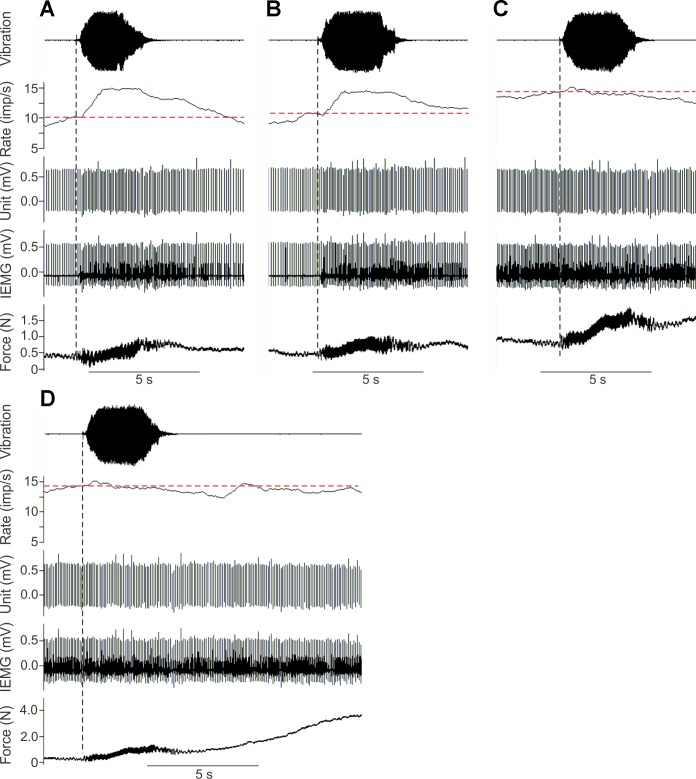
Figure 5 shows representative responses recorded in a single MU. When weak voluntary drive brought the discharge of the unit to ∼10 imp/s (Fig. 5A), the addition of tendon vibration caused a marked increase in firing rate to ∼15 imp/s. When the subject slightly increased drive to the muscle and increased the spike rate of the unit to ∼11 imp/s (Fig. 5B), tendon vibration again augmented firing rate, but the change in rate was not as great as in the first case. Finally, when drive to the muscle was increased so that the firing of the MU appeared to have saturated at a rate of ∼15 imp/s (Fig. 5C), tendon vibration had virtually no effect on the discharge of the unit. Nevertheless, tendon vibration remained an effective excitatory stimulus as it led to an increase in muscle force (see bottom trace, Fig. 5C), suggesting that other units were recruited or increased their rates in response to vibration.
Fig. 5.
Response of single biceps MU to tendon vibration. Subject voluntarily increased firing of unit to different initial rates (indicated by horizontal dashed lines): ∼10 (A), ∼11 (B), and ∼15 imp/s (C). Vibration was then applied to the biceps tendon (vertical dashed lines). Traces from bottom to top show elbow flexion force, intramuscular EMG signal, discriminated MU spikes, moving average (1-s window) MU firing rate, and noncalibrated acceleration signal indicating application of vibration. There was a marked increase in firing rate with vibration in A and B but no detectable change in rate in C despite clear evidence of vibration-mediated increase in muscle force. D: traces from C are redrawn and extended to show subsequent period of voluntary increase in muscle force. Once the unit reached a discharge rate of ∼15 imp/s, additional increases in firing rate were not observed in response to enhanced excitation mediated by tendon vibration or voluntary drive.
Immediately following the vibration trial shown in Fig. 5C, the subject was asked to voluntarily increase isometric force exerted by the biceps. Figure 5D redisplays the traces depicted in Fig. 5C showing the responses to tendon vibration and is extended to include the following period of increased voluntary force. Firing rate remained stable at ∼15 imp/s during both maneuvers suggesting that the MU was relatively unresponsive once saturated to increases in either peripheral or descending sources of excitatory drive. Although average firing rate (as shown in Fig. 5) rarely exceeded 15 imp/s when saturated, the instantaneous rate did fluctuate with a similar variability (e.g., Fig. 1A) as seen at lower rates. This indicates that firing rate can momentarily exceed the saturation level. Such temporary increases (and decreases) in firing rate likely reflect noisy fluctuations in membrane potential rather than systematic changes in the net driving current or intrinsic responsiveness of MNs.
For each unit recorded in experiment 2, firing rate was determined over a 2-s period immediately preceding tendon vibration and for a 2-s period 0.5 s after the onset of vibration (Fig. 6). These measurement windows were selected because they were associated with relatively stable periods of firing rate in response to tendon vibration. The difference in firing rate (Δrate) between that measured during vibration (vibration rate) and that before vibration (initial rate) represents the efficacy with which the supplemental excitation arising from peripheral receptors drove firing rate in the MU.
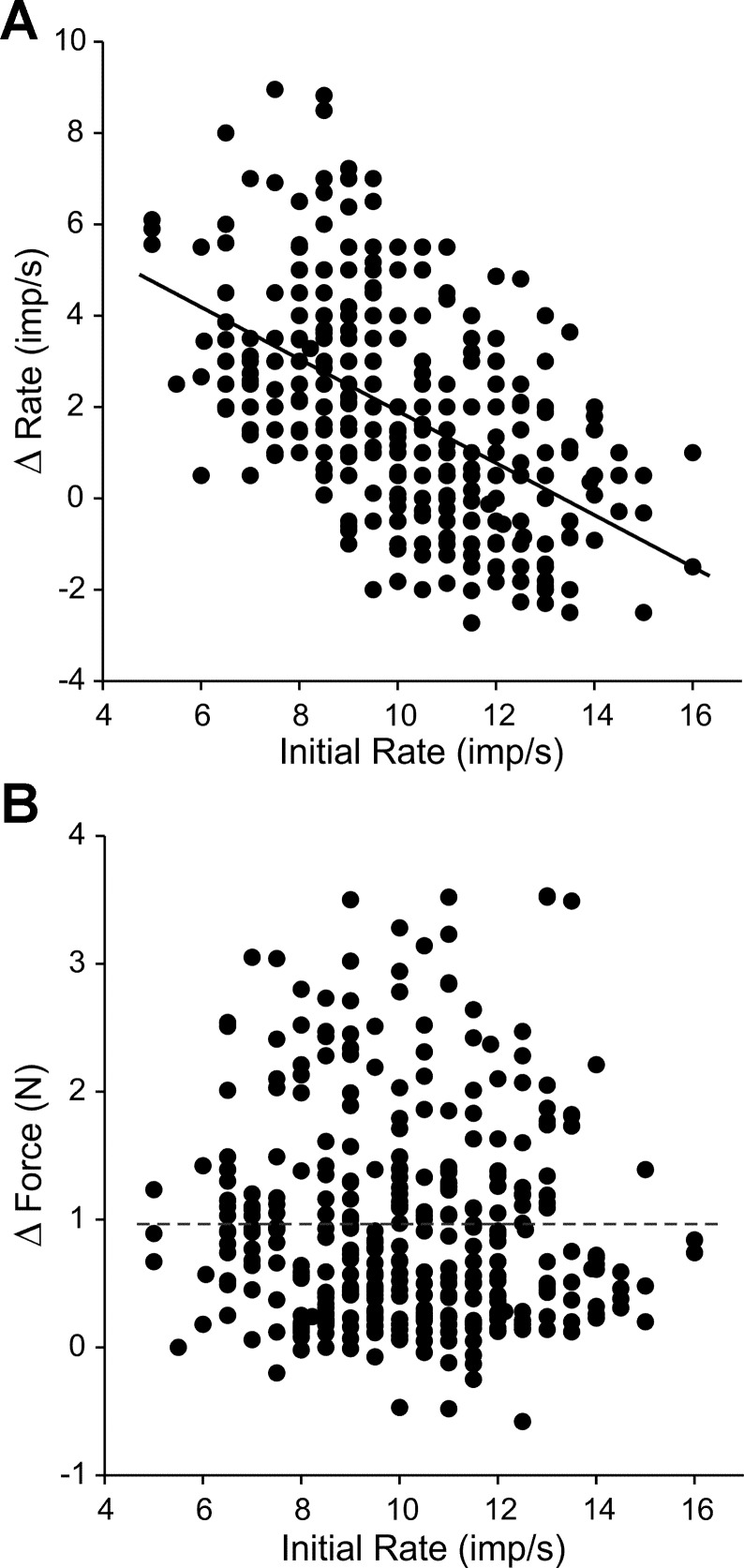
The vibration responses from a total of 96 MUs in 10 subjects were recorded and analyzed for this experiment. Each unit's response to vibration was evaluated, on average, at 4 ± 2 different initial firing rate levels. The changes in firing rate evoked by vibration are plotted as a function of firing rates immediately preceding vibration for all units and firing rate levels in Fig. 7A. Linear regression indicated a significant (P < 0.001, R2 = 0.28) negative relation between these two variables. For example, for an initial firing rate of ∼6 imp/s, vibration evoked on average about a 4 imp/s increase in firing rate, whereas for units discharging close to saturation frequencies (∼14 imp/s), vibration evoked little change in firing rate.
Fig. 7.
Vibration-mediated changes in firing rate as a function of initial rate. A: Δrate induced by tendon vibration for 362 total trials recorded in 96 MUs plotted as a function of the initial (i.e., previbration) firing rate. The firing rate change evoked by tendon vibration decreased as the initial rate on which the vibration was superimposed increased. Line shows linear regression (P < 0.001). B: average change in isometric force (Δforce) caused by tendon vibration for the same 362 trials shown in A. Linear regression was not significant (P = 0.16). Dashed line indicates average magnitude of vibration-induced force.
We were also interested to know whether the transient increase in firing rate often seen often at the outset of vibration (e.g., Fig. 6) was also diminished when units were discharging at saturation rates. We therefore calculated the change in firing rate in the 0.5-s window immediately following the onset of vibration (to capture the transient) for all trials for which the initial firing rate was ≥14 imp/s. These values were then compared with the change in rate for the subsequent 2-s period. Although the change in rate associated with the initial 0.5-s period was slightly greater on average (0.60 ± 0.86 imp/s) than for the subsequent 2-s period (0.40 ± 1.25 imp/s), these values were not significantly different from one another (P = 0.80, repeated-measures ANOVA). This implies that tendon vibration had little effect on MU discharge, either transiently or longer lasting, when MUs were already active near saturation rates.
One interpretation of the results in Fig. 7A is that MUs discharging at saturation frequencies are relatively unresponsive to additional synaptic excitation provided by tendon vibration, and as such, intrinsic cellular mechanisms likely limit firing rates of MUs during voluntary effort. This interpretation rests partially on the assumption that the vibratory stimulus provided roughly equivalent levels of additional excitatory synaptic input as initial firing rates (and associated contraction levels) increased. Our measure of the efficacy of supplemental excitatory drive provided to biceps MNs by vibration was the magnitude of the reflex contraction force superimposed on the voluntary force. The average magnitude of the vibration-induced force was 0.96 ± 0.92 N. Figure 7B shows a plot of the change in force elicited by vibration as a function of the initial firing rate for all 362 trials. Linear regression revealed no significant (P = 0.16) relation between these two variables, suggesting that the strength of the excitatory drive provided by vibration did not vary systematically with the initial firing rate.
DISCUSSION
In this study we have characterized the firing rate properties of low-threshold biceps MU during voluntarily graded isometric contractions. For most units recorded, firing rate initially increased steeply as a function of force and then leveled off (i.e., saturated) at relatively low rates, usually <20 imp/s. This result is qualitatively similar to that described previously by other investigators for biceps (Mottram et al. 2009, 2014) and in a variety of other human muscles (rectus abdominis, latissiumus dorsi, pectoralis major, triceps, and brachioradialis, Bracchi et al. 1966; genioglossus, Bailey et al. 2007; vastus lateralis, De Luca and Contessa 2012; deltoid, De Luca et al. 1982; soleus and tibialis anterior, Kiehn and Eken 1997; medial gastrocnemius, McGill et al. 2005; extensor digitorum, Monster and Chan 1977; first dorsal interosseus, Moritz et al. 2005; rectus femoris, Person and Kudina 1972). We have also shown in this work that augmenting descending excitatory drive to biceps MNs with peripheral excitation mediated by tendon vibration had little effect on the discharge of MUs that were firing at saturation frequencies but robustly increased firing rates of the same units when active at lower frequencies. These results suggests that firing rate saturation in human MNs is likely caused by intrinsic mechanisms that curtail increases in firing rate even in the presence of escalating synaptic excitation. Although the specific mechanisms responsible for saturation are presently unknown, we consider some possibilities below after first discussing limitations of this study.
Limitations
The major limitation of this study was that we were able to follow the firing rates of only the lowest threshold MUs (recruitment thresholds all <10% MVC). Furthermore, once recruited, we tracked subsequent changes in firing rates over only a small range of the entire force capacity of biceps. This was primarily due to the difficulty in isolating spikes of individual MUs because activities in other MUs contaminated the recordings as force increased. It is possible, therefore, that saturation of MU activity at low firing rates may be a feature of low-threshold MUs only. The recent development of high-density multielectrode surface arrays (Lapatki et al. 2004; Merletti et al. 2008; Nawab et al. 2010) using sophisticated decomposition algorithms (Holobar and Zazula 2007; Holobar et al. 2010; McGill et al. 2005) should enable reliable tracking of higher threshold MUs over a wider force range (Holobar et al. 2014). Indeed, using this approach, De Luca and Contessa (2012) recently showed saturation at rates <20 imp/s in vastus lateralis motor units recruited as high as 50% MVC.
A more general limitation of this study is that implicit comparisons between saturation firing rates generated in response to natural synaptic activation and those in response to intracellular current injection are made across species. As far as we are aware, no intracellular current injection studies have been carried out in human MNs. Likewise, only a few studies (e.g., Gorassini et al. 1999) have attempted to characterize MU activity during voluntarily graded isometric contractions in experimental animal models as is done so readily in human subjects. Future work in which both types of assessments are carried out within the same species will greatly aid in understanding the mechanisms underlying firing rate saturation.
Extrinsic Factors
Our argument that firing rate saturation is most likely caused by mechanisms intrinsic to individual MNs derives in part from consideration as to how extrinsic sources of synaptic input to a motor nucleus are organized. For example, anatomic and electrophysiological evidence suggests that individual muscle spindle Ia afferents arborize extensively to make excitatory synaptic connections on most alpha MNs of the homonymous motor nucleus (Brown and Fyffe 1978; Lüscher and Vadar 1989; Mendell and Henneman 1971). Likewise, individual corticospinal inputs (in primates) also appear to diverge extensively to provide excitatory input across many members of a MN pool (Lawrence et al. 1985; Mantel and Lemon 1987; Shinoda et al. 1981). Such broadly distributed excitation would seem unlikely to cause the restricted periods of firing rate increases in individual MUs characteristic of firing rate saturation.
It also seems improbable that the leveling off in firing rate seen to occur in different MUs at various time points and levels of excitatory drive (e.g., Fig. 2) could come about due to targeted increases in synaptic inhibition directed specifically to those MNs exhibiting saturation. This is not to say that an overall increase in synaptic inhibition may not occur as contraction strength increases. Indeed, recurrent inhibition, for example, mediated by Renshaw cells is likely to increase in intensity with increased MU activity. However, the distribution of inhibitory synaptic contacts made by Renshaw cells is widespread (Fyffe 1991) such that the overall degree of recurrent inhibition is more or less uniformly distributed across a pool of MNs (Lindsay and Binder 1991). Similar findings have also been reported for reciprocal inhibition mediated by Ia inhibitory interneurons (Heckman and Binder 1991b). As such, targeted inhibition from spinal interneurons to individual MNs seems unlikely.
Rubrospinal inputs, however, do appear to have a non-uniform distribution across motor nuclei supplying cat hindlimb muscles with such inputs tending to inhibit low-threshold MNs while facilitating higher threshold MNs (Powers et al. 1993). If the degree of engagement of rubrospinal inputs progressively increased with the intensity of muscle contraction, then aspects of firing rate saturation (e.g., leveling off of firing rate of low-threshold units while higher threshold units are recruited and increasing firing rate) might be affected (Heckman and Binder 1993). However, in humans, the rubrospinal tract is virtually absent (Massion 1988; Nathan and Smith 1982; Yang et al. 2011), making such a divergent source of synaptic input an unlikely cause of the saturation seen in the present experiments. Collectively, therefore, it seems unlikely that firing rate saturation in individual MUs could be due to a selective reduction in excitatory input or a targeted increases in inhibition.
Intrinsic Mechanisms
If, as we suggest, mechanisms intrinsic to individual MNs are primarily responsible for the limitation in firing rates observed in this and other studies of human MUs, then one must account for the relative absence of clear-cut saturation in studies involving current injection into individual MNs (e.g., Kernell 1965; Granit et al. 1966; Schwindt 1973; Schwindt and Calvin 1972; Schwindt and Crill 1982). As mentioned above, species differences might be an important consideration, because such current injection studies primarily involved cat MNs, whereas firing rate saturation during voluntary activation has mainly been reported for human MUs. As such, there could simply be species differences in the capacity of MNs to generate action potentials at high rates. However, substantial evidence of firing rate saturation has also been reported for cat MNs during synaptic activation mediated by reflex pathways (Alvord and Fuortes 1953; Bracchi et al. 1966; Burke 1968; Cordo and Rymer 1982; Denny-Brown 1929; Granit 1958; Granit et al. 1960; Kernell and Sjöholm 1975; Prather et al. 2002) and in response to stimulation of descending pathways (Brownstone et al. 1992; Kernell and Sjöholm 1975; Tansey and Botterman 1996; Zajac and Young 1980).
Despite numerous arguments to the contrary (Granit et al. 1966; Heckman and Binder 1991a; Kernell 1969, 2006; Kernell and Sjöholm 1973; Powers et al. 1992; Schwindt and Calvin 1973), some investigators have questioned the validity of using focal intrasomatic current injection to represent the complex processes by which neurons naturally integrate currents arising from hundreds of synaptic conductances distributed across elaborate dendritic arbors (Cushing et al. 2005; Destexhe and Paré 1999; Destexhe et al. 2003; Kuhn et al. 2004; Paré et al. 1998). For example, several investigators have pointed out that as synaptic activity increases, input resistance of the target neuron will progressively diminish due to increased opening of transmitter-gated channels (Binder et al. 1996; Destexhe and Paré 1999; Destexhe et al. 2003; Granit et al. 1966; Kernell 1969; Kuhn et al. 2004; Llinas and Terzuolo 1964; Paré et al. 1998; Powers and Binder 2000). Such a decrease in input resistance should have two consequences on neuron discharge. First, lowering of input resistance should lessen the effective change in membrane potential caused by a given increment in depolarizing current according to Ohm's law. Second, because membrane time constant is directly proportional to input resistance, lowering of input resistance will abbreviate the time course of postsynaptic potentials and thereby reduce their temporal summation. Because above-threshold changes in membrane potential are tightly linked to increments in discharge rate (Granit et al. 1966), such input resistance-related mitigation of membrane depolarization should subdue increases in firing rate, which in theory, could contribute to firing rate saturation.
One problem with this idea, however, is that the transitions into saturated states occurred at distinct times for different MUs. Because synaptic inputs are more or less uniformly distributed across a pool of MNs (Binder et al. 1998), changes in input resistance associated with increased synaptic activation should occur roughly in parallel, rather than independently, for members of an MU population.
Given the relatively discrete manifestation of saturation in individual MUs, the contributing mechanisms might be linked in some way to the extent of activity in each MN. For example, the magnitude of a slowly activating potassium conductance (Barraza et al. 2009; Partridge and Stevens 1976; Sawczuk et al. 1997) underlying “late” spike-frequency adaptation appears related to both the duration and intensity of MN activity (Gorman et al. 2005; Kernell and Monster 1982; Sawczuk et al. 1995). As such, spike-frequency adaptation might progressively undercut synaptic excitation acting on a MN as a ramp contraction progresses. However, the time constant for spike-frequency adaptation is typically found to be >20 s (Gorman et al. 2005; Kernell and Monster 1982; Sawczuk et al. 1995). Such a slowly developing conductance would therefore seem unlikely to account for the rapid transition into saturated states that can be seen in MUs even during slowly graded contractions (e.g., Fig. 2).
A third possibility, as elegantly explored in computer simulation by Cushing et al. (2005), is that progressive diminution of the electrochemical driving force at excitatory synapses could severely reduce synaptic currents as dendritic membranes become depolarized. This would limit the overall current delivered to the soma and thereby tend to abate increases in firing rate in the presence of increasing synaptic activity. Furthermore, the time course of this effect would occur somewhat independently for different neurons because change in membrane potential (and effect on driving potential) would depend on properties, such as size-related input resistance, specific to individual neurons.
A forth intrinsic factor that might underlie firing rate saturation is that associated with activation of persistent inward currents (PICs) (Heckman et al. 2008; Heckman and Enoka 2012; Hornby et al. 2002). PICs are mediated by intrinsic (i.e., nonsynaptic) voltage-gated conductances that slowly inactivate. PICs likely become activated near the recruitment threshold of MNs (Bennett et al. 1998) and then provide an additional potent source of depolarizing current to MNs (Bennett et al. 1998; Lee and Heckman 1998a, 2000). Such activation of PICs could contribute to a sharp rise in depolarizing current at the outset of firing in MNs and could contribute to the initial steep increase in firing rate characteristic of firing-rate saturation (Heckman et al. 2008; Hornby et al. 2002). Furthermore, because PICs primarily originate in the dendrites, dendritic membrane potential might shift rather abruptly into a depolarized state and thereby diminish synaptic driving potential (Cushing et al. 2005). As such, the dendritic membrane might approach a kind of voltage-clamp state and make the neuron relatively indifferent to additional synaptic input. Indeed, Powers and Binder (2000) demonstrated that synaptic excitation mediated by tendon vibration had virtually no effect on membrane potential when MNs were already exhibiting plateau potentials associated with activation of PICs. That finding closely parallels the absence of effect of tendon vibration on firing rate when MUs were saturated as shown in the present investigation. Similarly, under high-monoamine drive that likely promotes PICs, MNs can exhibit bistable firing in response to slow ramp increases of injected current (e.g., Lee and Heckman 1998b) that are not unlike the saturating responses of motor units in the present study.
It should also be said that framing possible mechanisms for saturation as a dichotomy between intrinsic and extrinsic factors may not be entirely constructive. Indeed, in an important computational study, Powers et al. (2012) have accurately simulated the type of firing rate saturation seen in human MUs but only under conditions in which the model incorporated some of the intrinsic properties described above combined with the extrinsic factor of time-varying synaptic inhibition.
Functional Consequences
Regardless of the underlying mechanisms, saturation in MU firing rate has functional consequences. The force exerted by a MU increases as a sigmoidal function of firing rate. In this study, we obtained an estimate of this relation by activating biceps at different stimulus rates (Fig. 4A). The stimulus rate needed to achieve near maximum force was ∼30 imp/s. Dalton et al. (2010) found somewhat higher stimulus rates (∼50 imp/s) to achieve maximal force in human biceps brachii. This difference may be due to the higher stimulus intensities or more flexed elbow posture used in that study.
Such electrical stimulation, however, is not selective for individual types of MUs. At best, the force-stimulus frequency relation like that depicted in Fig. 4A represents a weighted average of the subset of MUs whose axons were in the vicinity of the stimulating electrode. Nevertheless, it provides a rough indication of the firing rates needed to produce near maximum force in biceps MUs (>30 imp/s). The saturation firing rates recorded in this study and elsewhere (Mottram et al. 2014) for biceps during voluntary contraction were about half that estimated to be needed to produce maximal force (Fig. 4B). The firing rate needed to achieve maximum force, however, depends on the contractile properties of the MU, with slow units requiring lower rates (Botterman et al. 1986; Kernell et al. 1983). All the MUs recorded in the present study were low threshold and as such may have been slow twitch (cf. Fuglevand 2011) and therefore required somewhat lower activation rates to achieve maximum force than that indicated in Fig. 4A. Nevertheless, based on experiments involving intraneural microstimulation of single motor axons in humans, the stimulus rate needed to elicit maximum force in the slowest contracting MUs was never less than 30 imp/s (Thomas et al. 1991; Fuglevand et al. 1999). Collectively, these findings imply that muscle strength may be partially limited by an inability to drive MUs voluntarily with the high firing rates needed to attain maximum force (Enoka and Fuglevand 2001).
General Implications
Finally, because of their relative accessibility, MNs have long played an important role in shaping ideas about how neurons integrate synaptic information. In this regard, the present findings imply that the spiking output of a MN does not faithfully “report” the intensity of synaptic excitation received. Instead, once brought to threshold and following a brief and modest period of rate modulation, MNs seem to all but ignore additional synaptic excitation. As such, MNs appear to respond in an almost binary “on-off” fashion rather than as accurate integrators. The degree to which other neurons in the central nervous system respond in this way and the impact this might have on notions of information processing in the brain remain to be determined.
GRANTS
This work was supported by National Institutes of Health Grants NS079147 (to A. J. Fuglevand) and F31 DC012697 (to R. A. Lester).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.F. and R.K.J. conception and design of research; A.J.F., R.A.L., and R.K.J. performed experiments; A.J.F., R.A.L., and R.K.J. analyzed data; A.J.F., R.A.L., and R.K.J. interpreted results of experiments; A.J.F. prepared figures; A.J.F. drafted manuscript; A.J.F., R.A.L., and R.K.J. edited and revised manuscript; A.J.F., R.A.L., and R.K.J. approved final version of manuscript.
REFERENCES
- Alvord EC, Fuortes MG. Reflex activity of extensor motor units following muscular afferent excitation. J Physiol 122: 302–321, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol 97: 933–936, 2007. [DOI] [PubMed] [Google Scholar]
- Barraza D, Kita H, Wilson CJ. Slow spike frequency adaptation in neurons of the rat subthalamic nucleus. J Neurophysiol 102: 3689–3697, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JN, Crill WE. Influence of dendritic location and membrane properties on the effectiveness of synapses on cat motoneurones. J Physiol 239: 325–345, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998. [DOI] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am Physiol Soc, 1996, sect. 12, p. 1–53. [Google Scholar]
- Binder MD, Robinson FR, Powers RK. Distribution of effective synaptic currents in cat triceps surae motoneurons. VI. Contralateral pyramidal tract. J Neurophysiol 80: 241–248, 1998. [DOI] [PubMed] [Google Scholar]
- Botterman B, Iwamoto G, Gonyea W. Gradation of isometric tension by different activation rates in motor units of cat flexor carpi radialis muscle. J Neurophysiol 56: 494–506, 1986. [DOI] [PubMed] [Google Scholar]
- Bracchi F, Decandia M, Gualtierotti T. Frequency stabilization in the motor centers of spinal cord and caudal brain stem. Am J Physiol 210: 1170–1177, 1966. [DOI] [PubMed] [Google Scholar]
- Brown A, Fyffe R. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol 274: 111–127, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res 90: 441–455, 1992. [DOI] [PubMed] [Google Scholar]
- Burke RE. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol 30: 1114–1137, 1967. [DOI] [PubMed] [Google Scholar]
- Burke RE. Firing patterns of gastrocnemius motor units in the decerebrate cat. J Physiol 196: 631–654, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. Excitatory synaptic action in motoneurones. J Physiol 130: 374–395, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo PJ, Rymer WZ. Motor-unit activation patterns in lengthening and isometric contractions of hindlimb extensor muscles in the decerebrate cat. J Neurophysiol 47: 782–796, 1982. [DOI] [PubMed] [Google Scholar]
- Cushing S, Bui T, Rose PK. Effect of nonlinear summation of synaptic currents on the input-output properties of spinal motoneurons. J Neurophysiol 94: 3465–3478, 2005. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Jakobi JM, Allman BL, Rice CL. Differential age-related changes in motor unit properties between elbow flexors and extensors. Acta Physiol (Oxf) 200: 45–55, 2010. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107: 178–195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D. On the nature of postural reflexes. Proc R Soc Lond B Biol Sci 104: 252–301, 1929. [Google Scholar]
- Destexhe A, Paré D. Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol 81: 1531–1547, 1999. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Paré D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci 4: 739–751, 2003. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Fuglevand AJ. Motor unit physiology: some unresolved issues. Muscle Nerve 24: 4–17, 2001. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70: 2470–2488, 1993. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. J Neurophysiol 81: 1718–1729, 1999. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ. Mechanical properties and neural control of human hand motor units. J Physiol 589: 5595–5602, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M. Initiation of impulses in visual cells of Limulus. J Physiol 148: 14, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe R. Spatial distribution of recurrent inhibitory synapses on spinal motoneurons in the cat. J Neurophysiol 65: 1134–1149, 1991. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol 82: 709–717, 1999. [DOI] [PubMed] [Google Scholar]
- Gorman RB, McDonagh JC, Hornby TG, Reinking RM, Stuart DG. Measurement and nature of firing rate adaptation in turtle spinal neurons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191: 583–603, 2005. [DOI] [PubMed] [Google Scholar]
- Granit R. Neuromuscular interaction in postural tone of the cat's isometric soleus muscle. J Physiol 143: 387–402, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Haase J, Rutledge LT. Recurrent inhibition in relation to frequency of firing and limitation of discharge rate of extensor motoneurones. J Physiol 154: 308–328, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, Lamarre Y. Algebraical summation in synaptic activation of motoneurones firing within the “primary range” to injected currents. J Physiol 187: 379–399, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Hellsing G, Lofstedt L. TVR and vibration-induced timing of motor impulses in the human jaw elevator muscles. J Neurol Neurosurg Psychiatry 39: 719–728, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulation of the steady-state input-output function of the cat medial gastrocnemius motoneuron pool. J Neurophysiol 65: 952–967, 1991a. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Analysis of Ia-inhibitory synaptic input to cat spinal motoneurons evoked by vibration of antagonist muscles. J Neurophysiol 66: 1888–1893, 1991b. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Computer simulations of motoneuron firing rate modulation. J Neurophysiol 69: 1005–1008, 1993. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Enoka RM. Motor Unit. Hoboken, NJ: John Wiley & Sons, 2012. [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holobar A, Minetto MA, Botter A, Negro F, Farina D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Trans Neural Sys Rehabil Eng 18: 221–229, 2010. [DOI] [PubMed] [Google Scholar]
- Holobar A, Minetto MA, Farina D. Accurate identification of motor unit discharge patterns from high-density surface EMG and validation with a novel signal-based performance metric. J Neural Eng 11: 016008, 2014. [DOI] [PubMed] [Google Scholar]
- Holobar A, Zazula D. Multichannel blind source separation using convolution kernel compensation. IEEE Trans Signal Process 55: 4487–4496, 2007. [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG. Motoneurons: a preferred firing range across vertebrate species? Muscle Nerve 25: 632–648, 2002. [DOI] [PubMed] [Google Scholar]
- Kernell D. High-frequency repetitive firing of cat lumbosacral motoneurones stimulated by long lasting injected currents. Acta Physiol Scand 65: 74–86, 1965. [DOI] [PubMed] [Google Scholar]
- Kernell D. Synaptic conductance changes and the repetitive impulse discharge of spinal motoneurones. Brain Res 15: 291–294, 1969. [DOI] [PubMed] [Google Scholar]
- Kernell D. The Motoneurone and Its Muscle Fibres. Oxford: Oxford Univ. Press, 2006. [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA. Relation between isometric force and stimulus rate in cat's hindlimb motor units of different twitch contraction time. Exp Brain Res 50: 220–227, 1983. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res 46: 191–196, 1982. [DOI] [PubMed] [Google Scholar]
- Kernell D, Sjöholm H. Repetitive impulse firing: comparisons between neurone models based on “voltage clamp equations” and spinal motoneurones. Acta Physiol Scand 87: 40–56, 1973. [DOI] [PubMed] [Google Scholar]
- Kernell D, Sjöholm H. Recruitment and firing rate modulation of motor unit tension in a small muscle of the cat's foot. Brain Res 98: 57–72, 1975. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78: 3061–3068, 1997. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Aertsen A, Rotter S. Neuronal integration of synaptic input in the fluctuation-driven regime. J Neurosci 24: 2345–2356, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatki BG, van Dijk JP, Jonas IE, Zwarts MJ, Stegeman DF. A thin, flexible multielectrode grid for high-density surface EMG. J Appl Physiol 96: 327–336, 2003. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Porter R, Redman SJ. Corticomotoneuronal synapses in the monkey: light microscopic localization upon motoneurons of intrinsic muscles of the hand. J Comp Neurol 232: 499–510, 1985. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998a. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998b. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay AD, Binder MD. Distribution of effective synaptic currents underlying recurrent inhibition in cat triceps surae motoneurons. J Neurophysiol 65: 168–177, 1991. [DOI] [PubMed] [Google Scholar]
- Llinas R, Terzuolo CA. Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha-extensor motoneurons. J Neurophysiol 27: 579–591, 1964. [DOI] [PubMed] [Google Scholar]
- Lüscher HR, Vardar U. A comparison of homonymous and heteronymous connectivity in the spinal monosynaptic reflex arc of the cat. Exp Brain Res 74: 480–492, 1989. [DOI] [PubMed] [Google Scholar]
- Mantel G, Lemon RN. Cross-correlation reveals facilitation of single motor units in thenar muscles by single corticospinal neurones in the conscious monkey. Neurosci Lett 77: 113–118, 1987. [DOI] [PubMed] [Google Scholar]
- Massion J. Red nucleus: past and future. Behav Brain Res 28: 1–8, 1988. [DOI] [PubMed] [Google Scholar]
- McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods 149: 121–133, 2005. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol 34: 171–187, 1971. [DOI] [PubMed] [Google Scholar]
- Merletti R, Holobar A, Farina D. Analysis of motor units with high-density surface electromyography. J Electromyogr Kinesiol 18: 879–890, 2008. [DOI] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol 40: 1432–1443, 1977. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Heckman CJ, Powers RK, Rymer WZ, Suresh NL. Disturbances of motor unit rate modulation are prevalent in muscles of spastic-paretic stroke survivors. J Neurophysiol 111: 2017–2028, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol 102: 2026–2038, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain 105: 223–269, 1982. [DOI] [PubMed] [Google Scholar]
- Nawab SH, Chang SS, De Luca CJ. High-yield decomposition of surface EMG signals. Clin Neurophysiol 121: 1602–1615, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons in vivo. J Neurophysiol 79: 1450–1460, 1998. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Stevens CF. A mechanism for spike frequency adaptation. J Physiol 256: 315–332, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483, 1972. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Summation of effective synaptic currents and firing rate modulation in cat spinal motoneurons. J Neurophysiol 83: 483–500, 2000. [DOI] [PubMed] [Google Scholar]
- Powers RK, Elbasiouny SM, Rymer WZ, Heckman CJ. Contribution of intrinsic properties and synaptic inputs to motoneuron discharge patterns: a simulation study. J Neurophysiol 107: 808–823, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Robinson FR, Konodi MA, Binder MD. Effective synaptic current can be estimated from measurements of neuronal discharge. J Neurophysiol 68: 964–968, 1992. [DOI] [PubMed] [Google Scholar]
- Powers RK, Robinson FR, Konodi MA, Binder MD. Distribution of rubrospinal synaptic input to cat triceps surae motoneurons. J Neurophysiol 70: 1460–1468, 1993. [DOI] [PubMed] [Google Scholar]
- Prather JF, Clark BD, Cope TC. Firing rate modulation of motoneurons activated by cutaneous and muscle receptor afferents in the decerebrate cat. J Neurophysiol 88: 1867–1879, 2002. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J Neurophysiol 73: 1799–1810, 1995. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. J Neurophysiol 78: 2246–2253, 1997. [DOI] [PubMed] [Google Scholar]
- Schwindt PC. Membrane-potential trajectories underlying motoneuron rhythmic firing at high rates. J Neurophysiol 36: 434–439, 1973. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Calvin WH. Membrane-potential trajectories between spikes underlying motoneuron firing rates. J Neurophysiol 35: 311–325, 1972. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Calvin WH. Equivalence of synaptic and injected current in determining the membrane potential trajectory during motoneuron rhythmic firing. Brain Res 59: 389–394, 1973. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol 48: 875–890, 1982. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yokota JI, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett 23: 7–12, 1981. [DOI] [PubMed] [Google Scholar]
- Tansey KE, Botterman BR. Activation of type-identified motor units during centrally evoked contractions in the cat medial gastrocnemius muscle. II. Motoneuron firing-rate modulation. J Neurophysiol 75: 38–50, 1996. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Bigland-Richie B, Johansson RS. Force-frequency relationships of human thenar motor units. J Neurophysiol 65: 1509–1516, 1991. [DOI] [PubMed] [Google Scholar]
- Yang HS, Kwon HG, Hong JH, Hong CP, Jang SH. The rubrospinal tract in the human brain: Diffusion tensor imaging study. Neurosci Lett 504: 45–48, 2011. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Young JL. Discharge properties of hindlimb motoneurons in decerebrate cats during locomotion induced by mesencephalic stimulation. J Neurophysiol 43: 1221–1235, 1980. [DOI] [PubMed] [Google Scholar]