Abstract
Nigrostriatal dopamine denervation plays a major role in basal ganglia circuitry disarray and motor abnormalities of Parkinson's disease (PD). Studies in rodent and primate models have revealed that striatal projection neurons, namely, medium spiny neurons (MSNs), increase the firing frequency. However, their activity pattern changes and the effects of dopaminergic stimulation in such conditions are unknown. Using single-cell recordings in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated primates with advanced parkinsonism, we studied MSN activity patterns in the transition to different motor states following levodopa administration. In the “off” state (baseline parkinsonian disability), a burst-firing pattern accompanied by prolonged silences (pauses) was found in 34% of MSNs, and 80% of these exhibited a levodopa response compatible with dopamine D1 receptor activation (direct pathway MSNs). This pattern was highly responsive to levodopa given that bursting/pausing almost disappeared in the “on” state (reversal of parkinsonism after levodopa injection), although this led to higher firing rates. Nonbursty MSNs fired irregularly with marked pausing that increased in the on state in the MSN subset with a levodopa response compatible with dopamine D2 receptor activation (indirect pathway MSNs), although the pause increase was not sustained in some units during the appearance of dyskinesias. Data indicate that the MSN firing pattern in the advanced parkinsonian monkey is altered by bursting and pausing changes and that dopamine differentially and inefficiently regulates these behaviorally correlated patterns in MSN subpopulations. These findings may contribute to understand the impact of striatal dysfunction in the basal ganglia network and its role in motor symptoms of PD.
Keywords: basal ganglia, striatum, electrophysiology, bursts, pauses, Parkinson's disease, dyskinesias
the medium spiny neuron (MSN) is the striatal projection unit in the basal ganglia network and plays an important role in motor function. In Parkinson's disease (PD), the MSN activity is significantly dysregulated due to loss of dopamine (DA) modulation and adaptive changes in glutamate, GABA, acetylcholine, and other signaling mechanisms controlling striatal microcircuits (Cepeda et al. 1998; Shen et al. 2007; Tepper et al. 2004; Wang et al. 2006). Morphological and physiological studies have shown alterations of MSN dendritic terminals and changes in synaptic plasticity (Calabresi et al. 2014; Day et al. 2006). The spontaneous MSN firing frequency is substantially increased in rodent and primate models of PD (Gubellini et al. 2002; Ingham et al. 1998; Liang et al. 2008). Thus complex interacting mechanisms in the DA-denervated striatum may cause varied effects on MSN discharges that are likely to distinctively impact motor behaviors.
Notably, in the parkinsonian setting DA still maintains its dual modulation on MSN subpopulations that express D1 (excitatory) or D2 (inhibitory) receptors and project into the direct and indirect output pathways, respectively (Gerfen 1992; Surmeier et al. 1996). This dichotomous DA influence on MSN outputs is essential to reverse parkinsonian motor symptoms. However, DA inputs may also produce abnormal responses with typical “inversion of frequency changes” and the resulting imbalance of MSN discharges that is associated with involuntary movements, namely, levodopa-induced dyskinesias (Liang et al. 2008). Overall, the frequency analysis has shown profound MSN dysregulation in PD, ultimately generating altered responses to DA with significant motor effects. However, activity changes across basal ganglia stations in PD are characterized by altered firing patterns more conspicuously than frequencies (Boraud et al. 2001; Hammond et al. 2007; Mallet et al. 2008; Raz et al. 2000). Pathological bursting, pausing and synchronization of neuronal firing, or oscillations of local field potentials and single-unit spiking in the globus pallidus and subthalamic nucleus (STN) could be responsible for the release of abnormal motor commands in PD. These network changes are likely to correlate with striatal output alterations; indeed, MSNs play a role in the generation of altered bursting and pausing patterns in the external globus pallidus of rodents with dopaminergic lesions (Kita and Kita 2011). Nonetheless, the MSN activity patterns in PD and their behavioral correlates remain unknown.
We hypothesized that in PD, MSNs fire with bursts and pauses that, similarly to the described frequency changes, could be regulated by DA with various levels of efficiency, eventually generating abnormal responses and dyskinesias. To address this hypothesis, we recorded single-cell activity in the striatum of two severely parkinsonian rhesus monkeys and analyzed the MSN firing patterns in the transition to different motor states following levodopa administration. We found that pattern alterations developed in MSN subpopulations were differentially controlled by DA and correlated with motor responses. Burst/pause activity was present mostly in the subset of neurons increasing the firing rate in response to levodopa (D1 receptor-like response), and this bursting/pausing was almost eliminated by DA replacement. In turn, pausing with irregular (nonbursty) firing in the subset of MSNs decreasing the firing rate in response to levodopa (D2 receptor-like response) increased with the reversal of motor symptoms. Notably, levodopa response failed to normalize activity in both MSN subsets, thereby describing the inefficient effect of DA replacement.
MATERIALS AND METHODS
Animal preparation and behavior.
Two adult female rhesus monkeys (Macaca mulatta; 5–7 kg) were used in this study, which was conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” [DHEW Publication No. (NIH) 85-23, Revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205] and approved by the Institutional Animal Care and Use Committee of Emory University. Monkeys were rendered parkinsonian by intravenous administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) given weekly at variable doses across repeated injections ranging from 0.3 to 0.8 mg/kg (average dose was 0.4 mg/kg for a total duration of 13 and 15 wk for each monkey) and met the criteria for chronic, severe, and late-stage parkinsonism with exhibition of levodopa-induced dyskinesias as previously described (Cao et al. 2007). Parkinsonian motor disability and dyskinesias were measured with the standardized scale for MPTP-treated monkeys (Papa and Chase 1996). Doses of levodopa methyl ester plus benserazide given subcutaneously to elicit reproducible responses in successive recording sessions were screened and tested repeatedly in each monkey (Papa et al. 2004). The selected doses (50–75 mg sc) consistently reduced motor disability scores (MDS) from 21 ± 2 (mean ± SE) in the “off” state (parkinsonian baseline) to 7.4 ± 0.8 in the “on” state (reversal of disability at the peak of levodopa effects) and produced peak-dose dyskinesias of mild to moderate intensity for compatibility with the monkey's restraint in the primate chair during recordings (dyskinesia scores 6.4 ± 0.6 at the peak of levodopa effects). Regular oral levodopa was used for maintenance treatment at 50- and 100-mg total dose per day for 5 and 6 mo in each monkey before recording sessions started. After behavioral testing, monkeys were surgically implanted with a recording chamber and a head-holding device as previously described (Papa et al. 1999).
Electrophysiology.
The striatal areas were delimited by electrophysiological mapping of basal ganglia regions before recording experiments began (DeLong et al. 1985). On experiment days, oral levodopa treatment was withdrawn, and monkeys were transferred to the primate chair in the mornings, while parkinsonian disability was at its highest score, and prepared with a needle placed under the arm skin and connected to a tube for distal levodopa subcutaneous injection during the experiment. Motor behavior was continuously monitored through videotaping. The advanced parkinsonism in these monkeys produced a profound and invariable off state through successive experiment days. Tungsten microelectrodes (0.1–0.5 MΩ after reconditioning) were used for continuous recording, and single-cell activity was collected for 5–12 min in each state: off, on, and on-with-dyskinesias. To ensure the transition to the next state (the critical condition for storing neural data), a minimum of 5 min of behavioral changes was required (event marks were aligned with neuronal activity by time stamps). The onset of the on state, usually ∼20 min after the subcutaneous injection of levodopa, was typically recognized by rapid movements of the eyes, increased blinking, yawning, and stretching of the legs (all marked as voluntary movements or contamination if they introduced changes in recordings). Dyskinesias began ∼15 min after turning on and were distinguished from voluntary movements because of typical features, i.e., stereotyped or repetitive purposeless movements. Data were acquired at the sampling rate of 20 kHz (Plexon).
Data analysis.
Spike sorting with the use of strict criteria for stability of stationary activity based on waveform isolation and refractory period was performed off-line on the stored raw data. Isolated units compatible with MSN activity and complete data for all motor states were used for pattern analyses. A minimum of 3 min was finally analyzed in each state. After sorting, only units classified as MSNs were included in the study (Aosaki et al. 1994; Barnes et al. 2005). All other units that were classified as interneurons [fast-firing interneurons and tonically active neurons (TANs)] and units with unclear classification or uncertain location were excluded from the study (see details of unit classification in Aosaki et al. 1994; Barnes et al. 2005; Liang et al. 2008). Final acceptance of MSNs (n = 140) relied on matching of waveforms to confirm that activity of the same unit with stationary spiking was processed in each state. MSN location in the caudate or putamen was determined with reconstructed electrode tracks after histological verification of coordinates (Fig. 1). Firing frequency changes of MSNs associated with levodopa responses were used for analyses of different MSNs subsets. Frequency changes at the beginning of the on state indicated DA modulation in response to levodopa that may presumably correspond to different DA receptor subtypes (D1 or D2 receptors, as firing rate increases or decreases, respectively). Most studies agree about the predominant segregation of D1 and D2 receptors in MSN subpopulations (Gerfen and Surmeier 2011). There is a small number of MSNs with D1/D2 receptor coexpression, but their function and the impact of dopamine regulation on these neurons in PD models remain uncertain (Perreault et al. 2011). Progression of changes from off to on and from on to on-with-dyskinesias states indicated stable or inverted MSN firing rate changes in response to DA, as unidirectional or bidirectional responses, respectively (Liang et al. 2008).
Fig. 1.
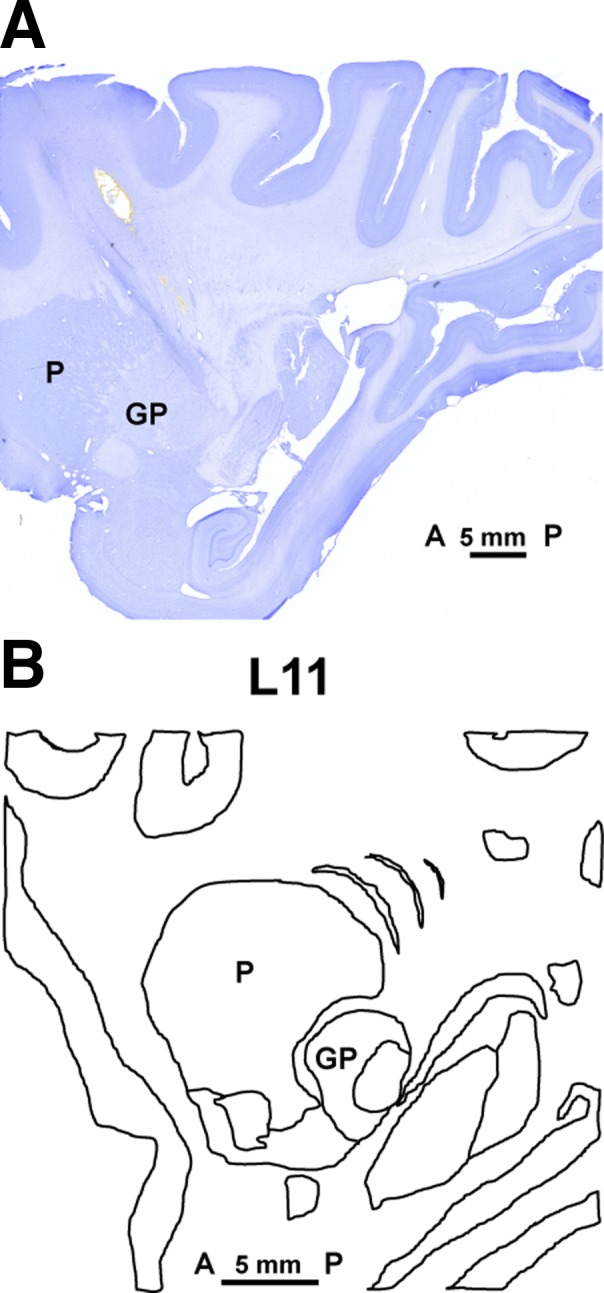
A: the Nissl-stained section of monkey brain shows the electrode track of a brain penetration made before euthanasia to show one of the recording sites. The electrode penetration targeted the posterior and lateral area of the putamen, passing through its edge according to coordinates taken from our electrophysiological mapping. The section matched the anatomy of lateral 11 (L11) in the sagittal plane of the monkey atlas (B). To confirm the location of recorded units, the anatomic position of electrode penetrations used in the recordings was then calculated with reference to the coordinates of this electrode track in L11. Bar shows the scale from anterior (A) to posterior (P). L, lateral; P, putamen; GP, globus pallidus.
The same normal MSN activity pattern as described originally in primates and rodents (Aosaki et al. 1994; Barnes et al. 2005; Crutcher and DeLong 1984a; Kimura 1992) has been confirmed in previous recordings, typically exhibiting very low activity with isolated spikes (0–2 Hz, nonbursty) and very irregular pattern including interspike intervals (ISIs) longer than 1 s. In the parkinsonian monkey, the MSN firing frequency is increased, but its irregularity is maintained (Liang et al. 2008) with overt burst and pause spiking. Therefore, the analysis of pattern changes included the firing of bursts and pausing of activity.
Burst analysis.
Burst activity was defined as a period with a significantly higher number of discharges by comparison with the typical discharge rates in the spike train. In all units after spike sort analysis, we applied an algorithm for variable burst detection (Fig. 2A) that is based in the discharge density histogram (DDH) instead of the ISI histogram (Kaneoke and Vitek 1996). Briefly, the DDH is constructed with the discharge rate over small intervals and usually follows a Poisson process. An interval t is defined as the reciprocal of the mean discharge rate m (t = 1/m), and the interval is used to construct the DDH with a bin width of unity (spikes/t) (Fig. 2, B and C). The distribution of the DDH is examined for positive skewness and compared with a Poisson distribution of mean 1 for a significant difference at P < 0.05 (χ2 test) to determine the presence of bursts. The threshold for the burst periods is determined by the turning point in the DDH or the point of slope change in the curve. Once the threshold density (d) is detected, the ISI threshold for bursts is calculated (t/d) and used on the spike train to detect bursts if more than two ISIs equal to or lower than the ISI threshold occur successively. The ISI threshold is then used to mark the beginning and end of the bursts and to calculate the number of spikes in the bursts as well as the intraburst ISIs (IB-ISI). Variability of IB-ISIs is examined by normalization of successive ISIs with the first IB-ISI to determine the consistency of serial ISI changes (see plots in Fig. 2, B and C). The critical parameters used by the algorithm were 1) interval t to calculate discharge density histogram, 2) minimal number of ISIs for burst classification (taken at 3 ISIs, 4 spikes), and 3) significance level for skewness and difference with the Poisson distribution (both at P < 0.05). With the exception of the interval t, other parameters were fixed in the analysis of all units. This burst detection method has an increased sensitivity for detecting different burst periods that depends on adapting the interval t according to the characteristics of the spike train and the type of bursts. The interval t (= 1/m) was varied within limits between 0.5 and 3 to avoid the binomial probability of spike frequencies and deviation of the histogram toward a normal distribution. The resulting DDH were thus compared with the corresponding Poisson distribution (mean 0.5–3). Results of the analyses using a different mean of the Poisson distribution were examined for a satisfactory curve in the DDH, changes of IB-ISIs, and consistency of the spike number in bursts. Bursty neurons had at a minimum 1 burst/10 s identified in one of the analyses, and neurons classified as nonbursty had no burst detection in any of the analyses. For computing the burst activity of the unit, the analysis with best detection of bursts was selected (see examples of burst detection changing the Poisson mean in Fig. 2, B and C). In each unit, the same parameters used for burst detection in the off state were used in the on and on-with-dyskinesias states for consistency and reliable analysis of burst changes in the transition to motor states. The transition to motor states increases or decreases the MSN firing frequency, which could potentially affect the burst formation. Thus postprocessing of data included the adjustment of burst rate (BR) by the firing rate (FR) in each motor state (off, on, or dyskinesia state) to account for the direct influence of frequency changes on burst formation, as follows:
Fig. 2.
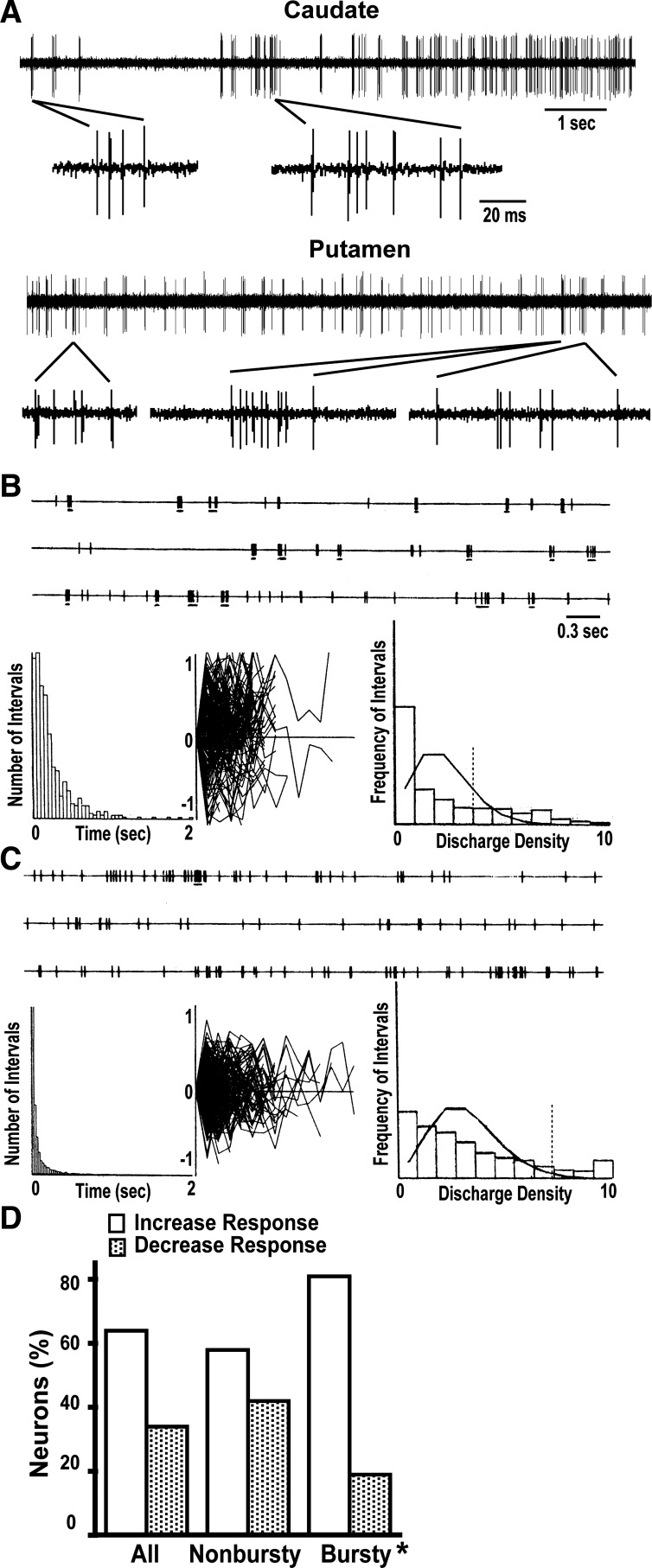
Examples of burst activity in medium spiny neurons (MSNs) recorded in the chronic parkinsonian state. A: selected spike trains of MSNs in the caudate and putamen exhibiting clear burst activity in the off state. Both traces display 10 s of raw data, and selected segments are presented in a decreased time scale to visualize the burst composition. B: example of the burst detection analysis showing the raster (top), where bursts are marked by underlining; interspike interval (ISI) histogram (bottom left); intraburst ISIs normalized by the first ISI and plotted in decimal log values (−1 to 1; bottom center); and discharge density histogram (DDH; bottom right) with the Poisson distribution curve (histogram shows the frequency of intervals t with a certain number of spikes starting from 0 at left and using a bin width of unity). The vertical dashed line in the DDH marks the threshold for burst detection. The analysis in this MSN (8.9-Hz firing frequency) shows a 1.1-Hz burst rate detected with t = 2/m (Poisson mean of 2) and a threshold bin of 4 for a significant deviation of the Poisson process (dashed line). Spike mean in a burst was 5.9 and mean ISI in bursts was 10.9 ms. The intraburst ISI plot does not show serial changes compatible with consistent increasing intervals as found in certain burst activity during sleep. C: example of another unit (37.1-Hz firing frequency) where a 1.82-Hz burst rate was detected using t = 3/m (Poisson mean of 3) and the threshold bin for a significant deviation of the Poisson process was 8 (dashed line). Spike mean in a burst was 5.4, and mean ISI in bursts was 5.5 ms. Notice the lack of evidence for burst activity in the ISI histogram compared with the DDH. Descriptions and conventions are the same as in B. D: distribution of the burst activity pattern in MSN subpopulations suggested by the change of firing frequencies in response to dopamine (increase or decrease of frequency is presumed to correspond to D1 or D2 receptor-mediated response, respectively). The bursty pattern of activity is predominantly exhibited in MSNs with dopamine-induced activity increases. *P < 0.001, observed vs. expected; χ2 test.
where ABR is the adjusted burst rate.
Pause analysis.
Pause of activity was defined as the time without spiking that is significantly longer than those typically found in the spike train. For detection of intervals classified as pauses we used the Poisson “pause” surprise. Based on the same principles of the surprise method for burst detection (Legendy and Salcman 1985), it calculates the probability that a certain interval containing a number of spikes or less is critically low (surprise) in relation to the average firing rate in the spike train to be considered a pause of activity. In this analysis, the parameters to detect pauses of the MSN activity were set using a methodology similar to that used for firing pauses of pallidal neurons (Elias et al. 2007). The maximum number of spikes in the pause is set to 2 (these were the spikes at the beginning and the end of the pause, because the actual sporadic firing within the pause was unpredictable for MSNs whose pausing was unknown, and contrarily, occasional spiking in a pause might represent simply a pause break). The interval used in the calculation of probability, t, is set at a minimum of 5 times the median ISI at the beginning of the algorithm for a low start point considering the variable range of mean firing rates of MSNs in our data set. Different intervals were tested in several trials with different spike trains to ensure that the initial t was never too long so that it could not begin with a high surprise value, leaving shorter pauses with a significant value unidentified. The program written in MATLAB calculates the probability, P, using the Poisson distribution and the surprise, S, as follows:
Thus three parameters are used to calculate P: n, number of spikes in pauses (0 to 2); r, firing rate (probability of emitting a spike in 1 ms); and t, 5 times the median ISI. After calculation of the surprise, S, the program evaluates whether the addition of one ISI to t results in a higher S, and it continues adding ISIs and recalculating P and S in successive iterations until pause detection reaches 0. Subsequently, the program selects the iteration with the level of acceptance for a significant surprise, which is set at S ≥ 3, and the t used in that iteration is the “duration of pauses” as detected in the spike train. The maximum limit of t is set at <1 s to prevent the possibility that occasionally long intervals caused by transient loss of signal may be identified as pauses of activity. The number of segments with t used in that iteration is counted on the spike train as the “number of pauses.” All segments analyzed for each unit in each motor state were ∼180 s (trains as short as 120 s were occasionally used). The algorithm also calculates the “pause rate” (number of pauses per second). Thus the criteria for pause detection were 1) maximum of 2 spikes, 2) surprise equal or higher than 3, and 3) duration of the interval no longer than 1 s. The same criteria for pause detection were used in the off, on, and on-with-dyskinesias states. Overall, this analysis results in the detection of pauses according to a significant surprise in the Poisson distribution and calculates the following parameters in each motor state: 1) duration of pauses, 2) number of pauses, and 3) pause rate. These results were examined for final acceptance of pausing in the spike train if a minimum number of 1 pause/60 s was detected (in most units: spike train ∼180 s, number of pauses ≥3, pause rate ≥0.17/10 s). The selection of a low limit was intended 1) to include different levels of pausing development and 2) to standardize the acceptance limit for all motor states because frequent pausing in the off state could decrease substantially in the on state. Similarly to burst analysis, postprocessing of pause data included the adjustment of pause parameters by the firing frequency in each motor state (i.e., adjusted pause rate, APR; adjusted pause duration, APD). To construct spectrograms using the time-stamped variable, the rate histogram was calculated with a bin width of 1 s and copied into the signal array to calculate discrete fast Fourier transform and the power spectrum (NeuroExplorer).
Both bursty and nonbursty MSNs were subgrouped for further analyses according to 1) location (caudate or putamen), 2) response to levodopa (increased or decreased firing rate), and 3) firing rate changes during the on-with-dyskinesias state (unidirectional or bidirectional response). Table 1 shows the percentage of units in each subgroup.
Table 1.
Distribution of bursty and nonbursty MSNs
| Bursty MSNs, % | Nonbursty MSNs, % | |
|---|---|---|
| Caudate | 48 | 49 |
| Putamen | 52 | 51 |
| Unclassified | ||
| Increased rate in on state | 80 | 57 |
| Decreased rate in on state | 18 | 41 |
| Unclassified | 2 | 2 |
| Unidirectional response | 52 | 41 |
| Bidirectional response | 46 | 48 |
| Unclassified | 2 | 11 |
All units (n = 48 bursty, n = 92 nonbursty) were classified as 1) exhibiting increased or decreased rate in the on state and 2) exhibiting unidirectional or bidirectional responses with dyskinesias on the basis of statistically significant changes in firing rate. Bursty medium spiny neurons (MSNs) correspond to neurons exhibiting associated bursts/pauses of firing; nonbursty MSNs correspond to neurons with significant detection of pauses without burst spiking (79 of 92, 86%; only 13 neurons lacked significant pauses).
Statistics.
One-way or two-way ANOVAs for repeated measures were applied to compare activity pattern changes in different motor states (off, on, and on-with-dyskinesias) and between MSN subgroups according to location (caudate/putamen), firing rate changes in response to DA inputs (increase/decrease), and stability of DA responses (unidirectional/bidirectional). Data were analyzed separately in bursty and nonbursty MSNs. Bonferroni's post hoc test was used for group differences if the ANOVA F value indicated significance. The level of significance was set at P < 0.05. Data are means ± SE.
RESULTS
Parkinsonism is associated with burst firing of MSNs with D1-like DA response.
In the parkinsonian state, MSN fired frequently with bursts in resting conditions, as opposed to the association of this pattern with movement execution in the normal animal (Crutcher and DeLong 1984b). The stationary burst firing of MSNs (total MSNs = 140; bursty MSNs = 48) had a prevalence of 34% in the chronic, advanced parkinsonian monkeys studied (between 30% and 43%) and was similarly distributed in putamen and caudate (25 and 23 bursty MSNs, respectively). MSN bursts were typically characterized by short spike trains (7 ± 0.5 spikes; 11 ± 1 ms IB-ISIs), and their frequency averaged 1.2 Hz throughout striatal areas (11.7 ± 1.2 bursts/10 s; Fig. 2, A–C).
Bursty MSNs fired at an average frequency of 23.5 ± 1.7 Hz at the baseline off state, which was slightly slower than the frequency observed in nonbursty MSNs (30.1 ± 1.6 Hz). DA loss in PD is predicted to differentially affect the MSN excitability depending on excitatory and inhibitory D1 and D2 receptors, respectively, leading to a relatively lower frequency in the D1 receptor subpopulation (Albin et al. 1989; DeLong 1990). The lower firing rate found in bursty MSNs compared with nonbursty MSNs is suggestive of a predominant development of burst activity in the D1 receptor subpopulation. Consistent with this notion, the majority of bursty neurons (80%; see Fig. 2D) exhibited frequency increases in the transition from the off to the on state, which is compatible with a D1 receptor-mediated response predominant of direct pathway MSNs. Thus spontaneous MSN burst activity in the chronic parkinsonian state largely predominated in units with a D1 receptor-like response.
MSN burst firing is highly regulated by levodopa.
Burst spiking was suppressed in 54% and markedly reduced in another 20% of MSNs in the transition to the on state produced by levodopa administration (Fig. 3A). Changes in burst rate started soon (∼3 min) before behavioral changes indicating the onset of the on state and continued until a significant reduction or disappearance was reached just before the on state data were saved at ∼23–28 min after levodopa injection. Analysis of burst rates after adjustment by the firing frequency in each motor state demonstrated a net reduction of bursts in the transition to the on state (Fig. 3B). In the small number of units with persistence of bursts during the on state, the spike composition of bursts and the IB-ISIs remained unchanged (Table 2). The robust levodopa regulation of burst activity was similar throughout striatal areas and in both MSN subsets, cells with D1- and D2-compatible responses (Fig. 3C). Notably, as the large majority of bursty units exhibited a D1 receptor-like response to levodopa, while levodopa influx increased their firing rates, it reduced burst spiking, suggesting a differential mechanism for burst regulation.
Fig. 3.
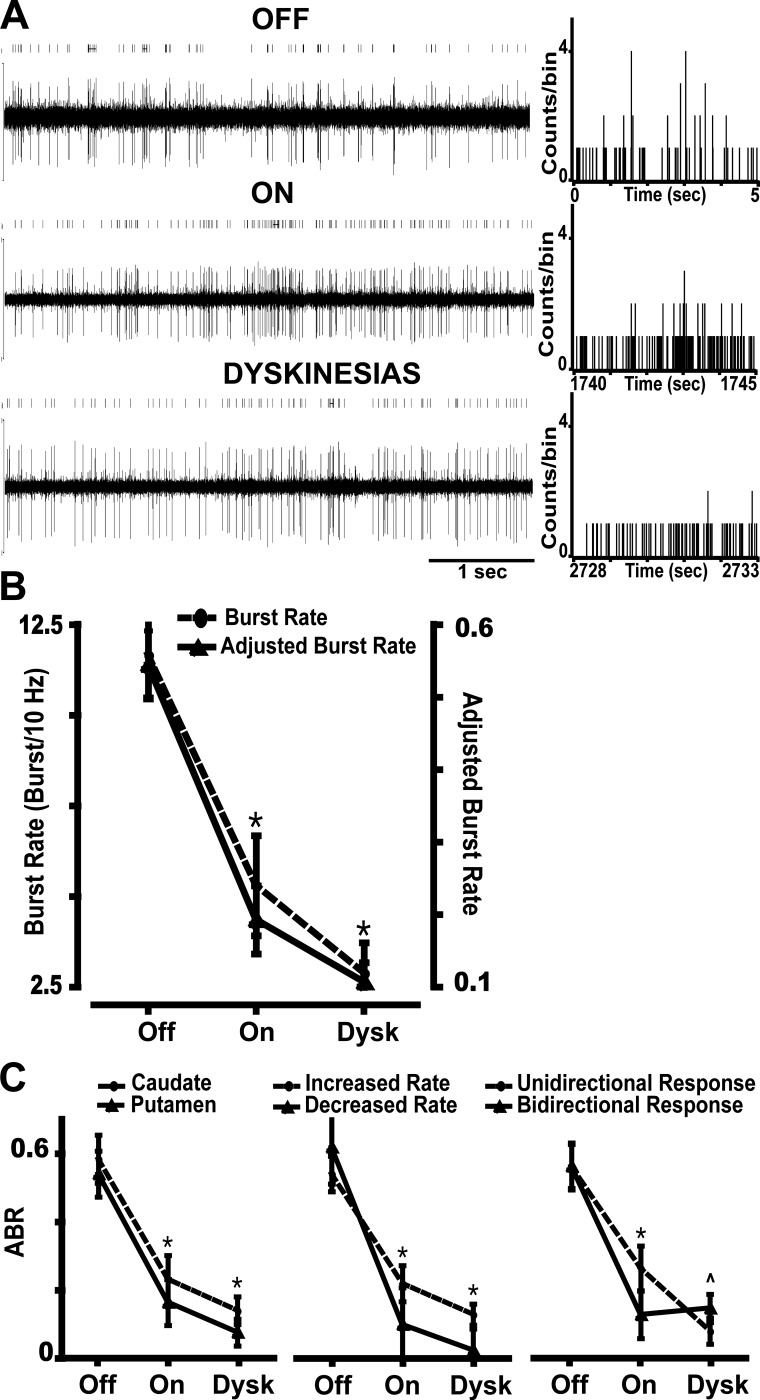
Changes of burst activity in MSNs during the transition to different motor states. A: example of a typical bursting decrease during transitions from the off to the on state and to the on-with-dyskinesias state. Five-second segments of spike trains of the same unit are presented for each motor state. Above each trace, the raster of the unit obtained after spike sorting analysis is depicted (bursts are marked by horizontal lines). At right of each trace, the rate meters show a decrease in the high counts per bin associated with the bursts in the on and on-with-dyskinesias states. B: burst activity changes in the transition to motor states in the total population of MSNs, as measured by burst rate and adjusted burst rate (frequency adjustment; ABR). The graph shows the decrease of bursting from the off to the on and on-with-dyskinesias states. *P < 0.001 vs. off state; ANOVAs for repeated measures followed by Bonferroni tests. C: burst activity changes in the transition to motor states in subpopulations of MSNs. The graphs show the ABR decrease from the off to the on state in both caudate and putamen MSNs (left), both MSN subsets as distinguished by the increase or decrease of activity in response to dopamine stimulation (middle), and both MSNs with unidirectional or bidirectional responses to levodopa as shown by firing frequency changes from off to on and on-with-dyskinesias states (right). During dyskinesias, bursting had a tendency to increase in bidirectional MSNs. Data points in all graphs are means ± SE. *P < 0.001 vs. off state; factorial ANOVAs for repeated measures followed by Bonferroni tests. P̂ = 0.06 for interaction. Dysk, on-with-dyskinesias state.
Table 2.
Characteristics of bursts in different motor states
| Off | On | Dyskinesias | |
|---|---|---|---|
| Number of spikes | 7.2 ± 0.7 | 5.9 ± 0.3 | 6.9 ± 1.4 |
| Intraburst ISI, ms | 12.1 ± 2.4 | 8.8 ± 2.4 | 9.2 ± 2.0 |
| Burst rate, bursts/10 s | 11.7 ± 1.2 | 5.3 ± 1.4* | 2.3 ± 0.8* |
| ABR, Hz | 0.5 ± 0.05 | 0.2 ± 0.05* | 0.1 ± 0.03* |
Data are means ± SE in the off, on, and on-with-dyskinesias states.
ISI, interspike interval; ABR, adjusted burst rate (adjusted by firing frequency).
P < 0.001 vs. off state.
Regulation of MSN burst firing correlates with reversal of parkinsonism.
The reduction of burst activity in the on state was consistent with clear improvement of parkinsonism, but levodopa responses in these advanced parkinsonian animals are also associated with the release of involuntary movements. In fact, the lowest burst rate after the onset of the on state was found during the dyskinesias (data from the on-with-dyskinesias state were saved at 45–60 min after levodopa injection for correlation with peak-dose dyskinesias; Fig. 3B). To determine the relationship between burst regulation and dyskinesias, burst changes were compared between MSNs with unidirectional and bidirectional changes of firing rates in response to levodopa, because the latter are associated with the appearance of dyskinesias (rate changes from the off to the on state are inverted during the on-with-dyskinesias state) (Liang et al. 2008). Although burst rate tended to slightly increase during dyskinesias in the MSN group with bidirectional responses (Fig. 3C), this change was not significant (burst rate difference across motor states at P < 0.001 was not affected by interaction from subgroups of unidirectional/bidirectional responses, P = 0.096; 2-way ANOVA for repeated measures), indicating that burst regulation is not particularly involved in the mechanisms of dyskinesia. Overall, levodopa regulation of MSN burst spiking is associated with the reversal of parkinsonian motor symptoms. Notably, the transition to the on state was followed by burst suppression but not completely normalized activity in bursty MSNs, which remained firing at significantly higher frequency than in the normal animal (Aosaki et al. 1994; Crutcher and DeLong 1984b; Gubellini et al. 2002; Kimura 1992).
Levodopa differentially regulates activity patterns in MSN subpopulations.
In the parkinsonian state, the large majority of MSNs (89%) fired with pauses at the significance level of detection for the spike train. Pausing was found to be equally distributed in caudate and putamen (P > 0.05) and in bursty (45/48) and nonbursty MSNs (79/92). However, pauses were longer (381 ± 38 vs. 268 ± 16 ms, P < 0.01) and more abundant (APR: 16.7 ± 1.6 vs. 3.5 ± 0.3 Hz, P < 0.001) in bursty than nonbursty neurons (see examples in Fig. 4, A and B). Because burst spiking confers an inherent pattern of ISI distribution (Hyland et al. 2002; Overton and Clark 1992), pauses in bursty neurons may depend on burst spiking, whereas pausing in nonbursty neurons may likely relate to different mechanisms. Conceivably, levodopa influx in the transition to motor states could regulate pauses differently in bursty and nonbursty MSNs (Fig. 4C). Thus pauses were analyzed separately in these MSN groups.
Fig. 4.
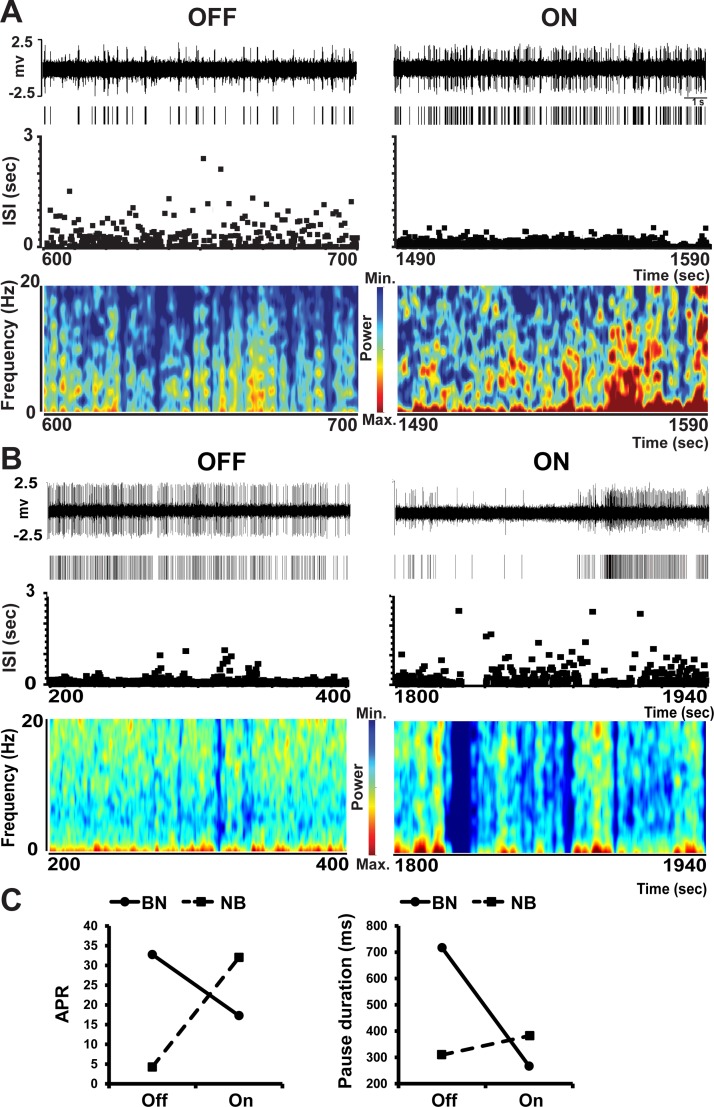
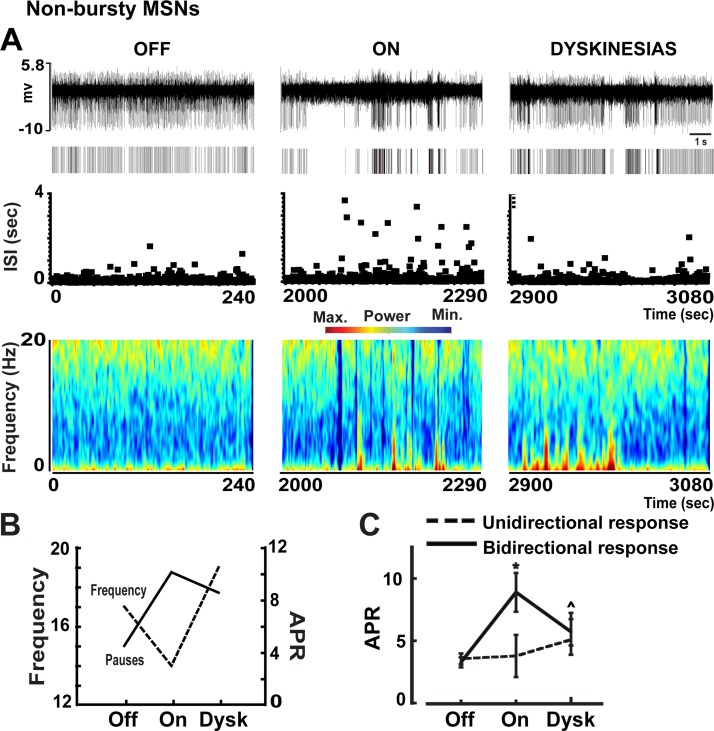
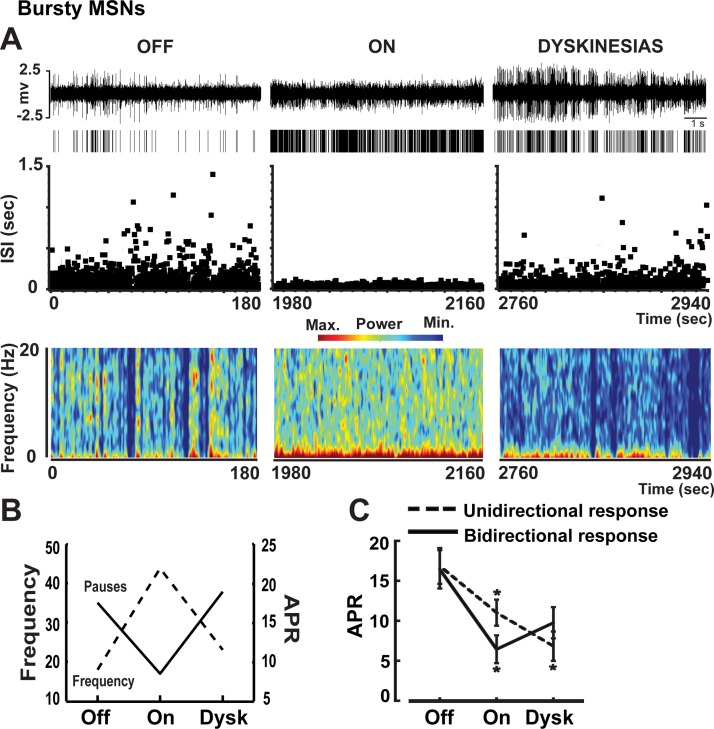
Examples of firing pauses in bursty (A) and nonbursty MSNs (B) in the off and on motor states. In A and B, traces, rasters, ISI distributions over time, and spectrograms are shown from each motor state. In the bursty MSN, the firing frequency increased (see the raster) but pausing decreased (see ISI distributions and spectrograms) in the on state induced by levodopa administration. In contrast, in nonbursty MSN, the firing frequency decreased but pausing increased in the on state. In both neurons, the number and duration of pauses changed in parallel in the on motor state. In C, adjusted pause rate (APR, left) and pause duration (right) are compared in the bursty and nonbursty MSNs presented in A and B. Pause rate was adjusted by the firing rate in both motor states to eliminate the direct influence of frequency changes on pausing. The present examples highlight differences between busty and nonbursty MSNs. BN, bursty MSN; NB, nonbursty MSN.
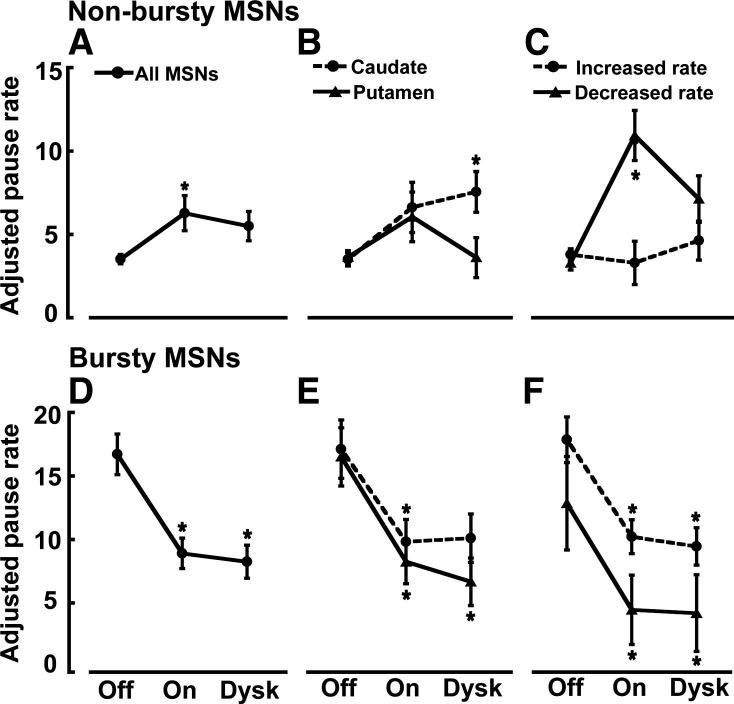
In nonbursty MSNs, firing pauses were significantly increased with the transition to the on state, and these increases were indistinctive in caudate and putamen areas (Fig. 5, A and B). However, pausing (as measured after adjustment by frequency changes, APR) increased mostly in units with reduced firing rates in the on state (D2 receptor-like responders) and remained unchanged in units with increased firing rates (D1 receptor-like responders), which also indicates that pause changes were independent of frequency changes (Fig. 5C). Contrarily, in bursty MSNs, firing pauses were globally reduced with the transition to the on state (Fig. 5D,) and the reductions were independent of the neuron location or DA receptor-like response (frequency increase/decrease, Fig. 5, E and F). This global APR decrease in both subgroups of MSN responders, those with frequency increases and those with decreases, also indicates that in bursty MSNs, APR changes are not secondary to frequency changes. DA may thus exert different regulatory mechanisms in units with and without burst firing development following DA denervation. Whereas levodopa influx reduced the firing pauses consistently across bursty MSNs (mostly D1 receptor-like responders), it increased them in nonbursty MSNs because of specific effects on units with firing rate decrease (D2 receptor-like responders). These findings indicate that activity patterns may be differentially regulated by DA in MSN subpopulations. In particular, the pause increase in MSNs with D2 receptor-like response led to a closer approximation of the normal activity (irregular and lower firing rates in this MSN subset) with the reversal of parkinsonian motor symptoms.
Fig. 5.
Changes of APR in nonbursty and bursty MSNs in the transition to different motor states. In nonbursty MSNs, APR increased in the on state (A), and this change was similar in caudate and putamen (B), but the analysis in MSN subsets as distinguished by the activity increase or decrease in response to dopamine stimulation (presumably D1 and D2 responders, respectively) (C) shows that pausing increased only in the D2 responders. In the transition to the on-with-dyskinesias state, the increased APR in nonbursty MSNs had a tendency to reverse back to the pausing level of the off state, particularly in the putamen and the D2 receptor-like MSNs (B and C). In bursty MSNs, APR decreased in the on state (D), and this change was similar in caudate and putamen (E) and in both MSN subpopulations with increased and decreased firing rate in response to dopamine (D1 and D2 receptor-like responders, respectively) (F). In the transition to the on-with-dyskinesias state, the decreased APR in bursty MSNs remained unchanged in all MSN subgroups (E and F). Data points in all graphs are means ± SE. *P < 0.05 vs. off state; factorial ANOVAs for repeated measures followed by Bonferroni tests.
The increased pause rate in the on state in nonbursty MSNs remained at similar levels during dyskinesias in all subgroups (Fig. 5, A–C). Although APR decrease was found in the putamen (motor regions) and D2 receptor-like responders during dyskinesias, these differences did not attain significance. However, the analysis of levodopa responses (unidirectional/bidirectional) showed that 1) the initial pause increase in the on state predominated in MSNs with bidirectional changes of firing rates, and 2) during dyskinesias there was a clear trend for reversal of the previous APR increase (P = 0.06; Fig. 6). The coherence between pause rate changes and the frequency changes specifically associated with dyskinesias suggests that pause regulation may participate in the mechanisms of dyskinesias.
Fig. 6.
Examples of firing pauses in a nonbursty MSN in the transition from the off to the on and the on-with-dyskinesias states. In A, traces, rasters, ISI distributions over time, and spectrograms are shown from each motor state. In B, the mean firing frequency changes are plotted with the APR changes of the MSN presented in A for comparison during the transition to motor states. As frequency decreases in the on state (MSN with D2 receptor-like response), pausing increases, but this is reversed during dyskinesias. In C, APR changes in the transition to motor states in all nonbursty MSNs are shown, analyzing subgroups of units with stable or inverted changes of firing frequency during the on state (unidirectional and bidirectional response, respectively). The increased APR from the off to the on state in nonbursty MSNs with bidirectional response had a clear trend to reverse during the on-with-dyskinesias state. Data points in all graphs are means ± SE. *P < 0.05 vs. off state; P̂ = 0.06 vs. on state; factorial ANOVAs for repeated measures followed by Bonferroni tests.
In bursty neurons, the reduced APR remained without significant changes during dyskinesias in all subgroups (Fig. 5, D–F), including the bidirectional response subgroup (Fig. 7). Nevertheless, firing pauses in bursty neurons may be part of the particular burst pattern, because the comparison of APR and ABR showed parallel changes throughout, i.e., marked reductions by levodopa influx in the on state and correlated responses in subgroup analyses (Figs. 3C and 5, D–F). This suggests that common mechanisms govern changes in the associated bursting/pausing of the MSN.
Fig. 7.
Examples of firing pauses in a bursty MSN in the transition from the off to the on and the on-with-dyskinesias states. In A, traces, rasters, ISI distributions over time, and spectrograms are shown from each motor state. In B, the mean firing frequency changes are plotted with the APR changes of the MSN presented in A for comparison during the transition to motor states. As frequency increases in the on state (MSN with D1 receptor-like response), pausing decreases, but this is reversed during dyskinesias. In C, APR changes in the transition to motor states in all bursty MSNs are shown, analyzing subgroups of units with stable or inverted changes of firing frequency during the on state (unidirectional and bidirectional response, respectively). The decreased APR from off to on states in both bursty MSN subgroups with unidirectional and bidirectional response remained unchanged during the on-with-dyskinesias state. Data points in all graphs are means ± SE. *P < 0.05 vs. off state; factorial ANOVAs for repeated measures followed by Bonferroni tests.
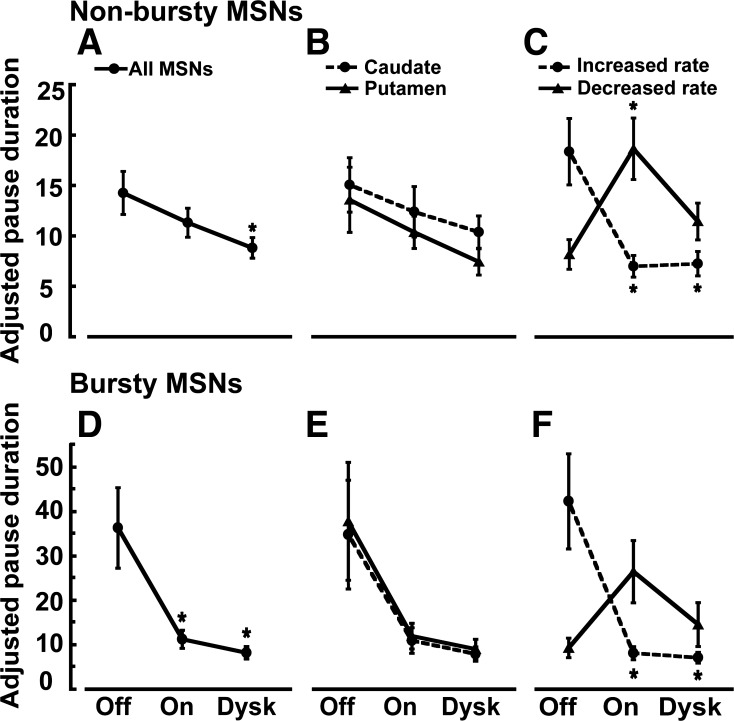
The duration of pauses significantly decreased in the on state in nonbursty and bursty MSNs, but these changes were consistent with the frequency responses in the on state, indicating the frequency dependence of this parameter (Fig. 8). Thus APD shortening in nonbursty or bursty MSNs is consistent with the preponderance of units with activity increases during the on state (62% and 82% in nonbursty and bursty MSNs with detected pauses, respectively).
Fig. 8.
Changes of adjusted pause duration (APD) in nonbursty and bursty MSNs in the transition to different motor states. In nonbursty MSNs, APD decreased in the on state (A), and this change was similar in caudate and putamen (B). Analysis in MSN subpopulations as distinguished by the activity increase or decrease in response to dopamine stimulation (D1 and D2 receptor-like responders, respectively) (C) shows that APD decreased in D1 receptor-like responders and increased in D2 responders in line with the effects of firing frequency changes. In the transition to the on-with-dyskinesias state, there are no significant changes of APD in any subgroup of nonbursty MSNs with respect to the on state. In bursty MSNs, APD decreased in the on state (D), and this change was similar in caudate and putamen (E). In MSN subpopulations with rate increase or decrease in response to dopamine stimulation (F), APD decreased in D1 receptor-like responders and increased in D2 receptor-like responders, again following the firing frequency changes. Also, in the transition to the on-with-dyskinesias state, there are no significant changes of APD in any subgroup of bursty MSNs with respect to the on state. Data points in all graphs are means ± SE. *P < 0.05 vs. off state; factorial ANOVAs for repeated measures followed by Bonferroni tests.
DISCUSSION
Technical considerations that are important to interpret the present data relate to the animal model and cell identification. The used macaques had an advanced, severe parkinsonism and sustained exposure to intermittent dopaminergic drugs to parallel the clinical late stage of PD (Potts et al. 2014). In this stage, changes in the MSN activity may be attributed to the interplay of DA depletion and chronic adaptive changes, including the effects of nonphysiological dopaminergic therapy (Chase et al. 1998; Obeso et al. 2000). Regarding the identification of cells, a set of strict criteria was used to classify the striatal neuronal activity and distinguish MSNs from interneurons (Aosaki et al. 1994; Barnes et al. 2005; Kimura 1992; Raz et al. 1996). Further classification of MSNs as D1 receptor/direct or D2 receptor/indirect pathway projecting units was not possible with the present data, obtained in large primates kept alert and relatively mobile during prolonged extracellular recordings to assess the pharmacological responses to drug administration in single units. Levodopa-induced increase or decrease of firing rate was considered suggestive of DA D1 or D2 receptor activation, respectively (Cepeda et al. 2001; Hernandez-Lopez et al. 2000; Kitai and Surmeier 1993; West and Grace 2002). Thus all data interpretation in relation to MSN subpopulations is based on the assumption that the recognized responses to dopaminergic stimulation are conserved in the present experimental conditions. Also, the predominant segregation of DA receptors in MSN subpopulations is widely accepted, leaving a small number of MSNs with receptor coexpression whose function and distribution remain uncertain (Gerfen and Surmeier 2011; Perreault et al. 2011). Clearly, the observed activity patterns in MSNs grouped by levodopa responses need to be reexamined using optogenetics or other methods for cell type identification in future primate studies.
The prominent burst activity of MSNs found at the baseline parkinsonian state at rest clearly contrasts with their phasic, single-spike firing at very low frequency (0–2 Hz) observed in normal animals (Adler et al. 2012, 2013; Barnes et al. 2005; Crutcher and DeLong 1984a; Kimura 1992). As described in other basal ganglia regions (Filion and Tremblay 1991; Heimer et al. 2002; Raz et al. 2000), this bursting pattern may be caused by DA loss. In fact, burst firing was nearly suppressed and replaced by more tonic, regular activity following the influx of levodopa with concomitant reversal of parkinsonian symptoms in the on state. Therefore, the present data show that the MSN burst spiking is strictly correlated with parkinsonian motor deficit. On the basis of the high proportion of bursting MSNs with DA D1 receptor-like response (80%), it could be reasoned that residual levels of DA in the parkinsonian state induced abnormal positive modulation at the D1 receptor, leading to burst spiking. However, there is profound DA depletion in this primate model of advanced PD, rather suggesting that other mechanisms contribute to burst firing (Blesa et al. 2010; Perez-Otano et al. 1994). In the parkinsonian state, the striatal glutamate signaling can be dysregulated, causing the rapid MSN spiking in bursts and the typical afterhyperpolarization (AHP) that separates bursts, as shown in other neurons (Wilson and Goldberg 2006). However, the strikingly high percentage of presumably D1 responders to levodopa among bursting MSNs supports the primary role of a D1 receptor mechanism, possibly acting on the slow AHP that controls the interburst pausing. In cholinergic interneurons (TANs), a slow AHP terminates bursts, generating long periods of hyperpolarization. Interestingly, this prolonged hyperpolarization can be evoked by subthreshold voltages if sufficient depolarizing inputs are above or below threshold (Bennett et al. 2000; Reynolds et al. 2004). Differently than TANs, MSNs lack autonomous activity, and likely, their bursting is associated with depolarizing inputs from increased basal glutamate activity (Calabresi et al. 2000), and the AHP following the bursts of firing causes the MSN interburst silences in such basal conditions. In the external pallidum, the increase of burst firing after dopamine depletion is thought to result from timely changes in membrane hyperpolarization and depolarization induced by glutamatergic and GABAergic inputs (Hashimoto and Kita 2006; Kita and Kita 2011). The MSN activity is also strongly influenced by cholinergic and GABAergic interneurons. Of note, the MSN “up” state silencing is controlled by slowly inactivating K channels of the KCNQ type, which are under the control of the muscarinic M1 receptor signaling (Shen et al. 2005). Likely, DA loss interacts with several MSN signaling mechanisms, generating burst spiking in the off state. The firing rate of presumed D1 receptor-bearing MSNs increased in the on state, but firing was more regular, nonbursty. Thus D1 receptor-mediated shifts from “down” and “up” states of membrane potentials (Kitano et al. 2002; Plenz and Kitai 1998; Wilson and Kawaguchi 1996) may be critical to reduce AHP and stabilize MSN firing. Nevertheless, the complex interplay between D1 receptor mechanisms, glutamatergic drive, and various interneuronal signaling in the generation of bursts of firing in the MSN after DA depletion remains to be examined. The present data suggest that burst spiking after DA loss may be particularly developed in the D1/direct pathway MSN subpopulation and may play a significant role in the expression of parkinsonian motor behaviors.
Burst firing of MSNs was also characteristically accompanied by spike pausing, and in parallel to bursts, pausing was reduced with the levodopa influx in the on state. In all bursty MSNs, i.e., subgroups of neurons with increases or decreases of firing rates in the on state, the pause rate was similarly reduced. This indicates that the regulation of pauses was independent of frequency changes. Instead, pausing and bursting changes were parallel in the transition to the on state, suggesting a relationship with AHP, which could have a variable time span and might often be detected as pausing in the spike train (Reynolds et al. 2004). The slow AHP in cholinergic interneurons is caused by an apamine-insensitive calcium-dependent potassium current (Wilson and Goldberg 2006). Also, the development of coupled bursting-pausing predominantly in MSNs with D1 receptor-like response suggests that loss of D1 receptor modulation may underlie this activity pattern in the parkinsonian state, and restoration of this mechanism in the on state may be responsible for the observed regularized spiking with low-variability ISIs that eliminated the associated bursting and pausing.
Firing pauses in nonbursty MSNs in the parkinsonian state increased with levodopa influx and the reversal of parkinsonian motor symptoms, but this effect occurred only in the D2 receptor-like responders to levodopa. Again, separate analysis in D1 and D2 receptor-like responders to levodopa showed unchanged and increased pause rates, respectively, arguing against a strict relationship between APR and the firing frequency changes in the on state. Pausing changes could not be explained by exaggerated responses to DA due to supersensitive denervation, since this occurs primarily as D1 receptor hypersensitivity in PD models (Gerfen et al. 2002). Particularly, levodopa influx tended to restore the “normal” activity pattern only in MSNs with D2 receptor-like response (MSNs with D1 receptor-like response fired more regularly but at a higher frequency in the on state). Very low activity with prolonged silence intervals is the signature of spontaneous MSN firing in the normal animal, which is more similar to the observed pattern in presumed D2 responders following levodopa influx in the on state. One plausible explanation for these DA differential effects on MSN subpopulations may be that supersensitive D1 receptor-bearing MSNs with increased responses send feedback inhibition through collateral terminals to neighbor D2 receptor-bearing MSNs (Guzman et al. 2003; Tepper and Plenz 2006). However, the collateral inhibition predominantly couples D1 or D2 MSNs and appears to be reduced after DA loss (Gerfen and Surmeier 2011; Taverna et al. 2008). The strong influence from interneurons may also play a role (Pisani et al. 2007; Zhou et al. 2002), but the effects of DA stimulation on striatal cholinergic and GABAergic interneurons selectively regulating MSN subpopulations are unclear. Overall, the present data suggest that, in advanced parkinsonism there are significant changes of firing patterns characterized by either burst development or loss of pausing in MSN subsets, which may contribute to symptomatology. In this context, DA stimulation results in asymmetrical effects on MSN subpopulations and inefficient restoration of normal MSN activity patterns.
The occurrence of dyskinesias was associated with a tendency to reverse the pause rate changes of the on state in the subset of nonbursty MSNs with inversion of the firing frequency changes during the on state (bidirectional responses that are typically associated with dyskinesias as previously described). Thus the concomitant reversal of pausing changes in those neurons could be interpreted as pausing participating in the mechanisms of dyskinesias. The release of involuntary movements has been attributed to imbalance of the striatal outputs, as occurring between the direct and indirect pathways (Calabresi et al. 2010; Jenner 2008; Papa 2008) or among single MSN discharges (Boldry et al. 1995; Liang et al. 2008). Pauses increased with levodopa influx in the on state, suggesting that pausing may be a constitutive MSN activity pattern in normal motor behavior, but its reversal to the reduced pausing rate of the off state in some MSNs (precisely the same neurons that do not maintain the DA-induced frequency changes) could create an imbalance of discharges and contribute to the expression of dyskinesias. By contrast, the MSN burst firing that significantly decreased in the on state had no further changes during the dyskinesias, although the persistence of abnormally high firing rates in MSNs with a D1 receptor-like response may also play a role in dyskinesias. Altogether, the data underscore the inefficient DA regulation of altered MSN activity patterns in the mechanisms of dyskinesias.
In summary, the data show marked MSN dysfunction in parkinsonism involving firing pattern changes to either burst/pause spiking in some units or more tonic (nonbursty) firing with pauses in other units and indicate that these dichotomous changes may be related to subpopulations of MSNs. Dopaminergic inputs that reverse parkinsonian symptoms are associated with burst/pause disappearance and irregularity/pausing increase. These effects could be construed as dopamine-induced modulation of the MSN activity toward normalization. However, the data also reveal that dopamine effects may not have the same impact on MSN subpopulations. Whereas burst elimination resulted in higher firing frequency, pause increase in nonbursty MSNs led to a better approximation of the normal pattern (irregular, low phasic activity), although this regulation often failed during dyskinesias. Thus, in the context of parkinsonian striatal dysfunction, dopamine modulation does not operate efficiently across MSNs, likely creating differential (“imbalanced”) striatal outputs.
GRANTS
This work was supported by National Institutes of Health grants NS045962 and NS073994 (to S. M. Papa) and RR000165 and OD011132 (to Yerkes National Primate Research Center). S. M. Papa has received research support from the National Institutes of Health, the Michael J. Fox Foundation, Pfizer, Inc., EnVivo Pharmaceuticals, Inc., FORUM Pharmaceuticals, Inc., GeneGrafts, Ltd., and Key Neurosciences. S. M. Papa is a consultant for Teva Neuroscience. X. Cao is supported by the National Natural Science Foundation of China grants NSFC 81171193 and 370700881.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S., L.L., Y.K., X.C., and S.M.P. conception and design of research; A.S., L.L., and S.M.P. analyzed data; A.S., L.L., and S.M.P. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S., L.L., Y.K., X.C., and S.M.P. approved final version of manuscript; L.L. performed experiments; S.M.P. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Jessica S. Whithear and Bhagya Laxmi Dyavar Shetty for valuable assistance in the care and maintenance of parkinsonian primates.
REFERENCES
- Adler A, Finkes I, Katabi S, Prut Y, Bergman H. Encoding by synchronization in the primate striatum. J Neurosci 33: 4854–4866, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A, Katabi S, Finkes I, Israel Z, Prut Y, Bergman H. Temporal convergence of dynamic cell assemblies in the striato-pallidal network. J Neurosci 32: 2473–2484, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci 14: 3969–3984, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437: 1158–1161, 2005. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci 20: 8493–8503, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa J, Juri C, Collantes M, Penuelas I, Prieto E, Iglesias E, Marti-Climent J, Arbizu J, Zubieta JL, Rodriguez-Oroz MC, Garcia-Garcia D, Richter JA, Cavada C, Obeso JA. Progression of dopaminergic depletion in a model of MPTP-induced Parkinsonism in non-human primates. An 18F-DOPA and 11C-DTBZ PET study. Neurobiol Dis 38: 456–463, 2010. [DOI] [PubMed] [Google Scholar]
- Boldry RC, Papa SM, Kask AM, Chase TN. MK-801 reverses effects of chronic levodopa on D1 and D2 dopamine agonist-induced rotational behavior. Brain Res 692: 259–264, 1995. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross CE. Dopamine agonist-induced dyskinesias are correlated to both firing pattern and frequency alterations of pallidal neurones in the MPTP-treated monkey. Brain 124: 546–557, 2001. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Marfia GA, Pisani A, Sancesario G, Bernardi G. Synaptic transmission in the striatum: from plasticity to neurodegeneration. Prog Neurobiol 61: 231–265, 2000. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Filippo MD, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson's disease: filling the bench-to-bedside gap. Lancet Neurol 9: 1106–1117, 2010. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17: 1022–1030, 2014. [DOI] [PubMed] [Google Scholar]
- Cao X, Liang L, Hadcock JR, Iredale PA, Griffith DA, Menniti FS, Factor S, Greenamyre JT, Papa SM. Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther 323: 318–326, 2007. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: contribution of calcium conductances. J Neurophysiol 79: 82–94, 1998. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor-deficient mice. J Neurophysiol 85: 659–670, 2001. [DOI] [PubMed] [Google Scholar]
- Chase TN, Oh JD, Blanchet PJ. Neostriatal mechanisms in Parkinson's disease. Neurology 51: S30–S35, 1998. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, DeLong MR. Single cell studies of the primate putamen. I. Functional organization. Exp Brain Res 53: 233–243, 1984a. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, DeLong MR. Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res 53: 244–258, 1984b. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9: 251–259, 2006. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285, 1990. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol 53: 530–543, 1985. [DOI] [PubMed] [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical properties of pauses of the high-frequency discharge neurons in the external segment of the globus pallidus. J Neurosci 27: 2525–2538, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res 547: 142–151, 1991. [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci 15: 133–139, 1992. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci 22: 5042–5054, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34: 441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agro A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci 22: 6900–6907, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Hernandez A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci 23: 8931–8940, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci 30: 357–364, 2007. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kita H. Slow oscillatory activity of rat globus pallidus neurons in vitro. Eur J Neurosci 23: 443–453, 2006. [DOI] [PubMed] [Google Scholar]
- Heimer G, Bar-Gad I, Goldberg JA, Bergman H. Dopamine replacement therapy reverses abnormal synchronization of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of parkinsonism. J Neurosci 22: 7850–7855, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLCβ1-IP3-calcineurin-signaling cascade. J Neurosci 20: 8987–8995, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 114: 475–492, 2002. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci 18: 4732–4743, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P. Molecular mechanisms of l-DOPA-induced dyskinesia. Nat Rev Neurosci 9: 665–677, 2008. [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods 68: 211–223, 1996. [DOI] [PubMed] [Google Scholar]
- Kimura M. Behavioral modulation of sensory responses of primate putamen neurons. Brain Res 578: 204–214, 1992. [DOI] [PubMed] [Google Scholar]
- Kita H, Kita T. Role of striatum in the pause and burst generation in the globus pallidus of 6-OHDA-treated rats. Front Syst Neurosci 5: 42, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai ST, Surmeier DJ. Cholinergic and dopaminergic modulation of potassium conductances in neostriatal neurons. Adv Neurol 60: 40–52, 1993. [PubMed] [Google Scholar]
- Kitano K, Cateau H, Kaneda K, Nambu A, Takada M, Fukai T. Two-state membrane potential transitions of striatal spiny neurons as evidenced by numerical simulations and electrophysiological recordings in awake monkeys. J Neurosci 22: RC230, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53: 926–939, 1985. [DOI] [PubMed] [Google Scholar]
- Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci 28: 7537–7547, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci 28: 4795–4806, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson's disease. Trends Neurosci 23: S2–S7, 2000. [DOI] [PubMed] [Google Scholar]
- Overton P, Clark D. Iontophoretically administered drugs acting at the N-methyl-d-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse 10: 131–140, 1992. [DOI] [PubMed] [Google Scholar]
- Papa SM. The cannabinoid system in Parkinson's disease: multiple targets to motor effects. Exp Neurol 211: 334–338, 2008. [DOI] [PubMed] [Google Scholar]
- Papa SM, Auberson YP, Greenamyre JT. Prolongation of levodopa responses by glycineB antagonists in parkinsonian primates. Ann Neurol 56: 723–727, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa SM, Chase TN. Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol 39: 574–578, 1996. [DOI] [PubMed] [Google Scholar]
- Papa SM, Desimone R, Fiorani M, Oldfield EH. Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Ann Neurol 46: 732–738, 1999. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Oset C, Luquin M, Herrero M, Obeso J, Del Rio J. MPTP-induced parkinsonism in primates: pattern of striatal dopamine loss following acute and chronic administration. Neurosci Lett 175: 121–125, 1994. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O'Dowd BF, George SR. The dopamine d1-d2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanat 5: 31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci 30: 545–553, 2007. [DOI] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. Up and down states in striatal medium spiny neurons simultaneously recorded with spontaneous activity in fast-spiking interneurons studied in cortex-striatum-substantia nigra organotypic cultures. J Neurosci 18: 266–283, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts LF, Wu H, Singh A, Marcilla I, Luquin MR, Papa SM. Modeling Parkinson's disease in monkeys for translational studies, a critical analysis. Exp Neurol 256: 133–143, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol 76: 2083–2088, 1996. [DOI] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci 20: 8559–8571, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. J Neurosci 24: 9870–9877, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci 25: 7449–7458, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci 10: 1458–1466, 2007. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16: 6579–6591, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J Neurosci 28: 5504–5512, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koós T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci 27: 662–669, 2004. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Plenz D. Microcircuits in the striatum: striatal cell types and their interaction. In: Microcircuits: The Interface Between Neurons and Global Brain Function (Dahlem Workshop Reports) Cambridge, MA: MIT Press, 2006, p. 127–135. [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 50: 443–452, 2006. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci 22: 294–304, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Goldberg JA. Origin of the slow afterhyperpolarization and slow rhythmic bursting in striatal cholinergic interneurons. J Neurophysiol 95: 196–204, 2006. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16: 2397–2410, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol 53: 590–605, 2002. [DOI] [PubMed] [Google Scholar]