Abstract
Rats use rhythmic whisker movements, called active whisking, to sense the environment, which include whisker protractions followed by retractions at various frequencies. Using a proxy of active whisking in anesthetized rats, called artificial whisking, which is induced by electrically stimulating the facial motor nerve, we characterized the neural responses evoked in the barrel cortex by whisking in air (without contact) and on a surface (with contact). Neural responses were compared between distinct network states consisting of cortical deactivation (synchronized slow oscillations) and activation (desynchronized state) produced by neuromodulation (cholinergic or noradrenergic stimulation in neocortex or thalamus). Here we show that population responses in the barrel cortex consist of a robust signal driven by the onset of the whisker protraction followed by a whisking retraction signal that emerges during low frequency whisking on a surface. The whisking movement onset signal is suppressed by increasing whisking frequency, is controlled by cortical synaptic inhibition, is suppressed during cortical activation states, is little affected by whisking on a surface, and is ubiquitous in ventroposterior medial (VPM) thalamus, barrel cortex, and superior colliculus. The whisking retraction signal codes the duration of the preceding whisker protraction, is present in thalamocortical networks but not in superior colliculus, and is robust during cortical activation; a state associated with natural exploratory whisking. The expression of different whisking signals in forebrain and midbrain may define the sensory processing abilities of those sensorimotor circuits. Whisking related signals in the barrel cortex are controlled by network states that are set by neuromodulators.
Keywords: somatosensory cortex, thalamus, sensory processing, artificial whisking, whisker, movement, vibrissa, active whisking, barrel cortex, trigeminal complex, acetylcholine, norepinephrine
rats use rhythmic whisker movements (active whisking) to sense the environment. During active whisking, the brain generates a motor command (efference), a copy of which is sent to other brain regions (efference copy), and also receives whisking movement signals (reafference) because trigeminal ganglion cells discharge during the movement. An additional (exafference) signal is produced by discharging ganglion cells when whiskers touch objects as they move (active touch) (Carvell and Simons 1990; Gao et al. 2001; Kleinfeld et al. 2006; Kleinfeld and Deschenes 2011).
A movement pattern similar to active whisking, called artificial or fictive whisking, can be produced in anesthetized rats by electrically stimulating the facial motor nerve (Brown and Waite 1974; Szwed et al. 2003; Zucker and Welker 1969). During artificial whisking in air (without object contact), the brain does not generate a motor command or the corresponding efference copy. Instead, it receives a signal that reflects the movement imposed by the electrical stimulation of the motor nerve. This reafference signal is caused by some trigeminal ganglion cells that discharge during artificial whisking in air (Szwed et al. 2003); more dispersed and less phase-locked ganglion cell discharges appear to occur during natural whisking in air in behaving animals (Khatri et al. 2009). While keeping these differences in mind, artificial whisking is a useful model to investigate whisker-evoked responses in neural circuits under highly controlled conditions that enable sophisticated experimental procedures that are technically difficult or unfeasible in freely moving animals.
During artificial whisking, cells in barrel cortex respond to whisking movement (reafference) and active touch (exafference) (Derdikman et al. 2006). In fact, population neural responses driven by artificial whisking have a characteristic large amplitude response to the onset of the whisking protraction, we call peakA (for simplicity), which is followed by a much smaller response during the offset of the protraction (retraction), called peakB (Bezdudnaya and Castro-Alamancos 2011). Interestingly, the cortical response evoked by the retraction, peakB, is enhanced by active touch compared with whisking in air (Bezdudnaya and Castro-Alamancos 2011; Derdikman et al. 2006; Yu et al. 2013). Here we further explored the nature of this retraction signal.
Neural activity evoked by artificial whisking has been previously studied in urethane anesthetized animals; a state characterized by the prevalence of synchronous slow oscillations (cortical deactivation), akin to slow wave sleep. However, natural active whisking occurs in aroused animals exploring the environment; a state characterized by the absence of synchronous slow wave oscillations (cortical activation). The impact of cortical activation on barrel cortex responses evoked by artificial whisking is unknown. Since natural active whisking occurs during cortical activation, it is logical to study artificial whisking responses during this state. In a recent study, we showed that cortical cholinergic and noradrenergic stimulation produces distinct activation states in the barrel cortex (Castro-Alamancos and Gulati 2014). Cortical activation is also induced by cholinergic stimulation of the somatosensory thalamus (Hirata and Castro-Alamancos 2011; 2010). Here we determined the effect of cortical activation states, produced by these three different neuromodulation methods, on barrel cortex responses evoked by artificial whisking.
METHODS
Fifty-seven adult Sprague-Dawley rats (300–350 g) were used in this study and cared for in accordance with National Institutes of Health guidelines for laboratory animal welfare. All experiments were approved by the Drexel University Institutional Animal Care and Use Committee. Rats were anesthetized with urethane (1.5 g/kg, i.p.) and placed in a stereotaxic frame. All skin incisions and frame contacts with the skin were injected with lidocaine (2%). Small craniotomies and small incisions of the dura were made over the target structures as necessary. Body temperature was automatically maintained constant with a heating pad at 37°C. The level of anesthesia was monitored with field potential (FP) recordings and limb withdrawal reflexes and kept constant at about stage III/3 (i.e., slow large amplitude FP cortical oscillations, absence of pinch withdrawal reflex, absence of whisker movements) using supplemental doses of urethane.
Electrophysiology.
In every case, a tungsten electrode was lowered into the depth of the barrel cortex (0.6–1 mm) to record multiunit (MUA) and FP activity. A second electrode was lowered adjacent to the first electrode (n = 51 rats) or into the ventroposterior medial (VPM) thalamus (n = 16 rats) to perform single-unit recordings. Single-unit extracellular recordings were obtained from high impedance (2–10 MΩ) glass electrodes filled with saline. MUA and FP recordings were obtained from tungsten electrodes (1–3 MΩ).
Artificial whisking stimulation protocols.
Once unit activity was isolated, a hand-held probe was used to determine the whiskers that activated the cells (receptive field). All the cells subjected to artificial whisking protocols responded to passive whisker stimulation using a hand-held probe and using an air-puff (puff) stimulus. After identification of the receptive field with the hand-held probe, puff stimulation was delivered by aiming a 2.5-mm diameter tube 15–25 mm away from the receptive field (slightly elevated and facing down with a slight angle from the front towards the whiskers) so that whiskers were pushed backwards by a 50-ms duration puff of pressurized air (40 PSI). Stimulus trials consisted of a 2-s period of no stimulation (1.5 s used to measure spontaneous firing) followed by 35 puffs at 0.5, 2, 5, or 10 Hz.
Puff stimulation was followed by artificial whisking in air. Artificial whisking was conducted as previously described (Bezdudnaya and Castro-Alamancos 2011). To trigger artificial whisking, we cut and positioned a pair of stainless steel wires in the buccal branch of the facial nerve (0.7–1 mm apart) and delivered a train of five pulses (100-μs duration) at 100 Hz. The stimulus intensity was adjusted (0.04- to 0.17-mA range) to produce a protraction with a 10–35° angle. In a few cases, we used synchronous video monitoring (500 frames per second; Motionscope, Red Lake Imaging, Morgan Hill, CA) to track whisker movement frame by frame (Bezdudnaya and Castro-Alamancos 2011). After tracking of each frame, the movements measured during each of the last 30 trains were averaged. Each train produced a whisker protraction (rising phase) that crested at around 50 ms from the train onset, and the whiskers returned back to the starting point (falling phase) within the next 30 ms. As per puff stimulation, each whisking in air trial consisted of a 2-s period of no stimulation (1.5 s used to measure spontaneous firing) followed by 35 stimuli at 0.5, 2, 5, or 10 Hz.
Whisking in air was followed by active touch consisting of whisking on three different surfaces. The surface was placed parallel to the rat's midline to mimic a wall that the whiskers brush against in the rostro-caudal direction. The distance from the wall to the whisker pad was adjusted to assure that most whiskers (except rostral microvibrissa, which are too short) made contact. We used three different sand paper surfaces that varied in coarseness based on grit size (g60, g220, and g600, coarse-to-smooth) and were placed on each of three sides of a rotating cube; rotating the cube 90° led to the presentation of a different surface. During whisking on surfaces, the electrical stimulus and the trial setup were identical to whisking in air but the whiskers contacted the surface. Once the whisking on surfaces trials ended, all stimulus trials were repeated several times (2–5), and the data from different trials were averaged together unless otherwise stated.
Peristimulus time histograms (PSTHs), rectified MUA, and FP responses were obtained by averaging the responses to the last 30 stimuli in each 35 stimulus trial. Puff-evoked responses were corrected by subtracting the time it took for the air to reach the whiskers, which was determined using a sensing piezo-electric device placed in the location of the whiskers. Stimulus artifacts created by the nerve stimulation were very distinct and easily removed. We measured the spontaneous firing rate of single-units or MUA from multiple 60-s periods before each trial of the different stimulus types (i.e., puff, whisking in air, and whisking on surfaces). To measure single-unit-evoked responses, we obtained the spike probability during several time windows and subtracted spontaneous firing. To measure MUA-evoked responses, we used both spike probability and rectified MUA. Rectified MUA-evoked responses are derived by obtaining the absolute value of the MUA and then measuring the baseline corrected area during the response time window. All data are expressed as means ± SE unless otherwise stated.
Microdialysis.
To apply drugs into the barrel cortex or somatosensory thalamus, a microdialysis cannula (250-μm outer diameter, 2-mm-long membrane) was placed adjacent (∼500 μm) to the recording electrodes in those areas. Artificial cerebrospinal fluid (ACSF) was continuously infused through the probe at 2–4 μl/min and drugs were dissolved in the ACSF. We have used this method to infuse drugs into various brain regions, including the thalamus and neocortex (e.g., Castro-Alamancos and Borrell 1993; Castro-Alamancos and Oldford 2002), and typically find that the effective doses during microdialysis are about 10 times higher than during direct application in slices. This is due to the fact that there is about a 10% reverse dialysis recovery of drugs in the extracellular medium as they dialyze down their concentration gradients across the dialysis membrane. Moreover, based on diffusion experiments using arrays of recording electrodes at different distances from the probe, we have estimated the spread to be ∼1 mm in the horizontal plane away from the membrane for the typical doses employed here. In a recent study, we specifically determined the effects of different doses of the cholinergic agonist carbachol (CA) and of norepinephrine (NE) on cortical activity in the barrel cortex (Castro-Alamancos and Gulati 2014). The doses used here were found to produce cortical activation (see results).
Analysis.
Statistical analyses consisted, for the most part, of paired comparisons of responses evoked in the same cells by different stimulus types (e.g., puff, whisking in air, and whisking on surfaces) and drugs. If the data were considered normally distributed, according to the Shapiro-Wilk normality test, we used parametric statistics. For two groups, we used the t-test (paired or independent). For more than two groups (and one factor), we tested for a significant main effect using the repeated-measures ANOVA followed by comparisons with Tukey's test. If the data were considered not normally distributed, we used nonparametric statistics consisting of the Wilcoxon signed ranks (paired comparisons) and the Mann-Whitney (independent comparisons) tests. When multiple comparisons were performed, P values were adjusted using a Bonferroni correction by multiplying the P value by the number of comparisons made. The alpha level used for significance was P < 0.05, or P < 0.01, as indicated.
Histology.
To assure that thalamic cells were located in VPM, we ejected Chicago Sky Blue (2% in saline) from the pipette using current (−5 μA, 5 s on/off; 5 min) to mark the recording site. These animals were euthanized with an overdose of pentobarbital and perfused through the heart with saline followed by fixative (paraformaldehyde, 4%). Coronal sections (100 μm) were obtained using a vibratome and stained with cresyl violet or neutral red.
RESULTS
Artificial whisking responses in barrel cortex.
It is worth noting that we did not intend to resolve if different cells in barrel cortex or thalamus are part of different pathways (channels) that disambiguate reafference and exafference signals (Derdikman et al. 2006; Yu et al. 2006; but see Masri et al. 2008). Instead, our main goal was to characterize population and single-cell responses driven by artificial whisking and to determine the effect of neuromodulation on these responses. Our single-cell data are pooled from different experiments; typically one cell per animal is tested before and during neuromodulation.
In urethane-anesthetized rats, we recorded single-unit, MUA, and FP responses in the barrel cortex during air-puff stimulation of stationary whiskers (passive touch), artificial whisking in air (whisking movement), and artificial whisking on three different surfaces varying in texture (active touch) as if the rat was brushing its whiskers on an adjacent wall. The single-unit recording electrode was placed either in the barrel cortex or the VPM thalamus, while the MUA and FP electrode was always placed in the barrel cortex. The cells were determined to be responsive to whisker stimulation by a hand-held probe and by an air-puff stimulus delivered at low frequency (0.5 Hz) aimed at the receptive field (i.e., whiskers that were determined to drive the cell using a hand-held probe). Puff stimulation was followed by artificial whisking in air (whisk) and whisking on three surfaces (g60, g220, and g600) at 0.5, 2, 5, and 10 Hz. While we employed three different textures during whisking on surfaces, in the present study we only focus on the differences between whisking in air and on surfaces because the differences between the different textures were not highly significant. This is likely because the barrel cortex only sparsely codes texture in behaving animals (Jadhav et al. 2009). As shown in Fig. 1A, gray trace in bottom panel, the whisker movement associated with artificial whisking in air consisted of a protraction with a rising phase and a falling phase (retraction). The rising phase crested at ∼50 ms, after which the protraction passively returns to baseline within the next ∼30 ms. In some cases, during the offset of the protraction, the whisker overshoots the original starting point retracting briefly; see example in Fig. 3C.
Fig. 1.
Artificial whisking responses in barrel cortex. A: average single-unit [barrel cortex and ventroposterior medial (VPM) thalamus], multiunit (MUA) rectified (barrel cortex), and field potential (FP) (barrel cortex) responses evoked by puff stimulation, artificial whisking in air (Whisk), and artificial whisking on a surface (g60) at 4 different frequencies. Bottom panel, gray trace: shows the whisker movement (angle in degrees) measured with video tracking during artificial whisking (positive values indicate that the whiskers protracted). Note the arrows pointing to peakA and peakB responses. Train refers to 5 pulses delivered at 100 Hz. B: lack of peakB responses in superior colliculus. Average single-unit (superior colliclus) and FP (barrel cortex) responses evoked by artificial whisking in air (Whisk, black trace) and artificial whisking on a surface (g60, red trace) at 0.5 Hz. Note the absence of peakB in superior colliculus despite its presence in the simultaneously recorded barrel cortex response.
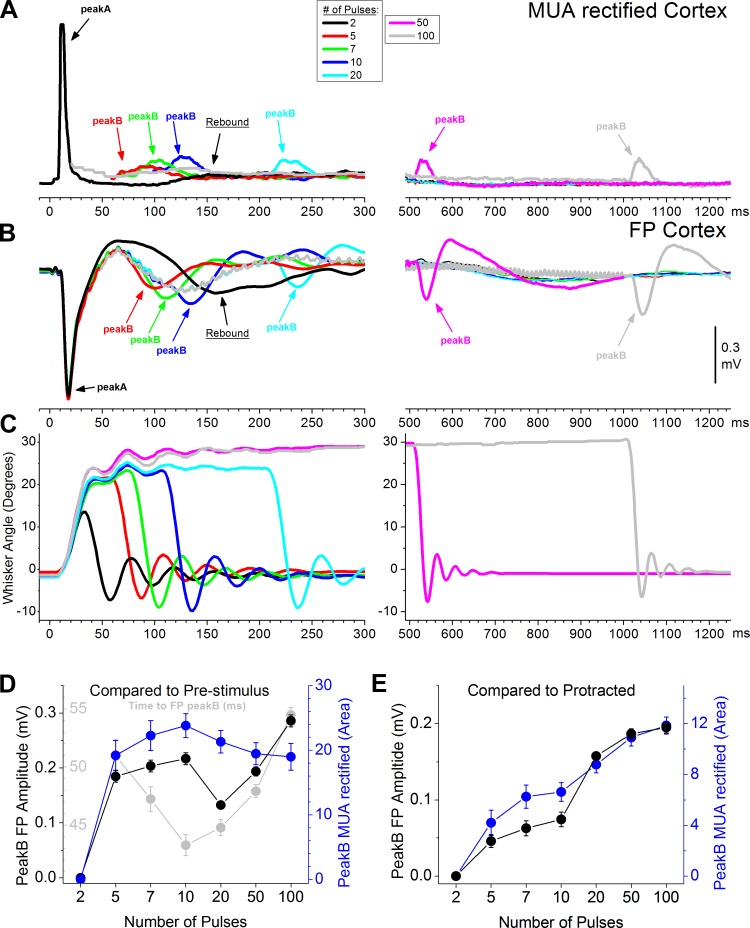
Fig. 3.
Effect of the duration of the protraction on peakB responses evoked by artificial whisking on a surface. Average MUA rectified (A) and FP responses (B) evoked in barrel cortex by artificial whisking on a surface (g60) with different protraction durations at 0.5 Hz. C: shows the whisker movement (angle in degrees) measured with video tracking during artificial whisking in air (positive values indicate that the whiskers protracted). To change the protraction duration, the number of pulses applied to the motor nerve (at 100 Hz) was adjusted between 2–100 pulses. This produced protractions lasting between 20–1,000 ms. Population data measuring FP (black) and MUA rectified (blue) peakB responses evoked by different protraction durations. In D, peakB responses were measured with respect to the prestimulus baseline (i.e., the prestimulus baseline was subtracted from the peakB response). The black plot shows the peakB FP amplitude and the blue plot shows the peakB MUA rectified. The gray plot shows the time (from the last pulse in the train) to the negative peak of the FP response used to measure peakB (measured for number of pulses 5–100). In E, peakB responses were measured with respect to the activity in the protracted state evoked by a longer lasting protraction (i.e., the activity evoked by a sustained protraction during the same time window was subtracted from the peakB response). See text for details.
Figure 1A shows average traces obtained from barrel cortex and VPM thalamus during puff, whisk, and g60 stimulation delivered at four different frequencies. The top panels show single-unit responses in barrel cortex and VPM. The bottom panels show MUA (rectified) and FP responses in barrel cortex. Responses consist in a short-latency peakA that occurs within the first 25 ms after the onset of the whisker movement (protraction). This is followed by a much smaller peakB that appears to be triggered by the offset of the whisker protraction (retraction). As the whisking movement frequency increases, both peakA and peakB adapt (depress) sharply. Thus, during artificial whisking at 5–10 Hz, peakB is not apparent.
The presence of peakB in VPM thalamus and barrel cortex contrasts with its absence in superior colliculus. Figure 1B shows average traces taken from a previous study (Bezdudnaya and Castro-Alamancos 2014). It shows neural activity simultaneously recorded in the barrel cortex (FP) and superior colliculus (single-units; n = 124) during artificial whisking in air and on a surface (g60). Neural activity during the movement protraction onset (peakA) is present in both barrel cortex and superior colliculus, but neural activity during the retraction (peakB) is notoriously absent in the superior colliculus. A statistical analysis compared spike probability between whisking in air and on a surface (0.5 Hz) for the peakB response window (65–100 ms) and found no significant difference (n = 124; P = 0.4), indicating that there was no significant peakB response in superior colliculus (as shown below a similar analysis in barrel cortex yields a significant difference). Thus retraction signaling appears to be a selective feature of the thalamocortical system.
Figure 2 shows population data measuring peakA and peakB responses in barrel cortex. For FP responses (Fig. 2A, black squares), we measured the negative peak amplitude. For MUA responses (Fig. 2A, blue circles), we measured the area of the rectified response. For single-unit responses (Fig. 2B; classified according to depth), we measured the spike probability of the responses corrected by spontaneous firing. Note that the evoked responses are strongly suppressed by frequency, reflecting robust rapid sensory adaptation. Note also that in a previous study (Bezdudnaya and Castro-Alamancos 2011) puff responses evoked in barrel cortex were smaller than artificial whisking responses, but in that case the puff was centered on the receptive field of cells recorded in superior colliculus. In the present study, puff responses are typically larger than artificial whisking responses because the puff is centered on the receptive field of the barrel cortex recordings. Nevertheless, statistical comparisons between artificial whisking and puff responses were avoided because these are difficult to equate between each other.
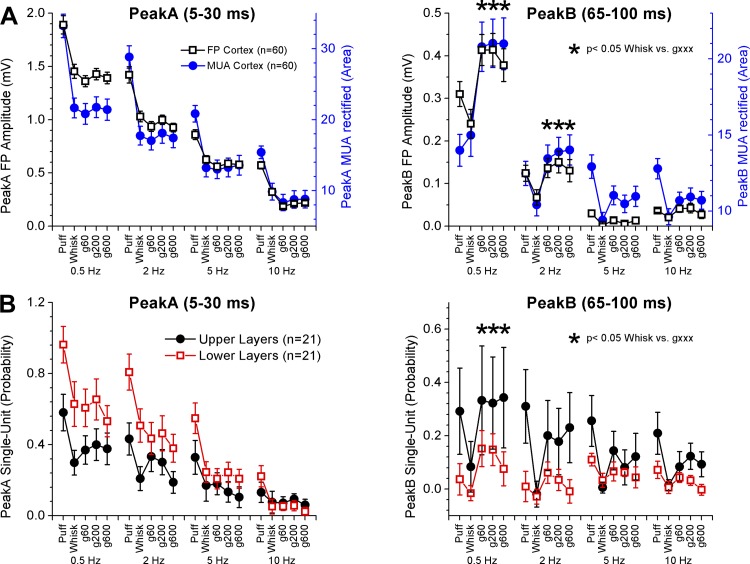
Fig. 2.
Population data measuring artificial whisking responses in barrel cortex. A: measurement of FP (black) and MUA rectified (blue) peakA and peakB responses evoked by puff and artificial whisking. Asterisks denote statistically significant differences between whisking in air and on a surface (Tukey). B: measurement of single-unit peakA and peakB responses classified by cortical depth as upper (black) and lower (red) layers (above or below 1 mm from the pia).
Here we compared responses evoked by artificial whisking in air and on surfaces. Taking together the whole population (n = 60), there was a small effect of active whisking on surfaces on peakA responses. In particular, we noted that peakA responses could be slightly suppressed by artificial whisking on surfaces compared with artificial whisking in air (Fig. 2A), but this effect was weak and seemed to be highly sensitive to spontaneous changes in the level of cortical activation; this effect is revealed later when the level of cortical activation is more carefully considered. On the other hand, there was a robust effect of active whisking on surfaces on peakB responses. At low artificial whisking frequencies (0.5 and 2 Hz), both FP and MUA peakB responses were significantly larger during whisking on surfaces than during whisking in air (Fig. 2A; Tukey). At higher artificial whisking frequencies (5–10 Hz), peakB responses are very adapted and virtually inexistent. Single-unit responses followed the same pattern as FP and MUA responses. Taken together (n = 42), we found that single-unit peakB responses were significantly larger during whisking on surfaces than during whisking in air at 0.5 Hz (Fig. 2B; Tukey). We also separated cortical cells into two groups according to cortical depth (above or below 1 mm from the pia) roughly representing infragranular or lower layers (layers 5–6) and supragranular or upper layers (layers 1–4). When the cells were separated into these two groups (n = 21 per group), we found that cells in the lower layers had significantly larger peakA responses than cells in the upper layers (ANOVA; P < 0.001). This main effect was primarily due to significant differences between the lower and upper layers for puff and whisking in air responses at low frequencies of 0.5–2 Hz (P < 0.05, Tukey). In contrast, peakB responses were significantly larger in upper layer cells than in lower layer cells (ANOVA; P < 0.001). However, variability in upper layer cells was high reflecting the fact that only a group of these cells had robust peakB responses. Cells in the VPM thalamus had robust peakA and negligible peakB responses during whisking in air (Fig. 1A). PeakB responses in VPM thalamus were strongly enhanced by whisking on a surface (Fig. 1A; P < 0.001; Tukey). Thus peakB responses in barrel cortex are driven by the VPM thalamus.
These results indicate that low frequency artificial whisking evokes a peakA response in barrel cortex during the onset of the protraction and a much smaller peakB response during the retraction. PeakB becomes apparent during artificial whisking on a surface and is most prominent in upper layer cells driven by VPM cells.
The nature of peakB triggered by artificial whisking.
We next explored the nature of peakB responses in neocortex. To determine if peakB signals the offset of the whisking movement protraction (retraction), we generated whisker protractions of varying durations by changing the number of pulses in the 100-Hz train applied to the motor nerve. We used trains of 2, 5 (standard train used), 7, 10, 20, 50, and 100 pulses, which produced similar amplitude protractions (except for 2 pulses, which is smaller in amplitude) lasting around 20, 50, 70, 100, 200, 500, and 1000 ms, respectively (whisker tracking in Fig. 3C). PeakA is virtually identical between all the whisker protraction durations and is not considered further in this section. If peakB is caused by the retraction, then it should shift in time following the duration of the protraction. If the magnitude of peakB changes as it shifts, then it could signal the duration of the protraction.
Figure 3, A and B, show MUA (rectified) and FP responses evoked in barrel cortex by protractions of different durations. Note that as the duration of the protraction increases, peakB shifts so that it occurs during the retraction. Notably, peakB is more salient at the long durations (e.g., 500- and 1,000-ms durations; right panels pink and gray traces). In fact, at the short durations (<200 ms) it mixes with a typical rebound produced by afferent thalamocortical activity; this thalamocortical rebound is state-dependent and typically peaks between 150–200 ms in anesthetized or quiescent/sleeping animals (see Castro-Alamancos and Connors 1996a,b).
At the shortest protraction duration (2 pulses; black trace) peakB merges completely with peakA or is simply too suppressed to be noticed. However, this duration (2 pulses) evokes an obvious rebound at ∼150 ms (particularly in the FP response; Fig. 3B). When five pulses are applied (red trace), the protraction lasts longer (∼50 ms) and peakB occurs during the retraction, but in this case the rebound (which usually occurs at ∼150 ms) is much smaller, indicating that it has been suppressed or that it has shifted to the left and is contained within peakB (more on this later). At 7 and 10 pulses (green and blue traces), peakB continues to track the retraction and its amplitude appears to increase. Importantly, the rebound (triggered by the initial pulses) now appears in the left shifted position before peakB and does not track the retraction. At 20, 50, and 100 pulses, peakB occurs completely after the rebound and is not contaminated by it. Thus peakB indeed reflects the retraction and appears to increase in magnitude with increases in the duration of the protraction. However, at protraction durations <200 ms (pulses 2–10), peakB mixes with the typical rebound driven by thalamocortical inputs.
We further explored how the duration of the protraction affects the magnitude of peakB by measuring it in two different ways. In the first measurement (Fig. 3D), the FP amplitude and the MUA area of the response window (5–70 ms after the last pulse) were obtained with respect to the prestimulus baseline. In the second measurement (Fig. 3E), we removed the contribution of the rebound by taking the same measures as above but with respect to the activity during the sustained whisker protraction, not the prestimulus baseline. In other words, we subtracted the activity evoked by the sustained protraction; keeping only the activity evoked by the retraction. For example, in the case of 10 pulses, this measurement would be the difference between the peakB amplitude (or area for MUA) in the blue trace minus the value at that same point in time from the light gray trace (note that the light gray trace corresponds to a protraction for up to 1,000 ms). Such an analysis subtracts the activities (e.g., rebound) triggered by the protraction, keeping only the activities evoked by the retraction. As expected, the first measurement of peakB (from baseline) was affected by the occurrence of the rebound and was not very revealing (Fig. 3D). A repeated-measures ANOVA of either the FP or MUA responses showed a significant effect of the number of pulses (n = 34; P < 0.01). However, this was primarily due to a highly significant difference (P < 0.01; Tukey) between the shortest protraction duration (2 pulses), which produces a negligible peakB, and the other durations (5–100 pulses). In Fig. 3D (light gray), we also show the time to the negative FP peakB measured from the last pulse in the train (for 5–100 pulses). This reveals that the time to peakB was decreasing during the trains that are contaminated by the rebound (5–10 pulses), but it actually significantly increases (20 vs. 100 pulses; P < 0.001) for trains that are not contaminated by the rebound (20–100 pulses). This effect is consistent with an increase in the FP response amplitude for the trains that are not contaminated by the rebound (20–100 pulses); the time to peak tends to become longer for larger FP responses.
In contrast, the second measurement (Fig. 3E), which measures more selectively the effect of the retraction, showed that peakB increases in magnitude with increases in the duration of the protraction. Thus a repeated-measures ANOVA of either the FP or MUA responses revealed a significant effect of the number of pulses (n = 34; P < 0.01). Multiple comparisons (P < 0.01; Tukey) revealed that each of the number of pulses was significantly different from all the other number of pulses, except for the most similar (adjacent) ones. Interestingly, the increase in peakB with the duration of the protraction can be explained by a recovery from rapid sensory adaptation (depression) (Castro-Alamancos 2004a,b; Castro-Alamancos and Connors 1996b; Chung et al. 2002; Khatri et al. 2004). Essentially, the farther away the retraction occurs from peakA, the larger peakB will be because it is given more time to recover from adaptation.
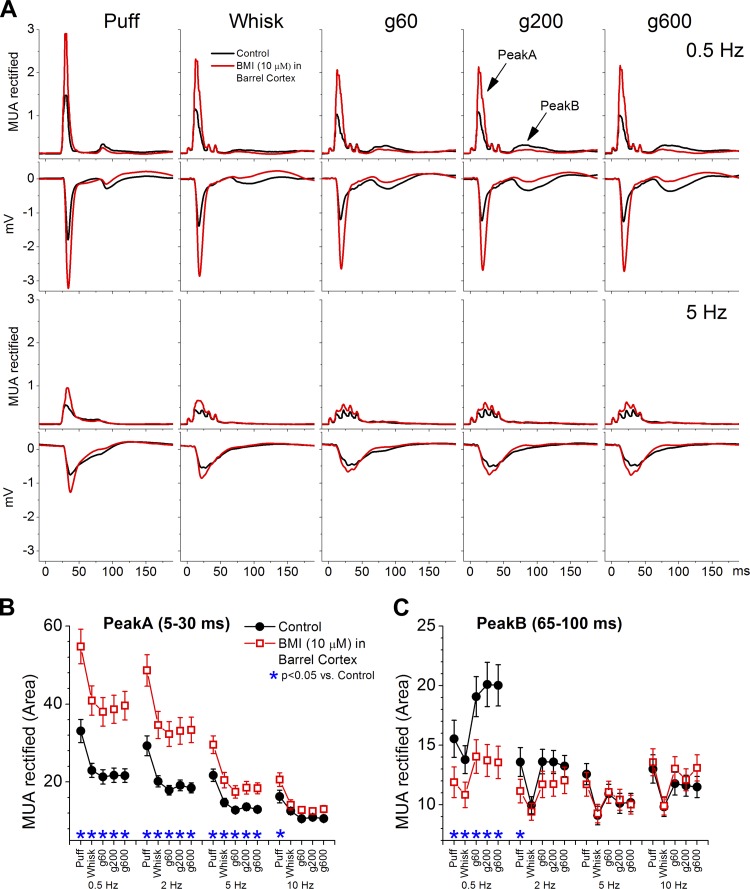
Next we tested the effect of enhancing peakA on peakB responses evoked by puff stimulation and artificial whisking (5 pulses). To enhance peakA we used a very low dose of the GABAA receptor antagonist bicuculline (BMI; 10 μM; n = 11). Indeed, this dose leads to a significant enhancement of peakA responses (Fig. 4, A and B) but without any spontaneous bursting or paroxysmal discharges (Castro-Alamancos 2000). At the same time, BMI significantly suppressed peakB responses (Fig. 4, A and C). The suppression of peakB caused by BMI may be due to a stronger peakA recruiting stronger feedforward inhibition (from unblocked GABAA receptors, GABAB receptors, and intrinsic afterhyperpolarizations) during the period when peakB occurs or due to stronger synaptic depression within the neocortex. Regardless, the results indicate that when peakA becomes stronger, by slight disinhibition, peakB becomes weaker. One interpretation is that peakB is controlled by inhibitory processes triggered by peakA.
Fig. 4.
Population data measuring the effect of peakA response enhancement on peakB responses evoked in barrel cortex by puff and artificial whisking. PeakA enhancement was caused by application of a low dose of bicuculline (BMI) into the barrel cortex. A: average MUA rectified and FP responses evoked in barrel cortex by puff stimulation and artificial whisking on different surfaces (g60, g200, and g600) at 0.5 and 5 Hz. Population data measuring peakA (B) and peakB (C) MUA rectified responses evoked by the different stimuli during control (black) and during application of BMI in barrel cortex (red). Asterisks denote statistically significant differences between control and BMI (Tukey).
In conclusion, peakB responses evoked in anesthetized animals by protractions lasting <200 ms contain two components; the activity from a shifted thalamocortical rebound, which is known to be state-dependent (see below), and the activity produced by the whisker retraction per se. PeakB responses driven by protractions lasting ≥200 ms occur after the thalamocortical rebound and are therefore caused purely by the retraction. The magnitude of PeakB signals the duration of the whisker protraction and is controlled by inhibitory processes triggered by the onset of the protraction.
Cortical neuromodulation produces cortical activation.
We next tested the effect of cortical cholinergic or noradrenergic stimulation on barrel cortex activity (FP, single-unit, and MUA). Cholinergic stimulation was produced by applying the cholinergic agonist CA. Noradrenergic stimulation was produced by applying NE. The doses used were based on a previous study that determined the doses of CA and NE that produce cortical activation (Castro-Alamancos and Gulati 2014). In that study, we found that application of CA (100 μM) into barrel cortex abolishes synchronous slow oscillations and shifts the firing rate to a tonic mode, which also activates the thalamus. Application of NE (500 μM) into barrel cortex also abolishes synchronous slow oscillations but suppresses overall firing, which deactivates the thalamus, and can lead to the common occurrence of spontaneous or whisker-evoked spindle oscillations in this state. First, we report the effects of these drugs on spontaneous cortical activity by measuring MUA and single-unit firing rate and autocorrelations, and the power spectrum of the FP. In the next section, we report the effects of the drugs on artificial whisking responses in barrel cortex.
During control conditions, MUA and FP activity shows spontaneous synchronous slow oscillations reflecting a deactivated or synchronized state. Application of CA (100 μM) into the barrel cortex produced a small but significant reduction of overall MUA spontaneous firing rate (P = 0.01; n = 22; Fig. 5A, left; Wilcoxon) and significantly suppressed the FP power spectrum between 0–10 Hz (P < 0.001; n = 22; Fig. 5B, top; Tukey). At the same time, CA significantly suppressed the high frequency (>50 Hz) peak in the MUA autocorrelation, reflecting the abolishment of synchronous slow oscillations (P < 0.001; n = 22; Fig. 5C; Wilcoxon). In contrast, application of NE (500 μM) into the barrel cortex produced a strong and highly significant reduction of overall MUA spontaneous firing rate (P < 0.001; n = 19; Fig. 5A, right; Wilcoxon) and significantly suppressed the FP power spectrum between 0–10 Hz (P = < 0.001; n = 19; Fig. 5B, bottom; Tukey). NE significantly suppressed the high frequency (>50 Hz) peak in the MUA autocorrelation, reflecting the abolishment of synchronous slow oscillations (P < 0.001; n = 19; Fig. 5D; Wilcoxon).
Fig. 5.
Population data showing the effect of neuromodulators applied in barrel cortex on spontaneous MUA, FP, and single-unit activity in barrel cortex. A: effect of cortical cholinergic stimulation [carbachol (CA)] or cortical noradrenergic stimulation [norepinephrine (NE)] on spontaneous MUA firing rate in barrel cortex. B: effect of CA and NE on the fast Fourier transform (FFT) power spectrum (10 ranges between 0–50 Hz) of the FP activity. Asterisks denote frequency ranges for which the drugs produced a significant effect compared with control. C and D: effect of CA and NE on autocorrelations of the spontaneous MUA. E: effect of CA and NE on firing rate of all single-units recorded in barrel cortex (see text for details). F: effect of CA and NE on firing rate of single-units classified in 2 groups according to their laminar location in the upper (>1 mm) or lower layers (<1 mm). The y-axis shows the mean ratio between the firing rate during the drug period and the control period.
Regarding single-unit firing, we analyzed the results in two ways. First, we considered all the cells in one group. Second, we separated the cells in two groups according to their cortical depth; cells below 1 mm were lower layer cells, and cells above 1 mm were upper layer cells. The first analysis revealed that CA did not have a significant effect on spontaneous firing when cells were considered together (P = 0.63; n = 22; Fig. 5E; Wilcoxon). In contrast, NE had a small but significant effect on spontaneous firing when all cells were considered together (P = 0.04; n = 19; Fig. 5E; Wilcoxon). However, it was clear that the vast majority of neurons were suppressed by NE and only two neurons (out of 19) showed an increase in firing during NE. Indeed, the level of significance of the effect of NE increased considerably when these two neurons that increased their firing were removed from the group (P = 0.0007; n = 17; Wilcoxon). This indicates that a small population of neurons in the neocortex may increase their firing during NE application, while the vast majority of neurons are suppressed.
The second analysis separated the cells in two groups according to their cortical depth. We then calculated a ratio of the effect of the drugs by dividing the firing rate during the drug by the firing rate during control. A value above 1 means that the firing rate increased while a value below 1 indicates that the firing rate was suppressed by the drug; a value of 1 indicates no change in firing rate. We then statistically compared the ratios (Mann-Whitney) of the cells in the lower and upper layers and found that these were significantly different for CA (Fig. 5F, left) but not for NE (Fig. 5F, right). Thus CA has a differential effect on cells in the upper (decreases firing) and lower layers (increases firing). Since the MUA electrode is in the upper layers, this may explain the small but significant suppression of MUA firing rate during CA described above (Fig. 5A, left). This agrees with a prior study that employed iontophoresis to deliver acetylcholine to different layers of the somatosensory cortex, which found that only 36% of cells were excited by it and they were located in lower layers (Lamour et al. 1988).
In contrast to CA, NE had a similar effect on cells in the upper and lower layers. In fact, the two cells that showed an increased firing during NE were distributed among both groups. The NE cell in the upper layer group that increased firing had a rather large ratio (11.9), and this skewed the mean of the ratios significantly. When these two cells were eliminated from the upper and lower layer groups, the NE ratios for both groups were below 1 (Upper = 0.40 ± 0.2, n = 7; Lower = 0.11 ± 0.04, n = 10) and still not significantly different from each other (P = 0.7; Mann-Whitney).
In conclusion, cholinergic or noradrenergic stimulation in barrel cortex produces cortical activation characterized by the absence of slow oscillations. Cholinergic activation differentially affects firing rate according to the depth of the neurons. It reduces firing in upper layers and enhances firing in lower layers. Noradrenergic activation reduced overall firing rate regardless of cortical layer, but a very small population of cells appear to be strongly excited by NE.
Cortical neuromodulation influences artificial whisking responses in barrel cortex.
We next explored the effects of cortical cholinergic (CA, 100 μM; n = 29) or noradrenergic (NE, 500 μM; n = 31) stimulation on puff and artificial whisking-evoked responses (5 pulses; ∼50-ms protraction). Figure 6 shows average responses evoked in barrel cortex and VPM thalamus by puff, whisk, g60, g200, and g600 stimulation (0.5 and 5 Hz) during control and during application of CA (Fig. 6A) or NE (Fig. 6B) into the barrel cortex. The panels in Fig. 6 show single-unit responses in barrel cortex and VPM, and MUA (rectified) and FP responses in barrel cortex.
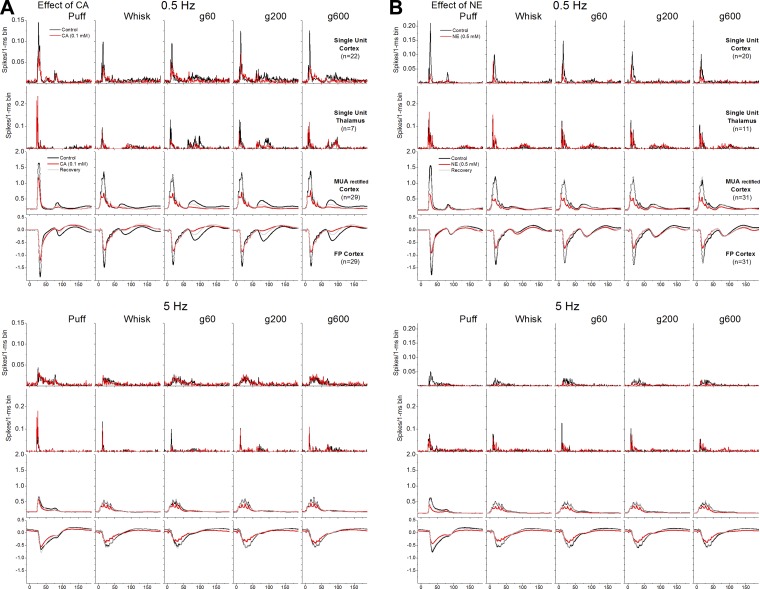
Fig. 6.
Effect of cortical cholinergic or noradrenergic stimulation on artificial whisking responses. Average single-unit (barrel cortex and VPM thalamus), MUA rectified (barrel cortex), and FP (barrel cortex) responses evoked by puff stimulation, artificial whisking in air (Whisk), and artificial whisking on different surfaces (g60, g200, and g600) at 0.5 and 5 Hz. Overlaid traces compare responses evoked during control (black) and during cortical cholinergic stimulation (A) or cortical noradrenergic stimulation (B) (red).
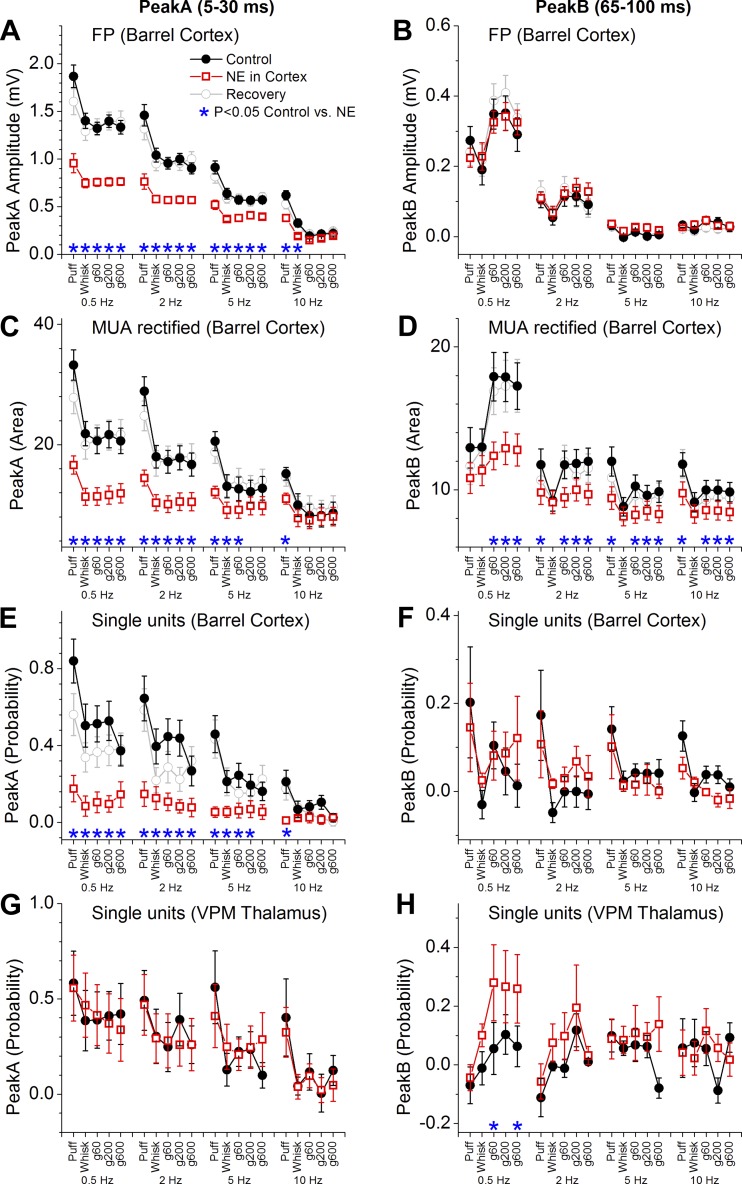
Figure 7 measures the effect of CA on peakA (5–30 ms) and peakB (65–100 ms) responses recorded from FP (Fig. 7, A and B), MUA (Fig. 7, C and D), and single-units in barrel cortex (Fig. 7, E and F) and from single-units in VPM thalamus (Fig. 7, G and H). Cortical cholinergic stimulation decreases peakA responses measured in the neocortex with FP, MUA (rectified), and single-unit (spontaneous activity corrected) recordings. The suppression occurs mostly for the largest responses evoked by low frequency puff or artificial whisking stimulation (see asterisks in Fig. 7); note that responses evoked by high frequency puff or artificial whisking are already strongly adapted. The suppressive effects of CA on peakA responses are largely reversible after washout of the drug (shown for FP and MUA). Since CA applied in barrel cortex also activates the thalamus (Castro-Alamancos and Gulati 2014), we would expect an effect on sensory adaptation in the VPM thalamus (Aguilar and Castro-Alamancos 2005; Castro-Alamancos 2002; Hirata et al. 2006). Indeed, during cortical cholinergic stimulation, peakA responses displayed virtually no rapid sensory adaptation in the VPM thalamus; i.e., peakA responses evoked by each of the frequencies are similar in VPM (particularly noticeable for puff responses). Cortical cholinergic stimulation suppressed peakB responses measured in the barrel cortex with FP, MUA, or single-unit recordings. Cortical cholinergic stimulation also tended to suppress peakB responses in the VPM thalamus, but this effect was not statistically significant. These results did not change much when the single-units in barrel cortex were separated by depth. PeakA responses were significantly suppressed for both upper (P = 0.003) and lower (P = 0.03) layer cells. PeakB responses were significantly suppressed for upper layer cells (P < 0.0001) but not for lower layer cells (P = 0.17) because lower layer cells have negligible peakB responses (as detailed above).
Fig. 7.
Population data measuring the effect of cortical cholinergic neuromodulation on peakA and peakB responses. PeakA responses were measured from FP (A), MUA rectified (C), and single-units (E) in the barrel cortex and from single-units in the VPM thalamus (G) during control (black) and cortical cholinergic stimulation (red). PeakB responses were measured from FP (B), MUA rectified (D), and single-units (F) in the barrel cortex and from single-units in the VPM thalamus (H) during control (black) and cortical cholinergic stimulation (red). Asterisks denote statistically significant differences between control and CA applied in barrel cortex (Tukey).
Figure 8 measures the effect of NE on peakA (5–30 ms) and peakB (65–100 ms) responses recorded from FP (Fig. 8, A and B), MUA (Fig. 8, C and D), and single-units in barrel cortex (Fig. 8, E and F) and from single-units in VPM thalamus (Fig. 8, G and H). Similar to cortical cholinergic stimulation, cortical noradrenergic stimulation decreases peakA responses in barrel cortex but not in VPM thalamus. The response suppression caused by NE was stronger and more widespread than the suppression caused by CA, involving higher frequencies. In contrast to cortical cholinergic stimulation, cortical noradrenergic stimulation did not suppress peakB FP (subthreshold) responses at all or single-unit responses (spontaneous corrected), but it did suppress overall MUA (rectified) responses in barrel cortex likely because of the effect of NE on spontaneous firing. In addition, cortical noradrenergic activation enhanced peakB responses in the VPM thalamus. Thus, even though overall MUA in barrel cortex is suppressed by NE, the thalamus compensates for this cortical suppression by enhancing its response to peakB. As a consequence, the subthreshold peakB response (FP) and single-unit responses in barrel cortex are not suppressed by NE. These results did not change much when the single-units in barrel cortex were separated by depth. PeakA responses were significantly suppressed for both upper (P < 0.0001) and lower (P < 0.0001) layer cells. PeakB responses were not significantly suppressed for either upper (P = 0.57) or lower layer cells (P = 0.75).
Fig. 8.
Population data measuring the effect of cortical noradrenergic neuromodulation on peakA and peakB responses. PeakA responses were measured from FP (A), MUA rectified (C), and single-units (E) in the barrel cortex and from single-units in the VPM thalamus (G) during control (black) and cortical noradrenergic stimulation (red). PeakB responses were measured from FP (B), MUA rectified (D), and single-units (F) in the barrel cortex and from single-units in the VPM thalamus (H) during control (black) and cortical noradrenergic stimulation (red). Asterisks denote statistically significant differences between control and NE applied in barrel cortex (Tukey).
These results indicate that both cortical cholinergic or noradrenergic activation suppress peakA responses in barrel cortex, but their effects on peakB responses are different. Cortical cholinergic activation suppresses peakB in barrel cortex, while cortical noradrenergic activation does not suppress peakB. During noradrenergic cortical stimulation, the peakB response in barrel cortex is supported by a larger peakB response in the VPM thalamus.
Thalamocortical activation influences artificial whisking responses in barrel cortex.
Cholinergic stimulation of the somatosensory thalamus induces cortical activation in barrel cortex (Hirata and Castro-Alamancos 2010) and affects sensory responses evoked by deflection of stationary whiskers (Hirata and Castro-Alamancos 2011). During this condition, the somatosensory thalamus also becomes activated and is a more effective sensory relay, particularly of high frequency inputs (Aguilar and Castro-Alamancos 2005; Castro-Alamancos 2002; Hirata et al. 2006). Here we tested the impact of thalamocortical activation caused by cholinergic stimulation of the somatosensory thalamus on artificial whisking responses in the barrel cortex.
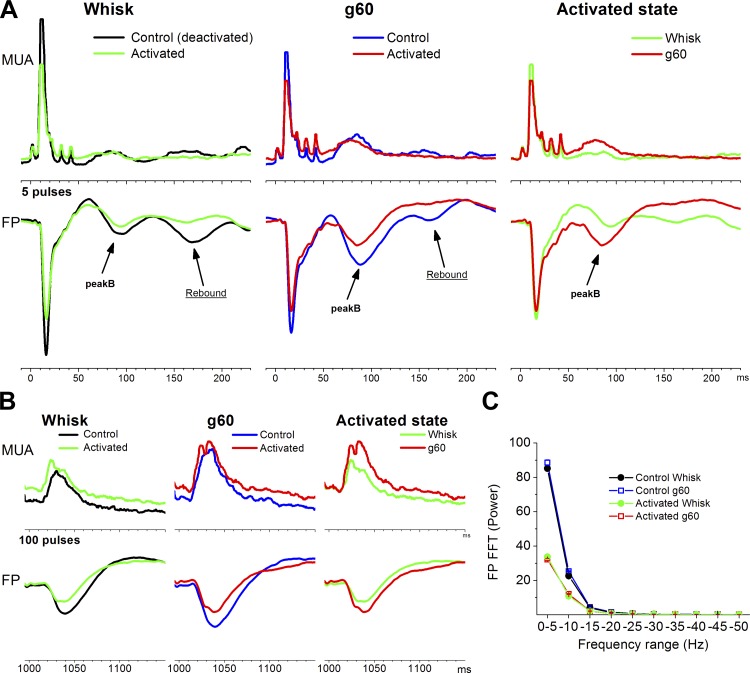
Figure 9 shows FP and MUA (rectified) responses evoked in barrel cortex by artificial whisking in air and on a surface during control and during cortical activation. We measured PeakA responses and peakB responses evoked by applying 5 pulses (∼50 ms protraction; Fig. 9A) or 100 pulses (∼1,000-ms protraction; Fig. 9B) to the motor nerve. Also, spontaneous FP activity recorded between stimulation trials served to demonstrate that the cortex was indeed activated (Fig. 9C) compared with control.
Fig. 9.
Effect of thalamocortical activation produced by thalamic cholinergic stimulation on artificial whisking responses in barrel cortex. Artificial whisking in air (Whisk) and on a surface (g60) responses were evoked by ∼50 ms (A, 5 pulses) or ∼1,000 ms (B, 100 pulses) protractions during control (deactivated) or during cortical activation (activated). A: shows FP and MUA rectified peakA and peakB responses evoked by the ∼50 ms protraction. B: shows peakB responses evoked by the retraction after the ∼1,000 ms protraction. C: shows the population power spectrum derived from spontaneous FP activity during the 4 conditions compared in A and B. Note the suppression of low frequency oscillations during activated compared with control.
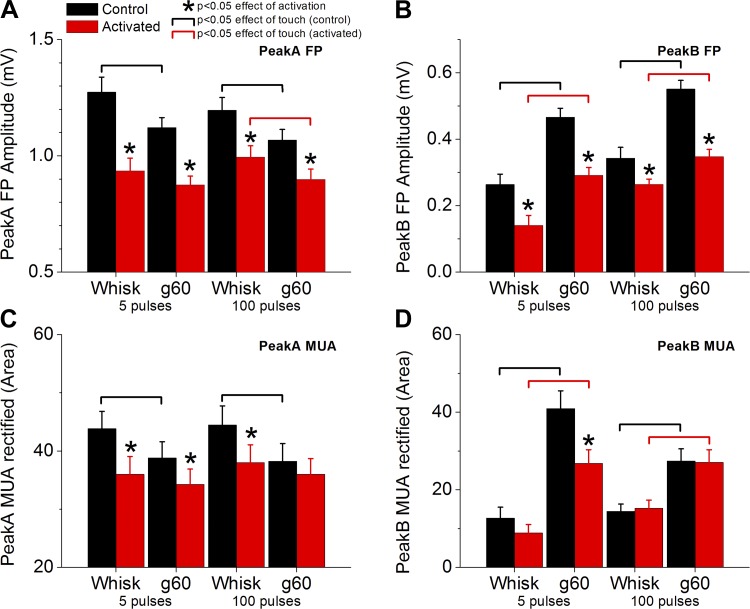
Figure 10 shows population data (n = 11) measuring the effects of activation and the effects of artificial whisking during deactivated or activated states. Regarding the effect of activation, we found that cortical activation significantly suppressed both peakA and peakB FP responses (Fig. 10, A and B). PeakA MUA (rectified) responses were also suppressed by activation (Fig. 10C). However, activation only suppressed peakB MUA responses evoked by whisking on a surface at short protraction durations (g60 at 5 pulses; Fig. 10D) likely because at this protraction duration peakB mixes with the thalamocortical rebound (as described above; Fig. 3), which is suppressed by activation. Regarding the effect of active touch (whisking on a surface vs. in air) on peakA responses, we found that active touch significantly suppressed both FP and MUA peakA responses during control (deactivated), but mostly not during activation (except for FP responses at 100 pulses) because these responses are already rather suppressed during activation (Fig. 10, A and C). Regarding the effect of active touch on peakB responses, we found that active touch significantly enhanced both FP and MUA peakB responses during both control and cortical activation (Fig. 10, B and D). Thus peakB continues to signal active touch during activation.
Fig. 10.
Population data measuring the effect of thalamocortical activation on peakA (A and C) and peakB (B and D) responses evoked by ∼50- and ∼1,000-ms protractions and measured with FP (A and B) and MUA rectified (C and D). Artificial whisking responses in air (Whisk) and on a surface (g60) were evoked by ∼50 ms (5 pulses) or ∼1,000 ms (100 pulses) protractions during control (black) or during cortical activation (red). For each stimulus, asterisks denote a statistically significant difference (Tukey) between control and activated states. Black brackets denote significant differences between artificial whisking in air and on a surface during the control state. Red brackets denote significant differences between artificial whisking in air and on a surface during the activated state.
These results indicate that during cortical activation, produced by cholinergic stimulation of the somatosensory thalamus, peakA and peakB responses are generally suppressed compared with cortical deactivation. Whisking on a surface (active touch) suppresses peakA responses compared with whisking in air during cortical deactivation, but mostly not during cortical activation because peakA responses are already suppressed during this state. Active touch enhances peakB responses regardless of cortical state.
DISCUSSION
A retraction response in neocortex that signals active touch and protraction duration.
Our population responses and pooled single-cell responses indicate that the barrel cortex contains both movement and touch signals and that these signals might be primarily disambiguated by their timing. Artificial whisking movement consists of a protraction with a rising phase and a falling phase (retraction). The onset of the protraction is associated with a peakA response that occurs during the first 25 ms. The retraction is associated with a peakB response that is much more prominent during artificial whisking on a surface (active touch) in upper layer cells of barrel cortex and in VPM cells. Both peakA and peakB adapt sharply with whisking frequency; during 5–10 Hz whisking, peakB is not evident. During low frequency whisking, the magnitude of peakB signals the occurrence of touch and the duration of the protraction.
Although peakB is prominent in barrel cortex and VPM thalamus during active touch, it is notoriously absent from another target of the trigeminal complex, the superior colliculus (Bezdudnaya and Castro-Alamancos 2014, 2011). In the superior colliculus, artificial whisking evokes two temporally defined responses (called peak1 and peak2) during the rising phase of the protraction but virtually no response during the retraction. We speculate that the absence of peakB in superior colliculus is caused by the very strong frequency-dependent suppression (adaptation) of whisker-evoked responses in this structure (Bezdudnaya and Castro-Alamancos 2014; Cohen et al. 2008), which seems more robust than in VPM thalamus (Castro-Alamancos 2002) or barrel cortex (Castro-Alamancos 2004a). Moreover, here we found that lower layer cells in barrel cortex, which encompass corticotectal cells, also do not express a robust peakB during the retraction. The selective presence of peakB in the upper layers of barrel cortex and VPM thalamus (not in midbrain) indicates that the thalamocortical system might be involved in processing information related to the duration of the protraction and the occurrence of the retraction during contact.
PeakB seems like a useful signal, computed by the thalamocortical system, to inform the brain that the protraction has ended, how long it lasted, and that contact occurred during the protraction. Signaling of the duration of the protraction by peakB can be explained by rapid adaptation (short-term depression) in the thalamocortical pathway (Castro-Alamancos 2004a,b; Castro-Alamancos and Connors 1996b; Chung et al. 2002; Khatri et al. 2004). The occurrence of peakA during the onset of the protraction triggers a number of inhibitory processes (synaptic depression, synaptic inhibition, and intrinsic inhibition) that coalesce to suppress the next afferent input. Gradual recovery from these processes over 10s to 100s of milliseconds allows peakB to increase in magnitude as the time from the occurrence of peakA (protraction onset) increases. During cortical deactivated states, which are the traditional states used to study artificial whisking responses, peakB responses can be contaminated by a thalamocortical rebound excitation that is also triggered by thalamic stimulation in vivo and is highly state-dependent (Castro-Alamancos and Connors 1996a). Indeed, this rebound was strongly suppressed or abolished by cortical activation. Hence, peakB should be free of the rebound during natural whisking in behaving animals.
Active whisking usually takes place when rodents are aroused, vigilant, and exploring the environment, a state when the neocortex is activated. Logically, it makes sense to study artificial whisking responses during cortical activation (Castro-Alamancos 2004b). We employed three different neuromodulation methods to induce cortical activation; cortical cholinergic stimulation, cortical noradrenergic stimulation (Castro-Alamancos and Gulati 2014), and thalamic cholinergic stimulation (Hirata and Castro-Alamancos 2010). For a discussion of these activation methods, please see those publications. A consistent finding is that peakA responses evoked by artificial whisking are suppressed by cortical activation induced by any of these methods. The type of neuromodulation determines the effect on artificial whisking responses, particularly for peakB. During cortical cholinergic activation peakB responses are strongly suppressed in the neocortex, in part because the thalamocortical rebound that merges with peakB is abolished. In contrast, during cortical noradrenergic activation overall cortical firing is suppressed but peakB is not suppressed because the thalamus compensates by enhancing its peakB response. These effects of cortical cholinergic and noradrenergic activation on artificial whisking responses were not different between upper and lower layer cells. During thalamic cholinergic stimulation both the thalamus and the neocortex are activated. During this state, peakB effectively signals active touch. The different thalamocortical states set by neuromodulators may define the quality of the signaling that takes place during whisking. This could serve to facilitate or impede tactile processing depending on the behavioral state of the animal. Hence, the varied effects of neuromodulators on cortical whisking related signals may be the basis for the distinct sensory processing abilities of animals in different attentive states.
Are the artificial whisking signals present during natural behavior?
During natural whisking, rats produce whisker movements of different durations, amplitudes, and frequencies (Carvell and Simons 1990; Gao et al. 2001; Grant et al. 2009; Kleinfeld et al. 2006; Kleinfeld and Deschenes 2011; Knutsen et al. 2008; Towal and Hartmann 2008; Wolfe et al. 2008), and both the protraction and the retraction are under muscular control (Berg and Kleinfeld 2003). A growing number of studies have recorded neural activity from the barrel cortex in unanesthetized rodents (rats and mice) during head-fixed or freely moving conditions (e.g., (Crochet and Petersen 2006; Curtis and Kleinfeld 2009; de Kock and Sakmann 2009; Fee et al. 1997; Hentschke et al. 2006; Jadhav et al. 2009; O'Connor et al. 2010; Wolfe et al. 2008). Neurons in the barrel cortex discharge weakly in phase with the natural whisking movement but much more briskly to contact with objects depending on when the contact occurred in the whisking cycle. A direct comparison between the present study and previous studies in behaving animals is difficult; for example, control over whisking frequency is not available during natural whisking. However, it seems that the large responses associated with the onset of the protraction (peakA) during low frequency artificial whisking in air are not typically observed during natural whisking in air. There are several reasons for the suppressed cortical responses during natural whisking in air compared with artificial whisking in air. First, artificial whisking activates mechanoreceptors in a different way than natural whisking; during artificial whisking in air trigeminal ganglion cell discharges are more phase-locked to the movement compared with natural whisking (Khatri et al. 2009). Evidently, the more synchronous afferent input will lead to stronger cortical responses during artificial whisking in air than during natural whisking in air. Second, during natural whisking in air animals are aroused and their thalamocortical system is activated. Here we show that peakA responses produced by artificial whisking in air are strongly suppressed by cortical activation, which brings them closer to the situation observed during natural whisking. Finally, neural activity during natural whisking in air has been typically evaluated during rhythmic whisking at 5–15 Hz, which produces cortical responses that are suppressed due to frequency adaptation. In fact, peakA responses are also strongly suppressed at these artificial whisking frequencies.
During artificial whisking, we also found a retraction signal (peakB) that appears to code the duration of the previous protraction. Importantly, this signal is only present during low frequency artificial whisking, equivalent to single whisking bouts in behaving animals, and is most obvious during long protractions. To our knowledge, a retraction signal that codes the duration of the previous protraction has not been reported during natural whisking. This may be because previous studies in behaving animals that measured cortical responses have focused mostly on rhythmic whisking (5–12 Hz), which occurs at frequencies during which this retraction signal is not present even during artificial whisking. Thus future work will need to evaluate if this signal is present during natural whisking by assessing single long-lasting whisking bouts.
It is worth noting that in our analysis of high frequency trains at whisking frequencies (5–10 Hz) we focused on steady state responses. This leaves out the dynamics that occur in the first few cycles (2–5) at the beginning of a stimulus train. Indeed, it is known that both artificial whisking (Derdikman et al. 2006; Yu et al. 2013) and passive whisker deflections (Castro-Alamancos and Gulati 2014; Hirata et al. 2009) evoke transient responses during the first few cycles (2–5) of a stimulus train. These transient responses may be relevant for whisking behavior during object coding since many object interactions last only a few cycles (Knutsen et al. 2006). On the other hand, these transient responses are state-dependent and may occur primarily during quiescent/anesthetized states (Castro-Alamancos and Gulati 2014).
In conclusion, peakA and peakB signals observed during artificial whisking in air and on surfaces code the onset of the movement protraction and the duration of the protraction, respectively. These signals are differently expressed in different brain areas. Thalamocortical activation, typical of arousal, modulates these signals in a way that is consistent with their presence during natural whisking.
GRANTS
This work was supported by the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.A.C.-A. conception and design of research; M.A.C.-A. and T.B. performed experiments; M.A.C.-A. and T.B. analyzed data; M.A.C.-A. interpreted results of experiments; M.A.C.-A. prepared figures; M.A.C.-A. drafted manuscript; M.A.C.-A. edited and revised manuscript; M.A.C.-A. approved final version of manuscript.
REFERENCES
- Aguilar JR, Castro-Alamancos MA. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states. J Neurosci 25: 10990–11002, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol 89: 104–117, 2003. [DOI] [PubMed] [Google Scholar]
- Bezdudnaya T, Castro-Alamancos MA. Neuromodulation of whisking related neural activity in superior colliculus. J Neurosci 34: 7683–7695, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Castro-Alamancos MA. Superior colliculus cells sensitive to active touch and texture during whisking. J Neurophysiol 106: 332–346, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AW, Waite PM. Responses in the rat thalamus to whisker movements produced by motor nerve stimulation. J Physiol 238: 387–401, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci 10: 2638–2648, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron 41: 455–464, 2004a. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol 539: 567–578, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol 74: 213–247, 2004b. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Origin of synchronized oscillations induced by neocortical disinhibition In vivo. J Neurosci 20: 9195–9206, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Borrell J. Motor activity induced by disinhibition of the primary motor cortex of the rat is blocked by a non-NMDA glutamate receptor antagonist. Neurosci Lett 150: 183–186, 1993. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term plasticity of a thalamocortical pathway dynamically modulated by behavioral state. Science 272: 274–277, 1996a. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Spatiotemporal properties of short-term plasticity sensorimotor thalamocortical pathways of the rat. J Neurosci 16: 2767–2779, 1996b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Gulati T. Neuromodulators produce distinct activated states in neocortex. J Neurosci 34: 12353–12367, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol 541: 319–331, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron 34: 437–446, 2002. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Hirata A, Castro-Alamancos MA. Vibrissa sensation in superior colliculus: wide-field sensitivity and state-dependent cortical feedback. J Neurosci 28: 11205–11220, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci 9: 608–610, 2006. [DOI] [PubMed] [Google Scholar]
- Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat Neurosci 12: 492–501, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock CP, Sakmann B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proc Natl Acad Sci USA 106: 16446–16450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D, Yu C, Haidarliu S, Bagdasarian K, Arieli A, Ahissar E. Layer-specific touch-dependent facilitation and depression in the somatosensory cortex during active whisking. J Neurosci 26: 9538–9547, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J Neurophysiol 78: 1144–1149, 1997. [DOI] [PubMed] [Google Scholar]
- Gao P, Bermejo R, Zeigler HP. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J Neurosci 21: 5374–5380, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RA, Mitchinson B, Fox CW, Prescott TJ. Active touch sensing in the rat: anticipatory and regulatory control of whisker movements during surface exploration. J Neurophysiol 101: 862–874, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke H, Haiss F, Schwarz C. Central signals rapidly switch tactile processing in rat barrel cortex during whisker movements. Cereb Cortex 16: 1142–1156, 2006. [DOI] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Influence of subcortical inhibition on barrel cortex receptive fields. J Neurophysiol 102: 437–450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26: 4426–4436, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Effects of cortical activation on sensory responses in barrel cortex. J Neurophysiol 105: 1495–1505, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol 103: 1147–1157, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Wolfe J, Feldman DE. Sparse temporal coding of elementary tactile features during active whisker sensation. Nat Neurosci 12: 792–800, 2009. [DOI] [PubMed] [Google Scholar]
- Khatri V, Bermejo R, Brumberg JC, Keller A, Zeigler HP. Whisking in air: encoding of kinematics by trigeminal ganglion neurons in awake rats. J Neurophysiol 101: 1836–1846, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri V, Hartings JA, Simons DJ. Adaptation in thalamic barreloid and cortical barrel neurons to periodic whisker deflections varying in frequency and velocity. J Neurophysiol 92: 3244–3254, 2004. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol 16: 435–444, 2006. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Deschenes M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron 72: 455–468, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsen PM, Biess A, Ahissar E. Vibrissal kinematics in 3D: tight coupling of azimuth, elevation, and torsion across different whisking modes. Neuron 59: 35–42, 2008. [DOI] [PubMed] [Google Scholar]
- Knutsen PM, Pietr M, Ahissar E. Haptic object localization in the vibrissal system: behavior and performance. J Neurosci 26: 8451–8464, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour Y, Dutar P, Jobert A, Dykes RW. An iontophoretic study of single somatosensory neurons in rat granular cortex serving the limbs: a laminar analysis of glutamate and acetylcholine effects on receptive-field properties. J Neurophysiol 60: 725–750, 1988. [DOI] [PubMed] [Google Scholar]
- Masri R, Bezdudnaya T, Trageser JC, Keller A. Encoding of stimulus frequency and sensor motion in the posterior medial thalamic nucleus. J Neurophysiol 100: 681–689, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67: 1048–1061, 2010. [DOI] [PubMed] [Google Scholar]
- Szwed M, Bagdasarian K, Ahissar E. Encoding of vibrissal active touch. Neuron 40: 621–630, 2003. [DOI] [PubMed] [Google Scholar]
- Towal RB, Hartmann MJ. Variability in velocity profiles during free-air whisking behavior of unrestrained rats. J Neurophysiol 100: 740–752, 2008. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Hill DN, Pahlavan S, Drew PJ, Kleinfeld D, Feldman DE. Texture coding in the rat whisker system: slip-stick versus differential resonance. PLoS Biol 6: e215, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol 4: e124, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Horev G, Rubin N, Derdikman D, Haidarliu S, Ahissar E. Coding of object location in the vibrissal thalamocortical system. Cereb Cortex 2013 Sept 22 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zucker E, Welker WI. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res 12: 138–156, 1969. [DOI] [PubMed] [Google Scholar]