seminal tracer experiments of the 1960s by Farquhar and Palade (8) using ferritin, a prototype of globular protein-like albumin, established that glomerular capillaries in mammals serve as a barrier to the transglomerular passage of circulating large-molecular-weight plasma proteins since this tracer was mainly seen in proximity to glomerular endothelial fenestrae with no permeation into the glomerular basement membrane (GBM). A subsequent study (3) with graded dextrans indicated that these capillaries or glomerular ultrafiltration units (GUUs) are endowed with size-selective properties since low-molecular-weight dextrans escaped into the urinary space, with no restriction at the slit diaphragm, wheras high-molecular-weight dextrans remained in the capillary lumina and did not enter the GBM; this suggested that the GBM may serve as the primary filtration barrier that discriminates their passage depending on their molecular weight or hydrodynamic radii. Simultaneous studies in the 1960s by Venkatachalam et al. (29, 30) using peroxidatic tracers suggested that the slit diaphragm, rather than the GBM, is the major barrier that restricts the passage of macromolecules across the GUU since some of them accumulated underneath the diaphragm. Thus, the controversy arose as to which component of the GUU, the GBM versus slit diaphragm, serves as the primary filtration barrier. Amidst this contentious issue, in the mid to late 1970s, Brenner and colleagues (2) performed various fractional clearance experiments using varying sizes of neutral, anionic, and cationic dextrans, and their results indicated that the glomerular capillary wall behaves as a size- as well as charge-selective barrier. At the same time, Venkatachalam and Farquhar and their colleagues (14, 25) performed studies using cationic ferritin(s) and observed that this modified tracer could permeate deeper into the GBM and localize within its lamina rarae, suggesting that the GBM, besides a size-selective barrier, has charge-selective properties. With respect to charge, Venkatachalam et al. (27) also demonstrated that intrarenal perfusion of solutions containing basic compounds, such as protamine sulfate (PS), led to a reversible fusion of the podocyte foot processes, suggesting that the podocyte surface also carries an electronegative charge and that its neutralization with PS led to their effacement. Thus, another controversy arose as to which component of the GUU, the GBM versus podocytes foot processes, is responsible for the charge-selective properties of the glomerular capillary, and this observation was later on further compounded by the fact that the endothelial cell surface also has a glycocalyx, which, in this equation, might also impart, to a certain extent, the electronegative charge (6). An article by Sverrisson et al. (28) explored to what extent the GUU exerts size versus charge selectivity in a PS perfusion model and if such properties are applicable to molecules with a wide range of hydrodynamic radii. In this regard, a brief description of the structural and biochemical composition of the GUU may be obligatory to comprehend these controversies.
The GUU of glomerular capillaries fractionates the blood into an ultrafiltrate that essentially includes small-molecular-weight proteins, amino acids, electrolytes, and plasma water. Large-molecular proteins and cells are retained within the capillary lumina. The GUU is a stratified structure that is made up of an attenuated fenestrated endothelium facing the capillary lumina, GBM, and interdigitating foot processes of podocytes attached to the GBM and suspended in the urinary space (Fig. 1A) (16). The endothelial fenestrae are large circular openings of ≈100-nm diameter and allows a bulk flow of intraluminal plasma solute to traverse without any impedance toward the GBM. The spaces between the interdigitating foot processes, with an interdistance of ≈39 nm, are spanned by thin membranes, known as slit diaphragms, that apparently restrict the transcapillary passage of macromolecules. They have a well-defined zipper-like substructure with 4 × 14-nm rectangular pores having effective restriction toward albumin, which has a hydrodynamic diameter of ≈7.2 nm (26). On the other hand, given the substructure dimensions of the zipper, its functionality is difficult to explain in terms of permeability of myoglobin, which has an effective diameter of ≈4 nm but a sieving coefficient close to unity. This may be related to its other biophysical characteristics, i.e., an isoelectric point (pI) of ≈7.2 versus ≈4.9 for albumin. Intriguingly, more recently, slit diaphragms have been shown to include central ellipsoidal and circular pores with an average radius of ≈12 nm (9). These pores, however, seem to be quite large to impart any major restriction to albumin. The GBM that occupies the space between the cellular elements is an amorphous extracellular matrix (ECM) scaffold of ≈300 nm width, and it is further stratified into a central dense layer, known as the lamina densa, that is flanked on either side by relatively loose electron lucent layers, described as the lamina rara interna and externa.
Fig. 1.
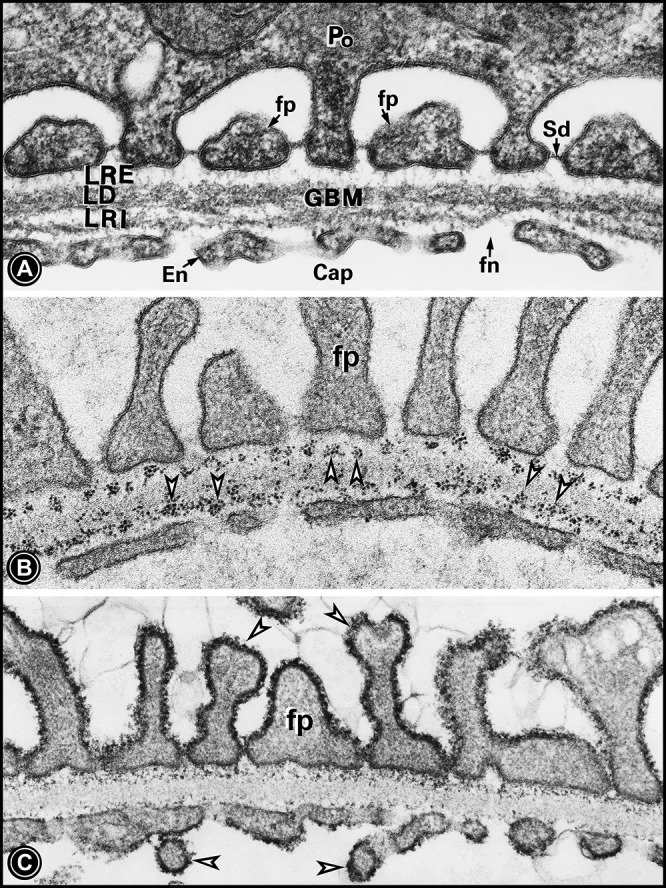
A: electron micrograph of the ultrafiltration unit. It is made up of the glomerular basement membrane (GBM), which is stratified into the lamina densa (LD), lamina rara interna (LRI), and lamina rara externa (LRE). The GBM is lined by the fenestrated (fn) endothelium (En) from the inside and with foot processes (fp) of podocytes (Po) from the outside. The space between the foot processes is occupied by the slit diaphragm (Sd). B: binding of cationic ferritin with GBM anionic sites in the LRE and LRI (arrowheads), which are enriched with heparan sulfate proteoglycans. C: colloidal iron-stained thick glycocalyx enriched with sialoglycoproteins (SGPs) covering the foot processes as well as the endothelium (arrowheads). US, urinary space.
All three layers of glomerular capillaries have distinct biochemical constituents that, along with their unique microscopic features, modulate ultrafiltration in mammals. The GBM is considered as the basic backbone of the GUU and is made up of high-molecular-weight proteins, including collagen type IV, laminins, entactin/nidogen, and sulfated proteoglycans (18). There are a variety of GBM proteoglycans, including agrin, perlecan, and collagen type XVIII, and, at times, they are chimeric molecules with a multitude of potential functional domains. Proteoglycans having sulfated glycosaminoglycans (GAGs), made up of either heparan sulfate (HS) or chondroitin sulfate (CS), are believed to supposedly impart electronegativity and thus the charge selectivity to GBMs (16). They are concentrated in the lamina rarae of the GBM and can be delineated with various probes, including cationic ferritin, ruthenium red, and polyethyleneimine (Fig. 1B) (14). Other high-molecular-weight proteins, i.e., laminin or collagen type IV, conceivably impart size selectivity to the GBM. The charge selectivity, in part, could be also contributed by the glycocalyx coating the plasmalemma of the attenuated glomerular endothelium (6, 10). The endothelial surface glycocalyx can be delineated with alcian blue, lysozyme, cupromeronic blue, cationic ferritin, and colloidal iron, and it is made up of a variety of sulfated proteoglycans, including syndecans, as well as hyaluronic acid and sialoglycoproteins (6, 10, 15). All these glycoproteins are amenable to impart electronegativity to the GUU. Likewise, the glycocalyx of podocytes can be delineated with basic dye probes and colloidal iron (Fig. 1C) (15, 16), and it is made up of podoendin, podoplanin, podocin, and podocalyxin, with the latter being a sialoglycoprotein (24). Another podocyte-secreted sialoglycoprotein, known as angiopoietin-like-4, has been described recently, and it has been incriminated in the enhanced passage of plasma albumin across the ultrafiltration unit (5). It is the only intrinsically expressed glycoprotein known to neutralize GBM charge when secreted in hyposialylated form in minimal change disease. Other plasmalemmal proteins that are expressed at the surface of the podocytes include α6β1- integrin and α- and β-subunits of dystroglycan along with sarcospan and sarcoglycans, and they mediate attachment of the foot processes to the GBM via the E3 and E8 domains of GBM laminin-1 (18). The major glycoproteins of the slit diaphragm, a prototype of shallow adherens, include nephrin and P-cadherins. Nephrins interact with CD2-associated protein, proteins with homolgy to nephrin, podocin, and zonula occludens-1, which, in turn, interact with the actin cytoskeleton of podocytes to maintain their dynamic geometry and thereby functionality of the GUU (22, 23, 24).
In an article by Sverrisson et al. (28) in a recent issue of the American Journal of Physiology-Renal Physiology, the authors described that the GUU maintains charge selectivity mainly toward “small molecules” like Ficoll with hydrodynamic radii of 20–35 Å, whereas PS and hyaluronidase infusions predominantly affect the glomerular sieving coefficient (θ) of large anionic Ficoll with hydrodynamic radii of 50–80 Å. They concluded that these properties of the GUU and changes in its θ are attributable to biochemical characteristics of the endothelial glycocalyx, enriched with hyaluronic acid, a nonsulfated proteoglycan. These conclusions are indeed supported by their carefully performed and elegant studies. Certainly, their studies supported previous observations that the GUU is a charge-selective barrier since inert dextran or Ficoll with similar hydrodynamic radii comparable with that of anionic albumin (pI ≈ 4.9, ≈36 Å) has an ≈100-fold higher θ value (0.02–0.1 vs. 0.0006) (11). The results of studies by Sverrisson et al. (28) were more or less similar to seminal observations made by Brenner et al. (2), and it seems that charge selectivity prevails over a relatively wide range of hydrodynamic radii, i.e., 20–35 vs. 18–44 Å. In this regard, results from a study (20) on the quantification of electrostatic properties suggested that although electrical charge makes a moderate contribution, it is capable of almost totally excluding albumin permeating via high-selectivity pathways in a charged-fiber matrix of the glomerular filtration barrier. Nevertheless, some caution needs to be exercised when comparing θ values of globular proteins like albumin versus branched polysaccharides like dextrans or Ficoll since the latter could be deformable under hydraulic force and thus can permeate through the GUU readily. This may be of some concern especially when one is dealing with modified dextrans or Ficoll since the modifications are amenable to alter the tertiary configuration of these macromolecules and thereby θ. In this regard, an anomalously “increased” transglomerular passage of “anionic” Ficoll was observed relative to “neutral” Ficoll, which in theory if this situation is applied to the globular proteins should have a “decreased” GUU θ value (1). In view of these concerns, Sverrisson et al. cautiously used “conformationally unchanged” or “intact Ficoll” with varying hydrodynamic radii as functional tracers for their experiments; however, minor changes in the tertiary conformation may still not be detectable. An interesting point that the authors alluded from their observations made in this and previous studies is that since PS and hyaluronidase increase the permeamebility of albumin (≈36 Å) and of relatively high-molecular-weight Ficoll (50–80 Å), it meant that albumin uses large pores (80–100 Å), whereas Ficoll with similar hydrodynamic radii permeate through relatively small pores (45–50 Å) of the GUU, according to the “two-pore model” proposed by Deen et al. (11). Extensive discussions on this complex theoretical issue have been the subject matter of many past and recent reviews (21).
Another major point the authors have made in this article is that the functionalities of the GUU mainly reside at the level of glomerular endothelial cell surfaces coated by a glycocalyx enriched with hyaluronic acid since treatment with PS and hyaluronidase affected size-selective permeability characteristics of the GUU (28). Besides hyaluronic acid, the glycocalyx has also been described to be enriched with sialoglycoproteins and sulfated proteoglycans, which can conceivably also contribute both charge and size selectivity to the GUU since a reduced volume of glycocalyx and increased expression heparanase is seen in proteinuric states and both neuraminidase or heparanase treatment leads to a similar reduction in the density/volume of glycocalyx (10, 15, 16). The fact that anionic ferritin with hydrodynamic radii of ≈61 Å is mostly seen at the endothelial fenestrae while cationic ferritin enters the GBM supports the conclusions of various recent studies that the glomerular endothelium could impart both size and charge selectivity to the GUU (Fig. 2) (6, 16). Here, one ask the following question: what about the role of the GBM in terms of size and charge selectivities of the GUU? The GBM is made up of large-molecular-weight proteins, such as collagen type IV, proteoglycans, and laminin, and it most likely has some size-selective properties, since mice with Lamb2 deficiency have a marked albuminuric response (13). However, mice lacking GBM agrin or perlecan do not develop proteinuria, and, likewise, mice with overexpression of heparanse are phenotypically normal (10, 12, 19). This refutes the idea that sulfated proteoglycans impart charge-selective properties to the GBM. The results of these gene disruption studies are difficult to reconcile with experiments with cationic ferritin, which binds to HS residues within the GBM and has relatively large hydrodynamic radii of ≈61 Å and has been clearly seen traversing the entire width of the GBM, perhaps via large pores (80–100 Å), and ultimately localizes underneath slit diaphragms (Fig. 2) (16). Since anionic ferritin does not enter the GBM, this can only be explained on the basis of charge selectivity of the GBM, and, ironically, these observations, at the same time, could raise the question in regard to the notion of size selectivity of the GBM of the GUU for circulating plasma proteins, that is, as to why and how such a large protein can readily traverses through the GBM.
Fig. 2.
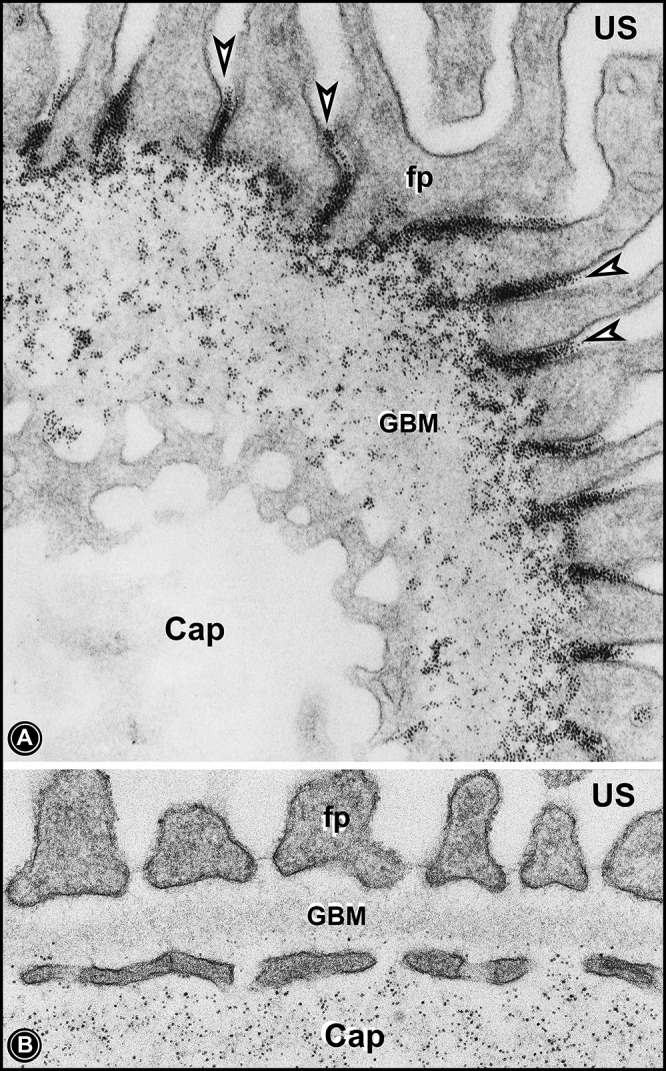
Electron micrographs of glomerular capillary loops of rats that received either intravenous injection of cationic ferritin (A) or anionic ferritin (B). Cationic ferritin can be seen traversing through the GBM and accumulating underneath slit diaphragms (arrowheads). Anionic ferritin can be seen mainly in the capillary lumina (Cap) or at the level of endothelial fenestrae. A very few particles of anionic ferritin can be seen in the innermost layer of the GBM.
The above discussion brings one to address the role of the most distal component of the GUU, i.e., podocyte foot processes, which have a glycocalyx enriched with sialoglycoproteins and cell surface proteoglycans, and the slit diaphragm, which contains a critical protein know as nephrin (23). It is likely that this slit diaphragm-podocyte glycocalyx complex has also dual size- and charge-selective properties, and there may be some correlation with the functionality of small- versus large-size theoretical pores of the GUU. In terms of the size selectivity of the slit diaphragm, the elegant structural analyses of Rodewald and Karnovsky suggested that the pore size in the slit diaphragm is very close to the size of hydrodynamic radii of serum albumin (≈36 Å) (26) and that any perturbations, such as genetic mutation in nephrin, are associated with heavy albuminuria or proteinuria (23). Intriguingly, a recent publication (9) has described the size of slit diaphragm pores to have radii of 110–120 Å by scanning microscopy while using high-sensitivity detectors. It is conceivable that despite the careful processing of tissues, they still may have undergone certain fixation artifacts since cationic ferritin, a tracer prototype of albumin with an Einstein-Stokes radius of ≈61 Å, clearly gets restricted underneath slit diaphragms and does not cross them (Fig. 2) (16). Thus, the pore size of the slit diaphragm has to be less than hydrodynamic dimensions of ferritin. Finally, the question whether of podocyte foot processes contribute to size- or charge-selective properties and influence the permeability characteristics or pore size of the GUU needs to be addressed. It is unlikely that podocyte foot processes influence the size selectivity of the GUU since they are not in the direct path of normal solute flow traversing the glomerular capillary wall. Nevertheless, no doubt, they are of enormous importance in the pathobiology of podocytes in maintaining the integrity of the GUU via the multitude of diverse transmembrane interactions between surface plasmalemmal and intracellular intracytoplasmic cytoskeletal proteins of the foot process (22, 24). This subject matter is not the emphasis of this editorial focus. In any event, the podocyte foot process could contribute to the charge selectivity of the GUU by virtue of having a glycocalyx enriched with sulfated proteoglycans and sialoglycoproteins, which are stainable with colloidal iron and other cationic probes or dyes (Fig. 3A) (15). With respect to sialoglycoproteins, the known well-characterized major ones are podocalyxin and angiopoietin-like-4, and both are hyposialylated in experimental nephrosis (5, 17). Hyposialylated angiopoietin-like-4 is now recognized as the major molecular mediator of minimal change disease, and correction of hyposialyation in vivo significantly improves proteinuria (4, 5). Moreover, the glycocalyx has been found to be reduced in nephrosis decades ago and more recently (Fig. 3B) (5, 15). Similar results have been observed after neuraminidase treatment, which results in the detachment of foot processes with subsequent bulk flow of the tracer proteins into the urinary space. All these old and recent observations indeed underscore the biology of podocyte foot processes with respect to the pathogenesis of proteinuria (15, 16, 22, 24).
Fig. 3.
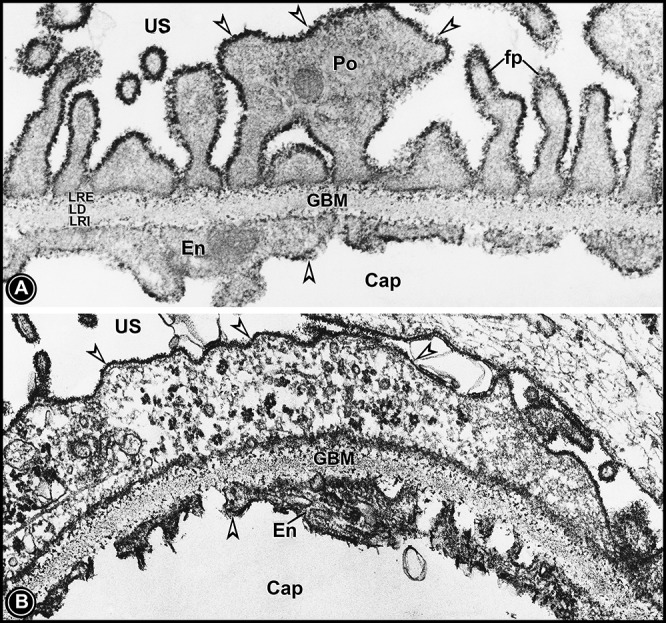
Electron micrographs of capillary loops of kidneys of a normal rat (A) and a nephrotic rat (B). Kidney tissues were subjected to colloidal iron staining to visualize the glycocalyx of podocytes and the endothelium. In the normal rat (A), a thick glycocalyx can be seen lining the podocyte foot processes and glomerular endothelium (arrowheads). The colloidal iron-stainable glycocalyx (arrowheads) was markedly reduced in the kidney section of the nephrotic rat (B). Normally, colloidal iron staining can also be seen in the lamina rara interna and externa of the GBM. The lamina densa is devoid of any staining.
In summary, this editorial focus highlights the controversies spanning four decades as to the structure-function relationship of glomerular mammalian capillaries. Taking into account the vast amount of literature data, it can be said that the integrated functions of all three stratified layers of the GUU are essential to maintain normal homeostasis of glomerular capillaries. In terms of all components of the glomerular capillary working in concert with one another to maintain glomerular ultrafiltration, it is best phrased by Deen and colleagues (7, 11) as follows: θGUU = θendothelium × θGBM × θpodocyte.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-60635.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.S.K. drafted manuscript; Y.S.K. edited and revised manuscript; Y.S.K. approved final version of manuscript.
REFERENCES
- 1.Asgeirsson D, Ventruroli D, Rippe B, Rippe C. Increased glomerular permeability to negatively charged Ficoll relative to neutral Ficoll in rats. Am J Physiol Renal Physiol 291: F1083–F1089, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Hostetter TH, Humes HD. Molecular basis of proteinuria of glomerular origin. N Engl J Med 298: 826–833, 1978. [DOI] [PubMed] [Google Scholar]
- 3.Caulfield JP, Farquhar MG. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J Cell Biol 63: 883–903, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chugh SS, Clement LC, Mace C. New insights into human minimal change disease: lessons from animal models. Am J Kidney Dis 59: 284–292, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clement LC, Avila-Casado C, Mace C, Soria E, Bakker WW, Kersten S, Chugh SS. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med 17: 117–123, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dane MJ, vande Berg BM, Avramut MC, Faas FG, vander Valg J, Rops AL, Rops Al Ravelli RB, Koster BJ, van Zonneveld AJ, Vink H, Rabelink TJ. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol 182: 1532–1540, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Deen WM. What determines glomerular capillary permeability? J Clin Invest 114: 1412–1414, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farquhar MG, Wissig SL, Palade GE. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J Exp Med 113: 47–56, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagliardini E, Conti S, Benigni A, Remuzzi G, Remuzzi A. Imaging of the porous ultra-structure of glomerular epithelial filtration slit. J Am Soc Nephrol 21: 2081–2089, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garsen M, Rops ALWMM, Rabelink TJ, Berden JHM, van der Vlag J. The role of heparanase and endothelial glycocalyx in the development of proteinuria. Nephrol Dial Transplant 29: 49–55, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Harvey SJ, Jarad G, Cunningham J, Rops Al Vlag JVD, Berde JH, Moeller MJ, Holzman LB, Burgess RW, Miner JH. Agrin mutant mice reveal the glomerular basement membrane is not a charge-selective filtration barrier. Am J Pathol 171: 139–152, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities in Lamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanwar YS, Farquhar MG. Presence of heparin sulfate in the glomerular basement membrane. Proc Natl Acad Sci USA 76: 1303–1307, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanwar YS, Rosenzweig LJ. Altered glomerular permeability as a result of focal detachment of visceral epithelium (podocytes). Kidney Int 21: 565–574, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Kanwar YS, Venkatachalam MA. Ultrastructure of glomerulus and juxtaglomerular apparatus. In: Handbook of Physiology. Renal Physiology. Bethesda, MD: Am. Physiol. Soc., 1992, sect. 8, vol. I, chapt. 1, p. 3–40. [Google Scholar]
- 17.Kerjaschki D, Vernillo AT, Farquhar MG. Reduced sialyation of podocalyxin–the major saiuloprotein of the rat kidney glomerulus–in aminonucleoside nephrosis. Am J Path 118: 343–349, 1985. [PMC free article] [PubMed] [Google Scholar]
- 18.Miner JH. The glomerular basement membrane. Exp Cell Res 318: 973–978, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita H, Yoshimura A, Inui K, Ideura T, Watanabe H, Wang L, Soininen R, Tryggvason K. Heparan sulfate of perlecan is involved in glomerular filtration. J Am Soc Nephrol 16: 1703–1710, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Oberg CM, Rippe B. Quantification of the electrostatic properties of the glomerular filtration barrier modeled as a charge fiber matrix separating anionic from neutral Ficoll. Am J Physiol Renal Physiol 304: F781–F787, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Oberg CM, Rippe B. A distributed two-pore model: theoretical implications and practical application to the glomerular sieving of Ficoll. Am J Physiol Renal Physiol 306: F844–F854, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Oh J, Reiser J, Mundel P. Dynamic (re)organization of the podocytes actin cytoskeleton in the nephrotic syndrome. Pedatr Nephrol 19: 130–137, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Patari-Sampo A, Ihalmo P, Holthofer H. Molecular basis of glomerular filtration: nephrin and the emerging protein complex at the podocyte slit diaphragm. Ann Med 38: 483–492, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Pavenstadt H, Kriz W, Ketzler M. Cell biology of the glomerular podocytes. Physiol Rev 83: 253–307, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Rennke HG, Cotran RS, Venkatachalam MA. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol 67: 639–646, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodewald R, Karnovasky MJ. Porous substructure of the glomerular slit diaphragm in rat and mouse. J Cell Biol 60: 423–433, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seiler MW, Venktachalam MA, Cotran RS. Glomerular epithelium: structural alterations induced by polycations. Science 189: 390–393, 1975. [DOI] [PubMed] [Google Scholar]
- 28.Sverrisson K, Axelsson J, Rippe A, Asgeirsson D, Rippe B. Dynamic, size-selective effect of protamine sulfate and hyaluronidase on the rat glomerular filtration barrier in vivo. Am J Physiol Renal Physiol 307: F1136–F1143, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Venkatachalam MA, Cotran RS, Karnovsky MJ. An ultrastructural study of glomerular peremeability in experimental nephrosis using catalase as a tracer tracer protein. J Exp Med 132: 1168–1180, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatachalam MA, Karnovsky MJ, Fahimi Glomerular permeability HD. Ultrastructural studies in experimental nephrosis using horseradish peroxidase as a tracer. J Exp Med 130: 381–399, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


