Abstract
Abnormally elevated postprandial glucose and triacylglycerol (TAG) concentrations are risk factors for cardiovascular disease in type 2 diabetes. The most effective time to exercise to lower postprandial glucose and TAG concentrations is unknown. Thus the aim of this study was to determine what time is more effective, either pre- or postdinner resistance exercise (RE), at improving postprandial risk factors in patients with type 2 diabetes. Thirteen obese patients with type 2 diabetes completed three trials in a random order in which they consumed a dinner meal with 1) no RE (NoRE), 2) predinner RE (RE → M), and 3) postdinner RE beginning 45 min after dinner (M → RE). Clinical outcome measures included postprandial glucose and TAG concentrations. In addition, postprandial acetaminophen (gastric emptying), endocrine responses, free fatty acids, and β-cell function (mathematical modeling) were measured to determine whether these factors were related to changes in glucose and TAG. The TAG incremental area under the curve (iAUC) was ∼92% lower (P ≤ 0.02) during M → RE compared with NoRE and RE → M, an effect due in part to lower very-low-density lipoprotein-1 TAG concentrations. The glucose iAUC was reduced (P = 0.02) by ∼18 and 30% during the RE → M and M → RE trials, respectively, compared with NoRE, with no difference between RE trials. RE → M and M → RE reduced the insulin iAUC by 35 and 48%, respectively, compared with NoRE (P < 0.01). The glucagon-like peptide-1 iAUC was ∼50% lower (P ≤ 0.02) during M → RE compared with NoRE and RE → M. Given that predinner RE only improves postprandial glucose concentrations, whereas postdinner RE improves both postprandial glucose and TAG concentrations, postdinner RE may lower the risk of cardiovascular disease more effectively.
Keywords: exercise timing, glycemic control, weight training, obesity, glucose metabolism, lipid metabolism
abnormally elevated postprandial glucose (17, 31) and triacylglycerol (TAG) (9, 24, 32) concentrations are strong risk factors for cardiovascular disease (CVD) in patients with type 2 diabetes. Therefore, interventions that reduce postprandial glucose and TAG concentrations should lower the risk of CVD (19, 25). Acute exercise typically lowers postprandial glucose (7, 12, 20, 21, 26, 28, 29, 37–40) and TAG concentrations (35) in patients with type 2 diabetes, but there is considerable heterogeneity in the responses, with some individuals not experiencing beneficial changes in these risk factors (11, 37). One potential explanation why some patients with type 2 diabetes do not have beneficial changes in postprandial glucose and TAG with acute exercise is because of the timing of the acute exercise session relative to meal consumption.
Limited evidence suggests that the timing of aerobic exercise around a meal may be important and might explain why some individuals are exercise “insensitive” or “nonresponders”. The only study to directly compare the effect of premeal and postmeal aerobic exercise on postprandial glucose concentrations in patients with type 2 diabetes showed that postdinner, but not predinner, walking lowered postprandial glucose concentrations (4). Although no study has directly examined the effect of exercise timing on postprandial TAG in patients with type 2 diabetes, there is evidence that exercise performed the day before a high-fat meal has no effect on postprandial TAG responses (5, 11), whereas postbreakfast aerobic exercise reduced the postprandial TAG response (35). Taken together, it appears that aerobic exercise may have its most powerful effect to lower postprandial glucose and TAG responses when performed after a meal, possibly because of slowed gastric emptying and/or greater skeletal muscle glucose and TAG uptake and utilization at this time.
The aforementioned studies suggest aerobic exercise timing is critically important, but no study, to our knowledge, has examined how resistance exercise (RE) timing relative to dinner alters postprandial glucose and TAG concentrations. From a practical perspective, many obese patients with type 2 diabetes may not enjoy or be able to perform aerobic exercise soon after a meal when the exercise session may have its most powerful effect to lower postprandial glucose and TAG concentrations. However, RE may be a practical alternative because it can be better tolerated in obese patients with type 2 diabetes as traditional RE consists of short work periods with long rests between sets. However, compared with aerobic exercise, RE is a different physiological stimulus (i.e., RE is anaerobic, does not involve continuous skeletal muscle contractions, and has lower energy expenditure), thus it is not clear if RE would alter postprandial glucose and TAG concentrations as robustly as aerobic exercise. Some studies report acute RE before meal(s) improves glycemic control (7, 38), while other studies report it does not (3, 8), and no study has assessed how acute RE alters postprandial TAG concentrations. In addition, most of these studies assessed the effects of RE on postprandial glucose concentrations after a breakfast or lunch meal, and not a dinner meal later in the day, a time when individuals typically eat their biggest meal and when glucose and TAG concentrations have been reported to be highest in patients with type 2 diabetes (1, 10). Therefore, the primary purpose of this study was to test the hypothesis that postdinner RE, compared with predinner RE, is more effective at improving two clinically important postprandial risk factors (glucose and TAG) for CVD at a time of day when they are typically highest in obese patients with type 2 diabetes. In addition, postprandial acetaminophen (gastric emptying), endocrine responses, free fatty acids (FFA), and β-cell function (mathematical modeling) were measured to determine whether alterations in these factors were related to changes in postprandial glucose and TAG concentrations. Subjective well-being was assessed to determine how feasible the different RE times were.
RESEARCH DESIGN AND METHODS
Participants
The University of Missouri Health Science Institutional Review Board approved this study protocol, and all participants provided written, informed consent. Participants in this study were obese (body mass index > 30 kg/m2), physician diagnosed with type 2 diabetes, receiving standard medical care, nonsmokers, not using insulin, had no history of surgery for weight loss, refrained from performing exercise or going on any special diets while participating, and were weight stable. The participants took their medications at the usual dose, frequency, and time while participating in this study. This study is registered at ClinicalTrials.gov: NCT02180620.
Experimental Design
Baseline testing included assessments of height, weight, body composition (assessed via BODPOD), resting energy expenditure, physical activity energy expenditure, and familiarization and strength testing (described in detail later). After baseline testing, all participants completed three, 3-day trials in a random order.
Day 1.
On day 1, a continuous glucose monitor was inserted into the abdomen of the participant, and the participants were instructed on how to take and record a finger stick blood glucose measure with a glucometer (Accu-Chek Compact Plus, Roche Diagnostics), which was used to calibrate the continuous glucose monitor. Following training, the participants were given their prepackaged study meals and were instructed on when to eat the meals the following day.
Day 2.
On day 2, the participants ate the standardized breakfast and lunch on their own and immediately before each meal recorded a finger stick blood glucose measure. In the evening, the participants reported to the laboratory for testing in the late afternoon, and, upon arrival, a venous catheter was inserted into a forearm vein, and frequent blood samples were taken (described in detail later). The participants consumed a dinner meal in the laboratory with 1) no RE (NoRE), 2) predinner RE (RE → M) ending ∼20–30 min before dinner, or 3) postdinner RE beginning 45 min after dinner (M → RE).
Day 3.
On day 3 of all trials (between 6:30 and 8:30 AM) and ∼12–15 h after the RE session, the participants reported to the laboratory overnight fasted, and a fasting blood sample was taken, followed by consumption of a standardized breakfast meal. After the participants consumed breakfast, they were allowed to leave the laboratory but were required not to consume any food for 4 h so that the 4-h postprandial glucose response to breakfast could be measured. The participants took their medications at the usual dose, frequency, and time while participating in this study.
RE
For baseline testing, each participant performed familiarization and strength testing so that they were not naive to RE before the study. The first familiarization was designed to teach the participants how to properly perform each exercise. During this visit, the weight for RE was light (∼10–40% of body weight), and the participants performed one to two sets of 10 repetitions of the following exercises (in this order): leg press, seated calf raises, seated chest flies, seated back flies, back extensions, shoulder raises, leg curls, and abdominal crunches. Within a week of this first visit, participants returned for strength testing, and their 10 repetitions maximum (10-RM) for each exercise described previously (except abdominal crunches) was determined. At least 3 days after 10-RM testing, a second familiarization was performed, during which the participants performed three sets (1–2 min rest between sets) of 10-RM for each RE. During this session, the first set for each exercise was a warm-up set, and the weight used was 50% of the participants' 10-RM. After the warm-up set, the weight for the next two sets was the participants' previously determined 10-RM. Following these visits, the participants completed the 3 study days, and the RE session during the study days was identical to the protocol for the second familiarization session.
Diet
During baseline testing, resting energy expenditure was measured using indirect calorimetry (ParvoMedics TrueOne 2400), and the average physical activity energy expenditure over a 2- to 3-day period was measured with a BodyMedia armband. The sum of these two measurements was used as an estimate of total daily energy expenditure, and the participants were provided with their respective energy needs during the day of testing. For all meals, the macronutrient composition was ∼50% carbohydrate, 35% fat, and 15% protein. The breakfast meal was 2,448 kJ and consisted of an English muffin, cheddar cheese, one large egg, ham, hash browns, ketchup, and apple or orange juice. The lunch meal was 2,439 kJ and consisted of white bread, ham, mayonnaise, cheddar cheese, a granola bar, and apple or orange juice. The dinner meal was spaghetti noodles, spaghetti sauce with beef added, garlic bread, a lemon-lime flavored soda, and 1.5 g of acetaminophen (to assess gastric emptying). The energy content of dinner was calculated by subtracting 4,887 kJ (from breakfast and lunch meals) from the estimated total daily energy expenditure for each participant.
Indirect Calorimetry, Heart Rate, Ratings of Perceived Exertion, and Well-being
Indirect calorimetry was used to measure energy expenditure and substrate oxidation during the time frame when RE was performed. Ratings of perceived exertion were measured at the end of every RE set using the Borg 6–20 scale. Subjective well-being was assessed using a 100-mm visual analog scale after every blood draw to determine how RE made the participants feel (2). The question was worded “How strong is your overall feeling of well-being (pleasure),” and the participants marked a single horizontal line through the vertical line on the scale in between the anchors “not at all” and “extremely”.
Blood Collection
Blood samples were taken every 5–10 min during the first 3.7 h of testing and every 30 min during the last 2 h. Blood samples were transferred immediately into chilled EDTA tubes, either with added aprotinin (ThermoFisher Scientific) only (for TAG samples), chilled EDTA tubes with added dipeptidyl peptidase-4 inhibitor (Millipore), Pefabloc SC (DSM Nutritional Products AG), and aprotinin (for hormone, glucose, and FFA analysis), or EDTA tubes without any protease inhibitors added (for acetaminophen assay). Blood was separated by centrifugation using an Eppendorf 5702R centrifuge at 3,000 rpm for 10 min at 4°C and then frozen at −80°C until analysis.
Separation of Lipoprotein Species
Separation of plasma chylomicron [Svedberg flotation index (Sf) > 400], very-low-density lipoprotein (VLDL)-1 (Sf 60–400), and VLDL-2 (Sf 20–60) was performed using density gradient ultracentrifugation, as described by Karpe and Hamsten (18), but with minor modification. KBr (0.1 g) was mixed into 1 ml of fresh plasma to bring the density up to ∼1.1 g/l in a polyallomer ultracentrifuge tube (14 × 89 mm tube, Beckman Coulter). Next, a density gradient consisting of 3 ml of KBr salt solution (1.1 g/l), 4 ml of NaCl solution (1.065 g/l), 3 ml of KBr solution (density 1.020 g/l), and 1 ml of NaCl solution (density 1.006 g/l) was layered above the plasma. Ultracentrifugation was performed using a TH-641 (ThermoFisher Scientific) swinging bucket rotor at 40,000 rpm and 10°C. For the separation of each lipoprotein species, consecutive ultracentrifugations of 32 min (chylomicrons), 3 h and 28 min (VLDL-1), and 16 h (VLDL-2) were performed. After each run, the top 1-ml layer was carefully removed and frozen at −80°C until analysis. Before the next run, the tube was refilled with 1 ml of NaCl solution of density 1.006 g/l. Only five time points were assessed for TAG due to limited space in the ultracentrifuge.
Biochemical Analyses
Whole blood glucose was assessed using a YSI 2700 Select (YSI). Plasma TAG (Infinity, ThermoFisher Scientific), FFA (Wako Chemicals), and acetaminophen (Cambridge Life Science) concentrations were determined using a colorimetric assay. Plasma hormone concentrations were determined using a MILLIPLEX magnetic bead-based immunoassay (Millipore). Hematocrit was measured after every blood draw, and samples were corrected for plasma volume shifts. Plasma volume variations (ΔVP) were calculated from hematocrit (Ht) variations (Ht1 = baseline Ht, Ht2 = sample after baseline Ht) using the following formula: %ΔVP = 100 × {(Ht1 − Ht2)/[Ht2 × (100 − Ht1)]}. Corrected plasma values were calculated using the following formula: corrected value = (initial value × 100)/(100 − ΔVP). Inter- and intra-assay coefficients of variation were < 10% for all variables.
β-cell Function
Model based β-cell function parameters (β-cell glucose sensitivity, potentiation factor ratio, and rate sensitivity) were calculated using a mathematical model developed by Mari et al. (22, 23). Briefly, β-cell glucose sensitivity is the slope of the dose-response relation between insulin secretion and glucose concentration. The potentiation factor represents relative potentiation or inhibition of insulin secretion during the test. The potentiation factor ratio is the ratio between potentiation at the end of the dinner meal (220–240 min) and the initial value (0–20 min). Rate sensitivity represents the enhancement of insulin secretion proportional to the rate of change of plasma glucose. Insulin secretion rates used in the model were calculated by de-convoluting C-peptide concentrations (36).
Calculations
Postprandial responses were quantified using incremental area under the curve (iAUC) (30). Gastric emptying was quantified using the acetaminophen iAUC. Insulin clearance was calculated as the molar ratio of insulin to C-peptide at each time point as a percent ({1 − [insulin/C-peptide (pmol/l)]} × 100) (14, 15), and then the iAUC was calculated to estimate insulin clearance over the entire testing period.
Statistical Analysis
Statistical analyses were performed using the SPSS statistical software, version 18.0 (IBM). A paired-samples t-test was used to compare metabolic, heart rate, and perceived exertion data between trials. A repeated-measures ANOVA with follow-up Bonferroni adjusted post hoc t-tests was used to determine specific differences between iAUC values. Alpha was set at P ≤ 0.05. All values are reported as means ± SE for all 13 subjects, unless otherwise noted.
RESULTS
Participant Characteristics and Metabolic and Perceived Exertion Data during Exercise
Thirteen obese men and women with type 2 diabetes completed this study (Table 1). The participants were weight stable during the study and did not change their medication usage. The average heart rate during exercise was 4 beats/min higher (P = 0.01) during M → RE compared with RE → M, while all other variables were similar (Table 2, P > 0.05).
Table 1.
Patient characteristics
| Means ± SD | |
|---|---|
| Age, yr | 48.5 ± 11.9 |
| Height, m | 1.67 ± 0.11 |
| Weight, kg | 103.2 ± 22.8 |
| Body mass index, kg/m2 | 36.7 ± 5.3 |
| Body fat, % | 39.5 ± 8.6 |
| Fasting glucose, mmol/l | 8.2 ± 2.3 |
| Fasting hemoglobin A1c, % (mmol/mol) | 7.2 ± 1.1 (55) |
| Diagnosed with type 2 diabetes, yr | 3.7 ± 3.9 |
| Patients with antidiabetes medication use, n | 12 |
n = 13 Subjects (5 men).
Table 2.
Metabolic data and RPE during resistance exercise session
| RE → M | NoRE-1 | M → RE | NoRE-2 | |
|---|---|---|---|---|
| Duration, min | 46 ± 1 | 46 ± 1 | 47 ± 1 | 47 ± 1 |
| Oxygen consumption, ml·kg−1·min−1 | 6.1 ± 0.4 | 2.9 ± 0.1* | 6.0 ± 0.3 | 2.9 ± 0.1* |
| Energy expenditure, gross kJ | 586 ± 59 | 264 ± 13* | 590 ± 59 | 276 ± 21* |
| Respiratory exchange ratio | 1.00 ± 0.01 | 0.83 ± 0.01* | 1.00 ± 0.01 | 0.87 ± 0.01*† |
| Heart rate, beats/min | 106 ± 4 | 110 ± 4‡ | ||
| Average RPE, Borg 6–20 scale | 12 ± 1 | 12 ± 1 |
Values are means ± SE. For duration, oxygen consumption, energy expenditure, respiratory exchange ratio, and heart rate, only data for n = 12 are reported. For ratings of perceived exertion (RPE), data for n = 13 are reported. RE → M, resistance exercise performed before dinner consumption; M → RE, dinner consumption before resistance exercise; NoRE, no resistance exercise. The data under NoRE-1 is during the same time frame (i.e., before dinner) as RE → M but during the NoRE trial, whereas the data under NoRE-2 is during the same time frame (i.e., after dinner) as M → RE but during the NoRE trial.
P < 0.05 compared with RE → M or M → RE.
P < 0.05 compared with heart rate during the RE → M trial.
P < 0.05 compared with NoRE-1.
TAG, Glucose, and Insulin Responses
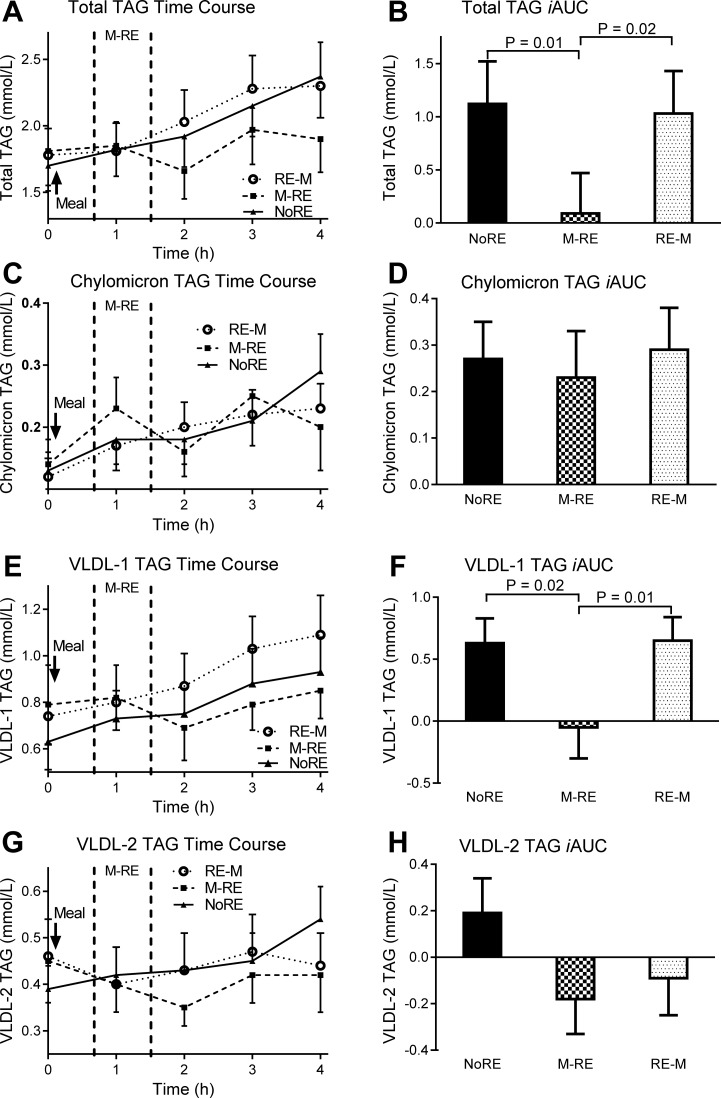
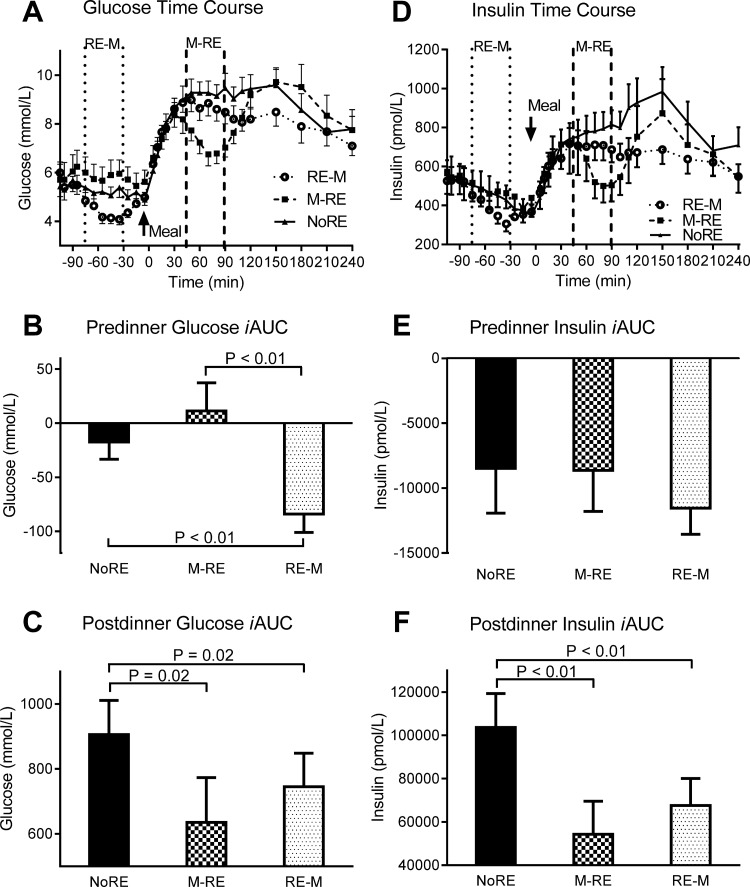
During M → RE, the postprandial total TAG iAUC was reduced by ∼92% (P ≤ 0.02) compared with NoRE and RE → M (Fig. 1, A and B), an effect due to reduced VLDL-1 TAG concentrations (Fig. 1, E and F). Neither chylomicron (Fig. 1, C and D) nor VLDL-2 TAG (Fig. 1, G and H) concentrations were different between trials (P > 0.05). The predinner glucose iAUC was significantly lower (P < 0.01) during RE → M compared with NoRE and M → RE (Fig. 2, A and B). The postprandial glucose iAUC was reduced by 30 and 18% during M → RE and RE → M, respectively, compared with NoRE (both P = 0.02), with no difference between exercise trials (Fig. 2C). Predinner insulin concentrations were not different between trials, but the postprandial insulin iAUC was 39 and 31% lower (P < 0.01) during M → RE and RE → M, respectively, compared with NoRE (Fig. 2, D–F).
Fig. 1.
Total, exogenous, and endogenous triacylglycerol (TAG) responses to the dinner meal with pre- or postdinner resistance exercise or no exercise. A, C, E, G: total, chylomicron, very-low-density lipoprotein (VLDL)-1, and VLDL-2 TAG time course, respectively. In the time course figures, the space in between the vertical dashed lines represents the time frame when resistance exercise was performed during the indicated trial. B, D, F, H: total, chylomicron, VLDL-1, and VLDL-2 TAG incremental area under the curve (iAUC), respectively. NoRE, no resistance exercise; RE → M, resistance exercise performed before dinner consumption; M → RE, dinner consumption before resistance exercise. Values are means ± SE; n = 13 subjects.
Fig. 2.
Glucose and insulin responses to the dinner meal with pre- or postdinner resistance exercise or no exercise. A and D: glucose and insulin time course, respectively. In the time course figures, the space in between the vertical dashed lines represents the time frame when resistance exercise was performed during the indicated trial. B and E: premeal glucose and insulin iAUC, respectively. C and F: postmeal glucose and insulin iAUC, respectively. Values are means ± SE; n = 13 subjects.
Insulin Kinetics, β-cell Function, and Gastric Emptying
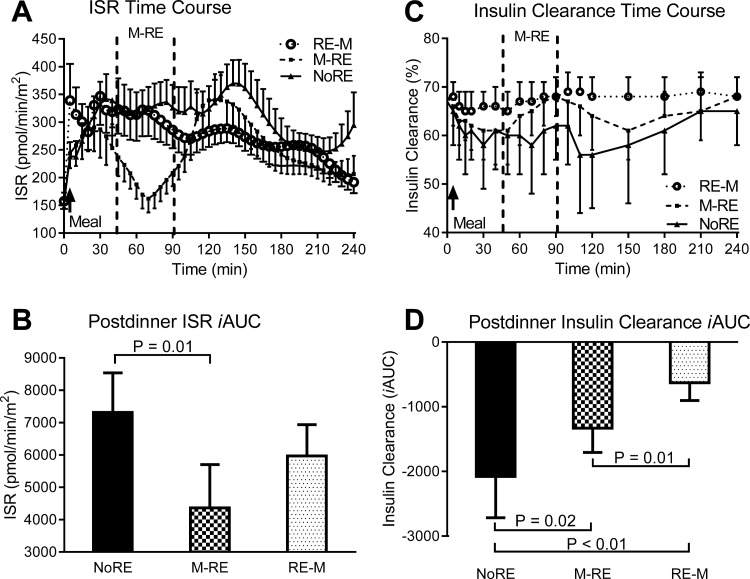
The postprandial insulin secretion rate iAUC was 40% lower (P = 0.01) during the M → RE trial compared with the NoRE trial and tended to be lower during the RE → M trial compared with the NoEX trial (P = 0.06) (Fig. 3, A and B). The postprandial insulin clearance iAUC was nearly three- and twofold greater (iAUC was less negative, P ≤ 0.02) during RE → M and M → RE, respectively, compared with NoRE, and nearly twofold greater (P = 0.01) during RE → M compared with M → RE (Fig. 3, C and D). There were no significant differences (P > 0.05) in gastric emptying, β-cell glucose sensitivity, rate sensitivity, or the potentiation factor ratio between trials (Table 3).
Fig. 3.
Insulin secretion or clearance responses to the dinner meal with pre- or postdinner resistance exercise or no exercise. A and C: insulin secretion response (ISR) and insulin clearance time course, respectively. In the time course figures, the space in between the vertical dashed lines represents the time frame when resistance exercise was performed during the indicated trial. B and D: postmeal ISR and insulin clearance iAUC, respectively. Values are means ± SE; n = 13 subjects.
Table 3.
β-cell function parameters and gastric emptying
| NoRE | RE → M | M → RE | |
|---|---|---|---|
| β-Cell glucose sensitivity, pmol·min−1·m−2·mM−1 | 39 ± 5 | 50 ± 8 | 50 ± 9 |
| Rate sensitivity, pmol·m−2·mM−1 | 306 ± 93 | 402 ± 141 | 307 ± 86 |
| Potentiation factor ratio, fold | 1.28 ± 0.14 | 0.99 ± 0.08 | 0.92 ± 0.10 |
| Acetaminophen iAUC, μmol/l | 17,777 ± 1,866 | 15,493 ± 1,792 | 19,001 ± 1,626 |
Values are means ± SE. iAUC, incremental area under the curve.
GIP, GLP-1, FFA, C-peptide, and Glucagon Responses
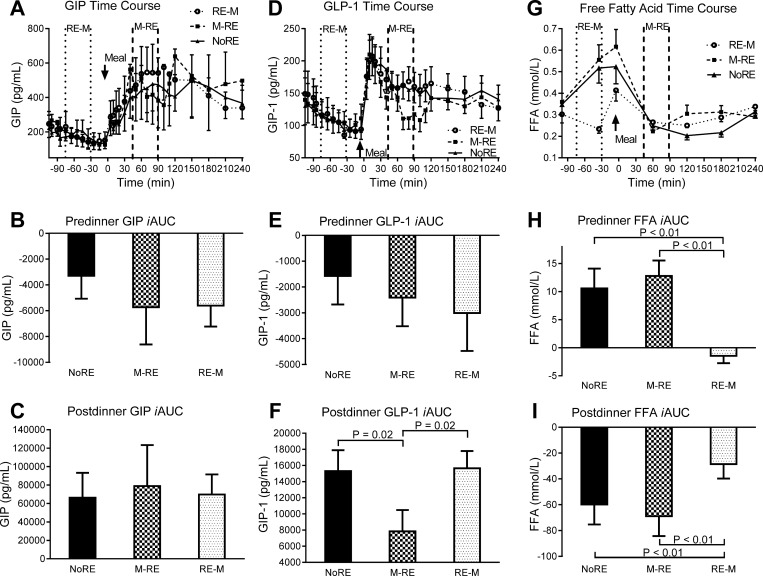
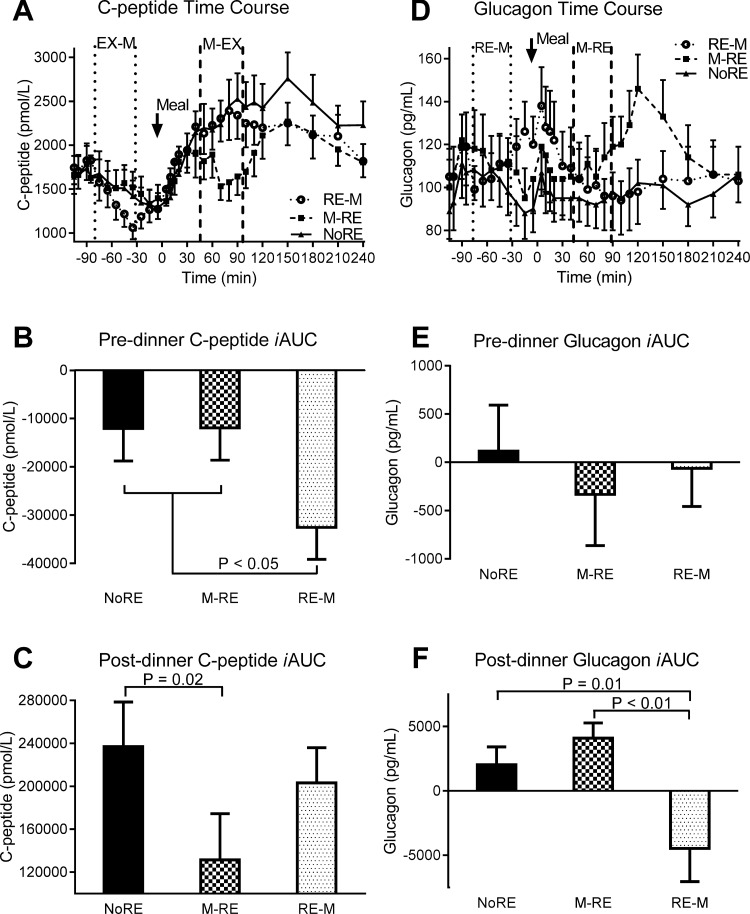
Neither premeal nor postprandial glucose-dependent insulinotropic polypeptide (GIP) concentrations were significantly different (P > 0.05) between trials (Fig. 4, A–C). During M → RE, the postprandial glucagon-like peptide-1 (GLP-1) iAUC was 50 and 49% lower (P ≤ 0.02) compared with RE → M and NoRE, respectively (Fig. 4, D–F). The rise in premeal FFA concentrations was significantly attenuated (P < 0.01) during RE → M compared with NoRE and M → RE (Fig. 4, G and H). Similarly, the drop in postprandial FFA concentrations was significantly less (P < 0.01) during RE → M compared with NoRE and M → RE (Fig. 4I). The predinner C-peptide iAUC was ∼2.7-fold lower (P = 0.01) during RE → M compared with M → RE and NoRE (Fig. 5, A and B). The postprandial C-peptide iAUC was 45% lower (P = 0.02) during M → RE compared with NoRE (Fig. 5C). Premeal glucagon responses were not different between trials, but the postprandial glucagon iAUC dropped significantly more (P ≤ 0.01) during RE → M compared with the other trials because it was higher at the start of the meal (Fig. 5, D–F).
Fig. 4.
Gut hormone and free fatty acid responses to the dinner meal with pre- or postdinner resistance exercise or no exercise. A, D, and G: glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), and free fatty acid (FFA) time course, respectively. In the time course figures, the space in between the vertical dashed lines represents the time frame when resistance exercise was performed during the indicated trial. B, E, and H: premeal GIP, GLP-1, and FFA iAUC, respectively. C, F, and I: postmeal GIP, GLP-1, and FFA iAUC, respectively. Values are means ± SE; n = 13 subjects.
Fig. 5.
C-peptide and glucagon responses to the dinner meal with pre- or postdinner resistance exercise or no exercise. A and D: C-peptide and glucagon time course, respectively. In the time course figures, the space in between the vertical dashed lines represents the time frame when resistance exercise was performed during the indicated trial. B and E: premeal C-peptide and glucagon iAUC, respectively. C and F: postmeal C-peptide and glucagon iAUC, respectively. Values are means ± SE; n = 13 subjects.
Nocturnal and Morning Glycemic Control and Insulin Sensitivity
Nocturnal or morning glycemic control (Table 4), fasting glucose, insulin, C-peptide, insulin clearance, homeostatic model assessment of insulin resistance, Quantitative Insulin Sensitivity Check Index, and TAG (Table 5) were not different between trials the next morning (P > 0.05).
Table 4.
Nocturnal and postbreakfast glucose responses
| NoRE | RE → M | M → RE | |
|---|---|---|---|
| Nocturnal glucose responses (12–6 AM) | |||
| Average blood glucose, mmol/l | 6.3 ± 0.5 | 5.9 ± 0.4 | 6.4 ± 0.6 |
| Time hyperglycemic (blood glucose >10 mmol/l), min | 0.4 ± 0.6 | 2.7 ± 3.9 | 14.6 ± 10.6 |
| Time hypoglycemic (blood glucose <3.9 mmol/l), min | 26.5 ± 30.5 | 17.7 ± 7.8 | 6.9 ± 3.7 |
| Morning glucose responses | |||
| Average morning blood glucose, mmol/l | 8.9 ± 0.6 | 8.7 ± 0.7 | 9.1 ± 0.9 |
| Breakfast 4 h iAUC, mmol·l−1·4 h−1 | 72 ± 17 | 83 ± 23 | 75 ± 22 |
| Time hyperglycemic (blood glucose >10 mmol/l), min | 80.8 ± 22.7 | 43.3 ± 19.0 | 66.3 ± 27.5 |
| Time hypoglycemic (blood glucose <3.9 mmol/l), min | 0 | 0 | 0 |
Values are means ± SE. The data for the nocturnal glucose response are for n = 13, whereas the data for the morning glucose responses are for n = 12.
Table 5.
Morning fasting measures the day after testing in the laboratory
| NoRE | RE → M | M → RE | |
|---|---|---|---|
| Blood glucose, mmol/l | 6.7 ± 0.4 | 6.7 ± 0.3 | 6.7 ± 0.6 |
| Insulin, pmol/l | 290 ± 43 | 256 ± 37 | 301 ± 53 |
| C-peptide, pmol/l | 1537 ± 181 | 1378 ± 113 | 1465 ± 194 |
| Insulin clearance, % | 81 ± 2 | 82 ± 2 | 80 ± 2 |
| HOMA-IR | 12.58 ± 1.98 | 10.98 ± 1.77 | 12.05 ± 1.92 |
| QUICKI | 0.28 ± 0.01 | 0.28 ± 0.01 | 0.28 ± 0.05 |
| Triacylglycerol, mmol/l | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 |
Values are means ± SE. HOMA-IR, homeostatic model assessment of insulin resistance; QUICKI, Quantitative Insulin Sensitivity Check Index.
Subjective Well-being
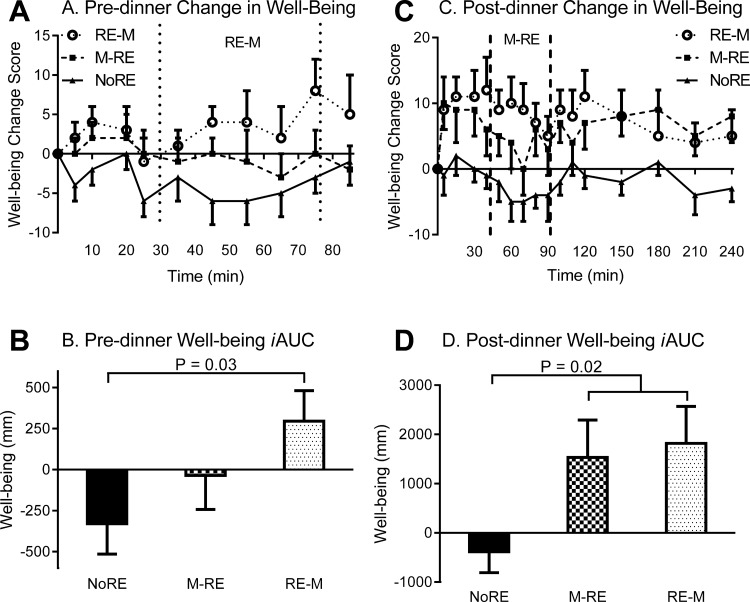
Baseline well-being was significantly different between trials (P = 0.02); thus, to better illustrate the change in well-being, change scores were used. The premeal subjective well-being iAUC was significantly greater during RE → M compared with NoRE (P = 0.03) and tended to be greater (P = 0.09) compared with M → RE (Fig. 6, A and B). The postprandial well-being iAUC was significantly greater (P = 0.02) with RE compared with NoRE, independent of RE timing (Fig. 6, C and D).
Fig. 6.
Subjective well-being responses to the dinner meal with pre- or postdinner resistance exercise or no exercise. A and C: premeal and postmeal change in well-being, respectively. In the time course figures, the space in between the vertical dashed lines represents the time frame when resistance exercise was performed during the indicated trial. B and D: premeal and postmeal well-being iAUC, respectively. Values are means ± SE; n = 13 subjects.
DISCUSSION
With a push toward personalized medicine, knowledge of the best time to perform exercise around a meal could provide health care professionals with a better understanding of how to personalize exercise prescription to optimize its metabolic health benefits. Thus the overarching goal of this project was to identify the most effective time, either before or after dinner, to perform RE to lower postprandial glucose and TAG concentrations, two important postprandial CVD risk factors in obese patients with type 2 diabetes. The key clinically significant findings of this study are as follows: 1) both pre- and postdinner RE reduces postprandial glucose concentrations, while only postdinner RE reduces both postprandial glucose and TAG concentrations; 2) both pre- and postdinner RE reduces insulin concentrations, but via different mechanisms, as predinner RE enhances estimated insulin clearance, whereas postdinner RE reduces estimated insulin secretion and enhances estimated insulin clearance; and 3) postdinner RE reduces postprandial GLP-1 concentrations, while predinner RE does not. Taken together, postdinner RE is more effective at improving postprandial CVD risk factors compared with predinner RE in obese patients with type 2 diabetes, although these benefits are short lived and do not last into the overnight period or into the next day. Importantly, RE improved well-being, making it a feasible option in this population.
Previous research is disparate as two studies have shown that acute RE prior to a meal(s) improves glycemic control (7, 38), but two other studies showed acute RE does not improve glycemic control (3, 8) in patients with type 2 diabetes. Similar to some of these findings, in the present study both pre- and postdinner RE equally improved glycemic control compared with NoRE. With predinner RE, postprandial glucose concentrations were lower from ∼1–3 h after the meal compared with NoRE. During postdinner RE, glucose concentrations were lower during exercise, from ∼45 min to 1.5 h after the meal, and rebounded at the cessation of exercise to NoRE levels. These drastic differences in glucose responses suggest different glucose-lowering mechanisms may be at play, depending on RE timing. It is possible that during the predinner RE trial, insulin-independent glucose uptake and insulin action should have been increased and thus increased glucose uptake and utilization during the subsequent meal. During the postdinner RE trial, elevated insulin concentrations due to the meal, as well as the skeletal muscle contractions, probably worked synergistically to increase skeletal muscle blood glucose uptake, as has been shown with aerobic exercise (20, 33, 34). Interestingly, once the postdinner RE session was over, blood glucose concentrations rebounded and were similar to those during the NoRE trial. This rebound in glucose may have been due to a simultaneous reduction in skeletal muscle glucose uptake at the cessation of exercise and a transient increase in hepatic glucose output, which has been shown to occur with postprandial aerobic exercise in patients with type 2 diabetes (20). The CGMS data suggest that these improvements in glycemia are short-lived and do not extend into the next day, thus daily RE may be required to maintain improvements in glycemia, at least in the early stages of training.
For the first time, this study shows that postdinner RE lowers postprandial TAG concentrations, whereas predinner RE does not. The available data with aerobic exercise in patients with type 2 diabetes somewhat support our findings, although comparisons between studies are difficult because of different study designs. For example, two studies have shown prior aerobic exercise performed the day before the test meal does not alter postprandial TAG concentrations (5, 11), while in another study postmeal aerobic exercise reduced postprandial TAG concentrations compared with no exercise (35). The majority of TAG in circulation originates from either exogenous sources and is in the form of chylomicron particles, or endogenous sources from the liver and is in the form of VLDL-TAG (13). We attempted to establish whether chylomicrons or VLDL-TAG were modified by RE timing and observed that neither premeal nor postmeal RE modified postprandial chylomicron or VLDL-2 TAG particles. Instead, the reduction in postprandial TAG concentrations with postdinner RE was mediated by reduced VLDL-1 TAG concentrations. Although not possible to determine from the data, the mechanism(s) for the reduction in VLDL-1 TAG with postdinner RE could have been mediated by enhanced hydrolysis of TAG by lipoprotein lipase in skeletal muscle and/or by reduced hepatic VLDL-1 TAG secretion (13).
In the present study, both pre- and postdinner RE reduced postprandial insulin concentrations, a finding that is in agreement with other work (8), but with potential differing physiological mechanisms. With predinner RE, the reduction in postprandial insulin concentrations was mediated by enhanced insulin clearance, and this has been shown previously for RE (8). Although predinner RE reduced glucose concentrations, this was not associated with significantly reduced insulin secretion (although insulin secretion was slightly lower), possibly because the insulin-potentiating hormones GLP-1 or GIP were not significantly reduced. This finding is similar to an aerobic exercise study in which premeal aerobic exercise did not alter postprandial GLP-1 or GIP responses in patients with type 2 diabetes (6). During postdinner RE, the reduction in insulin concentrations was mediated by both increased insulin clearance and reduced insulin secretion, although the increase in insulin clearance was not as great compared with the predinner RE trial. Lower postprandial insulin secretion with postdinner RE could have been mediated by reduced GLP-1 and glucose concentrations and not by changes in GIP or β-cell function since they were unchanged. We speculate the mechanism by which RE increases insulin clearance could potentially be due to increased skeletal muscle blood flow. Increased blood flow in skeletal muscle may increase blood flow through previously nonflowing capillaries, allowing more insulin to be exposed to and bind to the insulin receptor, internalized, and degraded. This hypothesis is supported by the work of Phillips et al. (27), who showed that prior RE enhanced postprandial leg blood flow responses, compared with no exercise. Another possibility may be that RE reduced portal vein glucose and/or FFA concentrations. Given that glucose and FFA have been shown to independently and synergistically impair insulin binding to receptors (16), it is possible that RE reduced these substrates in the portal vein, preventing glucose and FFA interference of insulin binding to receptors in the liver, thus allowing more insulin to bind to hepatic insulin receptors and be internalized and degraded (i.e., greater insulin clearance).
In conclusion, the key clinically significant finding of this study is that both pre- and postdinner RE reduces postprandial glucose concentrations, but only postdinner RE reduces both postprandial glucose and TAG concentrations. Thus, overall, postdinner RE, compared with predinner RE, more effectively improves two significant postprandial CVD risk factors in obese patients with type 2 diabetes. Future studies are needed to determine whether long-term postmeal exercise training would better reduce CVD risk more so than premeal exercise.
GRANTS
This project was supported by department funds and National Institutes of Health (NIH) Training Grant 5T32-AR-048523-10 (T. D. Heden). Salary support was provided by a Veterans Health Administration CDA2 award for R. S. Rector and NIH RO1-DK-088940 award for J. P. Thyfault.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.D.H., F.W.B., R.S.R., J.P.T., and J.A.K. conception and design of research; T.D.H., N.C.W., and J.A.K. performed experiments; T.D.H. and A.M. analyzed data; T.D.H., A.M., F.W.B., R.S.R., J.P.T., and J.A.K. interpreted results of experiments; T.D.H. prepared figures; T.D.H. drafted manuscript; T.D.H., N.C.W., A.M., F.W.B., R.S.R., J.P.T., and J.A.K. edited and revised manuscript; T.D.H. and J.A.K. approved final version of manuscript.
ACKNOWLEDGMENT
We thank Ying Liu for placing the catheters and helping with some of the data collection.
REFERENCES
- 1.Abbasi F, Chu JW, McLaughlin T, Lamendola C, Leary ET, Reaven GM. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism 53: 159–164, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med 62: 989–993, 1969. [PMC free article] [PubMed] [Google Scholar]
- 3.Bacchi E, Negri C, Trombetta M, Zanolin ME, Lanza M, Bonora E, Moghetti P. Differences in the acute effects of aerobic and resistance exercise in subjects with type 2 diabetes: results from the RAED2 Randomized Trial. PLos One 7: e49937, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colberg SR, Zarrabi L, Bennington L, Nakave A, Thomas Somma C, Swain DP, Sechrist SR. Postprandial walking is better for lowering the glycemic effect of dinner than pre-dinner exercise in type 2 diabetic individuals. J Am Med Dir Assoc 10: 394–397, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Dalgaard M, Thomsen C, Hermansen K. Effects of one single bout of low-intensity exercise on postprandial lipaemia in type 2 diabetic men. Br J Nutr 92: 469–476, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Eshghi SR, Bell GJ, Boule NG. Effects of aerobic exercise with or without metformin on plasma incretins in type 2 diabetes. Can J Diabetes 37: 375–380, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Fenicchia LM, Kanaley JA, Azevedo JL Jr, Miller CS, Weinstock RS, Carhart RL, Ploutz-Snyder LL. Influence of resistance exercise training on glucose control in women with type 2 diabetes. Metabolism 53: 284–289, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Fluckey JD, Hickey MS, Brambrink JK, Hart KK, Alexander K, Craig BW. Effects of resistance exercise on glucose tolerance in normal and glucose-intolerant subjects. J Appl Physiol (1985) 77: 1087–1092, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 300: 2142–2152, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Garg A, Bantle JP, Henry RR, Coulston AM, Griver KA, Raatz SK, Brinkley L, Chen YD, Grundy SM, Huet BA, et al. Effects of varying carbohydrate content of diet in patients with non-insulin-dependent diabetes mellitus. JAMA 271: 1421–1428, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Gill JM, Al-Mamari A, Ferrell WR, Cleland SJ, Perry CG, Sattar N, Packard CJ, Caslake MJ, Petrie JR. Effect of prior moderate exercise on postprandial metabolism in men with type 2 diabetes: heterogeneity of responses. Atherosclerosis 194: 134–143, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Gillen JB, Little JP, Punthakee Z, Tarnopolsky MA, Riddell MC, Gibala MJ. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes Obes Metab 14: 575–577, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg HN, Zhang YL, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res 36: 232–240, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Heden TD, Liu Y, Kearney ML, Kanaley JA. Weight classification does not influence the short-term endocrine or metabolic effects of high-fructose corn syrup-sweetened beverages. Appl Physiol Nutr Metab 39: 544–552, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Heden TD, Liu Y, Kearney ML, Park Y, Dellsperger KC, Thomas TR, Kanaley JA. Prior exercise and postprandial incretin responses in lean and obese individuals. Med Sci Sports Exerc 45: 1897–1905, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennes MM, Dua A, Kissebah AH. Effects of free fatty acids and glucose on splanchnic insulin dynamics. Diabetes 46: 57–62, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Liu W, Huang R, Zhang X. Postchallenge plasma glucose excursions, carotid intima-media thickness, and risk factors for atherosclerosis in Chinese population with type 2 diabetes. Atherosclerosis 210: 302–306, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Karpe F, Hamsten A. Determination of apolipoproteins B-48 and B-100 in triglyceride-rich lipoproteins by analytical SDS-PAGE. J Lipid Res 35: 1311–1317, 1994. [PubMed] [Google Scholar]
- 19.Krook A, Holm I, Pettersson S, Wallberg-Henriksson H. Reduction of risk factors following lifestyle modification programme in subjects with type 2 (non-insulin dependent) diabetes mellitus. Clin Physiol Funct Imaging 23: 21–30, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Larsen JJ, Dela F, Kjaer M, Galbo H. The effect of moderate exercise on postprandial glucose homeostasis in NIDDM patients. Diabetologia 40: 447–453, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Manders RJ, Van Dijk JW, van Loon LJ. Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes. Med Sci Sports Exerc 42: 219–225, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of β-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab 283: E1159–E1166, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 51, Suppl 1: S221–S226, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298: 299–308, 2007. [DOI] [PubMed] [Google Scholar]
- 25.O'Gorman DJ, Krook A. Exercise and the treatment of diabetes and obesity. Med Clin North Am 95: 953–969, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Oberlin DJ, Mikus CR, Kearney ML, Hinton PS, Manrique C, Leidy HJ, Kanaley JA, Rector RS, Thyfault JP. One bout of exercise alters free-living postprandial glycemia in type 2 diabetes. Med Sci Sports Exerc 46: 232–238, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips B, Williams J, Atherton P, Smith K, Hildebrandt W, Rankin D, Greenhaff P, Macdonald I, Rennie MJ. Resistance exercise training improves age-related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J Appl Physiol (1985) 112: 347–353, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Poirier P, Mawhinney S, Grondin L, Tremblay A, Broderick T, Cleroux J, Catellier C, Tancrede G, Nadeau A. Prior meal enhances the plasma glucose lowering effect of exercise in type 2 diabetes. Med Sci Sports Exerc 33: 1259–1264, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Praet SF, Manders RJ, Lieverse AG, Kuipers H, Stehouwer CD, Keizer HA, van Loon LJ. Influence of acute exercise on hyperglycemia in insulin-treated type 2 diabetes. Med Sci Sports Exerc 38: 2037–2044, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28: 916–931, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 23: 1830–1834, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Teno S, Uto Y, Nagashima H, Endoh Y, Iwamoto Y, Omori Y, Takizawa T. Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care 23: 1401–1406, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JF, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol Endocrinol Metab 277: E733–E741, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Thyfault JP. Setting the stage: possible mechanisms by which acute contraction restores insulin sensitivity in muscle. Am J Physiol Regul Integr Comp Physiol 294: R1103–R1110, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Tobin LW, Kiens B, Galbo H. The effect of exercise on postprandial lipidemia in type 2 diabetic patients. Eur J Appl Physiol 102: 361–370, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41: 368–377, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Van Dijk JW, Manders RJ, Canfora EE, Mechelen WV, Hartgens F, Stehouwer CD, Van Loon LJ. Exercise and 24-h glycemic control: equal effects for all type 2 diabetes patients? Med Sci Sports Exerc 45: 628–635, 2013. [DOI] [PubMed] [Google Scholar]
- 38.van Dijk JW, Manders RJ, Tummers K, Bonomi AG, Stehouwer CD, Hartgens F, van Loon LJ. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia 55: 1273–1282, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dijk JW, Tummers K, Stehouwer CD, Hartgens F, van Loon LJ. Exercise therapy in type 2 diabetes: is daily exercise required to optimize glycemic control? Diabetes Care 35: 948–954, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dijk JW, Venema M, van Mechelen W, Stehouwer CD, Hartgens F, van Loon LJ. Effect of moderate-intensity exercise versus activities of daily living on 24-hour blood glucose homeostasis in male patients with type 2 diabetes. Diabetes Care 36: 3448–3453, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]