Abstract
Abdominal pain and distention are major complaints in irritable bowel syndrome. Abdominal distention is mainly attributed to intraluminal retention of gas or solid contents, which may cause mechanical stress to the gut wall. Visceral hypersensitivity (VHS) may account for abdominal pain. We sought to determine whether tonic colon distention causes persistent VHS and if so whether mechanical stress-induced expression (mechanotranscription) of pain mediators in colonic smooth muscle cells (SMCs) plays a role in VHS. Human colonic SMCs were isolated and stretched in vitro to investigate whether mechanical stress upregulates expression of the pain mediator cyclooxygenase-2 (COX-2). Rat colon was distended with a 5-cm-long balloon, and gene expression of COX-2, visceromotor response (VMR), and sensory neuron excitability were determined. Static stretch of colonic SMCs induced marked expression of COX-2 mRNA and protein in a force- and time-dependent manner. Subnoxious tonic distention of the distal colon at ∼30–40 mmHg for 20 or 40 min induced COX-2 expression and PGE2 production in colonic smooth muscle, but not in the mucosa layer. Lumen distention also increased VMR in a force- and time-dependent manner. The increase of VMR persisted for at least 3 days. Patch-clamp experiments showed that the excitability of colon projecting sensory neurons in the dorsal root ganglia was markedly augmented, 24 h after lumen distention. Administration of COX-2 inhibitor NS-398 partially but significantly attenuated distention-induced VHS. In conclusion, tonic lumen distention upregulates expression of COX-2 in colonic SMC, and COX-2 contributes to persistent VHS.
Keywords: COX-2, visceral sensitivity, mechanical stress, irritable bowel syndrome
irritable bowel syndrome (IBS) is a functional bowel disorder, affecting ∼11% of the general population in the US (8, 26). Abdominal pain is one of the prominent symptoms in IBS (8, 14, 28). It is highly regarded that visceral hypersensitivity (VHS) is one of the main reasons for abdominal pain in IBS (8, 14, 28). However, the underlying mechanisms for VHS are not well understood. Abdominal distention and bloating are other major complaints in IBS (4, 9, 18, 51). Based on a comprehensive bowel symptom questionnaire, 76% of IBS patients reported abdominal bloating and 57% had abdominal distention (9). Objective measurements with an abdominal inductance plethysmography found that 60% of constipation-predominant IBS patients demonstrated measurable abdominal distention (16). The pathogenesis of abdominal distention is not well understood. However, it is attributed to excessive gas accumulation and impaired gas transit in the intestine and colon (15, 20, 36, 37). Other postulated mechanisms include intraluminal retention of fluid and solid contents and altered gut microflora (1, 9, 22, 47). Ineffective evacuation, resulting in fecal retention in the colon and rectum, may also contribute to abdominal distention in IBS (8, 46, 47). Thus abdominal distention in IBS is largely due to luminal retention of gas, solid, and liquid contents in the gut. However, it remains unknown whether abdominal distention is simply an accompanying sign in IBS or a part of pathogenic mechanisms of VHS in the gut (44).
In an attempt to determine whether distention modulates visceral sensitivity, several groups studied the acute effect of repetitive distentions of a balloon in the distal colon to noxious pressure (13, 29, 31). A noxious pressure is generally considered greater than 40 mmHg (31, 52). It was found in naive rats that colonic tonic distention at 60 mmHg for 10 min followed by a series of phasic distentions for another 10 min increased visceromotor response (VMR) (13, 29). This is similar to the findings in humans that repetitive colonic distention (60 mmHg, 30 s) for 10 times at 4-min intervals resulted in VHS and hyperalgesia in normal subjects (31). Moreover, Munakata et al. (30) found that repetitive sigmoid colon distention at 60 mmHg for 10 min led to rectal hypersensitivity and hyperalgesia in IBS patients. Lumen distention at noxious pressure (60 mmHg) induced spinal changes of neuropeptide expression and ERK 1/2 activation (27, 29). These data suggest that colon distention at noxious pressure may induce acute VHS via a central sensitization mechanism (30). It is not known whether lumen distention causes persistent VHS and if so whether peripheral sensitization plays a role in the development of distention-induced VHS.
In the present study, we sought to specifically address the following questions: 1) Does subnoxious colon distention (at or lower than 40 mmHg) lead to visceral sensitization in a force- and time-dependent manner? 2) If so, does the distention-induced visceral sensitization persist for 1, 3, or 7 days after distention is discontinued? 3) Is peripheral sensitization involved in the development of persistent VHS? Our hypothesis was that colon distention induces mechanotranscription (39) of pain mediator cyclooxygenase-2 (COX-2) (19) and that production of COX-2-derived PGE2 in colonic smooth muscle cells (SMCs) contributes to persistent VHS.
METHODS
Isolation of HCCSMCs.
Human colonic circular SMCs (HCCSMCs) were isolated and cultured as described previously (38, 41, 42). Human tissue was obtained from the descending and sigmoid colons of patients undergoing surgery for colorectal cancer with approval of the University of Texas Medical Branch Institutional Review Board. Full-thickness tissue was dissected from the disease-free margins of the resected segments. The circular muscle layer was separated from the taenia coli and lamina propria with a tissue slicer. Two successive digestions with papain and collagenase, as described previously by Shi and Sarna (38, 41, 42), were used to disperse SMCs. The cells were cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum in the presence of 100 units/ml of penicillin G, 100 μg/ml of streptomycin sulfate, and 0.25 μg/ml of amphotericin B. The culture medium was changed every 3 days. The cells in passages 3 to 5 were used in the experiments. Greater than 95% of the cells in culture stained with smooth muscle specific α-actin (41, 42). The cultures of colonic SMCs retained their contractile phenotype (41–43).
Application of mechanical stretch in the primary culture of human colonic SMCs.
The human colonic SMCs were seeded at 8 × 104 cells/well in six-well BioFlex culture plates coated with type I collagen (Flexcell, Hillsborough, NC) and allowed to grow to ∼80% confluence before subject to DMEM/1% FBS for 24 h prior to stretch (21, 23, 39). Cells were mechanically stretched by using the FX-4000 Tension Plus System (Flexcell) as described previously (21, 23, 39). This computer-regulated bioreactor system applies multiaxial strain to the cells cultured on flexible membrane plates through vacuum pressure. Cells incubated in parallel under identical conditions but without exposure to stretch served as controls. To mimic tonic lumen distention in vivo, cells were subjected to static stretch at 18% elongation in all the experiments of this study.
Lumen distention of the distal colon in rats.
Male Sprague-Dawley rats of ∼8–10 wk (Harlan Sprague Dawley, Indianapolis, IN) were used for the study. The rats were housed in a controlled environment (22°C, 12-h light-dark cycle) and allowed rodent pellet food and water ad libitum at all times unless stated otherwise. The Institutional Animal Care and Use Committee at University of Texas Medical Branch approved all procedures performed on the animals.
To apply balloon distention of the distal colon, rats were anesthetized with 2% isoflurane inhalation by an E-Z anesthesia vaporizer, and a 5-cm-long balloon attached to a catheter was inserted 7 cm into the distal colon from the anus. The catheter was secured to the tail with tape. Rats were then left conscious in a container (20 × 8 × 8 cm) for 30 min, before the application of static colon distention (CD) at different level of pressure (20, 30, or 40 mmHg) for various periods of time (10, 20, or 40 min) in separate rats. Pressure was closely monitored by using a sphygmomanometer connected to a pressure transducer as described (48, 53). The range of pressure levels of distention (∼20–40 mmHg) was chosen because distention at such levels is generally considered subnoxious (31, 52). Control rats were treated similarly in the laboratory with a balloon insertion, but without inflation.
In experiments involving in vivo administration of COX-2 inhibitor NS-398 (24, 25), animals were randomly assigned into control and distention groups to be treated with COX-2 inhibitor NS-398 (Cayman Chemical, Ann Arbor, MI) at 10 mg/kg ip in 250 μl of 20% DMSO (24, 25). NS-398 was administered one time immediately after balloon distention was completed, and rats were euthanized 24 h after the distention. NS-398 was not given before balloon distention to avoid any possible analgesic effect of NS-398 during the time of distention (19).
Measurement of visceromotor response.
Visceral sensitivity was measured by electromyographical (EMG) measurements of VMR to colorectal distention (CRD) as described previously (48, 53). Briefly, two electrodes were implanted in the external oblique muscle and externalized behind the head. Rats were allowed 1 wk to recover from the surgery. Under mild sedation with 2% isoflurane, a balloon (5 cm) was inserted 7 cm into the distal colon via the anus and held in place by taping the tubing to the tail. Rats were placed in a container and allowed to adapt for 30 min. CRD was performed by rapidly inflating the balloon to constant pressure. Pressure was measured via a sphygmomanometer connected to a pressure transducer. The balloon was inflated to various pressures (20, 30, 40, 50, 60, and 80 mmHg) for a 20-s stimulation period followed by a 2-min rest. EMG was recorded continuously during the experiment on a Biopac System EMG 100 C (Biopac Systems, Goleta, CA). EMG signals were amplified (5,000×), filtered with a 1-Hz high-pass filter and a 500-Hz low-pass filter, and digitized by use of Acknowledge (Biopac Systems). The area under the curve (AUC) for the EMG signal during each 20 s of distention was calculated by use of an in-house-written computer program (48). The net value for each distention was calculated by subtracting the baseline value derived from the AUC for the 20 s predistention period.
Labeling of colon specific sensory neurons in DRG.
Colon specific neurons in the dorsal root ganglia (DRG) were labeled for patch-clamp recordings by injecting 1,1′-dioleyl-3,3,3′,3-tetramethylindocarbocyanine methanesulfonate (DiI, Invitrogen, Carlsbad, CA) into the colon wall as described previously (48, 53). In brief, animals were anesthetized by 2% isoflurane with an E-Z anesthesia vaporizer. After a midline laparotomy, 2 μl of DiI (50 mg/ml in methanol) was injected into 10 sites on the exposed distal colon (∼5 cm in length). Animals were returned to normal housing and were treated for distention or as control before euthanasia for patch-clamp recordings ∼7∼10 days after DiI injection.
DRG neuron dispersion and patch-clamp study.
Isolation of DRG neurons from adult rats has been described previously (48, 53). Briefly, rats were euthanized by decapitation. The spinal column was removed and transferred to ice-cold, oxygenated fresh dissecting solution containing (in mmol/l) 130 NaCl, 5 KCl, 2 KH2PO4, 1.5 CaCl2, 6 MgSO4, 10 glucose, and 10 HEPES, pH 7.2 (osmolarity = 305 mosM). Thoracolumbar DRG (T13–L2) were obtained bilaterally. The ganglia were digested in dissecting solution containing collagenase D (∼1.5 mg/ml; Roche, Indianapolis, IN) and trypsin (∼1.2 mg/ml; Sigma, St. Louis, MO) at 34.5°C for 1.5 h. The DRG samples were washed in enzyme-free solution and triturated repetitively with glass pipettes to obtain single cell suspension.
Cells were plated onto acid-cleaned glass coverslips and perfused with normal external solution containing (in mmol/l) 130 NaCl, 5 KCl, 2 KH2PO4, 2.5 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.4 with NaOH (295–300 mosM). DiI-labeled neurons (bright red) were identified by using a fluorescence microscope (Olympus, Tokyo, Japan) with a rhodamine filter (excitation 546 mm, barrier filter at 580 mm). Whole-cell current and voltage were recorded by a Dagan 3911 patch-clamp amplifier (Dagan, Minneapolis, MN) (48, 50, 53). Capacitive transients were corrected by using capacitive cancellation circuitry on the amplifier that yielded the whole-cell capacitance and access resistance. Up to 90% of the series resistance was compensated electronically. The currents were filtered at ∼2–5 kHz and sampled at 50 or 100 μs per point. Data were acquired and stored on a Dell computer for later analysis by using pCLAMP 9.2 (Axon Instruments, Sunnyvale, CA).
Rat colon tissue collection.
The 5-cm-long distal colon (starting 2 cm from the anus) was collected from control and distended rats and placed immediately in carbogenated Krebs buffer (in mmol/l: 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1 NaH2PO4, 1.2 MgCl2, 11 d-glucose, and 25 NaHCO3). The mucosa/submucosa (M/S) and muscularis externa (ME) layers were separated by microdissection as described previously (21, 23, 38, 39, 40).
Protein extraction and Western blotting.
The colonic ME and M/S samples were homogenized on ice in lysis buffer supplemented with protease inhibitor cocktails (Sigma-Aldrich, St. Louis, MO) as described previously (38–42). After spinning at 12,000 g at 4°C for 15 min, the supernatant proteins were collected and resolved by a standard immunoblotting method (23, 39, 40, 49). Equal quantities (20 μg) of total protein were run on premade 4–12% Bis-Tris SDS-PAGE (Invitrogen, Carlsbad, CA). The primary antibody to COX-2 (1:1,000) was purchased from Cayman Chemical. β-actin antibody (1:5,000, Sigma) was used as loading control. The protein detection was done using ODYSSEY Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Enzyme immunoassay.
Rat colonic ME tissue was homogenized in cold PBS (in mmol/l 137 NaCl, 2.7 KCl, 10 Na2HPO4, KH2PO4, pH 7.4) supplemented with protease inhibitors for protein extraction. PGE2 was measured with the PGE2 enzyme immunoassay kit from Cayman Chemical by following the manufacturer's protocols (24).
RNA preparation and real-time qPCR.
Total RNA was extracted from colon ME samples by using the Qiagen RNeasy kit (Qiagen, Valencia, CA). One microgram of total RNA was reverse-transcribed with the SuperScript III First-Strand Synthesis System (Invitrogen) for quantitative RT-PCR with the Applied Biosystems 7000 real-time PCR system (Foster City, CA) (21, 23, 34, 39). The assay ID for TaqMan detection of rat COX-2 mRNA is Rn00568225-m1 (Applied Biosystems) (21, 23, 34, 39). For relative quantification of COX-2 gene transcription, real-time qPCR was performed with 40 ng of cDNA for the target gene and for the endogenous control 18S rRNA (part no. 4352930E, Applied Biosystems).
Statistical analysis.
All data points are expressed as means ± SE. Statistical analysis was performed by analysis of variance with nonrepeated measures (by Student-Newman-Keuls test) for comparisons of multiple groups and Student's t-test for comparisons of two groups. A P value of ≤0.05 was considered statistically significant.
RESULTS
Static mechanical stretch induces gene expression of COX-2 in HCCSMCs.
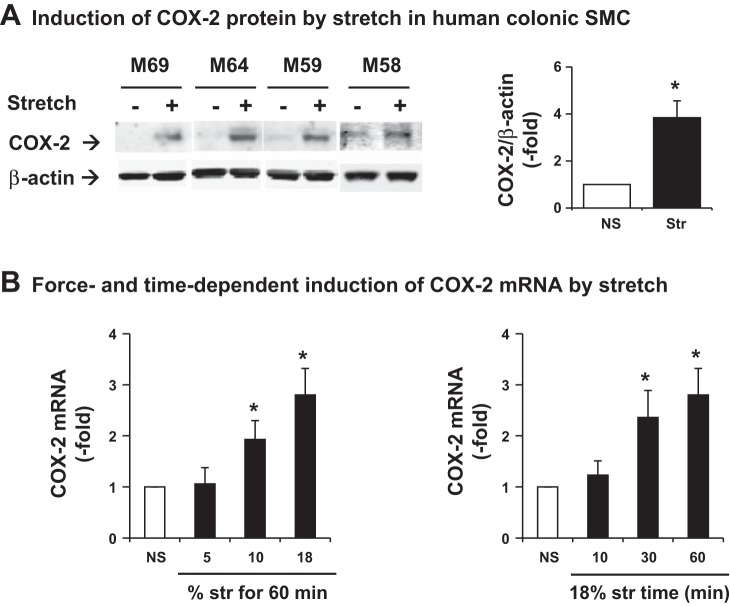
Sigmoid colon tissues from four male patients (58 to 69 years old) were obtained for preparations of HCCSMCs for culture. We found that static stretch of HCCSMCs at 18% elongation for 60 min significantly increased COX-2 protein expression by 3.83 ± 0.72-fold in 24 h (Fig. 1A). We harvested the cells 3 h after the start of stretch and further determined whether stretch induced expression of COX-2 mRNA in a force- and time-dependent manner. Static stretch for 60 min at 10 or 18% elongation, but not 5%, significantly upregulated COX-2 mRNA expression. On the other hand, stretch at 18% for 30 or 60 min, but not for 10 min, significantly induced COX-2. Stretch at 18% for 60 min increased COX-2 mRNA by 2.70 ± 0.40-fold (P = 0.02 vs. control, N = 4 with duplication) (Fig. 1B).
Fig. 1.
Mechanical stretch-induced gene expression of cyclooxygenase 2 (COX-2) in the primary culture of human colonic smooth muscle cells (SMCs). Human colonic SMCs were isolated from 4 male donors aged 58–69 (M69, M64, M59, and M58). A: cells were stretched at 18% elongation for 60 min and harvested 24 h later for Western blot detection of COX-2 protein expression. B: stretch-induced expression of COX-2 mRNA was determined by quantitative RT-PCR. To determine the force-dependent expression of COX-2 mRNA, cells were stretched at 5, 10, and 18% elongation for 60 min (left). To determine the time-dependent expression of COX-2 mRNA, cells were stretched at 18% elongation for 10, 30, and 60 min (right). Samples were harvested 3 h after the start of stretch (Str). Control cells were cultured the same condition with no stretch (NS). N = 4 with duplication. *P < 0.05 vs. NS.
Lumen distention of the distal colon time and force dependently upregulates expression of COX-2 in colonic smooth muscle.
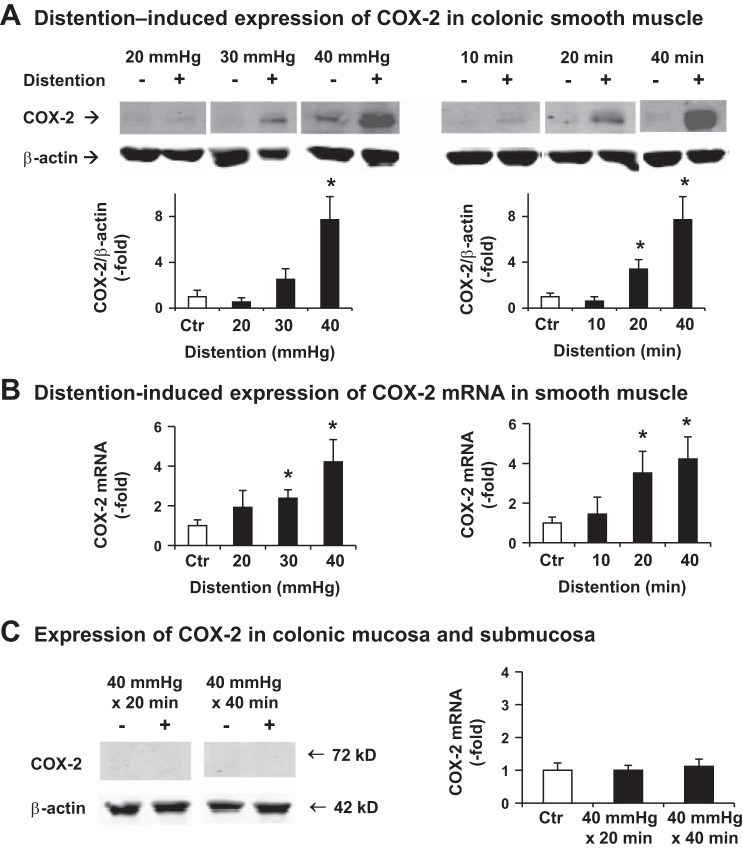
We then determined the effect of CD on gene expression of COX-2 in rats. Distention of the distal colon at 40 mmHg for 20 or 40 min significantly induced expression of COX-2 mRNA and protein in the muscularis externa (Fig. 2, A and B). Compared with sham controls, the COX-2 mRNA increased 3.52 ± 1.09-fold 24 h after CD for 20 min and 4.22 ± 1.12-fold when distended for 40 min (P < 0.05 vs. control, n = 5 or 6) (Fig. 2B). CD at 40 mmHg for 10 min did not lead to any significant change of COX-2 expression (Fig. 2, A and B). Interestingly, COX-2 expression in colonic mucosa and submucosa layer was not significantly altered by lumen distention up to 40 mmHg for 40 min (Fig. 2C).
Fig. 2.
Colon distention (CD)-induced gene expression of COX-2 in rats. A: Western blot results of 4 or 5 independent experiments detecting COX-2 protein expression in colonic muscularis externa. Distal colon (5 cm) was isolated 24 h after the colon was treated with balloon insertion only (Ctr) or balloon distention at various pressures (20, 30, 40 mmHg) for 40 min (left), or at 40 mmHg for various times of distention (10, 20, and 40 min) (right). Top: representative images. Bottom: quantitative results of COX-2/β-actin ratio. B: distention-induced expression of COX-2 mRNA in colonic muscularis externae was determined by quantitative RT-PCR. Colonic tissues were harvested 24 h after distention. N = 5 or 6. *P < 0.05 vs. control. C: expression of COX-2 protein and mRNA in colonic mucosa/submucosa layer. Colonic mucosa/submucosa tissues were harvested 24 h after distention. N = 5 or 6.
Distention of the distal colon for 40 min at 30 mmHg, like 40 mmHg, significantly induced expression of COX-2 mRNA in the muscularis externa (Fig. 2, A and B). Compared with sham controls, the COX-2 mRNA increased 2.38 ± 0.43-fold 24 h (P < 0.05 vs. control, n = 5). The COX-2 protein level showed a trend of being greater than in controls (2.51 ± 0.93 vs. 1.0 ± 0.56, P = 0.08 vs. control). Distention for 40 min at 20 mmHg did not cause a significant change of COX-2 mRNA and protein expression (Fig. 2, A and B).
Lumen distention induces VHS.
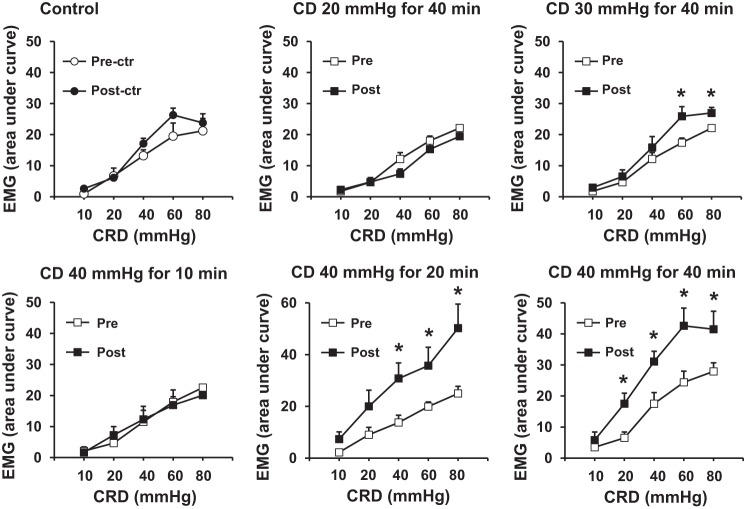
We next studied the persistent effect of tonic CD on visceral sensitivity. We measured VMR in rats before (Pre) and 24 h after (Post) CD at different pressure levels (20, 30, and 40 mmHg) for various times (10, 20, and 40 min). The results are shown in Fig. 3. In the control rats, the VMR values did not change 24 h after balloon insertion without inflation (Fig. 3A). However, the VMR significantly increased 24 h after CD at 40 mmHg for 20 or 40 min (Fig. 3, E and F, respectively), or at 30 mmHg for 40 min (Fig. 3C) (P < 0.05 vs. predistention self control, n = 5) (Fig. 3). Nevertheless, distention with pressure levels lower than 20 mmHg for shorter than 30 min did not induce significant VMR changes (n = 5 in each group). Moreover, the VMR remained significantly increased 3 days after the distention at 40 mmHg for 40 min (Fig. 4) but recovered to almost normal levels 7 days later.
Fig. 3.
Visceromotor response (VMR) determined by recording of the electromyography (EMG) of the abdominal muscle following graded colorectal distention (CRD, 10–80 mmHg, each 20 s). EMG was recorded before (Pre) or 24 h after (Post) colon distention (CD) of various levels (20, 30, and 40 mmHg) for different times (10, 20, and 40 min). N = 5 or 6. *P < 0.05 vs. the baseline level before the tonic distention.
Fig. 4.
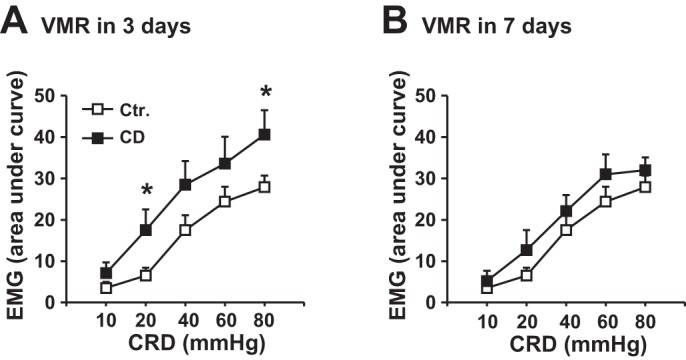
Visceromotor response (VMR) detected by recording of EMG in response to colorectal distention (CRD) 3 days (A) and 7 days (B) after a 1-time colon distention (CD) at 40 mmHg for 40 min. N = 5 or 6. *P < 0.05 vs. the baseline level before the tonic distention (Ctr).
Lumen distention sensitizes colon specific sensory neurons.
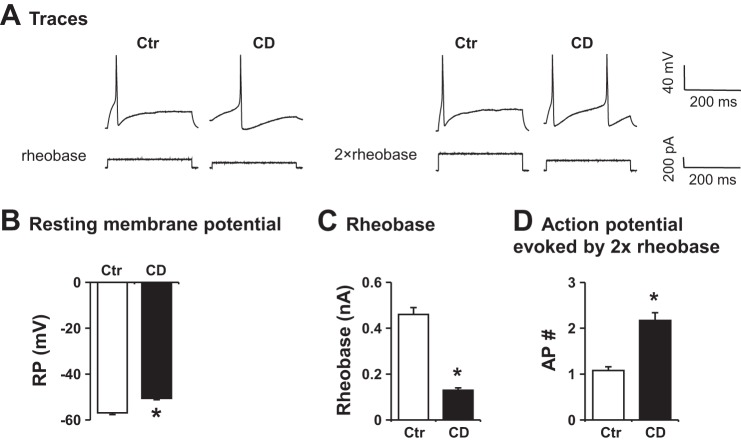
Twenty-four hours after a colon distention at 40 mmHg for 40 min, rats were euthanized for patch-clamp recording of the colon specific DRG neurons (Fig. 5). The resting membrane potential was significantly decreased from −56.7 ± 0.6 mV in control to −46.2 ± 0.9 mV in rats treated with distention (P < 0.01 vs. control, n = 5 or 6). The rheobase was lower in rats treated with distention than in control (0.18 ± 0.03 nA vs. 0.28 ± 0.02 nA, P < 0.05. n = 5 or 6). The number of 2× rheobase-evoked action potentials in the CD group was 2.3-fold of that in control (P < 0.05 vs. control). These data indicate a heightened excitability of the colon specific DRG neurons after distention.
Fig. 5.
Representative recordings (A) and quantitative data (B) of patch-clamp results in colon specific dorsal root ganglion (DRG) neurons. Neuronal cells were isolated from control (Ctr) and rats treated with colon distention (CD, 40 mmHg for 40 min). Rats were euthanized 24 h after the treatments. Resting membrane potential (RP), rheobase, and 2× rheobase-evoked action potentials (AP) were counted. N = 5 or 6 rats (∼8–10 neurons were studied in each rat). *P < 0.05 vs. Ctr.
COX-2 inhibitor attenuates lumen distention-induced VHS and neuron sensitization.
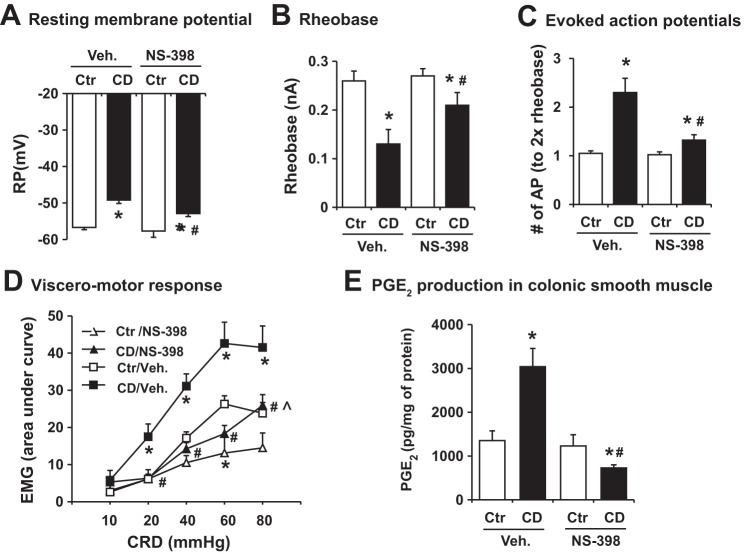
We then determined whether stretch-induced COX-2 in colonic smooth muscle is involved in the observed heightened neuron excitability and VHS 24 h after CD (at 40 mmHg for 40 min). As shown in Fig. 6, administration of COX-2 inhibitor NS-398 (10 mg/kg) significantly ameliorated distention-induced changes of cell excitability in colon specific DRG neurons (Fig. 6, A–C). In the NS-398 treated rats, the evoked action potential number was nearly the normal level (Fig. 6C). The resting membrane potential and rheobase were partially but significantly recovered in the distended rats treated with NS-398 (Fig. 6, A and B). NS-398 treatment in sham control rats did not change DRG neuron excitability (Fig. 6). Moreover, treatment with NS-398 significantly improved CD-induced changes of VMR, compared with CD rats treated with vehicle (Fig. 6D). When NS-398 was present, distention increased the EMG response only for the stimulation of 80 mmHg. NS-398 treatment in sham control rats decreased the EMG response to the 60 mmHg stimulation (Fig. 6D). Finally, our data showed that CD-induced COX-2 expression in the colonic muscularis externae was associated with increased production of PGE2, which was completely blocked by COX-2 inhibitor NS-398 treatment (Fig. 6E).
Fig. 6.
Effect of COX-2 inhibitor NS-398 administration on colon distention (CD)-induced sensory neuron hyperexcitability (A–C), visceromotor response (D) and PGE2 production in the colonic smooth muscle (E). A–C: NS-398 was administered 10 mg/kg ip immediately after balloon distention (40 mmHg for 40 min), and rats were euthanized 24 h after the treatments. Neuronal cells were isolated from control (Ctr) and rats treated with colon distention (40 mmHg for 40 min). Resting membrane potential, rheobase, and 2× rheobase-evoked action potentials were counted. N = 5 or 6 rats in each group, and ∼8–10 neurons were studied in each rat. D: EMG was recorded in different rats treated with NS-398 from those used for electrophysiology study. N = 5 rats in each group. E: PGE2 production was measured in colonic muscularis externa homogenates. N = 4 or 5 rats in each group. *P < 0.05 vs. control. #P < 0.05 vs. CD/vehicle (Veh.). ^P < 0.05 vs. Ctr/NS-398.
DISCUSSION
Our study showed that human colonic SMCs are very sensitive to mechanical stress. Static stretch of HCCSMCs at 10–18% elongation induced marked expression of COX-2, a key enzyme for synthesis of prostaglandins (PGs). PGs, especially PGE2, are well-recognized pain mediators and play an important role in the development of hyperalgesia (2, 19). PGE2 is a key component of nociceptive mediators involved in visceral pain in the gut and modulates colonic sensory neuron excitability (12, 45). Moreover, esophageal hyperalgesia in human subjects was attenuated by PGE2 receptor antagonist (35). These data suggest that COX-2-derived PGE2 contributes to human visceral pain and hypersensitivity.
In vivo experiments showed that lumen distention of the colon at 30 or 40 mmHg for as short as 20 min robustly induced gene expression of COX-2 and production of PGE2 in colonic smooth muscle in rats. Previous studies showed that COX-2 expression in colonic SMCs is markedly induced in the distended colon in bowel obstruction (23, 39). These data suggest that mechanical stress is an important stimulus of gene expression of COX-2 in gut SMCs. More importantly, the present study demonstrates that excitability of colon specific sensory neurons is significantly augmented as detected 24 h after a one-time colon distention at 40 mmHg for 40 min. Quantitative measurements of the VMR demonstrated that VHS was induced in rats undergoing colonic distention. We found that the visceral sensitivity remained increased for at least 3 days after lumen distention. Moreover, the increase of visceral sensitivity was partially but significantly attenuated by administration of COX-2 inhibitor NS-398. Thus our study suggests that lumen distention induces mechanotranscription of COX-2 and production of PGE2 in gut SMCs, which contribute to VHS. This is a novel mechanism of peripheral sensitization in the gut. It is of interest to note that although COX-2 inhibitor NS-398 completely blocked PGE2 production, it only partially improved distention-induced sensory neuron excitability and EMG response. This suggests that mechanical stress may induce expression of other nociceptive mediators, which could contribute to VHS in conditions with lumen distention.
The novel pathway of peripheral sensitization due to mechanotranscription of nociceptive mediators in gut SMCs may be relevant to abdominal pain in several gastrointestinal (GI) conditions. First, lumen distention is apparent in mechanical obstruction and pseudo-obstruction (10, 32, 34). Abdominal pain and visceral sensitization are present in obstructed patients and in animals with lumen distention (3, 10, 17, 32, 34). Our findings suggest that mechanotranscription of COX-2 in gut SMCs may play a critical role in the development of pain in obstructive bowel disorders. Second, our study may also have implications for VHS in functional bowel disorders such as IBS, in which abdominal distention and intraluminal gas and solid retention are well documented (16, 36). Although constant lumen distention may not be present in IBS, abdominal distention was demonstrated objectively in IBS patients, especially at the end of the day (16). Our results showed that constant lumen distention is not a requisite for increased VMR and for effective mechanotranscription of COX-2 in gut SMCs, since a subnoxious distention for as short as 40 min caused COX-2 expression and increased VMR. Thus abdominal distention and intraluminal retention are not just accompanying signs in IBS but may play a role in the development of VHS, as postulated by others (5, 33). Third, our study may help to explain, at least partly, why patients have abdominal pain after GI endoscopy, in which bowel distention is required. It is found that up to 50% of patients experience abdominal pain by 24 h after gas distention for endoscopy (6, 7, 11). Interestingly, if greater and longer gas retention was found in the GI tract after the procedure, more patients were likely to report pain (6, 7, 11).
Our studies show that mechanical stretch induces COX-2 expression in force- and time-dependent manners. Static stretch of human colonic SMCs for 60 min at 10 or 18%, but not 5%, increased COX-2 mRNA expression. On the other hand, stretch at 18% for 30 or 60 min, but not 10 min, significantly induced COX-2 gene expression in the human colonic SMCs. Further in vivo study found that colon distention at 40 mmHg for as short as 20 min led to mechanotranscription of COX-2 and VHS. However, the same pressure level for 10 min of distention did not cause detectable mechanotranscription or VHS 24 h later. We found that a minimal distention pressure at 30 mmHg was required to induce upregulation of COX-2 and VHS, since lumen distention at 20 mmHg for 40 min did not cause any significant change of COX-2 expression or visceral sensitivity. Given that colon distention with pressure of 40 mmHg is considered the nociception threshold (31, 52), our data indicate that mechanotranscription of COX-2 and visceral sensitization can be induced by lumen distention for relatively short period of time without patients' awareness.
Previous studies have shown that lumen distention with noxious stimulus pressures have acute effects on afferent neurons, spinal cord and the brain (27, 29). Million et al. (29) found that repetitive colon distention at 60 mmHg activated phosphorylation of MAPK in the DRG and spinal cord. This may help to explain the “chemical coding” of visceromotor response. Our study, on the other hand, applied subnoxious pressure to study the impacts of lumen distention on persistent VHS and gene expression in the colon. We detected elevated VMR for at least 3 days after the stimulation of lumen distention. Furthermore, we found that mechanical stress-induced COX-2 from gut SMCs is partially responsible for the persistent VHS.
Lumen distention due to gas, liquid, or solid contents represents a static circumferential mechanical stress to the colon wall. Thus we applied tonic lumen distention to the colon in vivo and static stretch of SMCs in vitro. We found that colonic SMC is selectively responsive to the static circumferential mechanical stress, as the expression of COX-2 in the mucosa/submucosa layer is not altered by distention. This is consistent with the findings in rat model of colon obstruction, in which a selective induction of COX-2 was found in colonic SMCs, but not in the mucosa or submucosa layers (39). The exact reason for the selective induction of COX-2 in the SMCs in lumen distention is not very clear. However, these studies suggest that gut SMCs, via mechanotranscription, may play a unique role in the pathogenesis of VHS in lumen distention-associated conditions. This role of gut SMCs had not been revealed previously because mechanotranscription is a molecular mechanism causing no apparent morphological damage to the SMCs (21, 23, 24, 39, 49). In addition, the smooth muscle layer, sitting deeply in the gut wall, is typically not a target of routine biopsy.
In summary, we found in the primary culture of human colonic SMCs that mechanical stretch induces gene expression of the pain mediator COX-2. The in vivo studies in rats demonstrated that lumen distention at a subnoxious level for as short as 20 min induces persistent visceral hypersensitivity and gene expression of COX-2 in colonic smooth muscle. Intervention studies showed that mechanical stress-induced COX-2 and COX-2-derived PGE2 play an important role in visceral sensitization in lumen distention. This novel peripheral sensitization pathway may underlie the mechanisms of abdominal pain in bowel disorders with lumen distention or intraluminal retention. Thus the pathway linking mechanical stress to production of pain mediators in the gut is a potential treatment target to ameliorate pain in bowel disorders with lumen distention.
GRANTS
This work was supported in part by National Institutes of Health (DK82563, AT05158) and a John Sealy Memorial Fund pilot grant to X.-Z. Shi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.-M.L., Y.F., C.C.W., G.-Y.X., and X.-Z.S. performed experiments; Y.-M.L., Y.F., C.C.W., G.-Y.X., and X.-Z.S. analyzed data; Y.-M.L., Y.F., C.C.W., L.-Y.M.H., and X.-Z.S. interpreted results of experiments; Y.-M.L. and Y.F. prepared figures; Y.-M.L., Y.F., C.C.W., L.-Y.M.H., and X.-Z.S. edited and revised manuscript; Y.-M.L., Y.F., C.C.W., G.-Y.X., L.-Y.M.H., and X.-Z.S. approved final version of manuscript; X.-Z.S. conception and design of research; X.-Z.S. drafted manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Sushil Sarna for critical reading and Dr. Linsey Yeager for helpful editing of the manuscript.
Present address for G.-Y. Xu: Institute of Neuroscience, Soochow University, Suzhou, Jiangsu, China 215006.
REFERENCES
- 1.Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol 104: 1998–2004, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Akbar A, Walters JR, Ghosh S. Review article: visceral hypersensitivity in irritable bowel syndrome: molecular mechanisms and therapeutic agents. Aliment Pharmacol Ther 30: 423–435, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 119: 1276–1285, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Azpiroz F, Malagelada JR. Abdominal bloating. Gastroenterology 129: 1060–1078, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Blalock JB. Chronic intestinal obstruction: a true “irritable bowel” syndrome. Ann Surg 161: 819–823, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bretthauer M, Lynge AB, Thiis-Evensen E, Hoff G, Fausa O, Aabakken L. Carbon dioxide insufflation in colonoscopy: safe and effective in sedated patients. Endoscopy 37: 706–709, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bretthauer M, Seip B, Aasen S, Kordal M, Hoff G, Aabakken L. Carbon dioxide insufflation for more comfortable endoscopic retrograde cholangiopancreatography: a randomized, controlled, double-blind trial. Endoscopy 39: 58–64, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol 592: 2967–2980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol 96: 3341–3347, 2001. [DOI] [PubMed] [Google Scholar]
- 10.De Giorgio R, Cogliandro RF, Barbara G, Corinaldesi R, Stanghellini V. Chronic intestinal pseudo-obstruction: clinical features, diagnosis, and therapy. Gastroenterol Clin North Am 40: 787–807, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES, Hawk JS, Grimm IS, Shaheen NJ. The use of carbon dioxide for insufflation during GI endoscopy: a systematic review. Gastrointest Endosc 69: 843–849, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E2 modulates TTX-RINa in rat colonic sensory neurons. J Neurophysiol 88: 1512–1522, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Gschossmann JM, Coutinho SV, Miller JC, Huebel K, Naliboff B, Wong HC, Walsh JH, Mayer EA. Involvement of spinal calcitonin gene-related peptide in the development of acute visceral hyperalgesia in the rat. Neurogastroenterol Motil 13: 229–236, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Hasler WL, Owyang C. Challenges of managing pain in constipation-predominant IBS: clinical perspectives on antinociceptive actions of linaclotide. Gastroenterology 145: 1196–1199, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Hernando-Harder AC, Serra J, Azpiroz F, Milà M, Aguadé S, Malagelada C, Tremolaterra F, Villoria A, Malagelada JR. Colonic responses to gas loads in subgroups of patients with abdominal bloating. Am J Gastroenterol 105: 876–882, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Houghton LA, Lea R, Agrawal A, Reilly B, Whorwell PJ. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology 131: 1003–1010, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Huang TY, Hanani M. Morphological and electrophysiological changes in mouse dorsal root ganglia after partial colonic obstruction. Am J Physiol Gastrointest Liver Physiol 289: G670–G678, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Shim L, Prott G, Hansen RD, Simmons LE, Kellow JE, Malcolm A. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol 105: 883–887, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata A. Prostaglandin E2 and pain—an update. Biol Pharm Bull 34: 1170–1173, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Koide A, Yamaguchi T, Odaka T, Koyama H, Tsuyuguchi T, Kitahara H, Ohto M, Saisho H. Quantitative analysis of bowel gas using plain abdominal radiograph in patients with irritable bowel syndrome. Am J Gastroenterol 95: 1735–1741, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Li F, Lin YM, Sarna SK, Shi XZ. Cellular mechanism of mechanotranscription in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 303: G646–G656, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA 292: 852–858, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Lin YM, Li F, Shi XZ. Mechano-transcription of COX-2 is a common response to lumen dilation of the rat gastrointestinal tract. Neurogastroenterol Motil 24: 670–677 e295–e296, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YM, Sarna SK, Shi XZ. Prophylactic and therapeutic benefits of COX-2 inhibitor on motility dysfunction in bowel obstruction: roles of PGE2 and EP receptors. Am J Physiol Gastrointest Liver Physiol 302: G267–G275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol 557: 191–205, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 10: 712–721, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Lu CL, Pasricha PJ, Hsieh JC, Lu RH, Lai CR, Wu LL, Chang FY, Lee SD. Changes of the neuropeptides content and gene expression in spinal cord and dorsal root ganglion after noxious colorectal distension. Regul Pept 131: 66–73, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med 358: 1692–1699, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Million M, Wang L, Wang Y, Adelson DW, Yuan PQ, Maillot C, Coutinho SV, Mcroberts JA, Bayati A, Mattsson H, Wu V, Wei JY, Rivier J, Vale W, Mayer EA, Taché Y. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut 55: 172–181, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology 112: 55–63, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain 43: 377–386, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Roeland E, von Gunten CF. Current concepts in malignant bowel obstruction management. Curr Oncol Rep 11: 298–303, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Robbins SE. A common cause of irritable bowel syndrome and diverticulitis: chronic distal colon distention from sedentary behavior and excessive dietary fiber. Expert Rev Gastroenterol Hepatol 7: 413–419, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Russell JC, Welch JP. Pathophysiology of bowel obstruction. In: Bowel Obstruction, edited by Welch JP. Philadelphia, PA: Saunders, 1990, p. 28–58. [Google Scholar]
- 35.Sarkar S, Hobson AR, Hughes A, Growcott J, Woolf CJ, Thompson DG, Aziz Q. The prostaglandin E2 receptor-1 (EP-1) mediates acid-induced visceral pain hypersensitivity in humans. Gastroenterology 124: 18–25, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut 48: 14–19, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafar J. The splenic flexure syndrome. Postgrad Med J 41: 148–150, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi XZ, Choudhury BK, Pasricha PJ, Sarna SK. A novel role of VIP in colonic motility function: induction of excitation-transcription coupling in smooth muscle cells. Gastroenterology 132: 1388–1400, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Shi XZ, Lin YM, Powell DW, Sarna SK. Pathophysiology of motility dysfunction in bowel obstruction: role of stretch-induced COX-2. Am J Physiol Gastrointest Liver Physiol 300: G99–G108, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi XZ, Lindholm PF, Sarna SK. NF-κB activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology 124: 1369–1380, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-κB. Gastroenterology 129: 1518–1532, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Shi XZ, Sarna SK. Transcriptional regulation of inflammatory mediators secreted by human colonic circular smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 289: G274–G284, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Shi XZ, Sarna SK. Cell culture retains contractile phenotype but epigenetically modulates cell-signaling proteins of excitation-contraction coupling in colon smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 304: G337–G345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shim L, Prott G, Hansen RD, Simmons LE, Kellow JE, Malcolm A. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol 105: 883–887, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol 80: 2632–2644, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan SN. Functional abdominal bloating. J Clin Gastroenterol 19: 23–27, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Sweetser S, Rao AS, Szarka LA. Constipation and recurrent abdominal distension in a 39-year-old woman with irritable bowel syndrome. Gut 61: 42, 107.2012. [DOI] [PubMed] [Google Scholar]
- 48.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology 138: 294–304 e3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu CC, Lin YM, Gao J, Winston JH, Cheng LK, Shi XZ. Are interstitial cells of Cajal involved in mechanical stress-induced gene expression and impairment of smooth muscle contractility in bowel obstruction? PLoS One 8: e76222, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, Gu Y, Xu GY, Wu P, Li GW, Huang LY. Adeno-associated viral transfer of opioid receptor gene to primary sensory neurons: a strategy to increase opioid antinociception. Proc Natl Acad Sci USA 100: 6204–6209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zar S, Benson MJ, Kumar D. Review article: bloating in functional bowel disorders. Aliment Pharmacol Ther 16: 1867–1876, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Zhang HQ, Al-Chaer ED, Willis WD. Effect of tactile inputs on thalamic responses to noxious colorectal distension in rat. J Neurophysiol 88: 1185–1196, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Zhou YY, Wanner NJ, Xiao Y, Shi XZ, Jiang XH, Gu JG, Xu GY. Electroacupuncture alleviates stress-induced visceral hypersensitivity through an opioid system in rats. World J Gastroenterol 18: 7201–7211, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]