Abstract
Painful neuropathy frequently develops as a consequence of commonly used chemotherapy agents for cancer treatment and is often a dose-limiting side effect. Currently available analgesic treatments are often ineffective on pain induced by neurotoxicity. Although peripheral administration of cannabinoids, endocannabinoids, and inhibitors of endocannabinoid hydrolysis has been effective in reducing hyperalgesia in models of peripheral neuropathy, including chemotherapy-induced peripheral neuropathy (CIPN), few studies have examined cannabinoid effects on responses of nociceptors in vivo. In this study we determined whether inhibition of fatty acid amide hydrolase (FAAH), which slows the breakdown of the endocannabinoid anandamide (AEA), reduced sensitization of nociceptors produced by chemotherapy. Over the course of a week of daily treatments, mice treated with the platinum-based chemotherapy agent cisplatin developed robust mechanical allodynia that coincided with sensitization of cutaneous C-fiber nociceptors as indicated by the development of spontaneous activity and increased responses to mechanical stimulation. Administration of the FAAH inhibitor URB597 into the receptive field of sensitized C-fiber nociceptors decreased spontaneous activity, increased mechanical response thresholds, and decreased evoked responses to mechanical stimuli. Cotreatment with CB1 (AM281) or CB2 (AM630) receptor antagonists showed that the effect of URB597 was mediated primarily by CB1 receptors. These changes following URB597 were associated with an increase in the endocannabinoid anandamide in the skin. Our results suggest that enhanced signaling in the peripheral endocannabinoid system could be utilized to reduce nociceptor sensitization and pain associated with CIPN.
Keywords: cisplatin, mouse, tibial nerve, allodynia, URB597, AM281, AM630
neurotoxicity is a major consequence of cancer treatment regimens that include chemotherapy agents. One of the difficulties in administering an adequate amount of chemotherapy drug is that painful peripheral neuropathy from the toxicity can necessitate diminishing the dose or discontinuing treatment prematurely (see Bhagra and Rao 2007 for review; Richardson et al. 2006). Symptoms of chemotherapy-induced peripheral neuropathy (CIPN) can appear early and progress over the course of treatment. Patients commonly experience sensory disturbances in the extremities, which range from numbness and tingling to painful burning or electrical sensations in the hands and feet (Krarup-Hansen et al. 2007; Quasthoff and Hartung 2002; Hamers et al. 1991). This syndrome can be severe and persist for months to years after treatment has ended (McWhinney et al. 2009; Quasthoff and Hartung 2002). In addition, pain is the primary dose-limiting side-effect of chemotherapy (Dworkin et al. 2003; Cherny et al. 1994).
Platinum-based chemotherapy compounds, including cisplatin, are used for a variety of cancers including breast, ovarian, and prostate (see Kelland 2007 for review). Cisplatin causes debilitating and painful peripheral neuropathy for a large proportion of patients, and studies have shown that patients treated with cisplatin and other platinum-based therapies are more likely to cease treatment due to CIPN than tumor progression itself (McWhinney et al. 2009; Richardson et al. 2006; McDonald et al. 2005; Diaz-Rubio et al. 1998). Cisplatin does not cross the blood brain barrier but diffuses easily into peripheral tissue, including the dorsal root ganglia (DRG; Quasthoff and Hartung 2002). Cisplatin exerts its cytotoxic effects by binding to nuclear and mitochondrial DNA and inhibiting transcription and synthesis of proteins involved in signal transduction (Huang et al. 1995; Melli et al. 2008; Ta et al. 2006; McDonald et al. 2005; Gill and Windebank 1998). Disruption of energy production from mitochondria dysfunction is associated with axonal degeneration in distal axons (Melli et al. 2008; Raff et al. 2002). Reactivity with structural proteins, including cytoskeletal microfilaments, may also contribute to axonal degeneration as well as apoptosis (Jordan and Carmo-Fonseca 2000).
Animal models of CIPN using oxaliplatin treatment showed decreased response thresholds and increased evoked activity of C fibers. Interestingly, hyperalgesia induced by oxaliplatin was blocked by the selective destruction of isolectin B4 (IB4)-positive nociceptors by the IB4-saporin conjugate, suggesting that this specific subtype of nociceptor may be more susceptible to the sensitizing effects of platinum compounds (Joseph et al. 2008). The effects of cisplatin on nociceptor activity have not been studied.
Analgesics typically used to treat neuropathic pain appear to be relatively ineffective in alleviating pain from CIPN (opioids: Cherny et al. 1994; gabapentin: Rao et al. 2007; amitriptyline: Kautio et al. 2008; for review, see Wolf et al. 2008). It is well known that cannabinoids reduce pain and hyperalgesia in models of inflammatory, neuropathic, and cancer pain (see reviews: Walker et al. 2001; Walker and Huang 2002; Burns and Ineck 2006; Rice et al. 2002). There is interest in whether the endogenous cannabinoids could be modulated in the periphery to attenuate or prevent CIPN. Peripheral administration of the endocannabinoid 2-arachidonoyl glycerol (2-AG), or compounds that inhibit its hydrolysis, attenuates hyperalgesia produced by inflammation (Guindon et al. 2007) and cancer (Khasabova et al. 2011) through cannabinoid receptors. It was shown that hyperalgesia following cisplatin treatment was associated with decreased levels of the endocannabinoid anandamide (AEA) in the skin, and intraplantar administration of AEA or URB597 {(3′-(aminocarbonyl)[1,1′-biphenyl]-3-yl)-cyclohexylcarbamate}, a compound that inhibits the breakdown of AEA by fatty acid amide hydrolase (FAAH), attenuates mechanical allodynia produced by cisplatin treatment through CB1 receptors (Khasabova et al. 2012). These studies provide a rationale to further examine the potential modulation of endocannabinoids in the treatment of painful CIPN.
In the present study, we examined changes in spontaneous activity and response properties of cutaneous nociceptors in mice treated with cisplatin and determined whether peripheral administration of URB597, which inhibits the breakdown of AEA by inhibiting FAAH, would attenuate nociceptor sensitization in cisplatin-treated mice.
MATERIALS AND METHODS
Subjects.
One-hundred forty-one adult (8–10 wk old) male C3H/HeJ mice (Jackson Laboratories), housed in cages of three to four on a 12-h light-dark cycle, were used. One-hundred and twelve mice received cisplatin treatment, 23 mice received normal saline only, and 6 mice were used as nontreated controls. All subjects had free access to food and water. Behavioral testing occurred between 8:00 AM and 5:00 PM. All protocols and procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and were conducted according to the guidelines established by the International Association for the Study of Pain (Zimmerman 1983) and the European Commission's Directive 86/609/EEC regarding the care and treatment of experimental animals.
Behavioral measurement of mechanical allodynia.
Mice were placed beneath individual glass containers (11 cm L × 6.5 cm W × 5.5 cm H) on a raised wire mesh surface and allowed to habituate for 30–40 min. Mechanical allodynia was evaluated using a calibrated von Frey monofilament (0.4 g, 3.9 mN) applied to the plantar surface of the hind paw through the mesh screen. Pressure was applied until the filament bent slightly, and it was held against the skin for 1–2 s. A withdrawal response was indicated by rapid removal of the hind paw from the monofilament, which was occasionally followed by rapid flinching and/or licking of the plantar surface. The monofilament was applied to each hind paw 10 times at intervals of at least 5 s, and the total number of withdrawal responses was recorded for each hind paw. The experimenter was blinded to the treatment condition.
Baseline measurements were taken over a 3-day period before the start of cisplatin or saline treatment. For cisplatin-treated mice, only subjects demonstrating consistent mechanical allodynia in both hind paws were used for electrophysiological studies. Mice that did not exhibit withdrawal response frequencies of at least 60% following treatment were not used (<10% of mice). All subjects who received saline injections alone were used for electrophysiology studies.
Surgical procedures and electrophysiological recording.
Mice were anesthetized using acepromazine maleate (20 mg/kg ip) and sodium pentobarbital (48 mg/kg ip). The level of anesthesia was evaluated by applying pressure to the right hind paw or tail and/or testing for corneal reflexes. Supplemental doses of sodium pentobarbital were administered as needed. At the completion of the experiment, mice were euthanized by an overdose of sodium pentobarbital.
Once mice were adequately anesthetized, the hair around the left hind leg was removed and an incision was made in the skin over the gastrocnemius muscle, which was dissected and removed to access the tibial nerve. The skin was then sutured to a stainless steel ring (1.3-cm inner diameter) to form a pool that was filled with mineral oil. Dental impression material (COE-FLEX; GC America) was applied to the skin and around the ring to prevent oil from leaking out of the pool during the experiment and to stabilize the hind paw. After the impression material had cured (∼10 min), the nerve was gently dissected from surrounding tissue and placed on a small mirror platform to perform fine dissection of nerve fibers. The epineurium was cut and removed allowing small bundles of fibers to be cut proximally, teased into fine filaments using fine forceps, and placed on a silver wire recording electrode. Action potentials from individual fibers were amplified, audiomonitored, and visualized on an oscilloscope and PC using Spike 2.0 software (CED, Cambridge, UK). Nociceptors were initially identified by mild pinching and/or applying pressure to the glabrous skin of the hind paw. Fibers responding to brushing and/or light touch and not responding to pinching of the skin were classified as mechanoreceptors and not studied. von Frey monofilaments were used to identify the precise location of the receptive field, which was marked on the skin with a felt-tip pen.
Conduction velocity (CV) was determined for each fiber. The fiber was stimulated electrically by insertion of two fine pin electrodes under the skin outside the receptive field. Beginning with a voltage below threshold, electrical pulses (200 μs) were delivered every 2 s until the response threshold was reached, and the conduction velocity was calculated using a stimulus 1.5 times the threshold value. The CV for each fiber was determined by dividing the conduction distance (distance from receptive field to recording electrode in mm) by the latency to the action potential. Fibers with CV of 1.3 m/s or less were classified as C fibers, those with CV between 1.3 and 13.6 m/s were classified as Aδ fibers, and those with CV >13.6 were considered Aδ fibers. For this study, C-fiber and Aδ-fiber nociceptors were preferentially studied.
Electrophysiological responses of nociceptors.
Once a nociceptor was identified, the rate of spontaneous activity was determined for a period of 2 min before any testing. Mechanical response thresholds were obtained using a set of calibrated von Frey monofilaments. The receptive field was stimulated multiple times with a single filament, and if no response was elicited, the next higher force (or lower force if there was a response) was applied. Response threshold was defined as the lowest force eliciting a response on 50% or more of the trials.
Responses evoked by suprathreshold mechanical stimuli were determined using a single suprathreshold von Frey monofilament that delivered a force of 147 mN. This monofilament was applied three times, each for a duration of 5 s with an interstimulus interval of 60 s. The response to the von Frey stimulus was defined as the mean number of evoked action potentials from the three trials.
A Peltier device (contact area 1 cm2) was used to deliver heat stimuli to the skin. Beginning at a base temperature of 32°C, stimuli of 34 to 50°C were delivered in ascending order of 2°C. Each stimulus was applied for 5 s, and a 60-s interstimulus interval was utilized. The temperature at which the fiber first responded was considered the heat threshold. Nociceptors that did not respond to any heat stimuli were classified as either C mechanonociceptors (CM) or Aδ mechanonociceptors (AδM) while those that responded to heat were classified as C-mechanoheat nociceptors (CMH) or Aδ- mechanoheat nociceptors (AδMH). We were unable to apply heat to some fibers due to the location of the receptive field.
Drug preparation and administration.
The platinum chemotherapy agent cis-dichlorodiammine platinum(II) (cisplatin; LKT Laboratories) was prepared in a 1 mg/ml solution in normal saline and administered intraperitoneally at 1 ml/kg. The FAAH inhibitor {3′-(aminocarbonyl)[1,1′-biphenyl]-3-yl}-cyclohexylcarbamate (URB597; Cayman Chemical) was prepared in a stock solution of 2.4 mg/ml using a vehicle consisting of DMSO and normal saline at a ratio of 7:3. The CB1 receptor antagonist 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide (AM 281; Tocris Bioscience) and the CB2 receptor antagonist 6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl(4- methoxyphenyl)methanone (AM 630; Tocris Bioscience) were prepared in stock solutions of 10 mg/ml in DMSO. Drugs were diluted to the appropriate concentration in sterile physiological saline before injection. URB597 was administered at a dose of 9 μg (in 10 μl), and the antagonists were administered at a dose of 10 μg in 10 μl. Vehicle injections also consisted of a 10-μl volume. All injections were made using a 0.3-ml syringe with a 28-gauge needle. Care was taken to insert the needle outside the receptive field to avoid damage to the receptive field.
Experimental design.
Naïve mice were tested for baseline mechanical paw withdrawal responses for 3 days before the start of treatment to ensure normal functioning before experimental manipulations. Following the third baseline assessment, mice were randomly assigned to a condition and received injections of either cisplatin (1 mg/kg ip) or an equivalent volume of normal saline (ip). Behavioral assessment of allodynia occurred daily before injections throughout the 7-day treatment period, with a final assessment occurring on postinjection day (PID) 8 followed immediately by electrophysiology experiments. For cisplatin-treated mice, only those that demonstrated consistent mechanical hypersensitivity were used for electrophysiology experiments. Mice were anesthetized and prepared for recording as described above.
To compare the characteristics of peripheral nociceptors in cisplatin-treated mice to saline- treated controls, all well-isolated C and Aδ fibers encountered during the experiment were studied. Once a fiber was isolated, the presence or absence of spontaneous activity was determined first by recording 2 min of activity in the absence of stimulation. This was followed by determining the mechanical response threshold, responses evoked by a suprathreshold mechanical stimulus (147 mN), heat threshold (if applicable), and CV.
The second part of the study focused on sensitized C-fiber nociceptors in cisplatin-treated mice only. For these experiments, only C fibers with spontaneous discharges during the initial 2 min of recording were studied. Once the fiber had been isolated, spontaneous activity and evoked responses were determined. Then, drug or vehicle was injected into the receptive field, and responses were determined every 30 min for 2 h.
Injections consisted of the FAAH inhibitor URB597 alone or with the CB1 (AM281) or CB2 (AM630) receptor antagonists or a vehicle control. Antagonists were administered 5 min before injection of URB597. In addition, a group of fibers was given an injection of vehicle alone 5 min before the injection of URB597 to determine whether multiple injections altered the impact of the drug.
Measurement of AEA, 2-AG, and palmitoylethanolamide in the skin.
To determine whether intraplantar injection of URB597 produced an increase in AEA locally, samples of plantar paw skin were collected from the ipsilateral paw of a group of cisplatin-treated mice following an intraplantar injection of URB597 or vehicle as well as a group of nontreated mice following an intraplantar injection of vehicle. Samples of paw skin were collected 1.5 h after injection of URB597 (9 μg in 10 μl) or vehicle (10 μl). Upon removal, samples were weighed, frozen in liquid nitrogen, and kept frozen at −80°C until the time of processing. To determine the selectivity and efficacy of URB597 on endogenous levels of AEA, 2-AG and palmitoylethanolamide (PEA) were also measured as previously described (Khasabova et al. 2008). On the first day of processing, samples were mixed with 5 vol of chloroform containing 5 pmol of d8-AEA, 100 pmol of d8-2-AG, and 5 pmol of d4-PEA (Cayman Chemical) as internal standards. Extraction of lipids occurred by incubation at 4°C overnight. Mixtures were then homogenized with an equal volume of methanol/Tris·HCl (50 mM) (1:1). Homogenates were centrifuged at 3,000 g for 15 min (4°C). The organic phase was evaporated with a gentle stream of nitrogen gas. Targeted isotope-dilution HPLC/atmospheric pressure chemical ionization/mass spectrometry was conducted on each sample. The AEA, 2-AG, and PEA levels in experimental samples were estimated from the ratio of the area of deuterated compounds and AEA (0.02–20 pmol), 2-AG (2–2000 pmol), or PEA (0.02–20 pmol) standards and were expressed as picomoles or nanomoles per gram of tissue.
Data analysis.
The frequency of withdrawal responses evoked by the 3.9-mN von Frey monofilament was expressed as the number of positive responses out of 10 trials for each hind paw and the average of both paws used for analysis. A two-way ANOVA with repeated measures was used to compare the difference between withdrawal responses before and after cisplatin treatment or saline. Fisher's least significant difference (LSD) post hoc tests were used to determine differences between the groups at specific time points.
The χ2-square tests were used to compare the distribution of spontaneously active C-fiber and Aδ-fiber nociceptors. Because of the variable rate of spontaneous activity among C fibers from cisplatin-treated mice and the lack of spontaneous activity in most fibers from control mice, the Mann-Whitney U-test was used to assess the difference in the median levels of spontaneous discharge of C fibers between groups. Mechanical response thresholds in millinewtons were log transformed to perform parametric statistical comparison between spontaneous and nonspontaneous fibers of both types between cisplatin- and saline-treated mice. Evoked responses of each fiber to the suprathreshold von Frey monofilament were determined by subtracting the number of spontaneous impulses during the 5 s immediately preceding each stimulus from the number of impulses evoked during the stimulus. The average evoked response over three trials was calculated for analysis. The mean numbers of evoked impulses were compared between cisplatin- and saline-treated mice using an independent t-test.
To determine changes in response characteristics produced by URB597 (alone or in combination with AM281 or AM630) compared with vehicle control, we compared spontaneous activity, mechanical response thresholds, and responses evoked by the suprathreshold von Frey monofilament before and after injection. A log transformation was performed on mechanical response thresholds (mN) to correct for positively skewed data among postinjection time points, and parametric analyses were performed. Data for the evoked response to a suprathreshold mechanical stimulus (147 mN) were expressed as percent change from preinjection values. Log-transformed mechanical response thresholds, the percent change in response to suprathreshold mechanical stimulus, and the rate of spontaneous discharge were evaluated using two-way ANOVA with repeated measures. Post hoc evaluations (Fisher's LSD) were performed to determine differences between the groups at specific time points and changes within each group over time. All data are expressed as means ± SE or median ± interquartile range.
One-way ANOVA or Mann-Whitney U-tests were performed to assess the difference in levels of AEA, 2-AG, and PEA in the plantar skin of the hind paw from cisplatin-treated vehicle and URB597-treated and nontreated vehicle groups.
RESULTS
Mechanical allodynia produced by cisplatin.
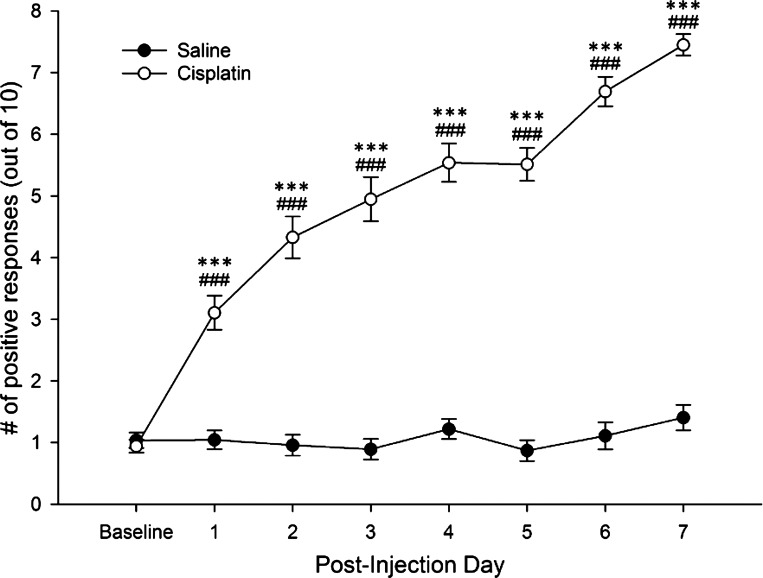
In the first experiment, 64 mice completed the 7-day treatment period, with 41 mice receiving cisplatin and 23 receiving saline. One cisplatin-treated mouse did not develop mechanical allodynia and was not included in the study. The frequency of paw withdrawal responses differed between cisplatin- and vehicle-treated mice (F1,61 = 200.32; P < 0.001). Post-hoc comparisons (Fisher's LSD) showed that while cisplatin- and vehicle-treated mice did not differ at baseline, mice treated with cisplatin had increased paw withdrawal frequency starting on PID1. Withdrawal frequency after cisplatin peaked at approximately PID7 and remained at this level for several weeks (unpublished observations). Response frequencies among control mice remained near baseline values throughout the treatment period (Fig. 1).
Fig. 1.
Cisplatin administration was associated with significantly increased paw withdrawal responses relative to both baseline assessments and saline-treated mice starting on postinjection day 1. Withdrawal responses in saline controls remained near baseline throughout the treatment period. ***P < .001 vs. saline; ###P < .001 vs. baseline.
Sensitization of nociceptors produced by cisplatin.
A total of 152 C-fiber and 59 Aδ-fiber nociceptors were studied for comparison between saline- and cisplatin-treated mice. A breakdown of response characteristics by fiber type is presented in Table 1. A large proportion of both C (44%) and Aδ fibers (45%) isolated in cisplatin-treated mice exhibited abnormal spontaneous discharge, while only three (8%) of C fibers and none of the Aδ fibers in saline-treated mice had spontaneous discharge. The proportion of fibers with spontaneous activity differed between saline- and cisplatin-treated mice for both C (χ2[1] = 32.48; P < 0.001) and Aδ fibers (χ2[1] = 16.72; P < 0.001). The rate of spontaneous activity for all C fibers in cisplatin-treated mice was higher compared with saline-treated mice (Mann-Whitney U-test = 1,127.50; n1 = 40; n2 = 112; P < 0.001; see Table 1). There were no differences between the saline- and cisplatin-treated groups in nerve fiber conduction velocities, mechanical response thresholds, or responses evoked by the suprathreshold von Frey monofilament when fibers were compared as a group regardless of the presence or absence of spontaneous activity. Similarly, heat response thresholds did not differ significantly among C fibers from cisplatin-treated mice (39.8 ± 1.3°C; n = 11) and those from saline-treated mice (42.0 ± 0.8°C; n = 6).
Table 1.
General characteristics of nociceptors in saline- and cisplatin-treated mice
| Group/Fiber Type | CV, m/s | Spontaneous Discharge? | SA Rate, Hz | Median Response Threshold, mN | Response to 147-mN Force, #imp/stimulus | |
|---|---|---|---|---|---|---|
| Saline | ||||||
| Aδ (n = 21) | 5.8 ± 0.7 | Yes | 0 (0%) | — | — | — |
| No | 21 (100%) | 0.00 ± 0.00 | 17.5 ± 4.9 | 71.4 ± 7.7 | ||
| C (n = 40) | 0.34 ± 0.03 | Yes | 3 (8%) | 0.20 ± 0.02 | 19.6 ± 25.5 | 36.1 ± 2.9 |
| No | 37 (92%) | 0.00 ± 0.00 | 13.7 ± 9.8 | 35.5 ± 8.8 | ||
| Cisplatin | ||||||
| Aδ (n = 38) | 7.4 ± 1.0 | Yes | 17 (45%)† | 0.32 ± 0.14 | 9.8 ± 35.3 | 70.0 ± 12.3 |
| No | 21 (55%) | 0.00 ± 0.00 | 9.8 ± 15.7 | 70.0 ± 10.5 | ||
| C (n = 112) | 0.41 ± 0.04 | Yes | 49 (44%)† | 0.39 ± 0.11† | 9.8 ± 13.7* | 47.4 ± 3.8 |
| No | 63 (56%) | 0.01 ± 0.00 | 19.6 ± 29.4 | 38.8 ± 4.0 | ||
Values are expressed as means ± SE or median ± interquartile range.
CV, conduction velocity; SA, spontaneous activity.
P < .001 SA vs. non-SA.
P < .001 cisplatin vs. saline.
Thus C and Aδ fibers isolated from cisplatin-treated mice were further divided into subgroups based on whether or not they exhibited spontaneous discharge that was consistently at or above 0.05 Hz (equivalent to 1 impulse every 20 s) to elucidate whether abnormal spontaneous discharge was associated with other signs of sensitization. No differences in log-transformed mechanical thresholds or mechanically evoked responses of Aδ nociceptors were found between those with and without spontaneously activity. However, C fibers with spontaneous activity (n = 49) had lower log-transformed mechanical response thresholds (2.42 ± 0.10 log mN) than those without spontaneous activity (2.97 ± 0.10 log mN; t101= 3.72; P < 0.001; n = 63). There was a trend for evoked responses to the suprathreshold von Frey monofilament to be greater among C fibers with spontaneous activity (47.4 ± 3.8 impulses) compared with those without spontaneous activity (38.8 ± 4.0 impulses), but this was not significant. Although evidence of sensitization (abnormal spontaneous discharge) was found for both C and Aδ fibers, lower response thresholds and a trend towards higher evoked responses suggested that sensitization among C fibers was more robust.
URB597 decreased spontaneous activity and responses to mechanical stimuli of C-fiber nociceptors.
Based on the data presented in Table 1, sensitization produced by cisplatin treatment was more marked in C fibers than in Aδ fibers. We therefore focused on C fibers with spontaneous discharge to determine whether URB597 would attenuate sensitization. Mice were divided into separate treatment groups that received injection of vehicle (n = 14), URB597 (n = 19), AM281 + URB597 (n = 10), and AM630 + URB597 (n = 6) into the receptive field. The magnitude of mechanical allodynia, as indicated by the frequency of paw withdrawal, did not differ for these groups of cisplatin-treated mice. A total of 54 C-fiber nociceptors were studied from 49 mice following 7 days of cisplatin treatment. Of these, 50 were classified as CM (92.5%) and 4 were classified as CMH (7.4%), with a mean heat threshold of 38.0 ± 0.8°C.
Before any injection, the mean rate of spontaneous discharge was 0.21 ± 0.02 Hz, the median mechanical response threshold was 19.61 ± 13.73 mN, and the mean number of impulses evoked by the suprathreshold von Frey stimulus was 43.6 ± 3.2 impulses. These values did not differ among the groups. Once a fiber was characterized, vehicle or drug in a volume of 10 μl was injected just outside the receptive field to avoid damaging the fiber. Injections of the antagonists preceded the injection of URB597 by 5 min. For a small number of fibers (n = 4), vehicle was injected into the skin followed 5 min later by injection of URB597 to determine whether multiple injections alone altered the effect of URB597. Since no differences were observed in the drug's impact whether vehicle was injected before URB597 or URB597 was given alone, data from these groups were pooled.
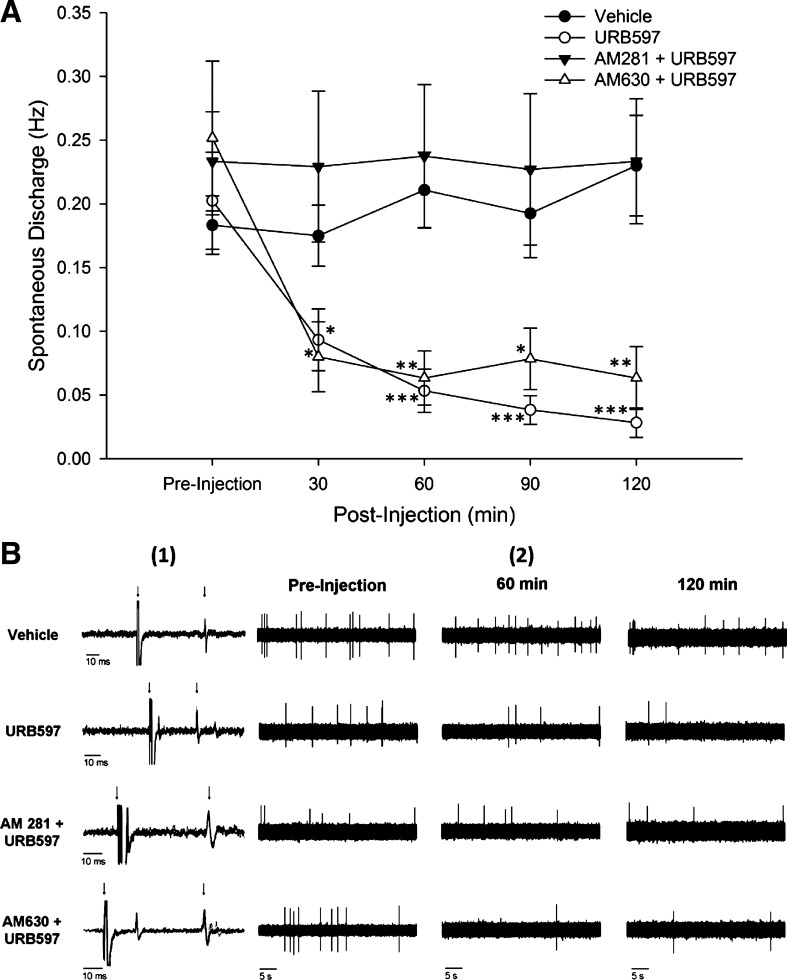
Spontaneous activity was decreased following injection of URB597 (see Fig. 2). Fibers with a baseline rate spontaneous discharge <0.10 Hz were not studied (n = 23). There was a significant difference in spontaneous discharge rate among the groups (two-way ANOVA; F3,25 = 7.10; P = 0.001). There were no differences in discharge rates among the vehicle (n = 10)-, URB597 (n = 10)-, AM281 + URB597 (n = 4)-, and AM630 + URB597 (n = 5)-treated groups before injection.
Fig. 2.
A: in C fibers isolated from cisplatin-treated mice, administration of URB597 significantly reduced spontaneous discharge, while vehicle treatment had no effect on spontaneous activity rates. The CB1 antagonist AM281 blocked the effect of URB597, while the CB2 antagonist AM630 failed to inhibit the effect of URB597. *P < 0.05; **P < 0.005; ***P < 0.001 vs. vehicle. B1: conduction latency of selected C-fiber nociceptors for each condition. Four individual traces were overlapped to show illustrate consistent conduction velocity. Time scale is equal to 10 ms. The left arrow indicates the onset of the stimulus, and the right arrow indicates the action potential of the fiber of interest. B2: representative samples of spontaneous activity over a 45-s period for each condition.
Administration of URB597 reduced the rate of spontaneous discharge relative to the vehicle-treated group at 30, 60, 90, and 120 min after injection. Vehicle treatment did not alter spontaneous discharge at any time. Pretreatment with AM281 blocked the effect of URB597, whereas pretreatment with AM630 did not alter the effect of URB597.
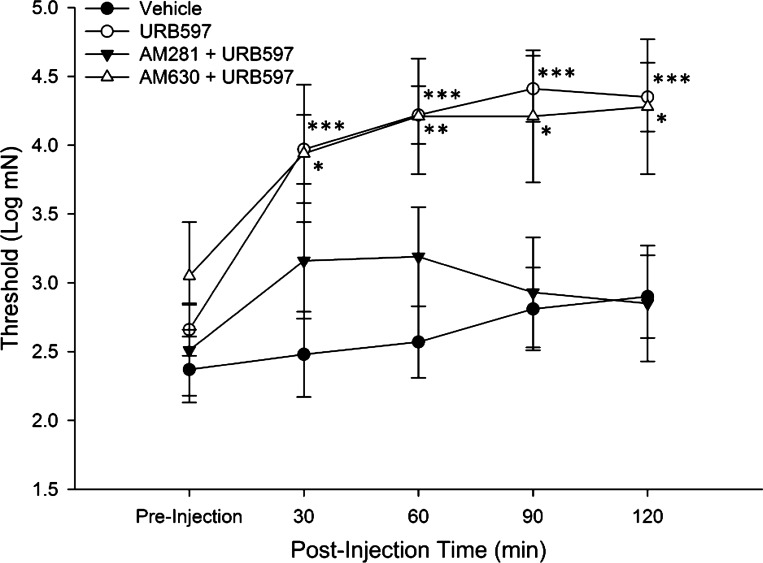
Log transformation of mechanical response thresholds were evaluated before and at 30, 60, 90, and 120 min after injection. Two-way ANOVA with repeated measures indicated a significant difference in threshold between the groups (F3,41 = 7.89; P < 0.001). Post hoc comparisons showed that while there were no differences in response thresholds among vehicle (n = 13)-, URB597 (n = 20)-, AM281 + URB597 (n = 7)-, and AM630 + URB597 (n = 5)-treated groups before injection, there were differences between the groups at each time point after injection. As shown in Fig. 3, vehicle treatment did not alter mechanical response thresholds at any time, whereas URB597 treatment was associated with a significant increase in threshold at each time point. Pretreatment with the CB1 receptor antagonist AM281 blocked this effect, and response thresholds of fibers in this group did not differ from the vehicle-treated group at any time point. In contrast, pretreatment with the CB2 receptor antagonist AM630 did not alter the effect of URB597, and mechanical response thresholds for these fibers were higher than those of the vehicle-treated group at all time points after injection. In addition, response thresholds of fibers treated with URB597 were increased at all time points after injection, and those pretreated with AM630 had higher thresholds relative to preinjection at 60, 90, and 120 min after injection.
Fig. 3.
In C fibers isolated from cisplatin-treated mice, URB597 administration significantly increased the mechanical response threshold at all postinjection time points relative to vehicle-treated fibers. The CB1 antagonist AM281 blocked the effect of URB597 while the CB2 antagonist AM630 had no effect. *P < 0.05; **P < 0.005; ***P ≤ 0.001 vs. vehicle.
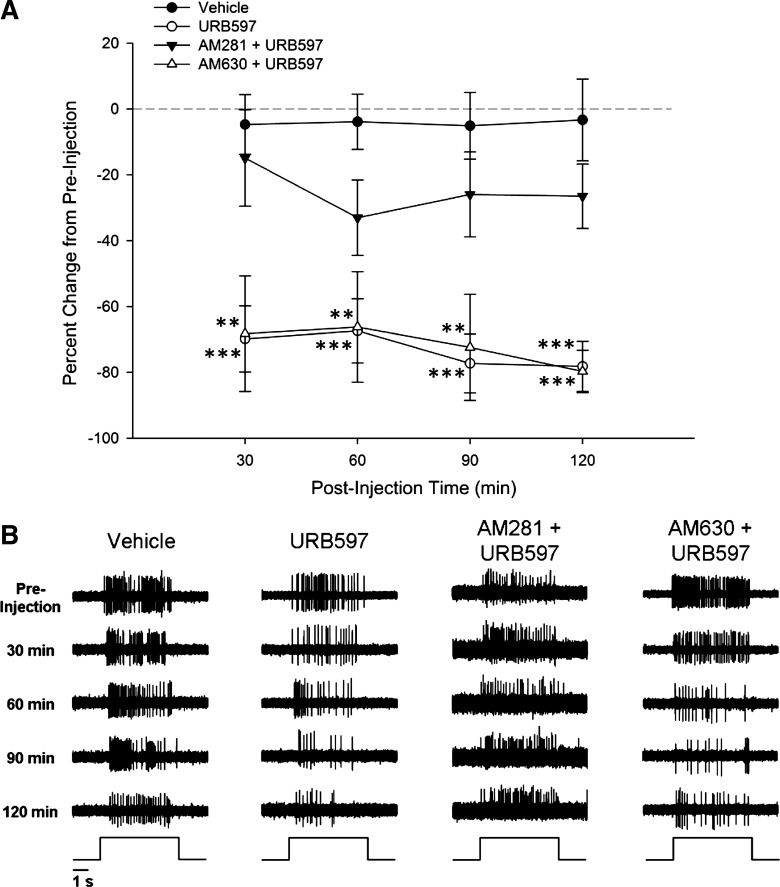
Responses to suprathreshold mechanical stimulation (147 mN for 5 s) were assessed by counting the number of action potentials evoked during the 5-s stimulus (the number of action potentials that occurred during the 5 s just before the stimulus was subtracted). The mean number of action potentials was determined over three trials and was used for analysis. Baseline responses in vehicle (n = 12)-, URB597 (n = 19)-, AM281 + URB597 (n = 9)-, and AM630 + URB597 (n = 5)-treated groups did not differ in the mean number of action potentials before injection (vehicle: 44.5 ± 6.1; URB597: 44.8 ± 7.2; AM281 + URB597: 38.1 ± 5.2; and AM630 + URB597: 42.5 ± 12.1 impulses). For the remaining analyses, the number of impulses evoked by the suprathreshold stimulus was expressed as a percent change from the baseline responses. Two-way ANOVA revealed a significant difference among the groups in the percent change in response (F3,41 = 13.27; P < 0.001). Post hoc comparisons (Fisher's LSD) indicated that responses were decreased (lower percent change in response) following the injection of URB597 at each time point after injection compared with those following injection of vehicle. Preinjection with AM281 blocked the effect of URB597, and responses did not differ at any time from those following injection of vehicle. Pretreatment with AM630 failed to block the effect of URB597, resulting in responses that were significantly lower than vehicle at all time points (see Fig. 4).
Fig. 4.
A: in C fibers isolated from cisplatin-treated mice, URB597 administration was associated with a significant decrease in the evoked response to a 147-mN force von Frey monofilament, while the evoked response from fibers treated with vehicle did not differ from preinjection. The CB1 antagonist AM281 attenuated the effect of URB597, while the CB2 antagonist AM630 failed to alter the effect of URB597. **P ≤ 0.005; ***P < 0.001 vs. vehicle. B: representative samples of raw data demonstrating the response to 147-mN force over 5 s for each condition.
Effects of URB597 on levels of endocannabinoids in the skin.
To determine whether attenuation of nociceptor sensitization produced by URB597 in cisplatin-treated mice was associated with increased AEA and not due to other endogenous lipids with similar physiological activity, the levels of AEA, 2-AG, and PEA were determined in samples of plantar skin from the hind paws of cisplatin-treated mice following intraplantar injections of URB597 (n = 10) or vehicle (n = 8) as well as a group of nontreated controls that received intraplantar injections of vehicle (n = 6). Paw tissue collected from cisplatin-treated mice injected with vehicle had significantly lower levels of AEA compared with tissue collected from nontreated mice injected with vehicle, while levels of AEA in the paw tissue of cisplatin-treated mice given URB597 did not differ from nontreated mice given vehicle (see Table 2). Levels of PEA were significantly higher in paw tissue from both cisplatin-treated groups relative to paw tissue from nontreated mice but no difference between tissues treated with URB597 and vehicle. Levels of 2-AG did not differ among the three treatment groups. This suggests that the effect of URB597 was specific to AEA.
Table 2.
Levels of endocannabinoids and PEA in plantar paw skin samples
| Treatment | AEA, pmol | PEA, nmol | 2-AG, nmol |
|---|---|---|---|
| Naïve + vehicle (6) | 20.1 ± 1.52* | 0.6 [0.34–0.68] | 21.4 ± 0.92 |
| Cisplatin + vehicle (8) | 11.0 ± 1.56 | 0.9 [0.76–1.17]† | 24.5 ± 2.35 |
| Cisplatin + URB597 (10) | 19.6 ± 1.95* | 0.9 [0.72–1.17]† | 20.1 ± 1.73 |
Data are presented as the means ± SE or the median with the 25th and 75th percentile range for palmitoylethanolamide (PEA) and expressed in picomoles or nanomoles per gram of tissue. Numbers in parentheses represent the sample size.
2-AG, 2-arachidonoyl glycerol.
Naïve mice received an intraperitoneal injection of vehicle (10 μl), and cisplatin-treated mice received an intraperitoneal injection of either vehicle (10 μl) or URB597 (9 μg in 10 μl) 1.5 h before tissue collection.
Different from cisplatin in anandamide (AEA) group at P = 0.003.
Different from vehicle in PEA group at P < 0.05 (one-way ANOVA with Bonferroni t-test).
DISCUSSION
The present study showed that allodynia produced by cisplatin treatment was associated with sensitization of cutaneous nociceptors and intraplantar administration of the FAAH inhibitor URB597 attenuated the sensitization of C-fiber nociceptors by reducing spontaneous activity, increasing mechanical response thresholds, and decreasing evoked responses to suprathreshold mechanical stimulation. While both Aδ- and C-fiber nociceptors exhibited abnormal spontaneous discharge, only spontaneously active C-fiber nociceptors appeared to have increased sensitivity to mechanical stimuli, as indicated by decreased response thresholds and enhanced responses to suprathreshold mechanical stimulation. Consistent with our data reported previously (Khasabova et al. 2012), seven daily injections of cisplatin resulted in the development of mechanical allodynia. The majority of cisplatin-treated subjects exhibited a consistent pattern of an increase in paw withdrawal frequency over the 7-day treatment period, whereas the frequency of withdrawal responses in saline-treated mice remained near baseline levels.
Earlier in vivo electrophysiological studies in control mice (Cain et al. 2001a,b) reported that very few cutaneous nociceptors exhibited spontaneous activity. In contrast, a large proportion (>40%) of both Aδ- and C-fiber nociceptors in cisplatin-treated mice had ongoing spontaneous discharge. The abnormal spontaneous activity may be related to ongoing pain, which was not measured in these studies. In addition, C-fiber nociceptors with spontaneous activity had decreased response thresholds and increased responses to suprathreshold mechanical stimuli. An increase in spontaneous activity and/or enhanced responses to mechanical stimuli also occurs in other models of peripheral neuropathy (Ali et al. 1999; Koltzenburg et al. 1994; Woolf and Ma 2007; Campbell and Meyer 2006; Shim et al. 2005; Tal and Eliav 1996; Zhang et al. 1999), including CIPN (Tanner et al. 1998; Xiao and Bennett 2008). The mechanisms that account for the nociceptor sensitization observed after cisplatin treatment may be related to increased signaling through certain ion channels since TRPV1, TRPA1 (Ta et al. 2010), TRPV2, P2X3, and ASIC3 (Hori et al. 2010) increase in DRG following cisplatin treatment. Moreover, antagonists of P2X3/2,3 and ASICs decreased cisplatin-evoked hyperalgesia (Hori et al. 2010).
Although our studies show that a portion of C-fiber nociceptors are sensitized after cisplatin treatment, it should be noted that central sensitization (Latremoliere and Woolf 2009) is also likely to contribute to ongoing pain and hyperalgesia following chemotherapy. It has been shown that spontaneous activity and/or responses of nociceptive dorsal horn neurons evoked by mechanical stimulation are increased following chemotherapy treatment, including cisplatin (Carozzi et al. 2013; Cata et al. 2008; Robinson et al. 2014; Weng et al. 2003).
Since cisplatin treatment led to more robust sensitization among C-fiber nociceptors, we focused on these fibers to examine the impact of the FAAH inhibitor URB597 on response characteristics over a 2-h period after injection. Injection of URB597 into the receptive field of sensitized C-fiber nociceptors decreased ongoing spontaneous activity, increased response thresholds to mechanical stimuli, and decreased responses to a standard suprathreshold mechanical stimulus. This effect was detected at 30 min following injection and persisted throughout the 2-h testing period. As reported previously (Khasabova et al. 2012), the effect of URB597 appeared to peak around 90–120 min after injection. Thus we conclude that the attenuation of cisplatin-induced mechanical allodynia following intraplantar administration of URB597 is a result of decreased sensitization of nociceptors, including C fibers. Similarly, we found through the use of specific cannabinoid receptor antagonists that the effect of URB597 is mediated primarily through CB1 but not CB2 receptors. Intraplantar administration of compounds that enhance peripheral cannabinoid activity have previously been shown to attenuate behavioral hyperalgesia in other models of chronic pain, including neuropathic pain (see Hohmann 2002; Rice et al. 2002 for review). In rats with neuropathic pain due to partial sciatic nerve ligation, intraplantar administration of cannabinoid agonists such as WIN 55,212–2 (Fox et al. 2001) or the endocannabinoid AEA (Guindon and Beaulieu 2006) reduced hyperalgesia and the effect was blocked by CB1, but not CB2, antagonists. Furthermore, in the case of WIN 55,212–2, intraplantar administration of the CB1 antagonist blocked its effect, but it was not impacted by intrathecal administration of the antagonist (Fox et al. 2001), indicating that the antihyperalgesic effect is dependent on peripheral mechanisms. Peripheral administration of the FAAH inhibitor URB597 attenuated responses of spinal neurons to mechanical stimulation following spinal nerve ligation, and this was blocked by pretreatment with a CB1 antagonist (Jhaveri et al. 2006). Similarly, activation of peripheral CB2 receptors with the CB2 receptor selective agonist JWH-133 inhibited activity of spinal neurons but through CB2 receptors (Elmes et al. 2004).
We also showed that the injection of URB597, but not vehicle, increased the level of AEA in the skin of the hind paw in cisplatin-treated mice. URB597 did not alter levels of 2-AG or PEA, indicating that the effect of inhibiting FAAH activity was specific to AEA metabolism and did not alter the levels of other endocannabinoids in the skin. Increased levels of AEA in the hind paw could result in increased CB1 receptor activity, which could decrease nociceptor sensitivity and response characteristics either directly (AEA binding to cannabinoid receptors located on the nociceptor itself) or indirectly (AEA binding receptors expressed in tissues surrounding the nociceptor). A large proportion of DRG neurons express mRNA for CB1, and CB1 receptors have been shown to colocalize with TRPV1 in a certain proportion of small-diameter DRG neurons (Ahluwalia et al. 2000; Bridges et al. 2003). Enhanced CB1 activity may counteract sensitization that is associated with increased TRPV1 activity following cisplatin treatment (Ta et al. 2010). CB1 receptors are also expressed on keratinocytes, and AEA has been shown to modulate the activity of immune cells (Cencioni et al. 2010; see Kupczyk et al. 2009 for review). The expression of CB1 receptors on nociceptors has been shown to be essential for cannabinoid analgesia. Loss of CB1 receptor tone in cisplatin-treated mice not only decreases response thresholds but also exaggerates hyperalgesia in experimental inflammatory pain conditions (Agarwal et al. 2007). Cisplatin treatment is associated with decreased levels of AEA in the skin of the hind paw (Khasabova et al. 2012), which could attenuate CB1 receptor activity in the periphery and thereby contribute to enhanced nociceptor sensitivity. Inhibiting FAAH to bring levels of AEA closer to those seen under normal conditions presumably contributes to a temporary return of basal cannabinoid tone in the periphery.
Interestingly, AEA has been identified as an endogenous ligand for the transient receptor potential vanilloid type one (TRPV1) receptor and is part of a growing class of endovanilloids (Melck et al. 1999; Zygmunt et al. 1999; Smart et al. 2000). Indeed, high concentrations of AEA excited isolated nociceptive dorsal root and trigeminal ganglion neurons through activation of TRPV1 receptors (Tognetto et al. 2001; Olah et al. 2001; Jerman et al. 2002; Roberts et al. 2002; Ahluwalia et al. 2003; Price et al. 2004; Fischbach et al. 2007). Consistent with these observations, we showed that AEA excited C-fiber nociceptors in vivo (Potenzieri et al. 2009). Although AEA can excite nociceptors, it is unlikely that AEA contributed to nociceptor sensitization following cisplatin since the levels of AEA in the skin were decreased following cisplatin treatment (Khasabova et al. 2012).
Drugs that inhibit the enzymes that degrade endocannabinoids show potential for use in clinical applications, especially for conditions that respond poorly to opioids (Petrosino and Di Marzo 2010). Enhancing endocannabinoid activity could help avoid some of the issues associated with using agonists that target cannabinoid receptors directly, including undesirable side-effects that affect functionality (Moreira et al. 2009). Future studies that explore the efficacy of endocannabinoid treatments are restricted to the periphery, such as the FAAH inhibitor URB937, for neuropathic conditions like CIPN whose effects begin in the distal extremities. Continued research into the analgesic and neuroprotective properties of compounds that enhance peripheral endocannabinoid activity could identify novel treatment strategies for a variety of chronic pain disorders.
GRANTS
These studies were supported by National Institute of Drug Abuse Grant DA-011471.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.L.U., I.A.K., and D.A.S. performed experiments; M.L.U. and I.A.K. analyzed data; M.L.U., I.A.K., and D.A.S. interpreted results of experiments; M.L.U. prepared figures; M.L.U. drafted manuscript; M.L.U., I.A.K., and D.A.S. edited and revised manuscript; I.A.K. and D.A.S. conception and design of research; D.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Glenn Giesler for reading an earlier version of the manuscript.
REFERENCES
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 10: 870–879, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci 17: 2611–2618, 2003. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 100: 685–688, 2000. [DOI] [PubMed] [Google Scholar]
- Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and α-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol 81: 455–466, 1999. [DOI] [PubMed] [Google Scholar]
- Bhagra A, Rao RD. Chemotherapy-induced neuropathy. Curr Oncol Rep 9: 290–299, 2007. [DOI] [PubMed] [Google Scholar]
- Bridges D, Rice AS, Egertova M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience 119: 803–812, 2003. [DOI] [PubMed] [Google Scholar]
- Burns TL, Ineck JR. Cannabinoid analgesia as a potential new therapeutic option in the treatment of chronic pain. Ann Pharmacotherapy 40: 251–260, 2006. [DOI] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol 85: 1561–1574, 2001a. [DOI] [PubMed] [Google Scholar]
- Cain DM, Wacnik PW, Eikmeier L, Beitz A, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve in a model of cancer pain in the mouse. Pain Med 2: 15–23, 2001b. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 52: 77–92, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozzi VA, Renn CL, Bardini M, Fazio G, Chiorazzi A, Meregalli C, Oggioni N, Shanks K, Quartu M, Serra MP, Sala B, Cavaletti G, Dorsey SG. Bortezomib-induced painful peripheral neuropathy: an electrophysiological, behavioral, morphological and mechanistic study in the mouse. PLoS One 12: e72995, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Dougherty PM. Behavioral and electrophysiological studies in rats with cisplatin-induced chemoneuropathy. Brain Res 1230: 91–98, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cencioni MT, Chiurchiù V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS One 5: e8688, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny NI, Thaler HT, Friedlander-Klar H, Lapin J, Foley KM, Houde R, Portenoy RK. Opioid responsiveness of cancer pain syndromes caused by neuropathic or nociceptive mechanisms: a combined analysis of controlled, single-dose studies. Neurology 44: 857–861, 1994. [DOI] [PubMed] [Google Scholar]
- Diaz-Rubio E, Sastre J, Zaniboni Labianca RA, Cortes-Funes H, de Braud F, Boni C, Benavides M, Dallavalle G, Homerin M. Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: A phase II multicentric study. Ann Oncol 9: 105–108, 1998. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell C, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain. Arch Neurol 60: 1524–1534, 2003. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci 20: 2311–2320, 2004. [DOI] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 9: 91–100, 2001. [DOI] [PubMed] [Google Scholar]
- Fischbach T, Greffrath W, Nawrath H, Treede RD. Effects of anandamide and noxious heat on intracellular calcium concentration in nociceptive drg neurons of rats. J Neurophysiol 98: 929–938, 2007. [DOI] [PubMed] [Google Scholar]
- Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest 101: 2842–2850, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Beaulieu P. Antihyperalgesic effects of local injections of anandamide, ibuprofen, rofecoxib and their combinations in a model of neuropathic pain. Neuropharmacology 50: 814–823, 2006. [DOI] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol 150: 693–701, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers FP, Gispen WH, Neijt JP. Neurotoxic side effects of cisplatin. Eur J Cancer 27: 372–376, 1991. [DOI] [PubMed] [Google Scholar]
- Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem Phys Lipids 121: 173–190, 2002. [DOI] [PubMed] [Google Scholar]
- Hori K, Ozaki N, Suzuki S, Sugiura Y. Upregulations of P2X3 and ASIC3 involve in hyperalgesia induced by cisplatin administration in rats. Pain 149: 393–405, 2010. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhu L, Reid BR, Drobny GP, Hopkins PB. Solution structure of a cisplatin-induced DNA cross-link. Science 270: 1842–1845, 1995. [DOI] [PubMed] [Google Scholar]
- Jerman JC, Gray J, Brough SJ, Ooi L, Owen D, Davis JB, Smart D. Comparison of effects of anandamide at recombinant and endogenous rat vanilloid receptors. Br J Anaesth 89: 882–887, 2002. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci 26: 13318–13327, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell Mol Life Sci 57: 1229–1235, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain 9: 463–472, 2008. [DOI] [PubMed] [Google Scholar]
- Kautio AL, Haanpaa M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage 35: 31–39, 2008. [DOI] [PubMed] [Google Scholar]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 7: 573–584, 2007. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Chandiramani A, Harding-Rose C, Simone DA, Seybold VS. Increasing 2-arachidonoyl glycerol signaling in the periphery attenuates mechanical hyperalgesia in a model of bone cancer pain. Pharmacol Res 64: 60–67, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Khasabov S, Paz J, Harding-Rose C, Simone DA. Cannabinoid type-1 receptor reduces pain and neurotoxicity produced by chemotherapy. J Neurosci 32: 7091–7101, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Khasabov SG, Harding-Rose C, Coicou LG, Seybold BA, Lindberg AE, Steevens CD, Simone DA, Seybold VS. A decrease in anandamide signaling contributes to the maintenance of cutaneous mechanical hyperalgesia in a model of bone cancer pain. J Neurosci 28: 11141–11152, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Kees S, Budweiser S, Ochs G, Toyka KV. The properties of unmyelinated nociceptive afferents change in a painful chronic constriction neuropathy. In: Proceedings of the 7th World Congress on Pain, Progress in Pain Research and Management, edited by Gebhart GF, Hammond DL, Jensen TS. Seattle, WA: IASP Press, 1994, vol. 2, p. 511–522. [Google Scholar]
- Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H. Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain 130: 1076–1088, 2007. [DOI] [PubMed] [Google Scholar]
- Kupczyk P, Reich A, Szepietowski JC. Cannabinoid system in the skin-a possible target for future therapies in dermatology. Exp Dermatol 18: 669–679, 2009. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10: 895–926, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: A potential mechanism for neurotoxicity. Neurobiol Dis 18: 305–313, 2005. [DOI] [PubMed] [Google Scholar]
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 8: 10–16, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, Di Marzo V. Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem Biophys Res Commun 262: 275–284, 1999. [DOI] [PubMed] [Google Scholar]
- Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, Taroni F, Lauria G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol 214: 276–284, 2008. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab 23: 133–144, 2009. [DOI] [PubMed] [Google Scholar]
- Olah Z, Karai L, Iadarola MJ. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J Biol Chem 276: 31163–31170, 2001. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Invest Drugs 11: 51–62, 2010. [PubMed] [Google Scholar]
- Potenzieri C, Brink TS, Simone DA. Excitation of cutaneous C nociceptors by intraplantar administration of anandamide. Brain Res 1268: 38–47, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol 141: 1118–1130, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol 249: 9–17, 2002. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science 296: 868- 871, 2002. [DOI] [PubMed] [Google Scholar]
- Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 110: 2110–2118, 2007. [DOI] [PubMed] [Google Scholar]
- Rice AS, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids 66: 243–256, 2002. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DI, Schuster M, Srkalovic G, Alexanian R, Rajkumar SV, Limentani S, Alsina M, Orlowski RZ, Najarian K, Esseltine D, Anderson KC, Amato AA. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 24: 3113–3120, 2006. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Christie MJ, Connor M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br J Pharmacol 137: 421–428, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CR, Zhang H, Dougherty PM. Altered discharges of spinal neurons parallel the behavioral phenotype shown by rats with bortezomib related chemotherapy induced peripheral neuropathy. Brain Res 1574: 6–13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience 132: 193–201, 2005. [DOI] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol 129: 227–230, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient receptor potential vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain 6: 15, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicity 27: 992–1002, 2006. [DOI] [PubMed] [Google Scholar]
- Tal M, Eliav E. Abnormal discharge originates at the site of nerve injury in experimental constriction neuropathy (CCI) in the rat. Pain 64: 511–518, 1996. [DOI] [PubMed] [Google Scholar]
- Tanner KD, Reichling DB, Levine JD. Nociceptor hyper-responsiveness during vincristine-induced painful peripheral neuropathy in the rat. J Neurosci 18: 6480–6491, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetto M, Amadesi S, Harrison S, Creminon C, Trevisani M, Carreras M, Matera M, Geppetti P, Bianchi A. Anandamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. J Neurosci 21: 1104–1109, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol Ther 95: 127–135, 2002. [DOI] [PubMed] [Google Scholar]
- Walker JM, Strangman NM, Huang SM. Cannabinoids and pain. Pain Res Manag 6: 74, 2001. [DOI] [PubMed] [Google Scholar]
- Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain 103: 131–138, 2003. [DOI] [PubMed] [Google Scholar]
- Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 44: 1507–1515, 2008. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors-noxious stimulus detectors. Neuron 55: 353–364, 2007. [DOI] [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Chemotherapy-evoked neuropathic pain: abnormal spontaneous discharge in A-fiber and C-fiber primary afferent neurons and its suppression by acetyl-l-carnitine. Pain 135: 262–270, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 82: 3359–3366, 1999. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain 16: 109–110, 1983. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Anderson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors in sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999. [DOI] [PubMed] [Google Scholar]