Abstract
Transcranial direct current stimulation (tDCS) is emerging as a versatile tool to affect brain function. While the acute neurophysiological effects of stimulation are well understood, little is know about the long-term effects. One hypothesis is that stimulation modulates ongoing neural activity, which then translates into lasting effects via physiological plasticity. Here we used carbachol-induced gamma oscillations in hippocampal rat slices to establish whether prolonged constant current stimulation has a lasting effect on endogenous neural activity. During 10 min of stimulation, the power and frequency of gamma oscillations, as well as multiunit activity, were modulated in a polarity specific manner. Remarkably, the effects on power and multiunit activity persisted for more than 10 min after stimulation terminated. Using a computational model we propose that altered synaptic efficacy in excitatory and inhibitory pathways could be the source of these lasting effects. Future experimental studies using this novel in vitro preparation may be able to confirm or refute the proposed hypothesis.
Keywords: transcranial electrical stimulation, gamma oscillations, tDCS, Izhikevich model, balanced excitation/inhibition
the number of studies on transcranial direct current stimulation (tDCS) has rapidly increased in recent years (Brunoni et al. 2012). Human studies have shown improvements in behavioral and cognitive performances after transcranial stimulation (Foerster et al. 2012; Javadi et al. 2011). Pharmacological interventions in human studies point to possible synaptic changes as well as changes in neuromodulator release as the cause for the aftereffects of the stimulation (Nitsche and Paulus 2000; Nitsche et al. 2003) (for a review, Stagg and Nitsche 2011). A few animal studies have also shown effects that outlast the stimulation period using evoked responses (Márquez-Ruiz et al. 2012; Cambiaghi et al. 2010; Fritsch et al. 2010; Ranieri et al. 2012; Gartside 1968). However, how these results may relate to the effects measured in human studies is not fully understood.
In recent years, brain oscillations, rhythmic neuronal activity reflecting coherent spiking, have become a target for transcranial electrical stimulation (Herrmann et al. 2013). Gamma oscillations in the range of 25–100 Hz, for example, are ubiquitous in the brain (Buzsáki and Draguhn 2004), play a role in neuronal coding (Fries et al. 2007; Wang 2010), and have been associated with attention and memory in humans (Jensen et al. 2007). Gamma rhythms in the 30-Hz range synchronize hippocampal activity during memory replay (Carr et al. 2012), and reduced gamma power leads to impaired spatial working memory and exploratory behavior (Fuchs et al. 2007). Therefore, modulation of gamma rhythms with weak currents could potentially affect brain function. While many human studies used transcranial alternating current stimulation (tACS), only few considered how DC stimulation could affect brain oscillations (Antal et al. 2004; Polanía et al. 2011).
The acute effects of DC stimulation on single neurons and networks of neurons have been extensively characterized in animals (Bikson et al. 2004; Fröhlich and McCormick 2010; Reato et al. 2010). However, no animal studies have shown the lasting effects of DC stimulation on brain oscillations. Here, we present an in vitro model of gamma oscillations and DC stimulation that shows lasting effects of stimulation.
Oscillations in the low-gamma-frequency range can be reliably induced in hippocampal slices using carbachol, a cholinergic agonist (Fisahn et al. 1998). We have shown previously that weak electric fields can modulate the magnitude of these in vitro oscillations acutely in an amplitude- and frequency-specific manner (Reato et al. 2010). Here we use the same slice model and report that modulation of gamma oscillations with weak constant electric fields applied for a prolonged time (10 min) outlasts the period of stimulation. Based on simulations with a previously validated computational model we propose that the aftereffects of stimulation are the result of balanced changes in excitatory and inhibitory synaptic strength.
MATERIALS AND METHODS
Hippocampal slice recordings.
Slice preparation and recordings from male 3- to 5-wk-old Wistar rats (City College of the City University of New York-Institutional Animal Care and Use Committee, approved Protocol 0846) followed the procedures of (Reato et al. 2010). Extracellular recordings were performed using three electrodes per slice placed in the CA3c stratum pyramidale area of the hippocampus at the distance of ∼250 μm. Perfusion with carbachol (20 μM) started 5 min after placement of the glass pipettes, and the recordings lasted for the following 2 h without moving the electrodes. Recordings were aligned in time across slices using the beginning of carbachol perfusion.
Electrical field stimulation.
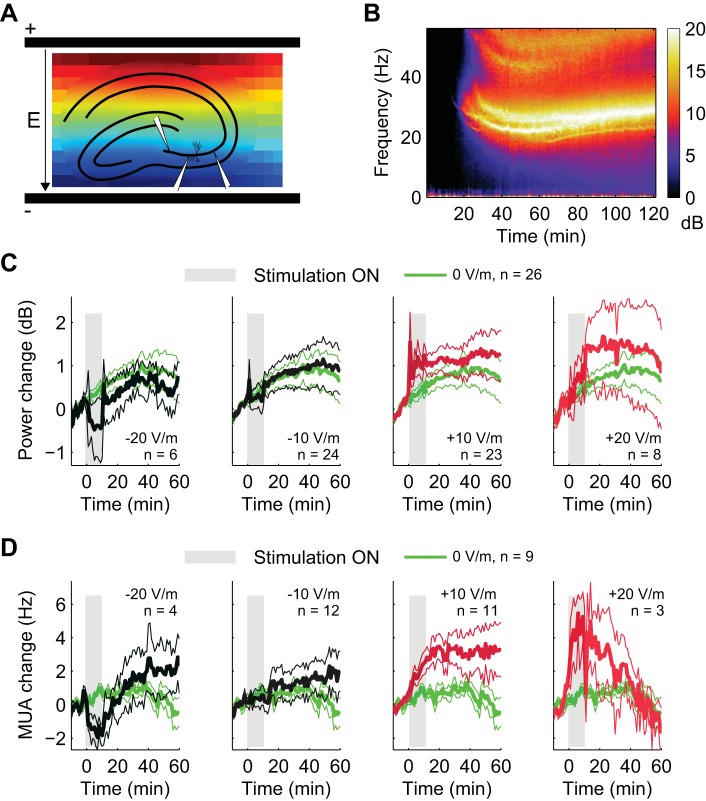
Spatially uniform electric fields were applied to slices with varying amplitudes by passing current between two parallel Ag-AgCl wires placed in the artificial cerebrospinal fluid across the slice (Gluckman et al. 1996; Bikson et al. 2004). All experimental results are reported as a function of this electric field magnitude. Slices were aligned in the chamber such that the induced uniform electric field was parallel to CA3c pyramidal neurons (Fig. 1A). Before every recording, electric fields were calibrated by passing current through the field wires and measuring the corresponding voltages between them (representative voltage measurements in Fig. 1A). Note that a linear voltage indicates a uniform value of the electric field. “Positive” (anodal) field polarity was defined as the positive electrode on the CA1 alveus side, and “negative” (cathodal) field polarity was defined as negative on the CA1 alveus side (Bikson et al. 2004). Weak positive field stimulation is thus typically expected to depolarize CA3 pyramidal cell somata, while weak negative field stimulation should hyperpolarize CA3 somata (Deans et al. 2007). DC stimulation commenced 60 min after the recordings started (55 min after application of carbachol). During this time, the oscillations largely stabilized in power and frequency, although a continued drift in power is often evident (Fig. 1C). Stimulation was applied for 10 min with amplitudes of −20 V/m (n = 6), −10 V/m (n = 24), 0 V/m (control, n = 26), +10 V/m (n = 23), and +20 V/m (n = 8). Each slice was stimulated with a single stimulation intensity.
Fig. 1.
Carbachol-induced gamma oscillations during electrical stimulation. A: extracellular recordings were performed in the CA3c area of rat hippocampal slices. Spatially uniform DC electric fields were applied using AgCl wire electrodes in the bath. Pseudocolors represent the voltage as recorded before a typical session. Linear voltage means constant electric field across the slice. B: average spectrogram of carbachol-induced gamma oscillations for control condition (n = 26 slices). C: average traces of gamma power in 5 different stimulation conditions (−20 V/m, −10 V/m, 0 V/m, +10 V/m, and +20 V/m; means ± SE). Shaded gray rectangle indicates the stimulation period (10 min). Average traces for control condition (green line) are reported in each figure for direct comparison. D: average traces of multiunit activity (MUA) during gamma oscillations in the same 5 stimulation conditions (means ± SE). Shaded gray rectangle indicates the stimulation period (10 min). Average traces for control condition (green line) are reported in each figure for direct comparison.
Power and frequency analysis.
Gamma power and frequency were estimated using multitaper spectral analysis. Power was computed in 1-min segments using the Chronux toolbox (http://chronux.org/; Mitra and Bokil 2008) with a time-bandwidth product of WT = 2 and using three tapers. The frequency range of carbachol-induced activity was then detected semiautomatically for each slice (location of the peak ±2σ but manually reduced to exclude, if present, electric noise contaminating that frequency range), and mean power was calculated for that frequency range. Gamma power was averaged across electrodes provided it was at least 5 dB above noise compared with the first 5 min of recording. To compare across slices, these mean values were normalized by the prestimulation gamma power (50–60 min). Gamma-peak frequency was estimated from the multitaper analysis for each 1-min segment by considering the location of the peak-power in the gamma band.
Multiunit activity detection.
Multiunit activity (MUA) was detected by thresholding the extracellular recordings after high-pass filtering (300 Hz cut-off frequency). The value of the threshold for automatic units detection was set to 7·median (Quiroga et al. 2004), where x was the high-pass filtered extracellular signal during the first 5 min of recording (before carbachol perfusion). A 1-ms dead time for detection was used. Our main results do not depend strongly on the specific threshold for MUA detection. A high threshold for detection, as we used here, was chosen to decrease false positive detections possibly due to electric artifacts [note that in this study we used conventional electrodes for local field potentials (LFP) recordings]. This simple method allowed to easily estimate MUA changes in our different stimulation conditions. Coherence between the candidate units and the extracellular LFPs was estimated using Chronux (http://chronux.org/; Mitra and Bokil 2008). Only electrodes that showed a strong unit-to-field-potential coherence (>0.3) in our frequencies of interest were considered for further analysis. When multiple electrodes detected MUA, the frequency of events was averaged across these electrodes in 1-min temporal windows. To compare across slices, estimated average rates (events per second) before the stimulation (50–60 min) were subtracted from each trace. Note that since the recordings started before the emergence of coherent activity in the slice and the electrodes were not moved throughout the experiment, a good MUA signal (high coherence with the LFP) was not always detected. Therefore, the number of slices for LFP and MUA analysis differs in the results.
Sensitivity of power and MUA to electrical stimulation.
We measured the sensitivity of the network oscillatory power to the applied field. In equations, ΔP = gpE, where gp represents how much power changes (in dB) per volts per meter electric field applied. MUA modulation follows a similar equation, ΔR = grE, where gr indicates how many hertz the estimated rate changes with stimulation intensity. These sensitivities were estimated with a linear fit as a function of the stimulation intensities using all available slices. Nonparametric statistics were obtained by randomizing the stimulation amplitudes and performing the linear fits to this random data. P values were then computed using these shuffled statistics. Statistical tests were performed for the 10 min of stimulation and the subsequent 10 min. This was based on previous literature showing that excitability changes in motor cortex outlast the stimulation period for durations comparable to the stimulation period (5 min in Nitsche and Paulus 2000, and 20 min in Nitsche and Paulus 2001).
Computational model.
Modeling of gamma oscillations induced by carbachol and its response to electric stimulation follow the methods of Reato et al. (2010). Briefly, the voltage behavior of single neurons is captured by Izhikevich's single-compartment neuron model (Izhikevich 2003). Here the network consists of 800 excitatory and 200 inhibitory neurons synaptically connected with all-to-all connections and 40% sparseness. Gamma oscillations in the model are generated by the interplay of increased excitation (simulating the effect of carbachol; Fisahn et al. 2002) and fast inhibitory feedback (Bartos et al. 2007). Weak electrical stimulation was implemented as a low-pass filtered current that polarizes all pyramidal neurons, according to experimental data (Deans et al. 2007). The parameters of the models are set such that a 1 V/m electric field induces a polarization of ∼0.1 mV, consistent with previous studies (Radman et al. 2007; Deans et al. 2007; Bikson et al. 2004; Fröhlich and McCormick 2010).
Here we tested how changes in the strength of synaptic connections affect power, frequency, and firing rate of excitatory neurons during simulated gamma oscillations. The form of synaptic connections in our model is wxx = ŵxx + [0, kxxw̄xx] if the connection is excitatory or wxx = ŵxx + [kxxw̄xx, 0] if inhibitory, and where xx = {ee,ei,ie,ii} indicates the type of connection (excitatory to excitatory, excitatory to inhibitory, inhibitory to excitatory, inhibitory to inhibitory). ŵxx Represents the baseline value of the connection, w̄xx the maximum value of the uniform distribution (from 0 to w̄xx), and kxx is a parameter that has been changed here to simulate changes in synaptic connections. As in Reato et al. 2010, we used here ŵee = ŵei = 0, ŵie = −0.8, ŵii = −0.3, w̄ee = 0.65, w̄ei = 2, w̄ie = −0.9, and w̄ii = −0.8. Throughout the text, the expression “balanced excitation/inhibition” indicates an equal level of excitatory and inhibitory inputs on single neurons during the gamma cycle. This definition is based on previous literature indicating per-neuron balanced excitatory and inhibitory current during gamma oscillations (Atallah and Scanziani 2009) and slow waves (Haider et al. 2006; Shu et al. 2003).
RESULTS
Extracellular recordings were performed with multiple electrodes located in the CA3c region of rat hippocampal slices (n = 87; Fig. 1A). Carbachol was perfused continuously beginning 5 min after the start of recording. Carbachol induced strong gamma oscillations (25–35 Hz, 5–25 dB over noise) that emerge ∼20 min after starting the perfusion, consistent with other studies (Colgin et al. 2003). In the average across slices, the oscillations became relatively stable in power and frequency after ∼60 min (average spectrogram in Fig. 1B; n = 26). Gamma power and MUA were measured over the whole duration of the recordings (2 h) and were normalized by their average value before the stimulation. Gamma power and MUA were not statistically different across the five stimulation conditions before the stimulation (ANOVA, n = 87, P = 0.61 for power and n = 39, P = 0.77 for MUA). Fifty-five minutes after the start of carbachol perfusion, slices were electrically stimulated with constant electric fields for 10 min. For each slice, only one stimulation intensity was used: −20 V/m (n = 6), −10 V/m (n = 24), 0 V/m (n = 26), +10 V/m (n = 23), and +20 V/m (n = 8). Figure 1, C and D, shows average traces of gamma power and MUA in the different stimulation conditions (the stimulation starts at 0 min). The significant variability observed over the recording period for individual traces required recording of a large number of slices (n = 87).
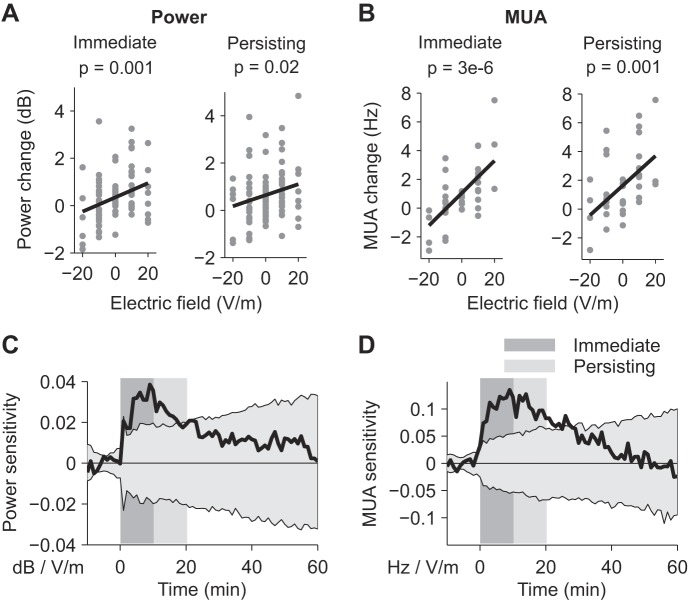
We tested whether electric fields modulated gamma oscillations and MUA in an intensity-dependent manner. We grouped the data from all the slices (n = 87 for power, and n = 39 for MUA) and performed a linear regression as a function of stimulation intensity for the 10-min interval during the stimulation (acute effects) and after stimulation (persisting effects). Combining data from all stimulation conditions was necessary to average out the strong fluctuation observed over time and across slices. The first minute in both intervals was excluded from the analysis to avoid possible transients or artifacts resulting from turning the stimulator on/off. Gamma power was significantly modulated during the stimulation [n = 87, estimated slope of the linear fit, gp = (0.03 ± 0.01) dB/(V/m), P = 0.001; Fig. 2A, left] and a similar effect was also measured for MUA [n = 39, estimated slope of the linear fit, gr = (0.11 ± 0.03) Hz/(V/m), P = 3 × 10−6; Fig. 2B, left]. The positive offset of the regression lines reflect the continuous strengthening of gamma oscillations even 55 min after carbachol perfusion. A positive slope implies that both gamma power and MUA are higher when positive (anodal) electric fields are applied and lower for negative (cathodal) fields. Importantly, the effects outlasted the stimulation in the subsequent 10 min for both power [n = 87, estimated slope of the linear fit, gp = (0.02 ± 0.01) dB/(V/m), P = 0.02; Fig. 2A, right] and MUA [n = 39, estimated slope of the linear fit, gr = (0.10 ± 0.03) Hz/(V/m), P = 0.001, Fig. 2B, right]. The same analysis performed on frequency changes did not reveal any significant effects of fields (P = 0.1 during and 0.3 after stimulation). To determine the exact progression of power and MUA changes, we then estimated power and MUA sensitivity to the electric field (considering data from all the slices, as previously done for Fig. 2, A and B) resolved in 1-min segments (Fig. 2, C and D). Both power and MUA sensitivity continuously increased during stimulation (dark gray shading) and then decayed after the end of the stimulation (light gray shading). The increasing confidence intervals reflect the substantial variability of the gamma oscillations (light gray area, 5 and 95% estimated by shuffle statistics). The time courses of power and MUA are strongly correlated (r2 = 0.65). Taken together, these results show that weak electrical stimulation can affect gamma oscillations and MUA and that the effects outlast the stimulation for at least 10 min.
Fig. 2.
Modulation of gamma power and MUA by weak electrical stimulation. A: gamma-power changes for each slice during (left: immediate) and after (right: persisting) the application of electrical stimulation (n = 87). Black line represents a linear fit. B: MUA changes for each slice during (left: immediate) and after (right: persisting) the application of electrical stimulation (n = 39). C: sensitivity of gamma power to the applied field as a function of time (black curve). D: sensitivity of MUA to the applied field as a function of time (black curve). The dark gray rectangles indicate the stimulation period, and the light gray rectangles indicate the interval considered for persisting effects. Gray shading represents 5 and 95% confidence interval.
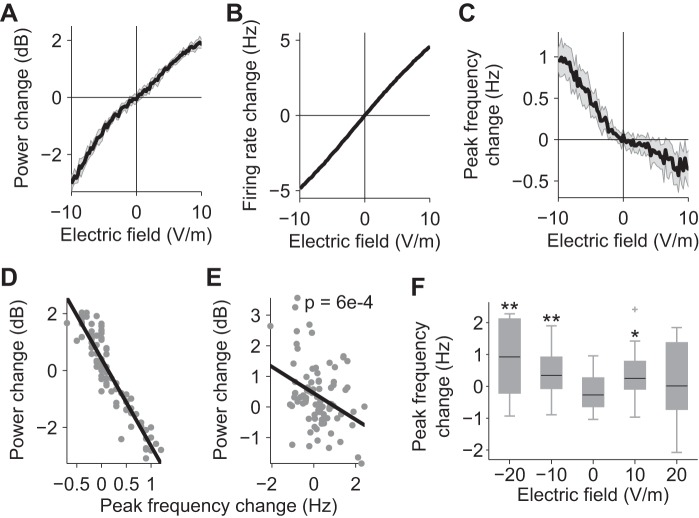
Next, we tried to determine possible causes for these lasting effects using an experimentally validated computational model for carbachol-induced gamma oscillations and their response to electric field stimulation (Reato et al. 2010). In the model, increasing field magnitudes leads to a monotonic increase of gamma power and firing rate (Fig. 3, A and B). Interestingly, increasing field intensity also leads to a monotonic decrease in gamma frequency (Fig. 3C) that correlates with changes in gamma power (r2 = 0.85; Fig. 3D). This relationship was confirmed with our present in vitro data during the stimulation (Fig. 3E; n = 87, P = 6 × 10−4) and is consistent with previous data showing that a balance between excitation and inhibition is the cause of this linear relationship (Atallah and Scanziani 2009). In the computational model, the relationship between electric field magnitude and gamma frequency is nonlinear (Fig. 3C). Thus we analyzed the changes in gamma peak frequency in the in vitro data separately for the different stimulation conditions and found significant effects in particular for negative field stimulation (Fig. 3F). To summarize, our experimental results showed acute effects for gamma power, frequency, and MUA, pointing to a balanced modulation of excitatory and inhibitory activity, yet persistent effects were only evident for oscillatory power and MUA.
Fig. 3.
Effects of electrical stimulation on gamma power, peak frequency (frequency of the peak power), and firing rate. A: gamma-power change as a function of electric field amplitude in the computational model. B: average firing rate change of excitatory neurons a function of electric field amplitude in the computational model. C: peak gamma-frequency change as a function of electric field amplitude in the computational model. Results indicate the average of 20 simulations (gray shading represents SD). D: acute gamma-power changes as a function of frequency changes in the model. Points reflect repeated simulations and stimulation amplitudes (as in A–C). E: acute gamma-power changes as a function of frequency changes during the stimulation in the experimental data. Each point represents a slice (n = 87) in the different conditions. F: frequency changes during the stimulation in the experimental data for the different conditions. *P < 0.05, **P < 0.01.
We then used the computational model to investigate whether the observed lasting changes could be explained by changes in synaptic strength, notably a change in gamma power and MUA but not frequency. We focused on synaptic changes, as they are often assumed to underlie the persistent effects observed in human studies (see discussion). We modulated the strength of excitatory-to-excitatory (e→e) synaptic connections, as well as the strength of the inhibitory feedback (excitatory-to-inhibitory, e→i, or inhibitory-to-excitatory, i→e, synaptic strength). Modulation of inhibitory synaptic strength was motivated by recent evidence for plasticity in inhibitory pathways (Kullmann et al. 2012). Modulating the strength of e→e synapses in the model did not strongly modulate the power of the oscillations (Fig. 4, A–D), while changing e→i or i→e synapses strongly modulated gamma power (Fig. 4, A and D). Both phenomena have previously been observed experimentally for endogenous neocortical gamma activity (Morita et al. 2008; Sohal et al. 2009). This provides further confidence in the present computational model. Here gamma frequency depends on both e→e and e→i connections but less on i→e (Fig. 4, B–E). Firing rate is more sensitive to changes in e→e connections (Fig. 4, C–F). Points in this parameter space that are consistent with the present experimental observation are indicated with an “0” for sham stimulation and “+”, “−” for positive and negative field stimulation. Along these diagonals, power and firing-rate change but not oscillation frequency. Therefore, the computational results suggest that the observed lasting changes may be explained by a lasting modulation of excitation matched by a corresponding change in inhibitory feedback.
Fig. 4.
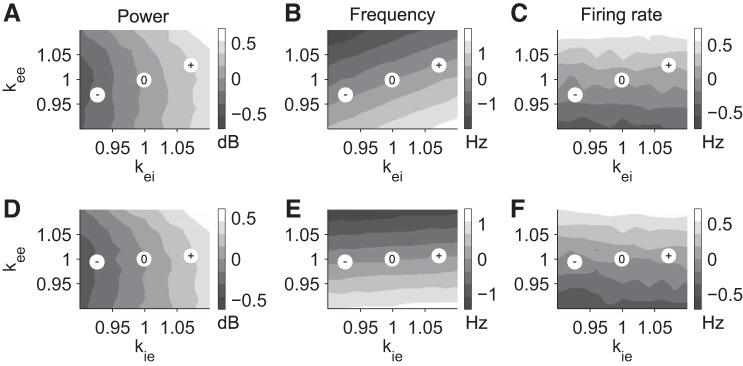
Gamma-power, frequency, and average firing rate changes as a function of changes of the strength of synaptic connections in the computational model. A–C: gamma-power, frequency, and firing rate changes as a function of changes in excitatory-to-excitatory (kee) and excitatory-to-inhibitory (kei) synaptic connections. D–F: gamma-power, frequency, and firing rate changes as a function of changes in excitatory-to-excitatory (kee) and inhibitory-to-excitatory (kie) synaptic connections. “0” indicates points in the parameter space corresponding to sham condition, while “+” and “−” indicate positive (depolarizing) or negative (hyperpolarizing) stimulation.
DISCUSSION
Transcranial electrical stimulation is a versatile tool to modulate brain activity (Nitsche and Paulus 2000; Fregni et al. 2006; Fecteau et al. 2007; Fridriksson et al. 2011). In vivo and in vitro studies have demonstrated that electric fields, whose amplitude is comparable to the one expected in tDCS, can modulate firing rate (Chan and Nicholson 1986), spike timing (Radman et al. 2007), and the magnitude of synaptic responses (Kabakov et al. 2012; Rahman et al. 2013). We have previously shown that acute effects of weak electrical stimulation can be amplified during endogenous oscillatory activity (Reato et al. 2010, 2013). These results suggest that brain oscillations may be a sensitive target for transcranial electrical stimulation with constant currents. Previous in vitro and in vivo studies have only shown acute effects of stimulation on oscillatory activity (Reato et al. 2010; Fröhlich and McCormick 2010; Ali et al. 2013; Ozen et al. 2010), and there are few reports on longer term effects in human studies (see Antal et al. 2004; Polanía et al. 2011). The majority of studies on oscillatory activity have used alternating current stimulation with the goal of enhancing brain oscillations (Marshall et al. 2006; Pogosyan et al. 2009; Kirov et al. 2009; Zaehle et al. 2010; Santarnecchi et al. 2013; Helfrich et al. 2014).
Here we found that weak constant current electrical stimulation applied for a longer period of time can induce lasting effects, measurable as altered gamma power and MUA. These lasting effects cannot be explained as persistent network activity in the absence of some adaptive process since in our previous work gamma power returned to baseline activity within 100 ms after short-lasting DC field stimulation (Reato et al. 2010). Importantly, the afterstimulation effect was consistent with the acute effect, reminiscent of Hebbian or activity-dependent plasticity and contrary to homeostatic plasticity (Fricke et al. 2011; Reato et al. 2013).
The field intensities used in this study are above those predicted to occur during tDCS, estimated to be maximum 1 V/m using conventional electrode montages (Datta et al. 2009; Ozen et al. 2010). These currents only induce a small polarization of the membrane (maximum 0.2 mV per V/m), which cannot lead to action potentials in quiescent neurons (Bikson et al. 2004). Previous in vitro studies have shown that for such low-intensity fields (subthreshold), most of the acute effects scale linearly with the change in field amplitude (Bikson et al. 2004; Deans et al. 2007; Reato et al. 2010), including changes in synaptic response (∼1% per V/m applied; Rahman et al. 2013). Therefore, the sensitivities observed here may also scale linearly with the field intensities. In this context we note that several factors may make the human brain more susceptible to electric fields, including larger sensitivity of individual neurons (due to size; Radman et al. 2009) and higher number of synaptic connections compared with our in vitro preparation (sensitivity to fields may increase with the number of synaptic inputs a neuron receives; Reato et al. 2013). Either way, our field amplitudes are still much below those generated with transcranial magnetic stimulation (Pascual-Leone et al. 2002) or deep brain stimulation (Perlmutter and Mink 2006), estimated in the order of 100 V/m (Salinas et al. 2009).
To generate a hypothesis for the possible cause of the experimental results, we turned to computational modeling. The model we used matches key features of weak-field stimulation on carbachol-induced gamma oscillations (Reato et al. 2010). Specifically, the model matches the firing properties of excitatory and inhibitory neurons and their timing within the gamma cycle (Hájos et al. 2004; Oren et al. 2006). The model successfully predicts firing rate and spike timing changes during AC or DC field stimulation in vitro. Without further modifications, this model reproduced the correlation observed in the present experiment between power and frequency changes due to DC stimulation. A variant of this model also successfully predicted the effects of weak transcranial stimulation on slow-wave oscillations in vivo (Ali et al. 2013). More complex models that capture physiological details such as gap junctions or the role of different neuronal compartments (Tiesinga et al. 2001; Traub et al. 2000) were not necessary to replicate the relevant experimental findings. Using the model, we focused on modulation of synaptic connections because tDCS is thought to modulate concentrations of neurotransmitters and neuromodulators (Stagg and Nitsche 2011; Nitsche et al. 2012), which in turn are known to affect synaptic efficacy. Based on the computational model we hypothesize that in gamma networks weakly depolarizing electric fields lead to a balanced increase of excitatory and inhibitory synaptic currents.
The lasting effects we measured experimentally could be mediated by a number of cellular mechanisms, which we discuss below.
Brain-derived neurotrophic factor.
It has been shown in humans (Antal et al. 2010) and in vitro (Fritsch et al. 2010) that the lasting effects of weak electrical stimulation can be mediated by brain-derived neurotrophic factor (BDNF) release. BDNF release is activity dependent (Park and Poo 2013) with a self-reinforcing feedback-loop involving acetylcholine (Knipper et al. 1994). Thus an acute increase in gamma activity due to electrical stimulation could be further enhanced by increased BDNF release, and this enhancement should outlast stimulation because of the longer time scale of BDNF release (Aicardi et al. 2004). Interestingly, BDNF affects both excitatory and inhibitory neurons (Park and Poo 2013) via the TrkB receptor, which has also been implicated in gamma activity (Zheng et al. 2011). Thus any enhancing effect resulting from increased BDNF release may strengthen both excitation and inhibitory feedback, as we have hypothesized here.
Acetylcholine.
Carbachol activates acetylcholine receptors leading to increased neuronal activity in hippocampus. It is well established that acetylcholine can induce hippocampal plasticity (Drever et al. 2011; Galey et al. 1994; Markevich et al. 1997; Fernández de Sevilla et al. 2008). Indeed, carbachol alone can induce lasting effects on the acetylcholine receptors (Auerbach and Segal 1994) and can facilitate hippocampal LTP (Auerbach and Segal 1996). Moreover, carbachol increases network responsiveness to external stimuli in vivo (Rodriguez et al. 2004; Rasmusson 2000) and can induce lasting effects on evoked responses (Rodriguez et al. 2004; Bröcher et al. 1992), presumably by increasing precision of spike timing in the network. Finally, long-lasting effects on cortical activity can be induced when sensory stimulation is paired with activation of cholinergic inputs from the basal forebrain in vivo (Froemke et al. 2013). It is thus possible that the increased activity due to electric fields in the presence of carbachol is translated also into increased carbachol-induced plasticity. Interestingly, a recent in vivo study reported that acetylcholine-mediated learning induces strengthening at both excitatory and inhibitory synapses (Mitsushima et al. 2013), supporting our hypothesis that weak electrical stimulation may affect both types of synapses.
Spike-timing-dependent plasticity.
The altered gamma activity with firing periods in the order of 10–30 ms may induce N-methyl-d-aspartate-mediated spike-timing-dependent plasticity (STDP) (Wespatat et al. 2004). Thus increased firing due to field stimulation could lead to altered synaptic efficacies via STDP, which outlast the period of stimulation.
Membrane excitability.
Finally, stimulation may affect membrane excitability (nonsynaptic; Ardolino et al. 2005). For example, stimulation-induced slow changes in neuromodulator release could lead to slow changes in neuronal membrane properties giving rise to changes in the population dynamics and the studied aftereffects (Augustin et al. 2013). Considering the nature of gamma oscillations in hippocampus, an increase/decrease in excitability of excitatory neurons (the most affected by electrical stimulation, Radman et al. 2009) could also lead to a balanced increase/decrease of inhibitory feedback.
In all instances, we propose that stimulation acutely affects ongoing activity, which then leads to lasting effects via endogenous plasticity mechanisms. We argue that using our slice preparation we will be able to test our specific hypothesis that balanced synaptic changes mediate the effects of weak electric fields on gamma oscillations.
GRANTS
This work was supported by a grant from National Institutes of Health/National Science Foundation/Bundesministerium für Bildung und Forschung/Collaborative Research in Computational Neuroscience (USA-German Collaboration in Computational Neuroscience Grant No.NIH-R01-MH-092926-05).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.R. and L.C.P. conception and design of research; D.R. performed experiments; D.R. analyzed data; D.R., M.B., and L.C.P. interpreted results of experiments; D.R. prepared figures; D.R., M.B., and L.C.P. drafted manuscript; D.R., M.B., and L.C.P. edited and revised manuscript; D.R., M.B., and L.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Fanny Cazettes for comments on the manuscript.
REFERENCES
- Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci USA 101: 15788–15792, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MM, Sellers KK, Fröhlich F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J Neurosci 33: 11262–11275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, Nitsche MA, Shoukier M, Ludwig H, Paulus W. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul 3: 230–237, 2010. [DOI] [PubMed] [Google Scholar]
- Antal A, Varga ET, Kincses TZ, Nitsche MA, Paulus W. Oscillatory brain activity and transcranial direct current stimulation in humans. Neuroreport 15: 1307–1310, 2004. [DOI] [PubMed] [Google Scholar]
- Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. J Physiol 568: 653–663, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 62: 566–577, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach JM, Segal M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol 72: 2034–2040, 1994. [DOI] [PubMed] [Google Scholar]
- Auerbach JM, Segal M. Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J Physiol 492: 479–493, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin M, Ladenbauer J, Obermayer K. How adaptation shapes spike rate oscillations in recurrent neuronal networks. Front Comput Neurosci 7: 9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56, 2007. [DOI] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol 557: 175–190, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröcher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res 573: 27–36, 1992. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 5: 175–195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science (New York, NY) 304: 1926–1929, 2004. [DOI] [PubMed] [Google Scholar]
- Cambiaghi M, Velikova S, Gonzalez-Rosa JJ, Cursi M, Comi G, Leocani L. Brain transcranial direct current stimulation modulates motor excitability in mice. Eur J Neurosci 31: 704–709, 2010. [DOI] [PubMed] [Google Scholar]
- Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75: 700–713, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol 371: 89–114, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Kubota D, Lynch G. Cholinergic plasticity in the hippocampus. Proc Natl Acad Sci USA 100: 2872–2877, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial DC stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul 2: 201–207, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans JK, Powell AD, Jefferys JG. Sensitivity of coherent oscillations in rat hippocampus to AC electric fields. J Physiol 583: 555–565, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drever BD, Riedel G, Platt B. The cholinergic system and hippocampal plasticity. Behav Brain Res 221: 505–514, 2011. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci 27: 12500–12505, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Núñez A, Borde M, Malinow R, Buño W. Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J Neurosci 28: 1469–1478, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 hz in the hippocampus in vitro. Nature 394: 186–189, 1998. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the m1 receptor to two mixed cation currents. Neuron 33: 615–624, 2002. [DOI] [PubMed] [Google Scholar]
- Foerster A, Rocha S, Wiesiolek C, Chagas AP, Machado G, Silva E, Fregni F, Monte-Silva K. Site-specific effects of mental practice combined with transcranial direct current stimulation on motor learning. Eur J Neurosci 37: 786–794, 2012. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, Nitsche MA, Pascual-Leone A. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 122: 197–209, 2006. [DOI] [PubMed] [Google Scholar]
- Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol 105: 1141–1149, 2011. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke 42: 819–821, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Nikolíc D, Singer W. The gamma cycle. Trends Neurosci 30: 309–316, 2007. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66: 198–204, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins AR, Zaika N, Bernstein H, Wachs M, Levis PA, Polley DB, Merzenich MM, Schreiner CE. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci 16: 79–88, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich F, McCormick DA. Endogenous electric fields may guide neocortical network activity. Neuron 67: 129–143, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 53: 591–604, 2007. [DOI] [PubMed] [Google Scholar]
- Galey D, Destrade C, Jaffard R. Relationships between septo-hippocampal cholinergic activation and the improvement of long-term retention produced by medial septal electrical stimulation in two inbred strains of mice. Behav Brain Res 60: 183–189, 1994. [DOI] [PubMed] [Google Scholar]
- Gartside IB. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: reverberating circuits or modification of synaptic conductance? Nature 220: 382–383, 1968. [DOI] [PubMed] [Google Scholar]
- Gluckman BJ, Neel EJ, Netoff TI, Ditto WL, Spano ML, Schiff SJ. Electric field suppression of epileptiform activity in hippocampal slices. J Neurophysiol 76: 4202–4205, 1996. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci 26: 4535–4545, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Pálhalmi J, Mann EO, Németh B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci 24: 9127–9137, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol 24: 333–339, 2014. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Rach S, Neuling T, Strüber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci 7: 279, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM. Simple model of spiking neurons. IEEE Trans Neural Netw 14: 1569–1572, 2003. [DOI] [PubMed] [Google Scholar]
- Javadi AH, Cheng P, Walsh V. Short duration transcranial direct current stimulation (tDCS) modulates verbal memory. Brain Stimul 5: 468–474, 2011. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30: 317–324, 2007. [DOI] [PubMed] [Google Scholar]
- Kabakov AY, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J Neurophysiol 107: 1881–1889, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov R, Weiss C, Siebner HR, Born J, Marshall L. Slow oscillation electrical brain stimulation during waking promotes EEG theta activity and memory encoding. Proc Natl Acad Sci USA 106: 15460–15465, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, da Penha Berzaghi M, Blöchl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci 6: 668–671, 1994. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron 75: 951–962, 2012. [DOI] [PubMed] [Google Scholar]
- Markevich V, Scorsa AM, Dawe GS, Stephenson JD. Cholinergic facilitation and inhibition of long-term potentiation of CA1 in the urethane-anaesthetized rats. Brain Res 754: 95–102, 1997. [DOI] [PubMed] [Google Scholar]
- Márquez-Ruiz J, Leal-Campanario R, Sánchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, Ruffini G, Gruart A, Delgado-García JM. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci USA 109: 6710–6715, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature 444: 610–613, 2006. [DOI] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. Oxford, UK: Oxford Univ. Press, 2008. [Google Scholar]
- Mitsushima D, Sano A, Takahashi T. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat Commun 4: 2760, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Kalra R, Aihara K, Robinson HP. Recurrent synaptic input and the timing of gamma-frequency-modulated firing of pyramidal cells during neocortical “UP” states. J Neurosci 28: 1871–1881, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553: 293–301, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527: 633–639, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57: 1899–1901, 2001. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Müller-Dahlhaus F, Paulus W, Ziemann U. The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol 590: 4641–4662, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren I, Mann EO, Paulsen O, Hájos N. Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci 26: 9923–9934, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen S, Sirota A, Belluscio MA, Anastassiou CA, Stark E, Koch C, Buzsáki G. Transcranial electric stimulation entrains cortical neuronal populations in rats. J Neurosci 30: 11476–11485, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Poo M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14: 7–23, 2013. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Davey N, Rothwell J, Wasserman E, Puri BK. Handbook of Transcranial Magnetic Stimulation (1st ed.). New York: CRC, 2002. [Google Scholar]
- Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci 29: 229–257, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. Boosting cortical activity at beta-band frequencies slows movement in humans. Curr Biology 19: 1637–1641, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R, Nitsche MA, Paulus W. Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp 32: 1236–1249, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput 16: 1661–1687, 2004. [DOI] [PubMed] [Google Scholar]
- Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in sub- and suprathreshold uniform electric field stimulation. Brain Stimul 2: 215–228, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman T, Su Y, An JH, Parra LC, Bikson M. Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J Neurosci 27: 3030–3036, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 591: 2563–2578, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P, Pilato F, Di Lazzaro V, Grassi C. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol 107: 1868–1880, 2012. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res 115: 205–218, 2000. [DOI] [PubMed] [Google Scholar]
- Reato D, Gasca F, Datta A, Bikson M, Marshall L, Parra LC. Transcranial electrical stimulation accelerates human sleep homeostasis. PLoS Comput Biol 9: e1002898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci 30: 15067–15079, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci 24: 10369–10378, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas FS, Lancaster JL, Fox PT. 3D modeling of the total electric field induced by transcranial magnetic stimulation using the boundary element method. Phys Med Biol 54: 3631–3647, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Polizzotto NR, Godone M, Giovannelli F, Feurra M, Matzen L, Rossi A, Rossi S. Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr Biol 23: 1449–1453, 2013. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature 423: 288–293, 2003. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist 17: 37–53, 2011. [DOI] [PubMed] [Google Scholar]
- Tiesinga PH, Fellous JM, José JV, Sejnowski TJ. Computational model of carbachol-induced delta, theta, and gamma oscillations in the hippocampus. Hippocampus 11: 251–274, 2001. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, Fisahn A, LeBeau FE, Whittington MA, Buhl EH. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci 12: 4093–4106, 2000. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90: 1195–1268, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wespatat V, Tennigkeit F, Singer W. Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J Neurosci 24: 9067–9075, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle T, Rach S, Herrmann CS. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One 5: e13766, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, An JJ, Yang F, Xu W, Xu ZQ, Wu J, Hökfelt TG, Fisahn A, Xu B, Lu B. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc Natl Acad Sci USA 108: 17201–17206, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]