Abstract
How do social interactions form and modulate the neural representations of specific complex signals? This question can be addressed in the songbird auditory system. Like humans, songbirds learn to vocalize by imitating tutors heard during development. These learned vocalizations are important in reproductive and social interactions and in individual recognition. As a model for the social reinforcement of particular songs, male zebra finches were trained to peck for a food reward in response to one song stimulus (GO) and to withhold responding for another (NoGO). After performance reached criterion, single and multiunit neural responses to both trained and novel stimuli were obtained from multiple electrodes inserted bilaterally into two songbird auditory processing areas [caudomedial mesopallium (CMM) and caudomedial nidopallium (NCM)] of awake, restrained birds. Neurons in these areas undergo stimulus-specific adaptation to repeated song stimuli, and responses to familiar stimuli adapt more slowly than to novel stimuli. The results show that auditory responses differed in NCM and CMM for trained (GO and NoGO) stimuli vs. novel song stimuli. When subjects were grouped by the number of training days required to reach criterion, fast learners showed larger neural responses and faster stimulus-specific adaptation to all stimuli than slow learners in both areas. Furthermore, responses in NCM of fast learners were more strongly left-lateralized than in slow learners. Thus auditory responses in these sensory areas not only encode stimulus familiarity, but also reflect behavioral reinforcement in our paradigm, and can potentially be modulated by social interactions.
Keywords: stimulus specific adaptation, lateralization, songbird, electrophysiology, individual recognition
in social species, individual recognition can make an important contribution to mediating social interactions between individuals who encounter each other repeatedly as members of a population. For example, birds use vocal signals in a range of reproductive and social interactions (Zann 1996), and vocalizations can provide a basis for individual recognition. In a songbird, the zebra finch (Taeniopygia guttata), young males learn a single song early in life from adult tutors through a process of vocal imitation with many parallels to speech acquisition (Doupe and Kuhl 1999); although the copies are good, they contain variations that make each song unique to the individual (Immelmann 1969; Miller 1979b). Behavioral studies show that adult zebra finches are able to recognize the unique songs and calls of individual conspecifics with which they have interacted socially, e.g., a tutor or mate (Miller 1979a; Riebel 2000; Vignal et al. 2004, 2008). Thus adult males form recognition memories of new songs that they hear, although they no longer copy new vocal signals. Songs used in various social contexts can become naturally associated with significant behavioral outcomes (e.g., success or failures in courtship and territorial defense). However, it is unknown how the neural representation of each specific vocalization is updated by these social interactions.
In this study, we explored the hypothesis that operant training modulates long-term neural memories for reinforcement-predictive stimuli in auditory regions of the adult brain. We combined established methods for creating and assessing auditory associations in the songbird brain to investigate how the behavioral relevance of auditory stimuli is reflected in the neural activity of sensory processing areas. First, zebra finches were trained to recognize individual songs and their behavioral relevance through operant conditioning, as a model of the way particular songs may be reinforced during natural social interactions. Then, neurophysiological responses to reinforced, familiar, and novel songs were recorded in awake birds to quantify the representations of familiar sensory stimuli and determine how differential reinforcement during training affects those representations in separate brain areas. Although sensory responses have traditionally been considered to be fixed, they are increasingly understood to be modulated by prior experience (Gentner and Margoliash 2003; Gilbert et al. 2009; Thompson and Gentner 2010; Weinberger 1998). Recordings were made in two auditory processing areas of the songbird forebrain where neuronal memories can be detected in immediate early gene and electrophysiological responses: the caudomedial mesopallium (CMM) and caudomedial nidopallium (NCM) (Menardy et al. 2012; Woolley and Doupe 2008). CMM and NCM receive auditory projections from primary auditory areas (Field L complex) and may be analogous to a secondary auditory cortex, or to superficial layers of mammalian A1 (Karten 1991; Vates et al. 1996; Wang et al. 2010). Neurons in these areas respond more strongly to conspecific vocalizations than other sounds, showing a response bias for stimuli that are behaviorally relevant to subjects (Chew et al. 1995, 1996; Mello et al. 1992). In addition, during awake neurophysiological recordings, neurons in NCM and CMM undergo a process of stimulus-specific adaptation; responses are robust to the initial presentations of each stimulus and then decrease over subsequent presentations to reach an asymptote (Chew et al. 1995; Smulders and Jarvis 2013). The rate at which multiunit responses to song stimuli decrease over repeated presentations can be used to assess the familiarity of stimuli (Chew et al. 1995; Phan et al. 2006). These song-specific changes in response to unreinforced exposure last hours to days (Chew et al. 1996; Phan et al, 2006). Furthermore, the expression of ZENK, an immediate early gene related to learning and the formation of memories (Bolhuis et al. 2001; Mello et al. 1992, 1995; Stripling et al. 2003), is increased during operant training in both NCM and CMM of zebra finches (Gentner et al. 2004). After training, increased ZENK expression (as well as increased electrophysiological activity) in CMM remains associated with playback of trained stimuli, while ZENK expression (and electrophysiological activity) in NCM habituates as stimuli become familiar (Gentner et al. 2004; Gentner and Margoliash 2003; Thompson and Gentner 2010).
In addition, NCM shows lateralized neural responses to auditory stimuli, and the direction of lateralization can be affected by changes in the acoustic environment (Moorman et al. 2012; Phan and Vicario 2010; Yang and Vicario 2012). This lateralization of neural activity is of specific interest because the human brain is also lateralized for language; both speech production and perception are predominantly left hemispheric processes, although both the mechanism and function of lateralized processing remain unclear. In NCM, hemispheric differences depend on developmental experience (Phan and Vicario 2010) and may be related to the quality of a songbird's auditory learning. When auditory information is blocked from reaching one hemisphere by lesioning the thalamic auditory relay nucleus of songbirds (nucleus ovoidalis), birds show differential deficits in auditory discrimination learning according to which hemisphere received the lesion (Cynx et al. 1992). Therefore, we hypothesized that auditory processing areas in the two hemispheres might contribute differently to performance in the operant conditioning paradigm.
Multiunit and single-unit responses were analyzed to identify effects of discrimination learning on responses to familiar and novel stimuli in NCM and CMM. We found individual differences in the speed of acquisition of the auditory discrimination, as others have reported (Atienza et al. 2002; Guillette et al. 2011; Katsnelson et al. 2011; Range et al. 2006; for review, Weinberger 2011). Therefore, responses were further analyzed to explore the relationship between speed of acquisition and the associated neural responses. The results shed light on how reinforcement-predictive memories of conspecific songs are represented by sensory neurons in the brain, altered by experience, and accessed during recognition.
METHODS
Subjects
Adult male zebra finches (n = 11, >120 days of age) that had been reared in our aviary (on a 14:10-h light-dark cycle), but were naive to discriminatory training, were used as subjects. For 5 days prior to start of operant training, the birds were isolated and acclimated to a custom-built wire chamber (45.72 × 29.21 × 27.94 cm) located inside of a sound-attenuated box (inside dimensions: 82.55 × 33.66 × 38.10 cm; outside dimensions: 91.44 × 40.64 × 48.26 cm), where they lived and were trained. Birds were food deprived overnight on days prior to training sessions (during which food was used as a reward for correct responses) and then given food ad libitum at the end of each daily training session; they had access to cuttlebone and water throughout training. During weekends, when the subjects were not training, they were given food ad libitum. All experiments were performed in accordance with protocols approved by the Rutgers University Institutional Animal Care and Use Committee (Protocol Number 02-217).
Training
Birds were trained 5 days a week for 6 h a day. After acclimation, the subjects were shaped to peck a sensor, breaking an infrared beam, to stimulate a food reward (as in Gess et al. 2011) using the ARTSy program (David Schneider, Columbia University, New York, NY). After 5 days of shaping, the birds began operant GO/NoGO training, during which subjects had to peck the sensor to hear a stimulus, and then respond correctly to that stimulus based on its GO/NoGO categorization. The two stimuli used in each discrimination were single-motif songs of unfamiliar male zebra finches; these stimuli were randomly assigned to the GO and NoGO categories. Each discrimination used a set of stimuli that were 50–70% different from one another and of similar length (as determined by an open source software program, SAP 2011; Tchernichovski). During a trial, if the GO stimulus was played, the correct response was to peck again for a food reward; however, for a NoGO stimulus, the correct response was to withhold pecking. Correct GO responses were rewarded with access to birdseed for 6 s, and incorrect NoGO responses were punished with the chamber lights being extinguished for 16 s. Each trial was concluded if there was no response within 6 s. Subjects were able to initiate trials immediately after one another, and thus could perform unlimited trials to reach the accuracy criterion, defined as at least 80% correct responses on two sequential sets of 100 trials. After meeting criterion, subjects learned to discriminate a second pair of stimuli. Before electrophysiological recording, birds were tested with all four song stimuli in a randomly interleaved set and had to meet criterion for both pairs of training stimuli.
Probe Trials
To investigate the behavioral strategies used to successfully perform the operant auditory discrimination, two novel unreinforced stimuli (probe stimuli) were added to the paradigm in a subset of birds (n = 4). After subjects reached criterion for both operant discriminations, correct GO responses and incorrect NoGO responses were reinforced 80% of the time (rather than 100%) to prepare for probe trials. After this, two probe stimuli, which were each equally different (within 10% similarity, Tchernichovski, SAP 2011) from the GO and NoGO stimuli, were added to the training trials. When these songs played as trial stimuli, the behavioral responses they evoked were not reinforced, either through reward or punishment. Each probe stimulus was played 10 times in every 100 trials, for a week. High or low levels of responding to probe stimuli can indicate a behavioral strategy in which only one stimulus category is truly learned by the subject during the discrimination learning.
Electrophysiology
At the conclusion of operant training, subjects underwent surgical preparation for the neural recording. Each bird was anesthetized (1.5–2.0% isoflurane in oxygen), and a craniotomy was performed to access the caudal forebrain around the bifurcation of the midsagittal sinus. A recording chamber was formed out of dental cement, and a metal pin was cemented to the skull for immobilizing the head during subsequent electrophysiological recordings. Allowing for complete recovery, 2 days later, the awake bird was comfortably restrained (in a plastic tube) and the head pin was clamped to a stereotaxic apparatus located in a soundproof booth (IAC, Bronx, NY). A multielectrode microdrive (Eckhorn, Thomas Recording, Giessen, Germany) was used to lower 16 tungsten microelectrodes (Type ESI2ec, impedance: 2–4 MΩ, Thomas Recording) bilaterally into the brain, targeting areas NCM and CMM (16 electrodes total, 4 in each area in each hemisphere). The microelectrodes were initially lowered to a depth of 500 μm and then slowly lowered from this depth while novel songs were played as search stimuli through a speaker placed directly (30 cm) in front of subjects. Once robust responses to song were located on all electrodes, experimental sets of song stimuli were played while recording multiunit neural responses (×19,000, band-pass filtered: 0.5–5 kHz; Spike 2 software, CED, Cambridge, UK). Stimulus sets consisted of novel (3 stimuli), trained (GO and NoGO) and probe songs equated for loudness (75-dB average; sampling rate: 44,444.4 Hz). Songs were presented for 25 repetitions each, in a shuffled order (8-s interspike interval).
Histology
At the conclusion of the neural recording, electrolytic lesions were made at recording sites (20 μA for 12 s). Subsequently, subjects were anesthetized with Nembutal and then perfused with saline followed by 4% paraformaldehyde. Postfixation, the brains were sectioned at 50 μm using a vibratome (Series 1000), placed onto slides, and stained with cresyl violet. Sections were visualized under a light microscope to confirm lesions and reconstruct recording sites (Fig. 1). Data from electrodes found to have been placed outside the areas of interest were excluded from analyses.
Fig. 1.
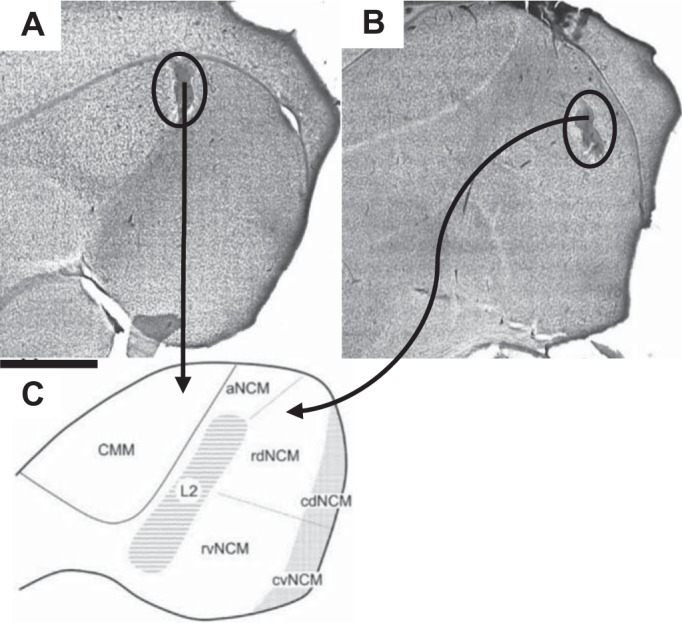
Histology and electrode placement in caudomedial nidopallium (NCM) and caudomedial mesopallium (CMM). Sites used for data analyses were confirmed to be in either CMM (A) or NCM (B) by sending electrolytic current (20 μA for 12 s) through electrodes at the conclusion of the electrophysiological experiment to produce lesions (scale bar = 500 μm). Brains were then sectioned, and slices were stained with cresyl violet and visualized under a light microscope to confirm placement. C: figure showing the boundaries of avian auditory areas (NCM, L2, CMM). aNCM, rdNCM, rvNCM, cdNCM, and cvNCM: apical, rostrodorsal, rostroventral, caudodorsal, and caudoventral NCM, respectively. [From Sanford et al. (2010). Reprinted with permission from Wiley.]
Isolation of Single Units
Single units were extracted from multiunit recording data, using Spike 2 software (CED, Cambridge, UK). For each electrode channel, an initial threshold for spike detection was set based on visual inspection. The shapes of spikes that crossed the threshold were used to create and update multiple waveform templates, each for spikes of similar shape. Principal components analysis and interval histograms were used to reclassify and group similar spikes together by their size and shape. To be accepted as a single unit, ≤2% of the interspike intervals had to be <2 ms.
Data Analysis
The neural response of each multiunit site to each stimulus repetition was quantified by subtracting the root mean square of activity during a control period (0.5 s) before stimulus onset from the root mean square of activity during stimulus playback. Absolute response magnitude (ARM), an index of response strength, was defined as the mean neural response on trials 2–6. The response adaptation rate, an index of “neuronal” memory, for each stimulus at each multiunit site, was calculated as the slope of responses from trials 6–25, divided by the mean ARM over the same trials to normalize for the response magnitude at each site. Sites were excluded from further analyses if: 1) they were not histologically verified to be within NCM or CMM; or 2) responses to two out of the three stimulus categories (GO, NoGO, novel) were not statistically different from responses during the control period. The latter criterion resulted in exclusion of only 5 sites (2 in NCM, 3 in CMM) of 152 sites total (67 in NCM, 85 in CMM).
Single-unit responses were quantified similarly to multiunit responses; spike rates were calculated by subtracting the spike rate (per unit time) during 0.5 s before stimulus onset from the spike rate during the stimulus period to get a response rate for each trial. The firing rates used in single-unit analyses were the average response spike rates to the first seven presentations of each stimulus. Response adaptation for single units was quantified for each stimulus by taking the slope of the regression line of spike-rate responses on trials 1–25 and dividing by the average response magnitude for those trials, to normalize. To compare single-unit responses to learned vs. novel stimuli, d′ values were calculated using a unit's average firing rate and the trial-to-trial variance in spike rates for stimulus trials (Green and Swets 1966; Theunissen and Doupe 1998). A d′ was computed by subtracting the average firing rate (FR) response to one stimulus category (i.e., novel) from the average FR response to another stimulus type (i.e., GO), multiplying by 2, and dividing by the square root of the sum of the variances for those two measures (as below).
Three sets of d′ values were computed for both NCM and CMM to compare: 1) GO stimuli to novel stimuli; 2) NoGO stimuli to novel stimuli; and 3) GO stimuli to NoGO stimuli. An additional set of d′ values was calculated by comparing single-unit spiking responses to pairs of novel conspecific stimuli, to get a baseline measure of the neural discriminability of an arbitrary pair of song stimuli in each brain area.
Statistical Analyses
To test for statistical differences between groups, nonparametric tests were performed when possible, and parametric analyses of variance (ANOVAs) were used only when repeated-measures and categorical factors (interactions) needed to be tested simultaneously. All statistical tests were run two-tailed with the criterion for statistical significance set at α = 0.05. Multiunit ARM and adaptation rate values were analyzed in both NCM and CMM on a site-by-site basis, using repeated-measures ANOVAs, to isolate any differences in responding based on familiarity of stimulus, valence of stimulus and subject speed of learning. When overall significant effects were detected, post hoc Bonferroni tests were conducted for comparisons of interest to detect which groups were significantly different. Nonparametric Friedman's tests were run on single-unit firing rate and adaptation rate data to test for effects of training on auditory responses. Significant effects detected by Friedman's tests were further investigated with post hoc Wilcoxon sign-rank tests (using Bonferroni-corrected P values as criterion for significant effects). Effects of training on single-unit d′ values were tested using nonparametric Kolmogorov-Smirnov tests to detect both differences in the central tendencies, as well as the variances of these values. Finally, regressions were run on d′ data to test the within-unit correlations between responses to GO, NoGO and novel stimuli.
Due to the variance in subjects' latencies to reach behavioral criterion, subjects were median-split into two groups (slow and fast learners) according to their average speed to acquire the auditory discriminations (Fig. 2). Neurophysiological data were analyzed with learning category as a factor, and direct comparisons were made between the two groups to investigate whether learning speed was related to neural responses to song in our two auditory processing areas of interest. Then, Kolmogorov-Smirnov tests were run to compare the multiunit ARMs and adaptation rates of slow to those of fast learners, as well as to test for lateralization of ARM and adaptation rate measures within these groups. In addition, a regression was run to test the hypothesis that probe trial responsiveness was related to the following: multiunit rates of adaptation (familiarity) to trained stimuli, or the speed with which subjects acquired auditory discriminations.
Fig. 2.
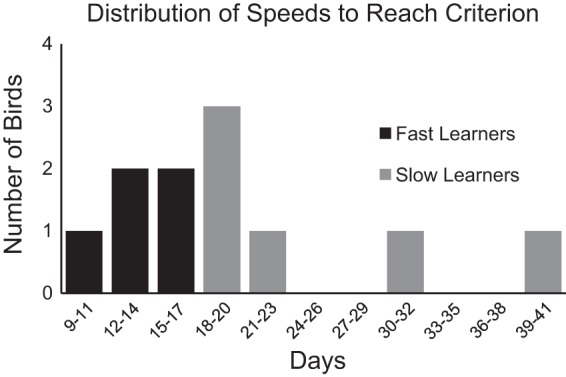
Speed of subject's acquisition of auditory discriminations. Individuals vary in the speed with which they reach behavioral criterion (80% accuracy) on the operant GO (pecking for food reward in response to one song stimulus)/NoGO (withhold responding for another) task. The distributions of days to reach criterion for discriminations 1 and 2, for all subjects, are shown. Subjects were median-split into two groups: faster (n = 5, shown in black) and slower (n = 6, shown in gray) learners, by their average speed to acquire both auditory discriminations.
RESULTS
Multiunit electrophysiological data were collected from 152 responsive recording sites histologically verified to be in NCM (67 sites) or CMM (85 in CMM) in 11 adult male zebra finches. A total of 86 single units were isolated offline from the multiunit recordings: 43 units in NCM, and 43 units in CMM.
Effects of Training on Multiunit Auditory Responses in NCM and CMM
Multiunit auditory responses in both NCM and CMM differed between stimuli reinforced during operant training and stimuli that were novel to these subjects. Training affected both ARMs and adaptation rates, but the effects differed between NCM and CMM. ARMs (trials 2–6) and adaptation rates (trials 6–25) were analyzed in ANOVAs using stimulus type (GO, NoGO, and novel) as a repeated measure at each site. Data from NCM and CMM were analyzed separately.
NCM.
In NCM recordings, there was a significant main effect of training on multiunit ARMs [F(2,104) = 3.12, P < 0.05]. Bonferroni post hoc tests revealed that this effect was driven by a significant difference between the mean (M) ARMs to GO (M = 84.04 ± 1.20) vs. NoGO stimuli (M = 78.43 ± 1.20; P < 0.05, Fig. 3A). NCM sites responded more strongly to positively reinforced GO stimuli than to punished NoGO stimuli. However, responses to novel stimuli were intermediate, not significantly different from either of these reinforcement-predictive stimuli. There was also a significant main effect of training on multiunit adaptation rates [F(2,104) = 7.45, P < 0.001] in NCM. This effect was driven by the significantly slower adaptation to NoGO stimuli (M = −0.33 ± 0.022) than GO stimuli (M = −0.43 ± 0.020) and novel stimuli (M = −0.47 ± 0.069; as shown by post hoc tests; Bonferroni P < 0.01, Fig. 3C). Slower adaptation to NoGO stimuli would suggest that subjects show a stronger memory for NoGO stimuli than novel or GO stimuli, based on earlier study of stimulus-specific adaptation (Chew et al. 1995; Phan et al. 2006; Fig. 3E for representative adaptation profiles).
Fig. 3.
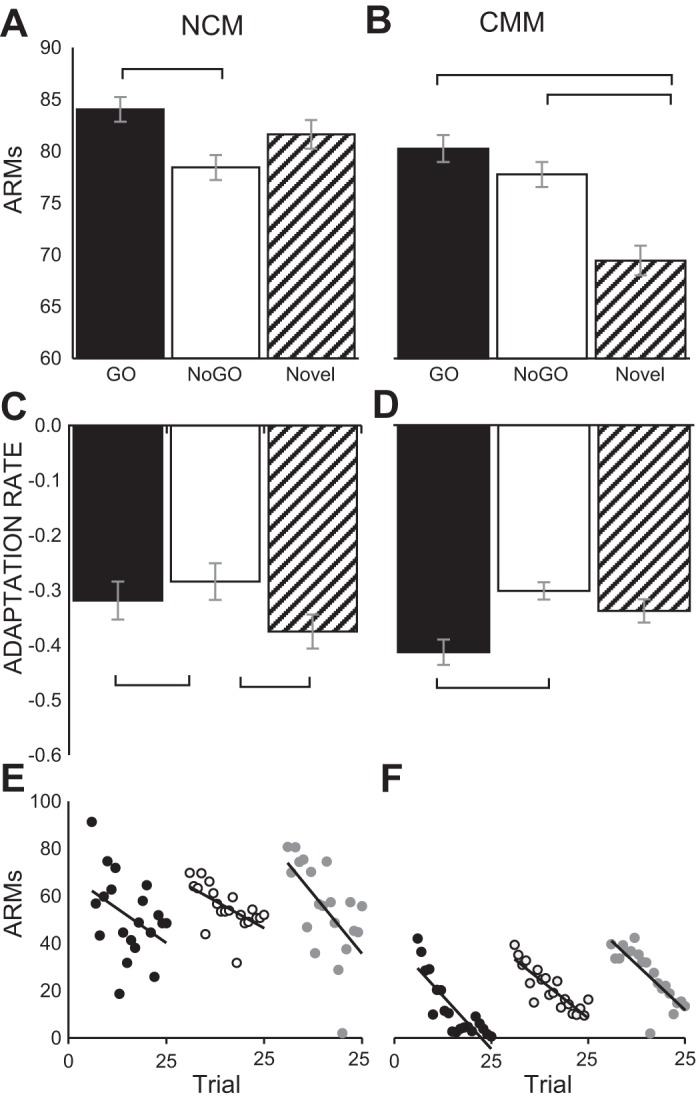
Effects of training on absolute response magnitudes (ARMs) and multiunit adaptation rates. The mean ARMs and adaptation rates of multiunit recording sites in NCM and CMM for subjects (n = 11) in response to GO, NoGO and novel auditory stimuli are shown. A: there was a significant main effect of training on ARMs in NCM (P < 0.05, repeated-measures ANOVA): responses were stronger for GO stimuli than NoGO stimuli (P < 0.05, Bonferroni post hoc test). B: there was also a significant main effect of training on ARMs in CMM (P < 0.0001, repeated-measures ANOVA). Here, novel stimuli evoked lower ARMs than reinforcement-predictive (GO and NoGO) stimuli did (P < 0.001 in both cases, Bonferroni post hoc test). In addition, multiunit adaptation rates showed main effects of training in NCM (P < 0.001, repeated-measures ANOVA) and CMM (P < 0.05, repeated-measures ANOVA). C: in NCM, NoGO stimuli were adapted to more slowly than GO (P < 0.01, Bonferroni post hoc test) and novel stimuli (P < 0.01, Bonferroni post hoc test). D: in CMM, NoGO stimuli were adapted to more slowly than GO stimuli (P < 0.05, Bonferroni post hoc test). Error bars depict within-subjects standard error. ARMs for representative multiunit responses (on trials 6–25) are shown for a novel, GO and NoGO stimulus for NCM (E) and CMM (F). Regression lines depict the adaptation of neural activity at each recording site. Brackets indicate significantly different comparisons.
CMM.
In the CMM recordings, there was a significant main effect of training on multiunit ARMs [F(2,120) = 11.05, P < 0.0001]. Bonferroni post hoc tests revealed that this effect was driven by significantly lower mean ARMs to novel (M = 69.46 ± 1.43) vs. both reinforcement-predictive stimuli [M(GO) = 80.27 ± 1.30; M(NoGO) = 77.77 ± 1.22], as shown by Bonferroni post hoc tests (P < 0.001, Fig. 3B). Multiunit adaptation rates in CMM also showed a significant main effect of training [F(2,120) = 3.77, P < 0.05]. As in NCM, this effect in CMM was driven by slower adaptation to NoGO stimuli (M = −0.32 ± 0.017) than GO stimuli (M = −0.44 ± 0.024), as shown by Bonferroni post hoc tests (P < 0.05, Fig. 3D). Slower adaptation, suggesting a stronger memory for NoGO compared with novel and GO stimuli, may have developed through a behavioral strategy in which subjects focus on learning one of the stimuli (NoGO) to complete the auditory discrimination (see Fig. 3F for representative adaptation profiles).
Effects of Speed of Learning on Multiunit Auditory Responses
Subjects were divided into slow and fast learners, based on a median split of the speed of acquisition of behavioral discrimination (Fig. 2). Slow learners (n = 6) took a greater number of trials to reach criterion on two discriminations (M = 5,660 ± 1,210) than fast learners (n = 5, M = 2,980 ± 606). Neurophysiological responses were compared between 73 multiunit sites in fast-learning subjects and 79 multiunit sites in slow-learning subjects. The multiunit results in both NCM and CMM showed an interesting pattern of differences in auditory responses between the learning groups.
ARMs and adaptation rates.
The speed with which birds learned the auditory discriminations was related to both the strength of neural responses and the rate at which these responses adapted during neurophysiological recording. In both NCM and CMM, faster learners showed significantly higher ARMs in Kolmogorov-Smirnov tests [M(NCM) = 89.24 ± 5.57, M(CMM) = 91.39 ± 8.71] to all song stimuli than slow learners [M(NCM) = 72.80 ± 4.65, M(CMM) = 68.85 ± 4.53; D(NCM) = 0.21, P < 0.05, D(CMM) = .26, P < 0.01; Fig. 4, A and B]. In parallel, faster learners showed faster adaptation [M(NCM) = −0.57 ± 0.079, M(CMM) = −0.76 ± 0.17] to all song stimuli than slow learners [M(NCM) = −0.32 ± 0.040, M(CMM) = −0.15 ± 0.039] in NCM and CMM [D(NCM) = 0.23, P < 0.05, D(CMM) = 0.40, P < 0.001, Fig. 4, C and D].
Fig. 4.
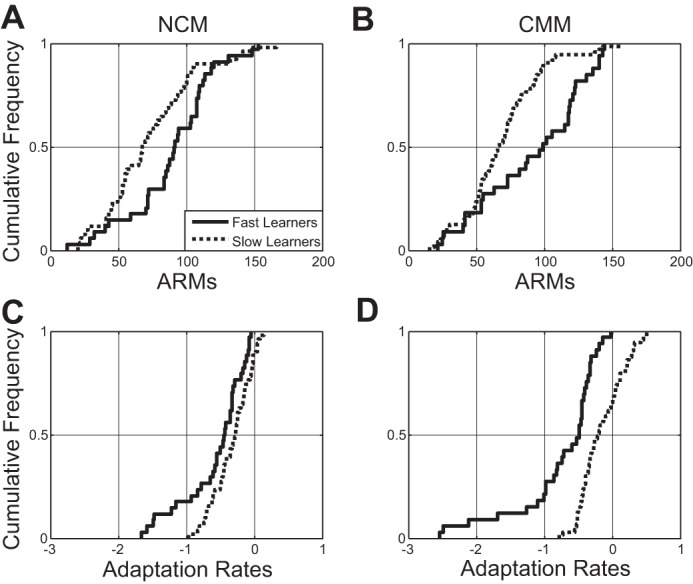
Speed of acquisition and neural responses in NCM and CMM. When subjects were median-split into two groups, according to their speed to reach criterion on the operant task, in NCM (A) and CMM (B), fast learners showed stronger ARMs to all song stimuli than slow learners [NCM, P < 0.01; CMM, P < 0.001, Kolmogorov-Smirnov (KS) test]. In addition, in NCM (C) and CMM (D), fast learners showed faster adaptation to all song stimuli than slow learners (NCM, P < 0.05; CMM, P < 0.001, KS test).
NCM lateralization.
In addition to the overall higher responses in faster learners, the speed to acquire auditory discriminations was related to the direction of lateralization of auditory responses in NCM. Animals that learned faster showed significantly higher ARMs in the left hemisphere (M = 114.27 ± 8.37) than in the right hemisphere (M = 64.21 ± 4.23) in response to all song stimuli (Kolmogorov-Smirnov, D = 0.65, P < 0.0001, Fig. 5B). On the other hand, animals that learned the discriminations more slowly showed no significant difference between the hemispheres (right: M = 85.50 ± 7.75; left: M = 68.73 ± 5.16) of NCM (Kolmogorov-Smirnov, D = 0.18, P = 0.32, Fig. 5B). Multiunit adaptation rates did not show this type of hemispheric interaction in NCM (fast: D = 0.18, P = 0.62, slow: D = 0.21, P = 0.16). In comparisons of CMM data, the fast- and slow-learning groups did not show significant lateralization of ARMs [Kolmogorov-Smirnov D(Fast) = 0.24, P = 0.25, D(Slow) = 0.18, P = 0.18] or adaptation rates, although there was a strong trend for the slow learners to have faster adaptation in the left hemisphere [D(Fast) = 0.27, P = 0.14; D(Slow) = 0.22, P = 0.05].
Fig. 5.
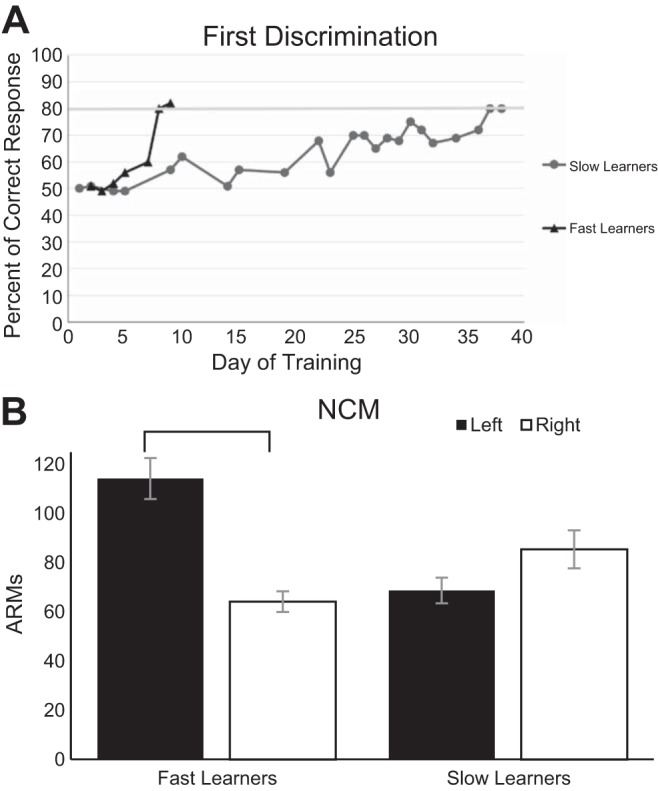
Speed of acquisition and lateralization of NCM. A: learning curves, showing the latency to reach behavioral criterion (80%) on the first discrimination, in examples from a fast and a slow learner. B: in NCM, fast learners showed stronger ARMs in the left hemisphere compared with the right (P < 0.001, KS test) for all stimuli. This asymmetry is the opposite from what has been reported in naive birds (Phan and Vicario 2010). In contrast, slow learners were not significantly lateralized. Lateral differences were not seen in CMM recordings.
Interaction between learning speed and training effects.
The speed with which the subjects learned the discriminations also interacted with the effects of training on multiunit neural responses. As detailed above, when repeated-measures ANOVAs were run to test the effects of learning on ARMs and adaptation rates, there were main effects of training on the ARMs and adaptation rates in NCM and CMM. However, when learning speed was used as a factor in the repeated-measures ANOVA, there were also significant interactions between speed of learning and the reinforcement-predictive value of stimuli in CMM ARM data [F(2,118) = 4.14, P < 0.05, Fig. 6A] and in NCM adaptation data [F(2,102) = 4.18, P < 0.05, Fig. 6B]. Fast learners showed slower adaptation to NoGO stimuli (M = −0.35 ± 0.024) than novel stimuli (M = −0.58 ± 0.017) in NCM (Bonferroni: P < 0.01). They also showed stronger ARMs to NoGO stimuli (M = 97.1 ± 2.15) than novel (M = −0.35 ± 0.024) stimuli in CMM (Bonferroni: P < 0.01). In contrast, slow learners showed stronger ARMs to GO stimuli (M = 74.25 ± 1.61) than novel stimuli (M = 62.72 ± 1.95) in CMM (P < 0.01). Therefore, if slower adaptation and stronger ARMs are indicative of neural memories for learned songs (as suggested by previous results), then these interactions between learning speed and the strength of memory for GO and NoGO stimuli suggest that the two groups may use different behavioral strategies to complete the behavioral task. Fast learners appear to attend more strongly to the NoGO stimulus, while slow learners attend to GO to perform the discrimination.
Fig. 6.
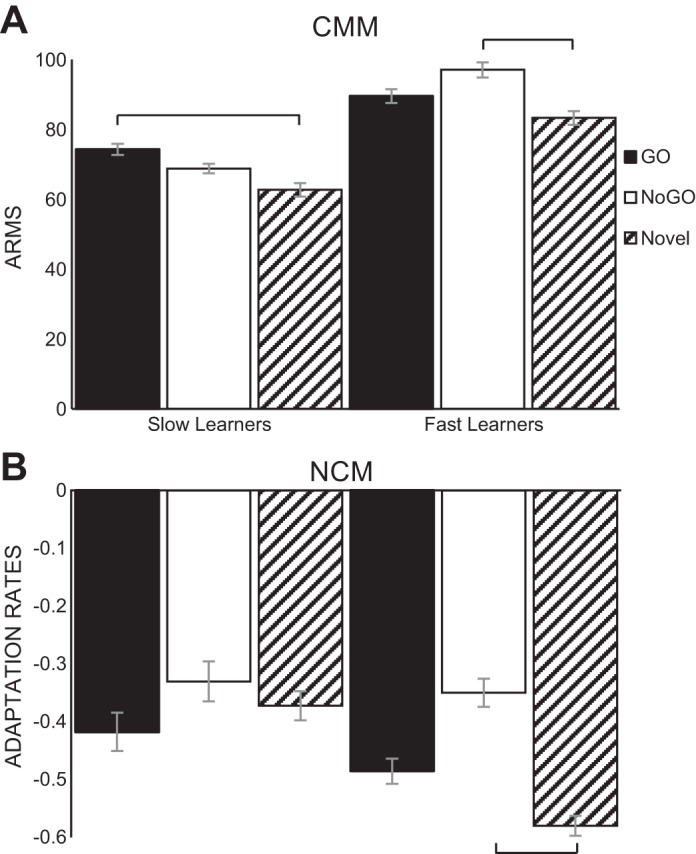
Speed of acquisition and resultant neural representations. Repeated-measures ANOVAs compared responses to GO (solid bars), NoGO (open bars) and novel (hatched bars) stimuli to test the effects of learning on ARMs and adaptation rates in NCM and CMM. There were main effects of training on the ARMs and adaptation rates in NCM and CMM. However, there were also significant interactions between speed of learning and stimulus reinforcement-prediction on the CMM ARM data (P < 0.05; A) and the NCM adaptation data (P < 0.05; B). Bonferroni post hocs revealed that fast learners showed slower adaptation to NoGO stimuli than novel stimuli in NCM (P < 0.01), as well as stronger ARMs to NoGO stimuli than novel in CMM (P < 0.01). Slow learners showed stronger ARMs to GO stimuli than novel stimuli in CMM (P < 0.01). Error bars denote within-subjects standard error values.
Behavioral and Neural Responses to Probe Stimuli
In a subset of animals (n = 4), performance on unreinforced probe trials was used to further explore the behavioral strategies employed in the auditory discriminations. High or low levels of responding to probe stimuli are hypothesized to reflect behavioral strategies in which only one stimulus category (GO vs. NoGO) is selectively learned by the subject during the discrimination training (cf., Morisaka and Okanoya 2008). For example, subjects that respond to probes more than 50% of the time should be more familiar with NoGO stimuli than GO stimuli, while those that seldom respond to probes are more familiar with GO than NoGO.
Individual subjects did show different tendencies to respond to probes, and these data allowed relationships between behavioral and neural responses to be assessed. Analysis of neural responses to probe stimuli demonstrated that the effects of training caused changes in absolute responses and adaptation rates that were not due (solely) to stimulus familiarity. Repeated-measures ANOVAs were run to compare neural responses to reinforcement-predictive (GO and NoGO), novel and probe stimuli, in both NCM and CMM.
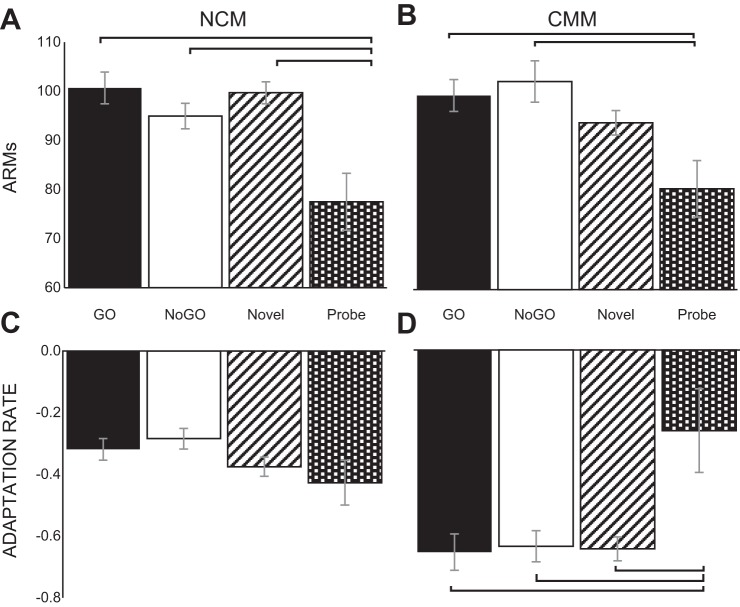
In NCM, there was a significant main effect of familiarity on ARMs [F(3,72) = 6.23, P < 0.001]. Probe stimuli (M = 77.50 ± 5.82) evoked significantly weaker responses than GO (M = 100.68 ± 3.21), NoGO (M = 94.95 ± 2.58) and novel (M = 99.70 ± 2.58) stimuli, as shown in Bonferroni post hoc tests (P < 0.05 for all comparisons, Fig. 7A). Multiunit adaptation rates in NCM, however, were unaffected by probe familiarity [F(3,72) = 1.32, P = 0.27, Fig. 7C].
Fig. 7.
Effects of passive familiarity on ARMs and multiunit adaptation rates. The mean ARMs and adaptation rates of multiunit recording sites in NCM and CMM for subjects exposed to probe stimuli (n = 4) in response to GO, NoGO, novel and probe auditory stimuli are shown. A: there was a significant main effect of familiarity on ARMs in NCM (P < 0.001, repeated-measures ANOVA): responses were stronger for GO, NoGO and novel stimuli than passively familiar probe stimuli (P < 0.05 for each Bonferroni post hoc). B: there was also a significant main effect of familiarity on ARMs in CMM (P < 0.01, repeated-measures ANOVA): in this structure (like in NCM), passively familiar probe stimuli evoked lower ARMs than reinforcement-predictive (GO and NoGO) stimuli did (P < 0.05 in both cases, Bonferroni post hoc). In addition, multiunit adaptation rates showed a main effect of familiarity in CMM (P < 0.001, repeated-measures ANOVA). D: probe stimuli were adapted to significantly slower than GO, NoGO and novel stimuli (P < 0.05 for all Bonferroni post hocs). C: in NCM, however, adaptation rates for probe stimuli were not significantly different than those to the other stimuli (P > 0.05, repeated-measures ANOVA). Error bars denote within-subjects standard error.
In CMM, there were significant main effects of familiarity on both ARMs and adaptation rates [F(3,60) = 4.17, P < 0.01; F(3,60) = 4.28, P < 0.01]. Bonferroni post hoc tests on CMM ARMs showed that probe stimuli elicited significant lower responses (M = 80.54 ± 5.75) than both reinforcement-predictive stimuli, GO (M = 99.53 ± 3.24) and NoGO (M = 102.38 ± 4.22; P < 0.05 for both comparisons, Fig. 7B). Similarly, the rates of adaptation of neural activity in CMM multiunits were significantly slower for probe stimuli (M = −0.26 ± 0.14) than GO (M = −0.66 ± 0.059), NoGO (M = −0.64 ± 0.050) and novel (M = −0.64 ± 0.039) stimuli (P < 0.05 for all comparisons, Fig. 7D). The results for unreinforced probe stimuli are consistent with previous studies which indicate that passively familiar song stimuli are adapted to more slowly than novel stimuli, due to stimulus-specific adaptation (Chew et al. 1995; Phan et al. 2006).
When the difference between GO and NoGO adaptation rates for each bird was plotted against that bird's behavioral responses on probe trials, there was a suggestive correlation between these two measures [r(4) = 0.90, P = 0.10, Fig. 8A]. Unexpectedly, birds with slower adaptation to GO stimuli than to NoGO stimuli (more familiarity) also responded to probe trials more frequently (as GO stimuli). Conversely, subjects that adapted to NoGO stimuli more slowly than GO stimuli responded to probe stimuli infrequently. Furthermore, when the relationship between speed to acquire auditory discriminations and response to probe stimuli was similarly assessed, there was a nonsignificant trend for the animals that learned more quickly to respond to the probe stimuli as NoGO stimuli, while slower learners responded to probes as GO stimuli [r(4) = 0.87, P = 0.13, Fig. 8B].
Fig. 8.
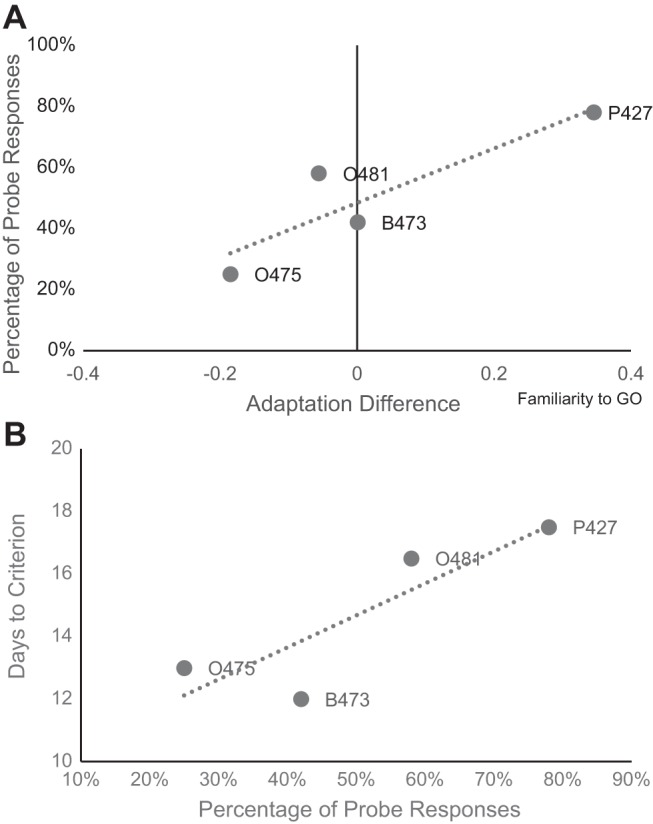
Familiarity of trained stimuli correlates with performance on probe trials. A: the difference between speed of multiunit adaptation to GO and NoGO stimuli (difference in familiarity) trended toward correlation with behavioral responses to probe trials. Slower adaptation to GO stimuli (indicating familiarity) appears as a positive value on the x-axis. Subjects that were more familiar with (adapted to more slowly) GO stimuli responded more often to unreinforced probe stimuli as GO stimuli, and vice versa for NoGO stimuli [r(4) = 0.8957, P = 0.104]. B: in addition, there was a trend for the animals that learned more quickly to respond to the probe stimuli as NoGO stimuli, while slower learners responded to probes as GO stimuli [r(4) = 0.868, P = 0.13].
Effects of Training on Single-Unit Auditory Responses in NCM and CMM
The effects of training on single-unit firing rates and adaptation rates were assessed with repeated-measure nonparametric Friedman's tests.
Firing rates and adaptation rates.
Although previous studies have shown that a majority of NCM sites show stimulus-specific adaptation (Chew et al. 1995), the single units isolated in this experiment were heterogeneous populations of both adapting and nonadapting neurons (Fig. 9). Single units recorded in NCM showed no effect of training on firing rates [χ2(2, N = 43) = 0.59, P = 0.76, Fig. 10A], or on adaptation rates [χ2(2, N = 43) = 1.81, P = 0.40, Fig. 10C]. However, in CMM, although the responses to GO, NoGO and novel stimuli did not differ in their firing rates (Fig. 10B), single units did adapt to trained stimuli differently than they adapted to novel stimuli [χ2(2, N = 43) = 3.40, P = 0.18; χ2(2, N = 43) = 12.47, P < 0.01]. This effect was driven by faster single-unit adaptation to novel stimuli (M = −0.024 ± 0.005) than to either GO stimuli (M = −0.013 ± 0.004) or NoGO stimuli (M = −0.015 ± 0.020), as shown by post hoc Wilcoxon signed-rank tests (P < 0.01 and P < 0.05, respectively, Fig. 10D).
Fig. 9.
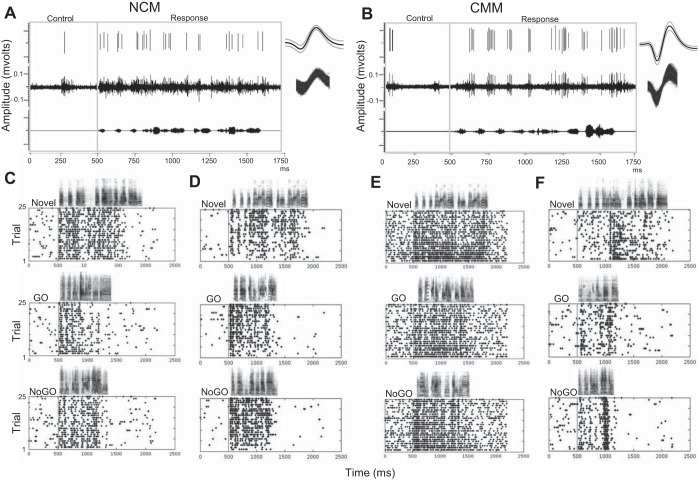
Response patterns of single units in NCM and CMM. Representative single-unit activity isolated from multiunit recordings in NCM (A) and CMM (B) is shown. A and B: bottom traces show the waveform of a novel song stimulus, middle traces show the multiunit response to that stimulus and top traces show the isolated single unit and its waveform. Single-unit firing rates were calculated by taking the neuron's spike rate during the 500-ms control window and subtracting it from the spike rate during the response window. Raster plots show the responses of two NCM (C and D) and two CMM (E and F) single units to example novel, GO and NoGO stimuli. The unit shown in C adapts and is the same as that shown in A, above; and the unit shown in E adapts and is the same as that shown in B, above. Units shown in D and F do not reliably adapt. The populations of single units isolated in both NCM and CMM contained both adapting (C: novel = −0.163, GO = −0.502, NoGO = −0.745; E: novel = −0.880, GO = −0.736, NoGO = −0.844) and nonadapting sites (D: novel = −0.448, GO = 0.290, NoGO = −0.387; F: novel = 0.703, GO = 0.778, NoGO = 0.791). Responses from trials 1–25 (y-axis) are plotted for each stimulus. The sonogram of the stimulus is represented above each plot along the x-axis (time).
Fig. 10.
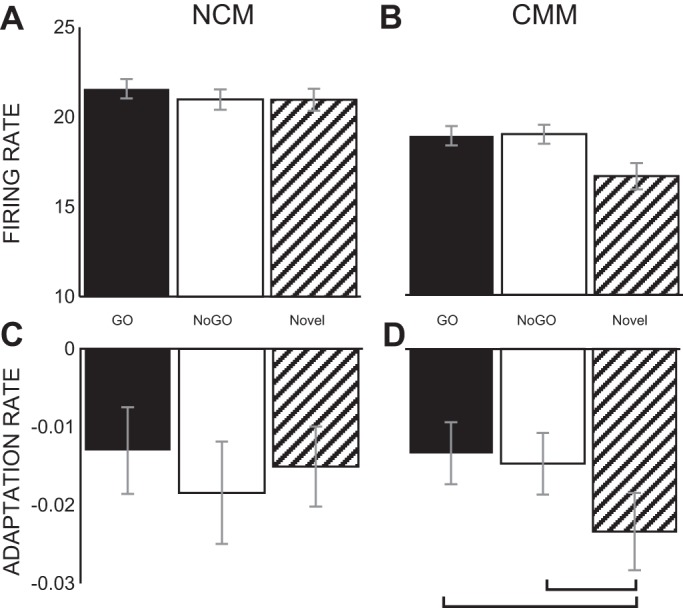
Effect of training on single-unit firing rates and adaptation rates. A and B: in both NCM and CMM, training had no effect on single-unit firing rates [nonsignificant (ns), Friedman's within-subjects test]. C: in NCM, there was no effect of training on single-unit adaptation rates (ns, Friedman's within-subjects test). D: in CMM, however, there was an effect of training on single-neuron adaptation rate (P < 0.001, Friedman's within-subjects test). Novel stimuli were adapted to faster than both GO stimuli and NoGO stimuli (P < 0.01 and P < 0.05, respectively, Wilcoxon signed-rank test).
d′ comparisons for single-unit responses.
In a further analysis, d′ values were calculated from single-unit spiking activity (see methods) to test how responses to reinforcement-predictive stimuli differed from novel (unreinforced) stimuli. Kolmogorov-Smirnov tests were performed to compare the baseline d′ values (between pairs of novel songs; see methods) to d′ values that compared responses to trained songs to those evoked by novel songs, in each structure. A Kolmogorov-Smirnov test was also performed for each structure comparing baseline d′ values to those attained by comparing responses to GO stimuli to those evoked by NoGO stimuli.
In NCM, firing-rate responses to GO stimuli were more discriminable from responses to novel stimuli than responses to two novel stimuli (baseline) were from one another (D = 0.30, P < 0.05, Fig. 11A). On the other hand, firing rate response to NoGO stimuli was no more discriminable from responses to novel than baseline (D = 0.14, P = 0.78, Fig. 11B). Firing-rate responses to GO stimuli also trended toward being more discriminable from responses to NoGO stimuli than responses to two novel songs were from one another (D = 0.27, P = 0.062, Fig. 11C), according to a Kolmogorov-Smirnov test.
Fig. 11.
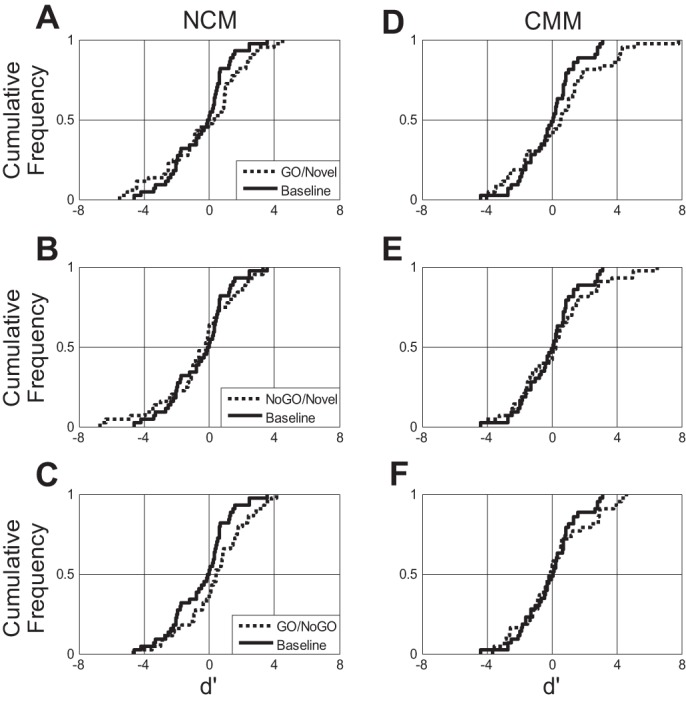
The d′ values of single-unit spiking responses to trained and novel stimuli. Plotted here are the cumulative distribution frequencies of d′ values in NCM and CMM comparing single-unit spike rate responses to trained stimuli (GO/NoGO) to those of novel songs (A, B, D, and E) and GO stimuli to those of NoGO stimuli (C and F). The d′ values calculated by comparing single-unit spiking responses to two novel conspecific songs (novel-novel) are also plotted with a dotted line for each area, as a baseline measure of discriminability of arbitrary song stimuli in that structure. A: in NCM, d′ values comparing responses to GO and novel stimuli were significantly different than baseline (D = 0.30, P < 0.05, KS test). D, E, and F: in CMM, however, there were no significant differences in d′ values of reward predictive and novel stimuli (ns, KS test).
In CMM, however, no differences were detected when the d′ values for learned songs were compared with baseline levels of discriminability for song stimuli in that structure. Baseline d′ values were not significantly different than d′ values calculated to compare responses to GO or NoGO to those evoked by novel songs [D(GO) = 0.23, P = 0.17; D(NoGO) = 0.14, P = 0.77, Fig. 11, D and E]. In addition, neural responses to GO stimuli were no more different from responses to NoGO stimuli than responses to two novel songs were from one another (D = 0.14, P = 0.77, Fig. 11F).
Within-unit correlations.
The lack of significant differences detected in the single-unit dataset, as well as the high level of variance in responses (as assessed by firing rates, adaptation rates and d′ values) suggested further analysis on the heterogeneity of the NCM and CMM single-unit populations recorded. It had already been established that the units isolated differed in the levels to which their firing rates adapted to auditory stimuli; therefore, tests were run to investigate the degree to which units differed in their response to trained songs, relative to novels. χ2 analyses were used to determine whether the individual single units showed enhanced or reduced responses (using d′ values, relative to novel) to both GO and NoGO stimuli more often than would be predicted by chance.
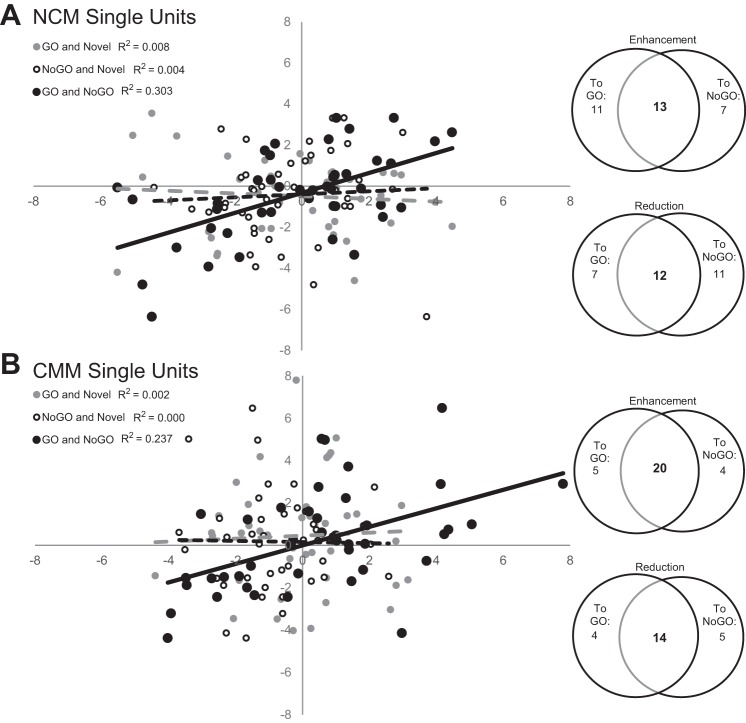
χ2 were run on the counts of units that showed either enhanced responses to both GO and NoGO, reduced responses to both GO and NoGO, or opposite effects for GO and NoGO. In NCM the χ2 was not significant [χ2(3, N = 43) = 1.93, P = 0.59]. However, CMM units tended to respond similarly to GO and NoGO (with enhancement or reduction) more often than would be predicted by chance [χ2(3, N = 43) = 16.26, P < 0.01, Fig. 12B]. In addition, when correlations were run to test the relationships between the d′ responses to GO relative to the d′ responses to NoGO, the units in both NCM and CMM showed significant positive correlations [r(NCM) (43) = 0.55, P < 0.001; r(CMM) (43) = 0.49, P < 0.001; Fig. 12]. However, when correlations were run to test the relationship between d′ values to the reinforcement-predictive and novel stimuli, there was no relationship between responses to GO and novel stimuli or NoGO and novel stimuli, in either brain area [NCM: r(GO) (43) = −0.088, P = 0. 57; r(NoGO) (43) = 0.059, P = 0.71; CMM: r(GO) (43) = 0.045, P = 0.78; r(NoGO) (43) = −0.017, P = 0.92; Fig. 12].
Fig. 12.
A single-unit's d′ values for GO songs are correlated to that unit's d′ values to NoGO stimuli, but not to d′ values for novel songs. A and B: Venn diagrams show the number of NCM and CMM units that show enhanced or reduced responses to both GO and NoGO (relative to novel), compared with the number of units that respond differently to GO and NoGO stimuli. Units that show a decrease in responsiveness to positively reinforced GO stimuli commonly show an increase in responsiveness to negatively reinforced NoGO stimuli, while those units which show reduced activity in response to GO also show reduced responses to NoGO (black data points) in both NCM (r = 0.5507, P < 0.001; A) and CMM (r = 0.4873, P < 0.001; B). Responses to novel stimuli are not correlated to responses to GO (gray) or NoGO (white) stimuli, in NCM or CMM. B: although the population acts as a heterogeneous mix of decreases and increases in neural activity in response to the playback of learned songs, a single unit shows the same direction of effect for GO and NoGO stimuli more often than would be predicted by chance (χ2 = 16.26, N = 43, P < 0.01).
DISCUSSION
Auditory processing areas NCM and CMM both showed effects of training on neural responses to playback of conditioned vs. novel songs. The data thus support the general hypothesis that operant training contributes to long-term neuronal memories for reinforcement-predictive stimuli observed in sensory regions of the adult brain. However, the patterns of differential neural activity for these stimuli differed between the two brain regions, between the two learning groups, and also between hemispheres.
Multiunit Responses to Operantly Trained and Novel Stimuli
In NCM, GO and NoGO stimuli elicited significantly different levels of responding from one another, but neither category evoked higher or lower responses than novel stimuli. In contrast, in CMM, training elicited higher absolute responding to all reinforcement-predictive stimuli (both GO and NoGO) with respect to novel stimuli. The training experience affected the neural responses to auditory stimuli in NCM and CMM in different ways, supporting the hypothesis that the two auditory areas serve different roles in auditory processing. As NCM and CMM have reciprocal projections, it is likely that they interact heavily with one another (Aiya et al. 2011). However, our results showed that CMM responses were more often influenced by the reinforcement-predictive values of auditory stimuli, while NCM responses were driven by stimulus familiarity, which was different for GO and NoGO stimuli in a way that reflected the behavioral strategies used by individual subjects. Similar differences between NCM and CMM were reported by Gentner and colleagues, based on ZENK and neurophysiological studies of operantly trained starlings (Gentner et al. 2004; Gentner and Margoliash 2003; Thompson and Gentner 2010).
The adaptation rate results showed a complex pattern of effects. Although there were effects of operant training on the speed with which NCM and CMM multiunit sites decreased responding over repeated stimulus presentations, the effects were different than those seen for ARMs. In both areas, NoGO stimuli were adapted to the most slowly: in NCM both GO and novel stimuli showed faster adaptation than NoGO stimuli, and in CMM adaptation was significantly faster for GO stimuli than for NoGO stimuli. Contrary to what was observed for ARMs, the significant differences in multiunit adaptation rate were driven by slow adaptation to one of the stimuli. If these results are interpreted in terms of stimulus-specific adaptation as a measure of familiarity, the songs that are adapted to most slowly are those that are the most familiar to subjects. Therefore, it is possible that subjects were more familiar with trained songs of negative valence (reinforced with punishment), perhaps due to the cognitive tactics used by subjects. However, familiarity alone does not explain the entirety of either the ARMs or adaptation rate effects, because passively familiar probe stimuli showed responses that differed from learned songs in both NCM and CMM.
Single-unit Responses to Trained and Novel Stimuli
Overall, single-unit responses to trained stimuli were neither higher nor lower than responses to novel stimuli in NCM and CMM. However, when d′ values were used to compare responses recorded in NCM, single-unit responses to GO stimuli were more discriminable from responses to novel stimuli than baseline. In CMM, d′ comparisons showed no differences. In light of the multiunit data, these results were unexpected because both GO and NoGO showed higher ARMs than novel, in CMM. However, the scarcity of learning effects seen in the single-unit population may be due to statistical limits of our single-unit sample size, or to the heterogeneity of unit responses. Because subpopulations of neurons may show differential responses to learned songs, further analyses were conducted to test the correlations between responses to GO and NoGO stimuli for each single unit. Results showed that, in both NCM and CMM, there were significant correlations between a single neuron's responses to GO and NoGO. In addition, although a single unit showed either enhanced or reduced responses to both learned songs more often than would be predicted by chance, responses to those stimuli were not correlated with that unit's responses to novel stimuli. This suggests that the neurons of NCM and CMM show differential activity to novel stimuli, and these responses are changed (by operant training) in a way that causes reinforcement-predictive stimuli to evoke similar responses, which can be used to signal important stimuli to downstream neural structures.
Although responses in some units are enhanced while others are reduced, within each unit both reinforcement-predictive stimuli (GO and NoGO) are responded to similarly. However, responses to different stimulus classes are variable and uncorrelated. These results suggest that the positive relationship between responses to GO and NoGO stimuli develops over the course of training, due to the learning process. Subpopulations of neurons include those that show enhancement to behaviorally relevant stimuli, those that show suppression to behaviorally relevant stimuli and those that discriminate and respond to the two behavioral valences in different ways. In fact, in NCM, the neurons that showed enhancement to both GO and NoGO tended to be those recorded in the left hemisphere, while the neurons that showed suppression to both tended to be those that were recorded in the right [χ2(3, N = 32) = 2.17, P = 0.14]. Therefore, the overall activity of the multiunit populations of NCM and CMM neurons may better represent the role of those structures in a circuit that processes relevant stimulus associations than the activity of any individual unit does. This finding supports the need for further neurophysiological studies of sensory processing, both at the multiunit and single-unit level.
Relationships Between Probe Trial Responses, Operant Behavior and Learning Speed
There were neurophysiological differences between subjects that mastered the task quickly and those that did not. In both structures NCM and CMM, animals that learned faster exhibited higher ARMs and faster multiunit adaptation to all song stimuli than animals that learned more slowly. This effect of learning speed on auditory responses may reflect enhanced neural plasticity in the faster learning individuals; these individuals not only showed stronger neural responses, but also faster adaptation of those responses, possibly related to memory formation for novel stimuli. The neural differences between fast- and slow-learning subjects in ARMs and adaptation rates may, in fact, have contributed to how those groups differed in their speeds to reach criterion. This is consistent with results from a classical conditioning experiment showing that ZENK induction patterns differ between fast and slow learners (Jarvis et al. 1995). Late in learning, slow learners show higher levels of ZENK induction than fast learners, suggesting slower adaptation of this immediate early gene. This may correspond to the slower rate of neural adaptation we have observed in slow learners.
The learning speed effects can also be interpreted as reflecting the behavioral strategies they used to acquire discriminations. When learning speed was included as a factor in repeated-measures analyses of training effects, there were interactions between training and learning speed in NCM adaptation data, as well as CMM absolute response data. These interactions suggest that individual subjects showed a stronger memory for one song valence or the other, favoring the stimulus they attended to more often to complete the auditory discrimination. In CMM, although the group data suggested that both GO and NoGO were responded to more vigorously then novel stimuli, only fast learners showed a significant difference between NoGO and novel stimuli, while slow learners showed different responses to GO and novel stimuli. Fast learners showed stronger memories for NoGO stimuli, while slow learners showed stronger memories for GO stimuli.
Responses on probe trials presented during operant training in a subset of birds provided a test of the behavioral strategies used by individual birds to perform auditory discriminations. When the neural data and probe trial data were analyzed together, there was a trend toward a correlation between the relative rate of adaptation (to GO and NoGO) and the rate of response to probe trials. The neural results suggest that novel probe stimuli were placed into the category (GO or NoGO) with which subjects were more familiar, based on adaptation rates. For example, subjects that showed slower adaptation to NoGO than to GO songs (indicating greater familiarity of the NoGO songs) also showed fewer behavioral responses to the probe trials during training (indicating they treated unfamiliar probes as NoGO stimuli). The opposite relationship was seen in birds that showed slower adaptation to GO songs.
An additional factor contributes to the interpretation. There was a nonsignificant correlation between speed of behavioral acquisition and probe responsiveness: the animals that learned more slowly were more likely to respond to probes as if they were GO stimuli. The data with probe stimuli are consistent with the interpretation that faster learners attend to NoGO stimuli to perform the auditory discrimination, while slower learners use identification of GO stimuli as their primary strategy. There are likely individual differences, between the subjects, in motivation and the internal value of reward and punishment. However, even without a complete explanation of these results, our data suggest that learning, and the plastic neural changes that occur during the learning process, reflect subject variables specific to each individual.
Lateralization
Although the effects of training were similar in left and right NCM for the majority of the analyses conducted, fast- and slow-learning groups did show significant differences in the degree to which their ARMs were lateralized in NCM. ARMs were significantly higher in the left NCM than in the right for fast learners, while slow learners exhibited a trend in the opposite direction, a pattern which is typical of naive birds (right higher than left, Phan and Vicario 2010). Ongoing research in the laboratory has further suggested the right-lateralization of auditory responses, seen in zebra finches, can be reversed by changes in the acoustic environment; the left hemisphere of NCM is activated by novel auditory experience, and neurogenesis in this area is involved in successful song-learning early in a songbird's life (Tsoi et al. 2014; Yang and Vicario 2015). Consequently, the experience of successful learning may cause responses in the left hemisphere of NCM to increase, while responses in the right hemisphere of NCM decrease; alternatively subjects that are more left lateralized for auditory responses may learn auditory discriminations more easily. Although the reason for the interaction between NCM lateralization and the speed of learning cannot be resolved with these data, this result does suggest that the two hemispheres of NCM serve different roles in auditory discrimination learning. Therefore, further study of the relationship between auditory learning, lateralization and neurogenesis in the songbird may help to identify variables that produce successful processing of communication signals. Ultimately, this research approach may have valuable implications for understanding deficits in the processing of communication signals such as language aphasias in humans.
In summary, our results indicate that animals show modified neural representations in the auditory forebrain for those sensory stimuli that they learned to discriminate behaviorally. This study is consistent with work showing that learned discriminations affect the neural responses of primary sensory areas of the brain, (Gentner and Margoliash 2003; Gilbert et al. 2009; Thompson and Gentner 2010; Weinberger, 1998), even extending out to the sensory periphery (Kass et al. 2013). NCM and CMM are sensory areas only three to four synapses beyond from the avian auditory thalamus, yet their activity is modulated in a way that reflects both the history of exposure to a stimulus and its behavioral relevance. It is too soon to know how much of this modulation arises locally and how much originates from “top-down” processes. Nonetheless, the current results show that sensory structures can do more than simply encode stimulus characteristics. If social reinforcement modulates neural responses to individual recognition cues as operant training does, then social experience should also affect the activity of neurons in these primary sensory cortices. This modulation could accelerate the discrimination of socially important sensory stimuli and thereby underlie faster behavioral decisions.
GRANTS
This work was funded by National Institute on Deafness and Other Communications Disorders Grants DC-008854 and DC-013174.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.A.B., M.L.P., and D.S.V. conception and design of research; B.A.B. performed experiments; B.A.B. analyzed data; B.A.B., M.L.P., and D.S.V. interpreted results of experiments; B.A.B. prepared figures; B.A.B. drafted manuscript; B.A.B., M.L.P., and D.S.V. edited and revised manuscript; B.A.B., M.L.P., and D.S.V. approved final version of manuscript.
ACKNOWLEDGMENTS
Comments and assistance were provided by Efe Soyman, Marina Sharobeam, and the rest of the Vicario Laboratory.
REFERENCES
- Aiya UV, Shukla PA, Pierce ML, Vicario DS, Phan ML. Using temporary inactivation to study auditory processing and interactions in the songbird forebrain. Program No. 517.07/ZZ14. In: 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2011. [Google Scholar]
- Atienza M, Cantero JL, Dominguez-Marin E. The time course of neural changes underlying auditory perceptual learning. Learn Mem 9: 138–150, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Hetebrij E, den Boer-Visser AM, De Groot JH, Zijlstra GGO. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur J Neurosci 13: 2165–2170, 2001. [DOI] [PubMed] [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci U S A 92: 3406–3410, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci U S A 93: 1950–1905, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J, Williams H, Nottebohm F. Hemispheric differences in avian song discrimination. Proc Natl Acad Sci U S A 89: 1372–1375, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci 22: 567–631, 1999. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Ball GF. Functional differences in forebrain auditory regions during learned vocal recognition in songbirds. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190: 1001–1010, 2004. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature 424: 669–674, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gess A, Schneider DM, Vyas A, Woolley S. Automated auditory recognition training and testing. Anim Behav 82: 285–293, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W, Piesch V. Perceptual learning and adult cortical plasticity. J Physiol 587: 2743–2751, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966, vol. 1. [Google Scholar]
- Guillette LM, Reddon AR, Hoeschele M, Sturdy CB. Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc R Soc Lond B Biol Sci 278: 767–773, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrilid finches. In: Bird Vocalization, edited by Hinde RA. Cambridge, UK: Cambridge Univ. Press, 1969, p. 61–74. [Google Scholar]
- Jarvis ED, Mello CV, Nottebohm F. Associative learning and stimulus novelty influence the song-induced expression of an immediate early gene in the canary forebrain. Learn Mem 2: 62–80, 1995. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Homology and evolutionary origins of the “neocortex”. Brain Behav Evol 38: 264–272, 1991. [DOI] [PubMed] [Google Scholar]
- Kass MD, Moberly AH, Rosenthal MC, Guang SA, McGann JP. Odor-specific, olfactory marker protein-mediated sparsening of primary olfactory input to the brain after odor exposure. J Neurosci 33: 6594–6602, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsnelson E, Motro U, Feldman MW, Lotem A. Individual-learning ability predicts social-foraging strategy in house sparrows. Proc R Soc Lond B Biol Sci 278: 582–589, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton DF. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene's response to that song in zebra finch telencephalon. J Neurosci 15: 6919–6925, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A 89: 6818–6822, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menardy F, Touiki K, Dutrieux G, Bozon B, Vignal C, Mathevon N, Del Negro C. Social experience affects neuronal responses to male calls in adult female zebra finches. Eur J Neurosci 35: 1322–1336, 2012. [DOI] [PubMed] [Google Scholar]
- Miller DB. Long-term recognition of father's song by female zebra finches. Nature 280: 389–391, 1979a. [Google Scholar]
- Miller DB. The acoustic basis of mate recognition by female Zebra finches (Taeniopygia guttata). Anim Behav 27: 376–380, 1979b. [Google Scholar]
- Moorman S, Gobes SM, Kuijpers M, Kerkhofs A, Zandbergen MA, Bolhuis JJ. Human-like brain hemispheric dominance in birdsong learning. Proc Natl Acad Sci U S A 109: 12782–12787, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaka T, Okanoya K. Cognitive tactics of Bengalese finch (Lonchura striata var. domestica) for song discrimination in a GO/NoGO operant task. J Ethol 27: 11–18, 2008. [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A 103: 1088–1093, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Vicario DS. Hemispheric differences in processing of vocalizations depend on early experience. Proc Natl Acad Sci U S A 107: 2301–2306, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range F, Bugnyar T, Schlögl C, Kotrschal K. Individual and sex differences in learning abilities of ravens. Behav Processes 73: 100–106, 2006. [DOI] [PubMed] [Google Scholar]
- Riebel K. Early exposure leads to repeatable preferences for male song in female zebra finches. Proc Biol Sci 267: 2553–2558, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford SE, Lange HS, Maney DL. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Dev Neurobiol 70: 73–86, 2010. [DOI] [PubMed] [Google Scholar]
- Smulders TV, Jarvis ED. Different mechanisms are responsible for dishabituation of electrophysiological auditory responses to a change in acoustic identity than to a change in stimulus location. Neurobiol Learn Mem 106: 163–176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripling R, Milewski L, Kruse AA, Clayton DF. Rapidly learned song-discrimination without behavioral reinforcement in adult male zebra finches (Taeniopygia guttata). Neurobiol Learn Mem 79: 41–50, 2003. [DOI] [PubMed] [Google Scholar]
- Theunissen FE, Doupe AJ. Temporal and spectral sensitivity of complex auditory neurons in the nucleus HVc of male zebra finches. J Neurosci 18: 3786–3802, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JV, Gentner TQ. Song recognition learning and stimulus-specific weakening of neural responses in the avian auditory forebrain. J Neurophysiol 103: 1785–1797, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi SC, Aiya UV, Wasner KD, Phan ML, Pytte CL, Vicario DS. Hemispheric asymmetry in new neurons in adulthood is associated with vocal learning and auditory memory. PLoS One 9: e108929, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello F, Nottebohm CV. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebrafinches. J Comp Neurol 366: 613–642, 1996. [DOI] [PubMed] [Google Scholar]
- Vignal C, Mathevon N, Mottin S. Audience drives male songbird response to partner's voice. Nature 430: 448–451, 2004. [DOI] [PubMed] [Google Scholar]
- Vignal C, Mathevon N, Mottin S. Mate recognition by female zebra finch: analysis of individuality in male call and first investigations on female decoding process. Behav Processes 77: 191–198, 2008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci U S A 107: 12676–12681, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Tuning the brain by learning and by stimulation of the nucleus basalis. Trends Cogn Sci 2: 271–273, 1998. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Reconceptualizing the primary auditory cortex: learning, memory and specific plasticity. In: The Auditory Cortex, edited by Winer JA, and Schreiner CE. New York: Springer, 2011, chapt. 22, p. 465–491. [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol 6: e62, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LM, Vicario DS. Exposure to a novel stimulus environment alters patterns of lateralization in avian auditory cortex. Neuroscience 285: 107–118, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann RA. Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford, UK: Oxford University Press, 1996. [Google Scholar]