Abstract
Cardiac troponin I (TnI) has an NH2-terminal extension that is an adult heart-specific regulatory structure. Restrictive proteolytic truncation of the NH2-terminal extension of cardiac TnI occurs in normal hearts and is upregulated in cardiac adaptation to hemodynamic stress or β-adrenergic deficiency. NH2-terminal truncated cardiac TnI (cTnI-ND) alters the conformation of the core structure of cardiac TnI similarly to that produced by PKA phosphorylation of Ser23/24 in the NH2-terminal extension. At organ level, cTnI-ND enhances ventricular diastolic function. The NH2-terminal region of cardiac troponin T (TnT) is another regulatory structure that can be selectively cleaved via restrictive proteolysis. Structural variations in the NH2-terminal region of TnT also alter the molecular conformation and function. Transgenic mouse hearts expressing NH2-terminal truncated cardiac TnT (cTnT-ND) showed slower contractile velocity to prolong ventricular rapid-ejection time, resulting in higher stroke volume. Our present study compared the effects of cTnI-ND and cTnT-ND in cardiomyocytes isolated from transgenic mice on cellular morphology, contractility, and calcium kinetics. Resting cTnI-ND, but not cTnT-ND, cardiomyocytes had shorter length than wild-type cells with no change in sarcomere length. cTnI-ND, but not cTnT-ND, cardiomyocytes produced higher contractile amplitude and faster shortening and relengthening velocities in the absence of external load than wild-type controls. Although the baseline and peak levels of cytosolic Ca2+ were not changed, Ca2+ resequestration was faster in both cTnI-ND and cTnT-ND cardiomyocytes than in wild-type control. The distinct effects of cTnI-ND and cTnT-ND demonstrate their roles in selectively modulating diastolic or systolic functions of the heart.
Keywords: cardiac troponin I, cardiac troponin T, isolated mouse adult cardiomyocyte, contractility, intracellular calcium transient
the contraction and relaxation of vertebrate striated muscles, i.e., the skeletal and cardiac muscles, are regulated by Ca2+. Contraction is initiated by the rise of cytosolic Ca2+ that binds to the troponin complex in the sarcomeric thin filaments and activates actomyosin ATPase and myofilament cross-bridge cycling (10). Troponin I (TnI), the inhibitory subunit, and troponin T (TnT), the tropomyosin-binding and thin filament anchoring subunit, of the troponin complex play central roles in the regulation of muscle contraction (24, 25, 27).
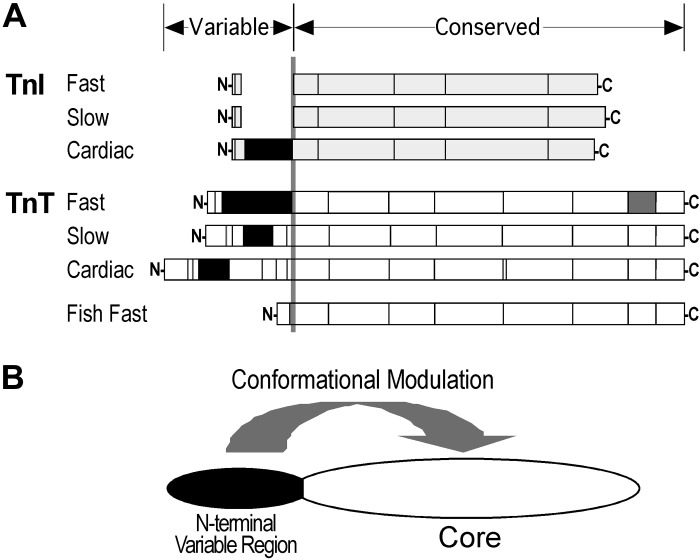
In contrast to the Ca2+-binding subunit of troponin, troponin C (TnC), which is a member of the calmodulin super family of calcium receptor proteins (22), TnI and TnT are striated muscle-specific proteins (18, 27). Three pairs of linked homologous genes have evolved in vertebrates to encode three muscle type-specific (cardiac, slow and fast skeletal muscle) isoforms of TnI and TnT. Molecular evolution studies have indicated that TnI and TnT genes emerged from duplication of the same ancestral gene (5). The common origin supports their shared structural feature of an NH2-terminal variable region that modulates the molecular conformation and function of TnI and TnT (1, 30) (Fig. 1).
Fig. 1.
Shared structural feature of troponin I (TnI) and troponin T (TnT). A: structures of the 3 muscle type-specific isoforms of TnI and TnT are outlined to demonstrate their common feature of an NH2-terminal variable region. Boxes represent segments encoded by different exons, with the alternative spliced exons represented by solid boxes. N, NH2 terminus; C, COOH terminus. B: a model illustrates that the molecular conformations of cardiac TnI and TnT are both modulated by NH2-terminal structural variations or modifications.
The NH2-terminal variable region of TnT isoforms has been extensively studied. The core structure of TnT is conserved during vertebrate evolution and among the muscle type-specific isoforms. The cardiac, fast skeletal, and slow skeletal muscle TnT isoforms are significantly different in the NH2-terminal variable region, whereas the middle and COOH-terminal regions are highly conserved (18, 31). The structure of the three isoforms of TnT is also regulated via alternative RNA splicing of multiple exons encoding the NH2-terminal variable region (27). NH2-terminal structural modifications modulate the molecular conformation of the middle and COOH-terminal regions with functional effects on the interactions with TnI, TnC, and tropomyosin and on the Ca2+ sensitivity and cooperatively of myofilaments (18).
Reflecting a similar structure-function relationship, the NH2-terminal segment of cardiac TnI is a late emerged structure specific to the adult cardiac muscle and is absent in the two skeletal muscle TnI isoforms (5). Embryonic hearts express solely slow skeletal muscle TnI that ceases expression after birth while the cardiac TnI gene is upregulated (12, 26). The NH2-terminal extension of cardiac TnI contains two Ser residues (Ser23/24) that are substrates of PKA. Phosphorylation of Ser23/24 of cardiac TnI on β-adrenergic stimulation lowers the Ca2+ affinity of troponin and increases the rate of cardiac muscle relaxation (28).
In addition to isoform diversity, alternative splicing, and phosphorylation regulations, the NH2-terminal segments of cardiac TnT and cardiac TnI can be selectively cleaved via restrictive proteolysis under physiological and pathophysiological conditions. Restrictive NH2-terminal truncation of cardiac TnT has been found in myocardial adaptations to ischemia-reperfusion (34) and pressure overload (6). Overexpression of NH2-terminal truncated cardiac TnT (cTnT-ND) in transgenic mouse hearts moderately lowered the contractile velocity, which significantly increased ventricular ejection time and stroke volume at high afterload (6). Restrictive NH2-terminal truncation of cardiac TnI is found in chronic adaptations to simulated microgravity (33) and β-adrenergic deficiency (7, 20). Overexpression of NH2-terminal truncated cardiac TnI (cTnI-ND) in transgenic mouse hearts selectively increased the relaxation velocity and facilitated ventricular filling to compensate for otherwise diminished cardiac function (7).
To understand the distinct functions of the NH2-terminal regulations of cardiac TnI and cardiac TnT, in this study we compared the effects of cTnI-ND and cTnT-ND in cardiomyocytes isolated from adult transgenic mice. We found that cTnI-ND, but not cTnT-ND, cardiomyocytes had significantly shorter resting length than wild-type cells with no change in sarcomere length. cTnI-ND, but not cTnT-ND, cardiomyocytes showed higher contractile amplitude and shortening and relengthening velocities in the absence of external load than wild-type cells, whereas isoproterenol diminished the difference. Ca2+ resequestration was faster in both cTnI-ND and cTnT-ND cardiomyocytes than in wild-type controls, in which cTnT-ND had less effect on the early phase. The distinct effects of cTnI-ND and cTnT-ND demonstrate their roles in selectively modulating diastolic or systolic functions of the heart.
MATERIALS AND METHODS
Genetically modified mice.
A transgenic mouse line overexpressing the NH2-terminal truncated cardiac TnT in the adult heart driven by a cloned α-myosin heavy chain (α-MHC) promoter was described previously (6). A transgenic mouse line overexpressing NH2-terminal truncated cardiac TnI in the heart driven by the α-MHC promoter was also described previously (2). By crossing the cTnI-ND transgenic line with heterozygotes of cardiac TnI gene (Tnni3) knockout mice (11), a double transgenic mouse line was developed to postnatally express solely cTnI-ND in the absence of endogenous intact cardiac TnI (8).
In addition to PCR genotyping, the expression of cTnT-ND and cTnI-ND in the adult cardiac muscle of the transgenic mice was confirmed using Western blot analysis. Mice of both sexes were used in the present study, and all animal protocols were approved by Institutional Animal Care and Use Committee.
SDS-polyacrylamide gel electrophoresis and Western blotting.
Isolated adult mouse cardiomyocytes were lysed in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer containing 2% SDS and 150 mM DTT, pH 8.8. The SDS-PAGE samples were heated at 80°C for 5 min and centrifuged in a microcentrifuge at top speed for 5 min, and the supernatant was loaded to SDS-gel with 14% acrylamide:bis-acrylamide at the ratio of 180:1, prepared in a modified Laemmli buffer system in which both stacking and resolving gels were casted in pH 8.8 buffer. The gels were run at constant current, and the protein bands resolved were stained with Coomassie blue R250. The amount of total protein in each lane was quantified with densitometry using ImageJ software to normalize the sample loading.
As described previously (32), duplicate gels were blotted on nitrocellulose membranes with the use of a Bio-Rad semidry electrotransfer apparatus and probed with the anti-TnI monoclonal antibody (mAb) TnI-1 (17) and the anti-cardiac TnT mAb CT3 (13, 16) under high-stringency conditions, including washes with Tris-buffered saline (TBS) containing 0.5% Triton X-100 and 0.05% SDS. With the use of alkaline phosphatase-labeled secondary antibody, cardiac TnI and cardiac TnT bands in the blots were detected via 5-bromo-4-chloro-3-indolylphosphate-nitro blue tetrazolium substrate reaction.
Isolation of adult mouse cardiomyocytes.
Cardiomyocytes were enzymatically isolated from the hearts of 2- to 8-mo-old wild-type, cTnI-ND, and cTnT-ND transgenic mice using a protocol described previously (32). Mice were injected intraperitoneally with 100 units of heparin and pentobarbital (100 mg/kg). After 20 min, the heart was rapidly removed, cannulated through the aorta, and mounted on a modified Langendorff perfusion system. The heart was first perfused at constant flow of 3 ml/min for 3 min with a buffer containing (mM) 120 NaCl, 5.4 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 5.6 glucose, 20 NaHCO3, and 5 taurine, with or without 10 mM 2,3-butanedione monoxime (BDM), oxygenated with 95% O2 and 5% CO2. The perfusion was then switched to a recirculating enzyme digestion solution, consisting of 50 ml of perfusion buffer plus 12.5 μM CaCl2, 2.5 mg of Liberase DL research grade (Roche), and 0.278 ml of 2.5% trypsin (Invitrogen), at 37°C for 15–20 min until the heart became pale and flaccid.
After being removed from the perfusion apparatus, the heart was dissected to remove atria and large vessels. The ventricular muscle was disaggregated with forceps and gentle pipetting with the use of a transfer pipette in a petri dish containing 10 ml of enzyme stopping buffer [perfusion buffer plus 2.5% bovine serum albumin (BSA) and 12.5 μM CaCl2]. The cell suspension was filtered through a 200-μm nylon mesh and settled with gravity in a 15-ml conical tube for 15 min. After the old medium was removed, the isolated cells were resuspended in 12 ml of fresh stopping buffer. CaCl2 was slowly added from a 100 mM stock to the final concentration of 1 mM (in 4 steps over 20 min). The isolated cardiomyocytes were used within 4 h for morphological and functional studies. For cardiomyocytes used in contractility and Ca2+ transient studies, BDM was omitted from the isolation buffer.
Morphological measurements.
After being stabilized in medium with a restored physiological level of Ca2+, the isolated mouse cardiomyocytes were photographed using a Zeiss AXIO Observer A1 microscope attached with a digital camera (ProGres C3; Jenoptik, Jena, Germany). With the stage micrometer image photographed at the same magnifications (×50 for cell length and ×400 for cell width and sarcomere length), phase-contrast images of large numbers of unfixed cardiomyocytes were manually measured on the photographs for resting cell length, cell width, and sarcomere length. During the measurements, the width of each cardiomyocyte was obtained by averaging measurements in three positions.
Contractility analyses of isolated adult mouse cardiomyocytes.
Cardiomyocytes were loaded into a perfusion chamber of ∼0.25-ml volume, mounted on the stage of a Nikon eclipse ST100 inverted microscope using a heating adapter with feedback temperature control. After settling at the bottom of the chamber, the cells were superfused at 1 ml/min with oxygenated buffer containing (mM) 132 NaCl, 4.8 KCl, 1.2 MgCl2, 10 HEPES, 15 glucose, 2 sodium pyruvate, and 1.8 CaCI2, pH 7.4, at 36.5–37°C.
Rod-shaped cardiomyocytes in a stable, quiescent state with sharp edges, clear sarcomeric striations, and a positive frequency response to pacing were selected for contractility studies. Contractions were induced by field electrical pacing with a MyoPacer stimulator (IonOptix, Milton, MA) using 10-V, 4-ms pulses at 0.5, 1, 2, 5, and 10 Hz in the absence or presence of 30 nM isoproterenol. Cell shortening and relengthening were recorded using a charge-coupled device video camera (IonOptix). Edge detection data were acquired at a sampling rate of 240 Hz and analyzed using the SoftEdge computer program (IonOptix).
Measurement of intracellular Ca2+ transient.
To examine intracellular Ca2+ transient in the cardiomyocytes during contractile cycles, isolated cells in superfusion buffer containing 1.8 mM CaCl2 were incubated with 2 μM fura 2-AM (Invitrogen) in the dark at room temperature for 30 min. After the loading of fura, the cells were washed twice with the superfusion buffer containing 1.8 mM CaCl2 and 500 μM probenecid and kept in the same buffer in the dark at room temperature for 30 min. Loaded in the superfusion chamber and paced as described above, the cells were examined for cytosolic Ca2+ transient during contraction and relaxation using a photomultiplier tube (PMT-300; IonOptix). Cytosolic free Ca2+ was measured as the 360- to 380-nm (360/380) ratio of fura 2 fluorescence excited at 510 nm. The effect of isoproterenol was also examined.
Pro-Q diamond phosphoprotein staining.
Isolated cardiomyocytes were treated with 30 nM isoproterenol for 10 min and immediately frozen at −80°C. Total protein was extracted by homogenization in SDS-gel sample buffer and processed for SDS-PAGE as described above. The gel was prefixed with 50% methanol and 10% acetic acid, washed with deionized water, and incubated with Pro-Q Diamond phosphoprotein staining reagent (Invitrogen) in a dark box for 90 min. The gel was then destained in 20% acetonitrile and 50 mM sodium acetate, pH 4.0, for 3 changes of 30 min each, and washed twice with deionized water for 5 min each in a dark box. The destained gel was imaged using a Typhoon 9410 fluorescence scanner (GE Healthcare) with excitation at 532 nm and the emission recorded at 560 nm. The gel was then stained with Coomassie blue R250 to determine the total protein contents. The relative amount of a phosphoprotein band was quantified with densitometry using ImageJ software with normalization to the amount of total protein, with the latter determined from the Coomassie blue stain.
Data analysis.
SDS-gel and Western blot images were scanned at 600 dpi for densitometry analysis using ImageJ software. Contractility and Ca2+ transient data were calculated by averaging a train of consecutive pulses during 0.5–1.0 min of stable recording. Data are means ± SE. Statistical significance of differences was determined using unpaired two-tailed Student's t-test.
RESULTS
cTnI-ND, but not cTnT-ND, cardiomyocytes from young transgenic mice had shorter slack length with no change in width or sarcomere length.
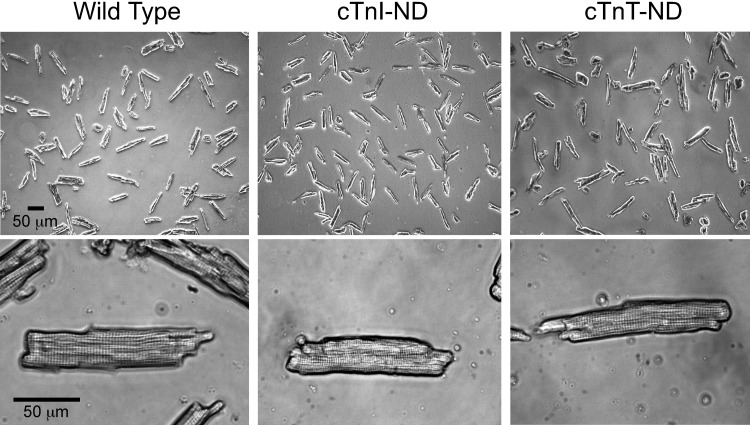
The representative microscopic images in Fig. 2 show cardiomyocytes isolated from 2- to 3-mo-old cTnI-ND and cTnT-ND mice. The cells had similar morphology and striation pattern to that of cardiomyocytes isolated from wild-type control mice. This observation is consistent with the nonhypertrophy and nonfailing heart phenotypes of these transgenic mouse lines (6, 7). The results also support the use of the transgenic mouse cardiomyocytes at early age for studies on the functional effects of NH2-terminal truncated cardiac TnI and cardiac TnT avoiding secondary effects from myocardial hypertrophy.
Fig. 2.
Morphology of cardiomyocytes isolated from adult transgenic and wild-type mice. Representative microscopic images show that NH2-terminal truncated cardiac TnI (cTnI-ND) and NH2-terminal truncated cardiac TnT (cTnT-ND) mouse cardiomyocytes had similar morphology to that of wild-type cardiomyocytes, except for a slightly shorter length of the cTnI-ND cells. Cells from 10 wild-type, 5 TnI-ND, and 5 TnT-ND hearts were examined. At least 100 cells from each heart were measured and pooled for statistical analysis (Table 1).
The results in Table 1 further show that cTnI-ND cardiomyocytes had shorter resting (slack) length than wild-type and cTnT-ND cells. In the meantime, the cell width and resting sarcomere length were not changed. In contrast, cTnT-ND cardiomyocytes had similar slack length, width, and resting sarcomere length to that of wild-type controls (Table 1).
Table 1.
Decreased cell length of cTnI-ND, but not cTnT-ND, cardiomyocytes with no change in cell width and resting sarcomere length
| Wild Type | cTnI-ND | cTnT-ND | |
|---|---|---|---|
| Cell length, μm | 124.53 ± 1.77 | 111.66 ± 5.67* | 131.34 ± 3.32 |
| Cell width, μm | 25.51 ± 1.03 | 24.41 ± 0.52 | 24.94 ± 0.78 |
| Sarcomere length, μm | 1.82 ± 0.01 | 1.82 ± 0.01 | 1.80 ± 0.01 |
The resting length of young adult NH2-terminal truncated cardiac troponin I (cTnI-ND) mouse cardiomyocytes was shorter than that of wild-type or NH2-terminal truncated cardiac troponin T (cTnT-ND) cells (*P < 0.05). No significant difference was found in cell width and resting sarcomere length among the 3 groups. Data are means ± SE (n = 10 hearts for wild type and n = 5 hearts each for cTnI-ND and cTnT-ND groups). At least 100 cells from each heart were measured and pooled for statistical analysis.
cTnI-ND, but not cTnT-ND, increased contractility of isolated cardiomyocytes in the absence of external load.
The results in Table 2 show that compared with wild-type controls for paced contractions at 2 Hz, cTnI-ND cardiomyocytes had significantly higher contractile amplitude and shortening and relengthening velocities. In contrast, these values in cTnT-ND cardiomyocytes were not significantly different from those in the wild-type cells.
Table 2.
cTnI-ND, but not cTnT-ND, increased contractility of isolated cardiomyocytes in the absence of external load
| No Iso |
30 nM Iso |
|||
|---|---|---|---|---|
| Wild Type | cTnI-ND | Wild Type | cTnI-ND | |
| Shortening amplitude, % | 3.45 ± 0.55 | 6.90 ± 0.64** | 13.52 ± 1.24‡ | 15.49 ± 0.81‡ |
| Shortening velocity, μm/s | 121.56 ± 21.47 | 235.00 ± 32.68* | 433.21 ± 56.10‡ | 509.14 ± 43.36‡ |
| Relengthening velocity, μm/s | 87.24 ± 15.32 | 205.99 ± 31.83* | 402.69 ± 58.23‡ | 486.56 ± 35.04‡ |
| No Iso |
30 nM Iso |
|||
|---|---|---|---|---|
| Wild Type | cTnT-ND | Wild Type | cTnT-ND | |
| Shortening amplitude, % | 9.06 ± 1.11 | 6.88 ± 1.11 | 18.49 ± 2.28‡ | 23.45 ± 6.46‡ |
| Shortening velocity, μm/s | 329.13 ± 38.29 | 266.92 ± 45.70 | 582.85 ± 93.79‡ | 566.29 ± 85.32‡ |
| Relengthening velocity, μm/s | 294.36 ± 36.02 | 224.67 ± 41.36 | 587.57 ± 103.34‡ | 497.78 ± 74.66‡ |
cTnI-ND cardiomyocytes paced at 2 Hz had significantly increased contractile amplitude and maximal shortening and relengthening velocities in the absence of isoproterenol (Iso) compared with wild-type controls (
P < 0.05;
P < 0.01), whereas cTnT-ND cardiomyocytes paced at 2 Hz showed no significant change in the baseline contractile amplitude and maximal shortening and relengthening velocities compared with wild-type controls. Iso treatment increased the contractility of all 3 groups (
P < 0.01) and diminished the difference between cTnI-ND and wild-type cells. Data are means ± SE (for cTnI-ND data, n = 4 hearts in each group; for cTnT-ND data, n = 6 hearts in wild-type group and n = 7 hearts in cTnT-ND group). At least 3–5 cells were studied for each heart and pooled for statistical analysis.
Treatment with 30 nM isoproterenol significantly increased the contractility in all three groups (Table 2), indicating retained physiological functions. Isoproterenol treatment diminished the baseline difference between cTnI-ND and wild-type cells (Table 2), consistent with the previous observation that restrictive NH2-terminal truncation and PKA-catalyzed NH2-terminal phosphorylation at Ser23/24 had similar and nonadditive downstream effects on enhancing cardiac function (2).
cTnI-ND and cTnT-ND had no effect on the resting and peak levels of cytosolic Ca2+ in cardiomyocytes of transgenic mice.
The results in Table 3 show that during paced contractions at 2 Hz, cTnI-ND and cTnT-ND cardiomyocytes had no significant differences from wild-type cells in the levels of resting and peak intracellular Ca2+. Isoproterenol treatment significantly increased peak cytosolic Ca2+ similarly in all three groups (Table 3). The results indicate no significant secondary change in the Ca2+ handling system of the transgenic mouse cardiomyocytes, validating their use in the study of primary functional effects of cTnI-ND and cTnT-ND.
Table 3.
cTnI-ND and cTnT-ND cardiomyocytes had no change in baseline and peak cytosolic Ca2+ but shorter times of Ca2+ resequestration
| No Iso |
30 nM Iso |
|||
|---|---|---|---|---|
| Wild Type | cTnI-ND | Wild Type | cTnI-ND | |
| Baseline 360/380 ratio | 0.76 ± 0.01 | 0.77 ± 0.03 | 0.76 ± 0.01 | 0.78 ± 0.03 |
| Peak 360/380 ratio | 1.06 ± 0.04 | 1.14 ± 0.10 | 1.18 ± 0.05‡ | 1.25 ± 0.10‡ |
| TR25, ms | 45.48 ± 3.10 | 36.23 ± 1.96* | 36.52 ± 2.06‡ | 30.00 ± 1.60*‡ |
| TR75, ms | 156.56 ± 9.26 | 131.85 ± 5.00* | 115.11 ± 7.44‡ | 95.54 ± 4.03*‡ |
| No Iso |
30 nM Iso |
|||
|---|---|---|---|---|
| Wild Type | cTnT-ND | Wild Type | cTnT-ND | |
| Baseline 360/380 ratio | 0.73 ± 0.02 | 0.76 ± 0.03 | 0.73 ± 0.02 | 0.78 ± 0.03 |
| Peak 360/380 ratio | 1.00 ± 0.06 | 1.04 ± 0.05 | 1.10 ± 0.06‡ | 1.15 ± 0.05‡ |
| TR25, ms | 46.87 ± 4.17 | 41.73 ± 3.29 | 36.80 ± 2.81‡ | 38.20 ± 3.09 |
| TR75, ms | 181.53 ± 10.64 | 143.33 ± 10.25* | 130.73 ± 8.37‡ | 101.13 ± 5.81**‡ |
Paced at 2 Hz, cTnI-ND and cTnT-ND cardiomyocytes showed no significant difference in levels of resting (baseline) and peak cytosolic Ca2+ (measured as 360/380 fluorescence ratio) from wild-type controls. Iso treatment significantly increased peak cytosolic Ca2+ in all 3 groups (
P < 0.01). The times to 25% (TR25) and 75% Ca2+ resequestration (TR75) in cTnI-ND cardiomyocytes were significantly shorter than in wild-type controls (
P < 0.05). TR75, but not TR25, in cTnT-ND cardiomyocytes was significantly shorter compared with those in wild-type controls (
P < 0.05,
P < 0.01). Iso treatment significantly shortened TR75 in all 3 groups. Wild-type and cTnI-ND cardiomyocytes had significant responses to Iso by shortening TR25 (
P < 0.01 compared with baseline), whereas cTnT-ND cardiomyocytes did not. Data are means ± SE (for cTnI-ND data, n = 4 hearts for wild-type group and n = 3 hearts for cTnI-ND group; for cTnT-ND data, n = 3 hearts for wild-type group and n = 4 hearts for cTnT-ND group). At least 3–5 cells from each heart were studied and pooled for statistical analysis.
cTnI-ND and cTnT-ND shortened the time of Ca2+ resequestration in adult cardiomyocytes.
The results in Table 3 reveal that when paced at 2 Hz, the times from peak Ca2+ to 25% and 75% resequestration were significantly shorter in cTnI-ND cardiomyocytes than in wild-type control. cTnT-ND cardiomyocytes showed a shorter time to 75%, but not 25%, Ca2+ resequestration compared with wild-type control. By enhancing Ca2+ resequestration into sarcolemma reticulum (3), isoproterenol treatment significantly shortened the time to 75% Ca2+ decay in all three groups, although cTnI-ND and cTnT-ND cardiomyocytes remained different from the wild-type control (Table 3). In contrast to cTnI-ND cells that retained a shorter time for 25% Ca2+ resequestration on isoproterenol treatment, cTnT-ND cardiomyocytes showed no significant response to 30 nM isoproterenol in the time of 25% Ca2+ resequestration. The altered early phase of Ca2+ resequestration suggests decreased Ca2+ release from the myofilaments. This, in turn, suggests that cTnT-ND may have had an effect on the Ca2+ off rate of troponin in the transgenic mouse cardiomyocytes.
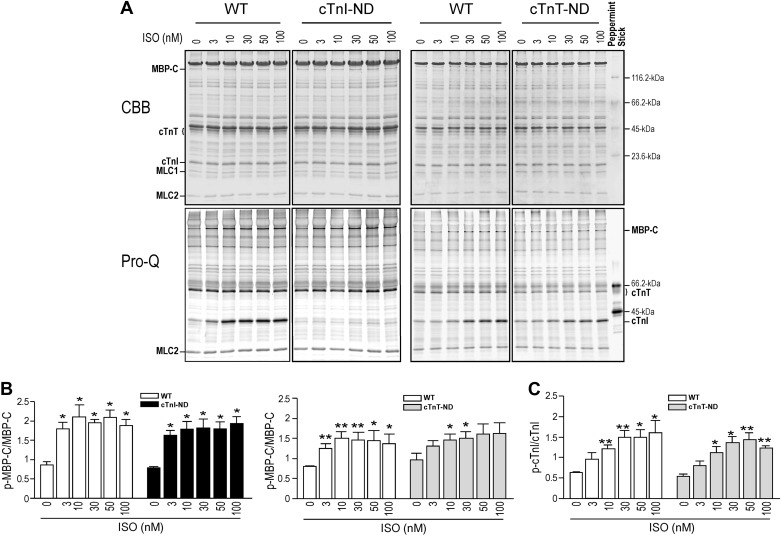
Phosphorylation of myosin binding protein-C and cardiac TnI was preserved in cTnI-ND and cTnT-ND mouse cardiomyocytes.
Pro-Q phosphoprotein staining of SDS-PAGE gels and densitometry quantification showed that isoproterenol treatment increased the phosphorylation of myosin binding protein-C (MBP-C) in cTnI-ND, cTnT-ND, and wild-type cardiomyocytes in similarly dose-dependent manners (Fig. 3, A and B). Isoproterenol also induced phosphorylation of endogenous (intact) cardiac TnI in wild-type and cTnT-ND cardiomyocytes (Fig. 3, A and C), whereas transgenic mouse cardiomyocytes containing solely cTnI-ND that lacks the NH2-terminal Ser23/24 showed no detectable phosphorylation of cardiac TnI before and after isoproterenol treatment (Fig. 3A).
Fig. 3.
Phosphorylation of cardiac TnI and myosin binding protein-C (MBP-C) is unchanged in cTnI-ND and cTnT-ND mouse cardiomyocytes. Pro-Q staining of SDS-PAGE gels (A) and densitometry quantification (B) showed that isoproterenol (Iso) treatment increased the phosphorylation (p) of MBP-C in wild-type, cTnI-ND, and cTnT-ND cardiomyocytes similarly in a dose-dependent manner. C: Iso-stimulated phosphorylation of intact (endogenous) cardiac TnI in cTnT-ND cardiomyocytes was also similar to that in wild-type cells. *P < 0.05; **P < 0.01 compared with baseline; n = 3 hearts in each group. Experiments were repeated 3 times. CBB, Coomassie brilliant blue; MLC-1 and -2, myosin light chain 1 and 2; WT, wild type.
DISCUSSION
Molecular evolution and similarity of cardiac TnI and cardiac TnT in the regulatory function of their NH2-terminal segments.
Troponin I and TnT are subunits of the troponin complex. Although research data suggest that they emerged from the same ancestor gene (5), TnI and TnT have highly diverged structures and functions. On the other hand, based on their common evolutionary origin (5), an inhered structural feature of TnI and TnT is that their NH2-terminal segments are regulatory structures that were added during molecular evolution (5). Modifications in the NH2-terminal segments of cardiac TnI and cardiac TnT both produce conformational effects on other regions of the molecule with functional effects (Fig. 1) (1, 30).
A primary physiological mechanism that modifies the NH2-terminal structure of cardiac TnI is PKA-catalyzed phosphorylation of Ser23/24 downstream of β-adrenergic stimulation (23, 28). Regulation of cardiac TnI function via restrictive NH2-terminal truncation was a more recent finding (33) that produces a similar and nonadditive effect to that of PKA phosphorylation on the molecular conformation (1) and function (2) of cardiac TnI.
The NH2-terminal region of cardiac TnT is regulated during development via alternative RNA splicing (14, 15). Splice forms of TnT varying in the NH2-terminal region have altered overall molecular conformation (21, 30) and function (4, 9, 21). The more recent finding of the restrictive NH2-terminal truncation of cardiac TnT demonstrates a posttranslational regulation in adult cardiac muscle in adaptation to stress conditions (6, 34).
Demonstrated in ex vivo working hearts, restrictive deletion of the NH2-terminal segments of cardiac TnI and cardiac TnT selectively affects diastolic and systolic functions, respectively (6, 7, 19). This intriguing observation led to a hypothesis that these two posttranslational modifications may be specific submolecular targets for therapeutic modifications for selective treatment of systolic and diastolic heart failure conditions. Our present study directly compared for the first time the contractility and calcium dynamics of cardiomyocytes isolated from transgenic mice, and the results add several new insights into the specific functions of cTnI-ND and cTnT-ND.
Restrictive NH2-terminal truncation of cardiac TnI enhances the relaxation of cardiomyocytes in the absence of external load.
Previous studies have demonstrated that cTnI-ND increases relaxation velocity of the ventricular muscle in ex vivo working hearts (7). Our new data further show that in isolated adult cardiomyocytes, cTnI-ND enhanced the relaxation velocity in the absence of external load (Table 2). This cellular phenotype provided novel evidence that the effect of cTnI NH2-terminal extension on the diastolic function of the heart remains in the absence of external preload, i.e., not relying on the filling pressure to the ventricular chambers. This feature indicates that cTnI-ND is a myofilament-based mechanism that regulates the diastolic function of cardiac muscle independently of hemodynamic feedback and thus may be targeted for the treatment of diastolic heart failure with preserved ejection fraction in which preload could remain normal (29).
cTnI-ND cardiomyocytes further showed increased contractile velocity and increased shortening amplitude (Table 2). This cellular-level positive effect on contractility suggests that the effect of cTnI-ND on increasing relaxation is not simply to decrease the Ca2+ affinity of troponin but to selectively facilitate Ca2+ release during diastole without decreasing Ca2+ binding during systole. The shortened time of early Ca2+ resequestration (Table 3) in cTnI-ND cardiomyocytes supports increased release of Ca2+ from troponin. This notion is consistent with the results that cTnI-ND had no significant effect on the baseline and peak levels of cytosolic Ca2+ transient in the transgenic mouse cardiomyocytes (Table 3) or on the time to reach peak cytosolic Ca2+ level during contraction (data not shown).
Restrictive NH2-terminal truncation of cardiac TnT did not slow the contractile velocity of cardiomyocytes in the absence of external load.
Consistent with the observations that engineered TnT constructs with a shortened NH2-terminal variable region produced lower myofilament Ca2+ sensitivity (4, 21), organ-level studies showed that cTnT-ND produced moderately slower contractile velocity in ex vivo working hearts with a beneficial effect on prolonging ventricular ejection time to increase stroke volume at high afterload (6). Our new results show that in contrast to the retained phenotype of cTnI-ND at the cellular level, cTnT-ND did not have any detectable effect on relaxation and also did not produce significant changes in contractile velocity or shortening amplitude in isolated mouse cardiomyocytes in the absence of external load.
This observation supports the notion that modifications in the NH2-terminal segment of cardiac TnT modulate systolic function of cardiac muscle in response to afterload demands. Therefore, the contractility of isolated cardiomyocytes in the absence of external afterload would not reflect such modulatory effect. This hypothesis is supported by our previous finding that the effect of cTnT-ND on increasing stroke volume is only prominent at increased afterload (6). This afterload-dependent function of cTnT-ND may be further established as a valuable submolecular target for the development of a selective treatment of systolic heart failure.
Although cardiac TnT NH2-terminal deletion had no effects on most of the parameters that were measured, the resequestration of cytosolic Ca2+, especially the later phase, was faster in cTnT-ND cardiomyocytes (Table 4). A previous study demonstrated that the negatively charged NH2-terminal segment of TnT binds Ca2+ (35). Therefore, the absence of the NH2-terminal segment of cardiac TnT in cTnT-ND cardiomyocytes would result in a reduced amount of Ca2+ bound to the myofilaments during systole and, subsequently, the faster decay of cytosolic Ca2+ during diastole. This observation supports a direct role of the NH2-terminal segment of TnT in regulating calcium dynamics in muscle cells and worth further investigation.
Table 4.
Percent change of functional parameters of cTnI-ND and cTnT-ND hearts relative to wild-type control
| No Iso |
30 nM Iso |
|||
|---|---|---|---|---|
| cTnI-ND | cTnT-ND | cTnI-ND | cTnT-ND | |
| Resting cell length | −12.0 ± 4.5* | +5.8 ± 2.7# | N/A | N/A |
| Resting cell width | −4.2 ± 2.0 | −2.4 ± 0.1 | N/A | N/A |
| Resting sarcomere length | −1.0 ± 0.4 | −1.4 ± 0.4 | N/A | N/A |
| Amplitude of cell shortening | +100.1 ± 18.4** | −24.1 ± 12.2## | +14.6 ± 6.0 | +26.8 ± 35.0 |
| Shortening velocity | +93.3 ± 26.9* | −18.9 ± 13.9## | +17.5 ± 10.0 | −2.8 ± 15.0## |
| Relengthening velocity | +136.1 ± 36.5* | −23.7 ± 14.1## | +20.8 ± 8.7 | −15.3 ± 12.7## |
| Baseline cytosolic Ca2+ | +1.4 ± 4.5 | +3.3 ± 3.5 | +2.4 ± 4.2 | +6.7 ± 3.6 |
| Peak cytosolic Ca2+ | +7.7 ± 9.1 | +4.4 ± 4.8 | +6.0 ± 9.1 | +4.7 ± 4.5 |
| TR25 | −20.3 ± 4.3* | −11.0 ± 7.6 | −17.9 ± 4.4* | +3.8 ± 8.4# |
| TR75 | −15.8 ± 3.2* | −21.0 ± 5.7* | −17.0 ± 3.5* | −22.6 ± 4.4** |
From the primary data shown in Tables 1–3, the effects of cTnI-ND and cTnT-ND on cardiomyocyte function were calculated as %change relative to wild-type controls (+, increase; −, decrease). Data are means ± SE. cTnI-ND but not cTnT-ND cardiomyocytes had shorter slack length with no change in sarcomere length and increased baseline contractile amplitude and shortening and relengthening velocities. cTnI-ND and cTnT-ND cardiomyocytes both had significantly shorter times to TR75 at baseline or in the presence of 30 nM Iso. cTnI-ND but not cTnT-ND cardiomyocytes also had shorter times to TR25 at baseline or in the presence of 30 nM Iso.
P < 0.05;
P < 0.01 compared with wild-type control.
P < 0.05;
P < 0.01, cTnI-ND compared with cTnT-ND.
Posttranslational modifications of two subunits of troponin selectively regulate the systolic and diastolic functions.
It is an interesting observation that posttranslational modifications of two subunits of the same protein complex, troponin, in cardiac muscle thin filament can separately modulate systolic and diastolic functions in a highly selective manner. Together with our previous studies using integrative muscle tissue and organ systems, results of the present study using isolated cardiomyocytes and avoiding the influences of external load, extracellular matrix, and systemic feedbacks indicate that the regulatory effects of cTnI-ND and cTnT-ND are myofilament-based mechanisms and thus may be further investigated as attractive novel targets for the development of new treatment of heart failure to specifically and more effectively correct systolic and diastolic dysfunction of the cardiac muscle.
Our unpublished results from multiple experiments showed that no single condition or treatment was able to induce the restrictive NH2-terminal deletions of both cardiac TnI and cardiac TnT at the same time. The apparently separate regulatory mechanisms of these two posttranslational modifications of troponin indicate their physiological roles in separate functional adaptations, further supporting their value as specific targets for therapeutic regulation of diastolic and systolic functions of the heart.
GRANTS
This study was supported by National Institutes of Health Grants HL098945 and AR048816 (to J.-P. Jin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.W. performed experiments; H.W. and J.-P.J. analyzed data; H.W. and J.-P.J. interpreted results of experiments; H.W. and J.-P.J. prepared figures; H.W. and J.-P.J. drafted manuscript; H.W. and J.-P.J. edited and revised manuscript; H.W. and J.-P.J. approved final version of manuscript; J.-P.J. conception and design of research.
ACKNOWLEDGMENTS
We thank Hui Wang for technical assistance and Dr. M. Moazzem Hossain for maintenance of the transgenic mouse lines.
REFERENCES
- 1.Akhter S, Bueltmann K Jr, Huang X, Jin JP. Restrictive cardiomyopathy mutations demonstrate functions of the C-terminal end-segment of troponin I. Arch Biochem Biophys 552–553: 3–10, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Barbato JC, Huang QQ, Hossain MM, Bond M, Jin JP. Proteolytic N-terminal truncation of cardiac troponin I enhances ventricular diastolic function. J Biol Chem 280: 6602–6609, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol 76: 107–127, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Chandra M, Montgomery DE, Kim JJ, Solaro RJ. The N-terminal region of troponin T is essential for the maximal activation of rat cardiac myofilaments. J Mol Cell Cardiol 31: 867–880, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Chong SM, Jin JP. To investigate protein evolution by detecting suppressed epitope structures. J Mol Evol 68: 448–460, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng HZ, Biesiadecki BJ, Yu ZB, Hossain MM, Jin JP. Restricted N-terminal truncation of cardiac troponin T: a novel mechanism for functional adaptation to energetic crisis. J Physiol 586: 3537–3550, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng HZ, Chen M, Weinstein LS, Jin JP. Removal of the N-terminal extension of cardiac troponin I as a functional compensation for impaired myocardial β-adrenergic signaling. J Biol Chem 283: 33384–33393, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng HZ, Hossain MM, Huang XP, Jin JP. Myofilament incorporation determines the stoichiometry of troponin I in transgenic expression and the rescue of a null mutation. Arch Biochem Biophys 487: 36–41, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes AV, Venkatraman G, Davis JP, Tikunova SB, Engel P, Solaro RJ, Potter JD. Cardiac troponin T isoforms affect the Ca2+ sensitivity of force development in the presence of slow skeletal troponin I: insights into the role of troponin T isoforms in the fetal heart. J Biol Chem 279: 49579–49587, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev 80: 853–924, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Pi Y, Lee KJ, Henkel AS, Gregg RG, Powers PA, Walker JW. Cardiac troponin I gene knockout: a mouse model of myocardial troponin I deficiency. Circ Res 84: 1–8, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Jin JP. Alternative RNA splicing-generated cardiac troponin T isoform switching: a non-heart-restricted genetic programming synchronized in developing cardiac and skeletal muscles. Biochem Biophys Res Commun 225: 883–889, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Jin JP, Chen A, Ogut O, Huang QQ. Conformational modulation of slow skeletal muscle troponin T by an NH2-terminal metal-binding extension. Am J Physiol Cell Physiol 279: C1067–C1077, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Jin JP, Lin JJ. Isolation and characterization of cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T. J Biol Chem 264: 14471–14477, 1989. [PubMed] [Google Scholar]
- 15.Jin JP, Lin JJ. Rapid purification of mammalian cardiac troponin T and its isoform switching in rat hearts during development. J Biol Chem 263: 7309–7315, 1988. [PubMed] [Google Scholar]
- 16.Jin JP, Root DD. Modulation of troponin T molecular conformation and flexibility by metal ion binding to the NH2-terminal variable region. Biochemistry 39: 11702–11713, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Jin JP, Yang FW, Yu ZB, Ruse CI, Bond M, Chen A. The highly conserved COOH terminus of troponin I forms a Ca2+-modulated allosteric domain in the troponin complex. Biochemistry 40: 2623–2631, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Eukaryot Gene Expr 18: 93–124, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Charles PY, Nan C, Pinto JR, Wang Y, Liang J, Wu G, Tian J, Feng HZ, Potter JD, Jin JP, Huang X. Correcting diastolic dysfunction by Ca2+ desensitizing troponin in a transgenic mouse model of restrictive cardiomyopathy. J Mol Cell Cardiol 49: 402–411, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McConnell BK, Popovic Z, Mal N, Lee K, Bautista J, Forudi F, Schwartzman R, Jin JP, Penn M, Bond M. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J Biol Chem 284: 1583–1592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogut O, Granzier H, Jin JP. Acidic and basic troponin T isoforms in mature fast-twitch skeletal muscle and effect on contractility. Am J Physiol Cell Physiol 276: C1162–C1170, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Parmacek MS, Leiden JM. Structure, function, and regulation of troponin C. Circulation 84: 991–1003, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Parmacek MS, Solaro RJ. Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis 47: 159–176, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Perry SV. Troponin I: inhibitor or facilitator. Mol Cell Biochem 190: 9–32, 1999. [PubMed] [Google Scholar]
- 25.Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 19: 575–602, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Saggin L, Gorza L, Ausoni S, Schiaffino S. Troponin I switching in the developing heart. J Biol Chem 264: 16299–16302, 1989. [PubMed] [Google Scholar]
- 27.Sheng JJ, Jin JP. Gene regulation, alternative splicing, and posttranslational modification of troponin subunits in cardiac development and adaptation: a focused review. Front Physiol 5: 165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumandea MP, Burkart EM, Kobayashi T, De Tombe PP, Solaro RJ. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann NY Acad Sci 1015: 39–52, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Udelson JE. Heart failure with preserved ejection fraction. Circulation 124: e540–e543, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Jin JP. Conformational modulation of troponin T by configuration of the NH2-terminal variable region and functional effects. Biochemistry 37: 14519–14528, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Wei B, Jin JP. Troponin T isoforms and posttranscriptional modifications: evolution, regulation and function. Arch Biochem Biophys 505: 144–154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei H, Jin JP. A dominantly negative mutation in cardiac troponin I at the interface with troponin T causes early remodeling in ventricular cardiomyocytes. Am J Physiol Cell Physiol 307: C338–C348, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu ZB, Zhang LF, Jin JP. A proteolytic NH2-terminal truncation of cardiac troponin I that is up-regulated in simulated microgravity. J Biol Chem 276: 15753–15760, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Biesiadecki BJ, Jin JP. Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia reperfusion by myofibril-associated mu-calpain cleavage. Biochemistry 45: 11681–11694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Jin JP, Root DD. Binding of calcium ions to an avian flight muscle troponin T. Biochemistry 43: 2645–2655, 2004. [DOI] [PubMed] [Google Scholar]