Abstract
Endoplasmic reticulum (ER) stress was previously reported to contribute to neurogenic hypertension while neuronal angiotensin-converting enzyme type 2 (ACE2) overexpression blunts the disease. To assess which brain regions are important for ACE2 beneficial effects and the contribution of ER stress to neurogenic hypertension, we first used transgenic mice harboring a floxed neuronal hACE2 transgene (SL) and tested the impact of hACE2 knockdown in the subfornical organ (SFO) and paraventricular nucleus (PVN) on deoxycorticosterone acetate (DOCA)-salt hypertension. SL and nontransgenic (NT) mice underwent DOCA-salt or sham treatment while infected with an adenoassociated virus (AAV) encoding Cre recombinase (AAV-Cre) or a control virus (AAV-green fluorescent protein) to the SFO or PVN. DOCA-salt-induced hypertension was reduced in SL mice, with hACE2 overexpression in the brain. This reduction was only partially blunted by knockdown of hACE2 in the SFO or PVN, suggesting that both regions are involved but not essential for ACE2 regulation of blood pressure (BP). DOCA-salt treatment did not increase the protein levels of ER stress and autophagy markers in NT mice, despite a significant increase in BP. In addition, these markers were not affected by hACE2 overexpression in the brain, despite a significant reduction of hypertension in SL mice. To further assess the role of ER stress in neurogenic hypertension, NT mice were infused intracerebroventricularlly with tauroursodeoxycholic acid (TUDCA), an ER stress inhibitor, during DOCA-salt treatment. However, TUDCA infusion failed to blunt the development of hypertension in NT mice. Our data suggest that brain ER stress does not contribute to DOCA-salt hypertension and that ACE2 blunts neurogenic hypertension independently of ER stress.
Keywords: neurogenic hypertension, central nervous system, blood pressure, angiotensin-converting enzyme type 2, deoxycorticosterone acetate
the brain renin-angiotensin system (RAS) plays a critical role in the regulation of blood pressure (BP) via its actions in various brain regions, such as subfornical organ (SFO), paraventricular nucleus (PVN), rostral ventrolateral medulla (RVLM), and nucleus tractus solitarius (NTS) (17). An overactivated brain RAS leads to the development of neurogenic hypertension at least in part by increasing sympathetic outflow, blunting the baroreflex sensitivity and stimulating vasopressin secretion (17). Angiotensin-converting enzyme type 2 (ACE2), a pivotal RAS component, has been found in many regions in the bran, including SFO and PVN (8). Numerous studies have shown that ACE2 has opposite properties to that of the classic RAS via its conversion of angiotensin (ANG) II into ANG-(1–7) (20). We previously reported that overexpression of ACE2 in the brain blunts the development of hypertension in several animal models (9, 31). However, it is not clear which brain regions might be more important for the preservation of ACE2 compensatory activity.
Recently, endoplasmic reticulum (ER) stress has been suggested as a result of overactivation of the RAS (18, 27, 33). The ER is an important cellular organelle responsible for the folding, sorting, and transportation of synthesized proteins. Stress, which impairs ER function, leads to an accumulation of unfolded or misfolded proteins, known as ER stress. ER stress triggers an evolutionarily conserved response, called unfolded protein response (UPR) (22, 24). Three ER stress transducers have been identified: protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor (ATF) 6 (13, 36). The initial activation of these sensors is dependent on the dissociation of Bip [also called glucose regulated protein 78 (GRP78)], the ER chaperone (13, 22). Activation of PERK promotes eukaryotic iniation factor 2α phosphorylation and the translation of ATF4, leading to the enhanced transcription of target genes such as CCAAT/enhancer-binding protein homologous protein (CHOP), an apoptotic transcription factor. Activation of IRE1 induces the splicing of X-box-binding protein 1 (XBP1) mRNA. XBP1 translated from the sliced XBP (s-XBP) mRNA activates the transcription of ER stress-related genes, including CHOP and Bip (13, 36). The initial intent of UPR is to adapt to the environmental changes and reestablish normal ER function through increasing protein folding capacity and activation of protein degradation to remove misfolded proteins (22, 24). However, when ER stress is prolonged, UPR is unable to rescue cells and eventually leads to cell death (22, 24, 32). ER stress has been implicated in neurodegenerative diseases, cancer, obesity, and diabetes (13). Very recently, ER stress has been suggested in the development and maintenance of neurogenic hypertension (7, 37). However, the mechanisms involved remain to be determined.
It is well known that the deoxycorticosterone acetate (DOCA)-salt hypertension model exhibits increased brain RAS activity, such as increased ANG II and ANG II receptor type 1 (AT1R) levels (21). ANG II has been shown to induce ER stress in both periphery and brain (33, 37). Here, we asked whether ER stress is involved in the development of hypertension in the DOCA-salt model. In addition, we previously reported that ACE2 overexpression in the brain blunts DOCA-salt-induced hypertension in mice (31). However, the role of ACE2 on ER stress has not been reported. In this study, we aimed to 1) identify the brain regions supporting the ACE2-mediated reduction of hypertension and 2) investigate whether the reduction of ER stress is involved in this process.
MATERIALS AND METHODS
Transgenic mice and animal husbandry.
Experiments were performed in adult (males, 14–16 wk of age, 25–30 g) nontransgenic (NT) and transgenic mice harboring a floxed human ACE2 (hACE2) transgene in neurons (synapsin-LoxP-hACE2, SL; Fig. 1A). SL mice were generated in collaboration with Dr. Curt D. Sigmund at The University of Iowa. Briefly, the linearized transgene was microinjected into fertilized C57BL/6J×SJL/J (B6SJLF2) mouse embryos. Founders were bred to establish transgenic lines with floxed hACE2 in neurons (SL), allowing for specific knockdown of hACE2 following introduction of Cre recombinase. Animals were fed standard mouse chow and water ad libitum. All procedures were approved by the Louisiana State University Health Sciences Center Animal Care and Use Committee and are in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
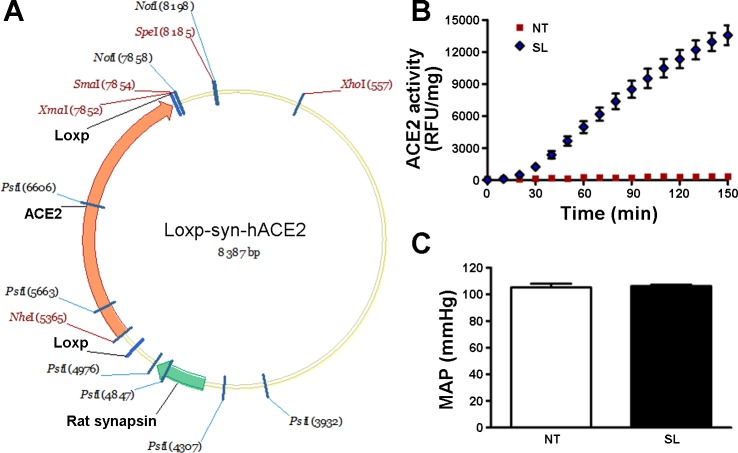
Fig. 1.
Characterization of synapsin-loxp-hACE2 (SL) transgenic mice. A: schematic of the human (h) angiotensin-converting enzyme type 2 (ACE2) transgene flanked by LoxP sites and driven by a rat synapsin promoter. B: ACE2 activity assay showing that SL mice present significantly higher enzyme activity in the brain compared with nontransgenic (NT) mice. C: baseline mean arterial blood pressure (MAP) was not different in SL mice compared with NT mice.
Telemetry implantation, microinjection, DOCA-salt treatment, and BP recordings.
In the first set of experiments, SL and NT mice were implanted with telemetry probes for conscious BP monitoring, as described previously (31). After 1 wk of recovery, baseline BP was recorded during 3 days, and then mice were implanted subcutaneously either with a DOCA-silicone sheet (DOCA-silicone = 1:3; DOCA: 50 mg/mouse) or an empty silicone sheet (Smooth-On). At the same time, AAV-green fluorescent protein (GFP) [108 viral genome-containing particles (vg)/200 nl] or AAV-Cre (108 vg/200 nl; University of Iowa Gene Transfer Vector Core) was microinjected to the SFO (0 mm lateral, 0.3 mm caudal, 2.7 mm ventral from bregma) or bilaterally to the PVN (±0.3 mm lateral, 0.6 mm caudal, 5.0 mm ventral) of these animals. BP was recorded for three more weeks. Mice were then killed, and brains were collected and cryosectioned to examine GFP fluorescence and ACE2 expression in the SFO and PVN.
Immunofluorescence.
Anesthetized mice (ketamine/xylazine 100/10 mg/kg) were perfused transcardially with 4% paraformaldehyde in 0.1 mol/l phosphate buffer (PB), as described previously (9). Brains were removed, postfixed for 1 h, and then transferred to 20% sucrose in PB overnight. For GFP fluorescence, cryostat sections (30 μm, coronal) containing SFO or PVN were visualized directly using a fluorescence microscope (Olympus U-TB190). For ACE2 expression, cryostat sections were incubated with a rabbit anti-ACE2 antibody (sc-20998; 1:200 dilution; Santa Cruz) for 24 h at 4°C. Sections were washed with PB and incubated with the secondary antibody AlexaFluor 594 Goat anti-rabbit IgG (A-11–12, 1:500; Invitrogen) for 2 h in the dark. Immunofluorescence was visualized using a fluorescence microscope, as above.
Real-time RT-PCR and Western blotting for ER stress and autophagy biomarkers.
In a second set of experiments, NT and SL mice (n = 8/group) underwent a similar DOCA-salt/sham treatment. After 3 wk, mice were killed, and both SFO and PVN regions were micropunched. One-half of the collected tissue was used for quantitative real-time RT-PCR, the other one-half was used for Western blotting.
Quantitative real-time RT-PCR.
Total RNA was isolated using TRI REAGENT (MRC), and cDNA was synthesized using the High Capacity cDNA Reverse Transcription kit (AB Applied Biosystems). Real-time RT-PCR amplification reactions were performed with SYBR Green master mix (Roche) using a Light Cycler 480 II real-time PCR machine (Roche) to detect the expression of ER stress markers. The primer sequences used for real-time RT-PCR are listed in Table 1. Data were normalized to β-actin expression by the ΔΔCT comparative method and expressed as a fold change compared with the NT group.
Table 1.
List of primers used for real-time RT-PCR
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| sXBP | GCTTGGGAATGGACACGCT | GCCTGCACCTGCTGCAGAGG |
| ATF4 | GCAACCCCCACCGGCCTAAG | CCCGACTGGTCGAAGGGGGA |
| Bip | CCTGCGTCGGTGTGTTCAAG | AAGGGTCATTCCAAGTGCG |
| CHOP | TGTTGAAGATGAGCGGGTGGCA | CCATGACTGCACGTGGACCAGG |
sXBP, sliced X-box-binding protein; ATF4, activating transcription factor 4; CHOP, CCAAT/enhancer-binding protein homologous protein.
Western blotting.
Proteins (20–40 μg) extracted from SFO and PVN (n = 4/group) were processed for Western blotting to detect the expression of ER stress and autophagy markers, using rabbit anti-ATF4 (sc-200, 1:100; Santa Cruz), goat anti-Bip (sc-1050, 1:1,000; Santa Cruz), rabbit anti-LC3A/B (no. 4108, 1:1,000; Cell Signaling), and rabbit anti-lysosome-associated membrane protein-2 (LAMP2, NB300–591, 1:500; Novus Biologicals) antibodies. Specific bands were detected by chemiluminescence according to the manufacturer's instructions and quantified by laser densitometry. Equal loading was determined using either α-actin, α-tubulin, or γ-tubulin loading controls.
ER stress inhibitor tauroursodeoxycholic acid intracerebroventricular infusion.
In a third set of experiments, NT mice were implanted with telemetry probes, and baseline BP was recorded as above. Mice were then divided into four groups (n = 8/group), for the following treatments: 1) sham; 2) tauroursodeoxycholic acid (TUDCA) intracerebroventricular infusion [5 μg/day (37), 21-day osmotic pumps]; 3) DOCA-salt + artificial cerebral spinal fluid (aCSF) intracerebroventricular infusion; and 4) DOCA-salt + TUDCA intracerebroventricular infusion. BP was recorded for three more weeks.
Statistical analysis.
Data are expressed as means ± SE. Data were analyzed, when appropriate, by Student's t-test, repeated-measures ANOVA, or one-way ANOVA (after Bartlett test of homogeneity of variance) followed by Tukey-Kramer correction for multiple comparisons between means. Statistical comparisons were performed using Prism5 (GraphPad Software, San Diego, CA). Differences were considered statistically significant at P < 0.05.
RESULTS
Characterization of SL transgenic mice.
Using standard transgenic technology, we generated floxed hACE2 transgenic (SL) mice (Fig. 1A), amenable for site-specific knockdown of hACE2. Following RT-PCR in various tissues, we observed that the highest levels of hACE2 expression were located in the brain and spinal cord (data not shown), confirming that the synapsin promoter drove hACE2 expression prominently to neurons. To further confirm this, ACE2 activity was assessed in the brain. Figure 1B shows that the enzyme activity in the central nervous system was dramatically increased in SL compared with NT mice. To test whether the transgene affects baseline BP, we recorded these animals using radiotelemetry. As previously observed in related SA mice (9, 31), mean arterial pressure (MAP) was not different in SL compared with NT mice (Fig. 1C), suggesting that floxed hACE2 expression in the brain does not affect baseline BP.
Brain ACE2 overexpression-mediated reduction in DOCA-salt hypertension was partially blunted by SFO or PVN targeted knockdown of ACE2.
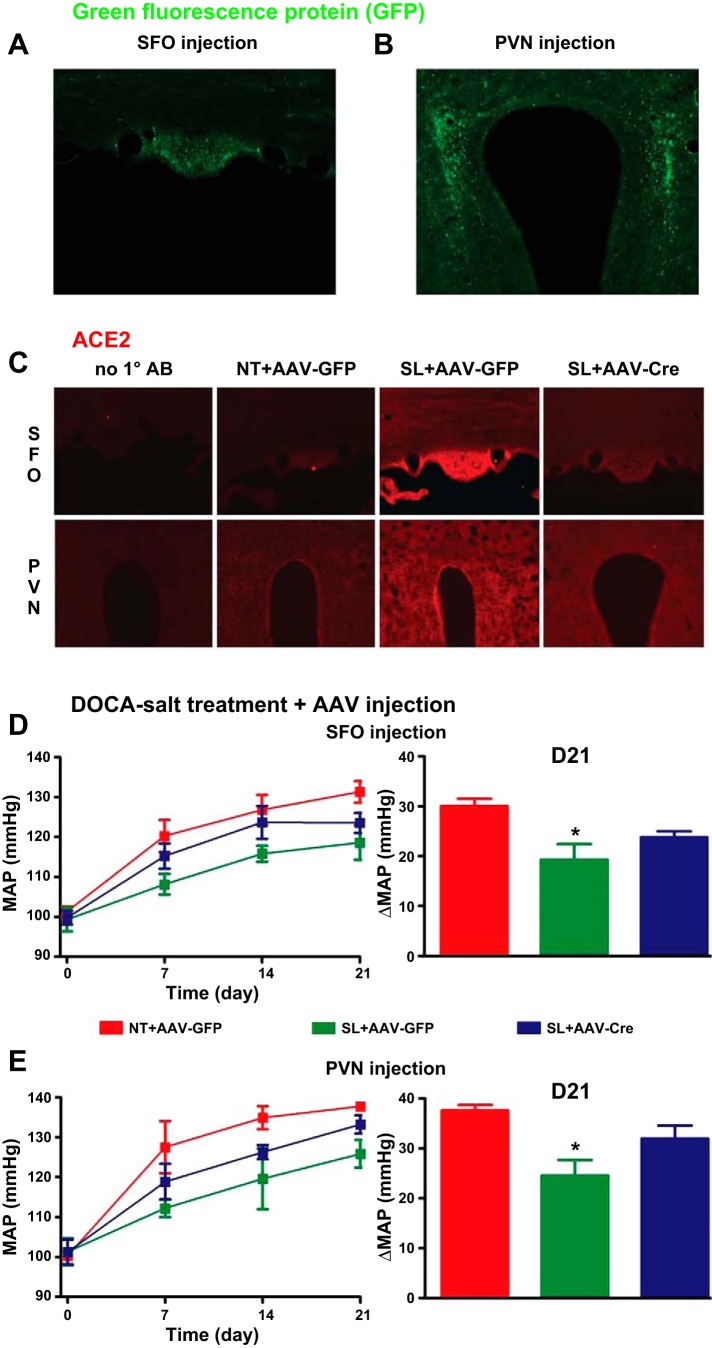
We previously reported that neuron-targeted ACE2 overexpression reduces DOCA-salt-mediated development of hypertension in mice (9, 31), suggesting a compensatory effect of ACE2 on the brain RAS. To determine whether SFO or PVN are critical for ACE2 compensatory activity, we deleted the exogenous hACE2 in SFO or PVN in SL mice, using AAV-Cre microinjection to these regions, and assessed BP changes in these animals. Figure 2, A and B, shows the typical GFP fluorescence in SFO and PVN, respectively, following microinjection with AAV-GFP, demonstrating the successful targeting of these regions. To determine whether hACE2 is knocked down by AAV-Cre infection, we performed immunofluorescence staining in brain sections using a nonselective ACE2 antibody. As shown in Fig. 2C, no fluorescence was observed in the negative control sections (from SL + AAV-GFP mice), where the primary antibody was omitted. Total ACE2 expression was higher in the SFO and PVN of SL mice compared with NT mice with control GFP virus transfection, confirming the overexpression of ACE2 in our transgenic mice. However, with AAV-Cre, ACE2 expression was reduced in the SFO or PVN in SL mice 3 wk after microinjection in these specific regions (Fig. 2C), suggesting that ACE2 was knocked down by AAV-Cre. To investigate whether the changes of ACE2 in these regions affect DOCA-salt hypertension, we analyzed BP. Similar to what our group (31) and others (12) previously reported, DOCA-salt treatment for 3 wk significantly increased mean BP in NT mice receiving the control virus AAV-GFP in either SFO (ΔMAP: +30.0 ± 1.5 mmHg; Fig. 2C) or PVN (ΔMAP: +37.6 ± 1.1 mmHg; Fig. 2D), confirming that AAV-GFP infection does not affect BP in these animals. As expected, DOCA-salt-mediated hypertension was significantly blunted in AAV-GFP-infected SL mice [SFO: +19.3 ± 3.0 mmHg (Fig. 2D); PVN: +24.6 ± 3.0 mmHg (Fig. 2E), P < 0.05 vs. NT + AAV-GFP]. However, knockdown of ACE2 using AAV-Cre in the SFO or PVN of SL mice contributed to the increase in BP in these mice. DOCA-salt-mediated-changes in BP in SL mice following AAV-Cre infection to the SFO (ΔMAP: +23.8 ± 1.2 mmHg; Fig. 2C) or PVN (ΔMAP: +30.0 ± 1.5 mmHg; Fig. 2D) was not significantly different from either the NT or SL group post-AAV-GFP infection (P > 0.05; Fig. 2, C and D). This suggests that, while ACE2 activity in the SFO and PVN is important, deletion of ACE2 in one of these regions is not a limiting factor for the beneficial effect on BP.
Fig. 2.
Knockdown of ACE2 in the subfornical organ (SFO) or paraventricular nucleus (PVN) of SL mice blunted ACE2 overexpression-mediated reduction of deoxycorticosterone acetate (DOCA)-salt hypertension. A and B: representative images showing green fluorescent protein (GFP) staining in the SFO (A) and PVN (B) following microinjection with adenoassociated virus (AAV)-GFP [108 viral genome-containing particles (vg)/200 nl] in these regions. C: representative immunoflurorescence images for ACE2 in the SFO (top) and PVN (bottom) of NT and SL mice following AAV-GFP or AAV-Cre injection. ACE2 expression was much higher in the SFO and PVN in SL mice compared with NT mice with the control virus AAV-GFP infection to these regions. The expression was knocked down in SL mice in the SFO and PVN following AAV-Cre infection in the specific regions. D and E: after 3 wk treatment, DOCA-salt-induced increase in BP was significantly reduced in SL mice compared with NT mice with AAV-GFP infection to the SFO (D) or PVN (E; *P < 0.05 vs. NT + AAV-GFP). However, these effects were partially blunted by AAV-Cre infection in the SFO (D) or PVN (E). D21, day 21.
ER stress markers protein levels are not altered in the SFO or PVN in DOCA-salt hypertensive mice.
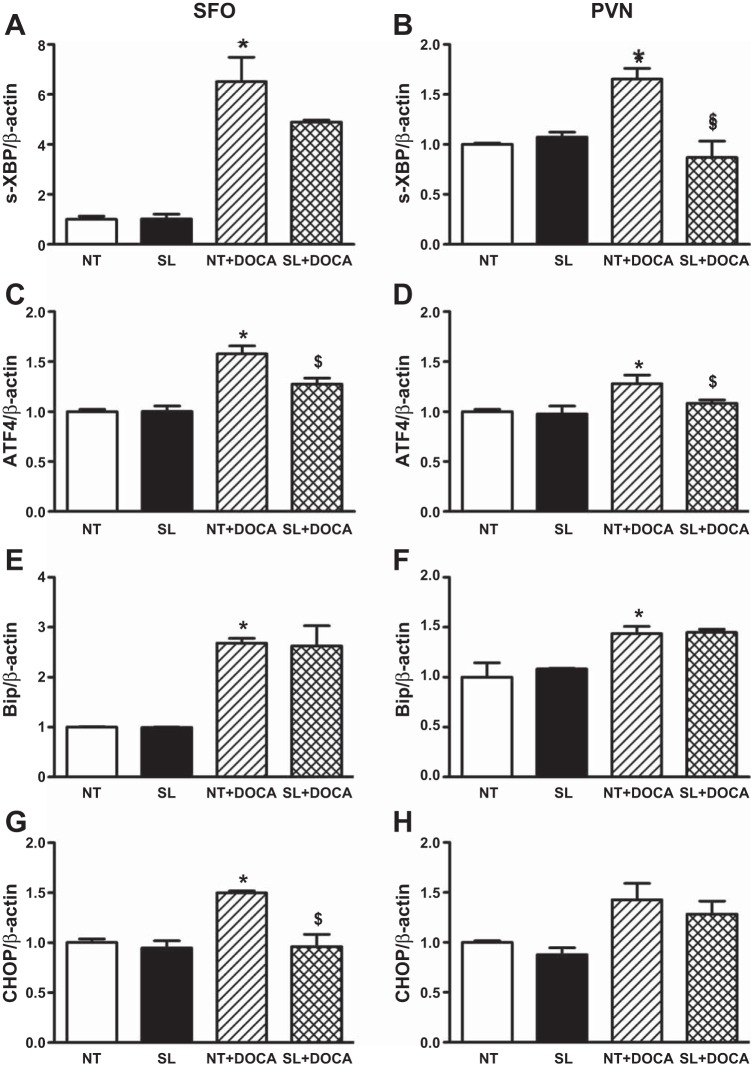
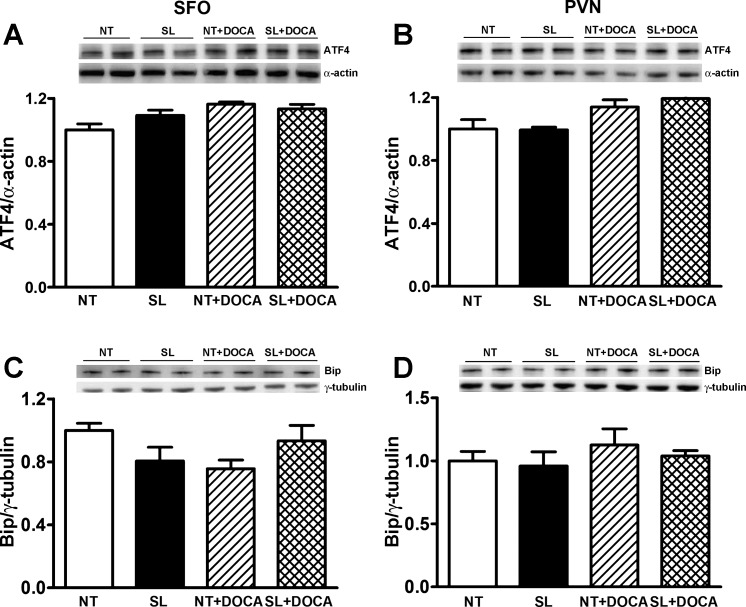
ER stress has previously been implicated in hypertension (7, 37). To determine whether brain ER stress contributes to DOCA-salt hypertension, and whether ACE2 blunts DOCA-salt hypertension through prevention of ER stress, we measured ER stress marker mRNA and protein levels in the SFO and PVN regions. Real-time PCR data show that s-XBP, ATF4, Bip, and CHOP mRNA levels in the SFO or PVN were not different between untreated NT and SL mice (Fig. 3, A–H). After 3 wk of DOCA-salt treatment, mRNA levels of s-XBP, ATF4, and Bip were significantly increased in both SFO (Fig. 3, A, C, and E) and PVN (Fig. 3, B, D, and F) regions of NT mice (P < 0.05 vs. NT), and some of the increases were significantly blunted (Fig. 3, B, C, and D) in the same regions of SL mice (P < 0.05 vs. NT + DOCA). CHOP mRNA level was increased in the SFO but not PVN of NT mice after DOCA-salt treatment, but it was normalized in SL mice with the same treatment (Fig. 3, G and H). These data seem to indicate that ER stress is induced in the SFO and PVN at some levels during DOCA-salt hypertension, and ACE2 overexpression in the brain could blunt some of the effects. Interestingly, Western blotting data show that protein levels of ATF4 or Bip were not significantly changed in DOCA-salt hypertensive mice in either SFO or PVN (Fig. 4, A–D), and ACE2 overexpression in the brain did not affect the protein expression of these markers. In addition, the ER stress marker CHOP protein was not detected by Western blot in any of the groups (data not shown), supporting the lack of ER stress in the brain of DOCA-salt-treated mice. These data also indicate that ACE2 overexpression reduces DOCA-salt hypertension independently of ER stress.
Fig. 3.
Endoplasmic reticulum (ER) stress biomarker mRNA levels in the SFO and PVN are increased in DOCA-salt-treated NT mice and blunted in SL mice. Spliced (s) X-box-binding protein 1 (XBP), activating transcription factor (ATF) 4, Bip, and CCAAT/enhancer-binding protein homologous protein (CHOP) mRNA levels in the SFO or PVN are not different between NT and SL mice (A–H). Following DOCA-salt treatment, the mRNA levels are significantly increased (A–G) or tend to increase (H) in the SFO and PVN (*P < 0.05 vs. NT). However, ACE2 overexpression in the brain blunts (A and C) or prevents (B and D) the increases in s-XBP and ATF4 in the SFO and PVN and normalizes CHOP expression in the SFO (G; $P < 0.05 vs. NT + DOCA). It does not affect Bip mRNA levels in either SFO or PVN, or CHOP mRNA in the PVN.
Fig. 4.
ER stress biomarker protein levels are not altered in the SFO and PVN of NT and SL mice following DOCA-salt administration. Western blotting shows that protein levels of ATF4 (A and B) and Bip (C and D) in the SFO or PVN were similar between NT and SL mice. The levels are not significantly altered by DOCA-salt treatment in either NT or SL mice (A–D).
Autophagy is not induced in the SFO or PVN in DOCA-salt hypertensive mice.
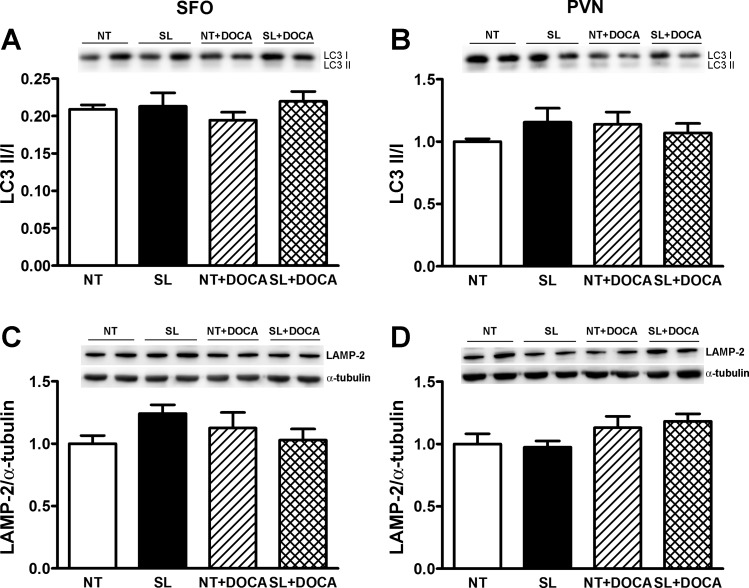
Because ER stress leads to autophagy, we further looked at autophagy markers in the SFO and PVN in DOCA-salt hypertensive mice. Similarly, the ratio of the membrane-bound form of LC3 (LC3 II) to the cytosolic form of LC3 (LC3 I) (Fig. 5, A and B) or LAMP2 (Fig. 5, C and D) protein levels was not significantly changed in either SFO or PVN in NT mice following DOCA-salt treatment, and not altered by ACE2 overexpression in the brain, suggesting that autophagy is not induced in these regions during DOCA-salt hypertension.
Fig. 5.
Autophagy biomarker protein levels are not altered in the SFO and PVN of NT and SL mice following DOCA-salt administration. Western blotting shows the ratio of the protein level of the membrane-bound form of LC3 (LC3 II) to the cytosolic form of LC3 (LC3 I) (A and B) and lysosome-associated membrane protein-2 (LAMP-2; C and D) in the SFO or PVN were similar between NT and SL mice. They are not significantly altered by DOCA-salt treatment in either NT or SL mice.
Inhibition of ER stress in the brain does not prevent the development of DOCA-salt hypertension.
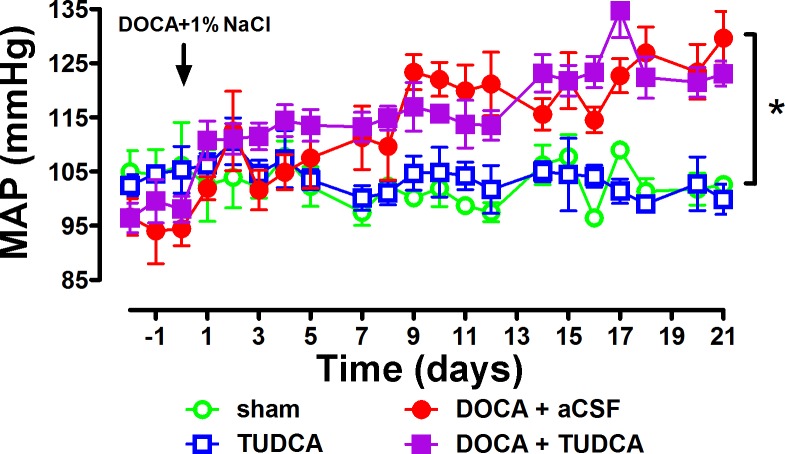
To further confirm our findings, we infused NT mice intracerebroventricularly with TUDCA, an ER stress inhibitor, during DOCA-salt treatment and monitored BP changes for 3 wk. Sham surgery or TUDCA intracerebroventricular infusion alone did not change MAP in these mice (Fig. 6). Again, similar to what our group (31) and others (12) reported previously, DOCA-salt administration for 3 wk significantly increased MAP (P < 0.05 vs. baseline; Fig. 6). However, coadministration with TUDCA (icv) did not reduce DOCA-salt-induced hypertension in these mice (P > 0.05, DOCA + TUDCA vs. DOCA + aCSF; Fig. 6), confirming that ER stress is not involved in this model.
Fig. 6.
DOCA-salt-induced increase in blood pressure (BP) in NT mice was not reduced by ER stress inhibitor tauroursodeoxycholic acid (TUDCA) infusion to the brain. Mean arterial pressure (MAP) was not affected in sham or artificial cerebral spinal fluid (aCSF) icv-infused mice. DOCA-salt administration for 3 wk significantly increased MAP in the mice (*P < 0.05 vs. sham), and this was not reduced by coinfusion (icv) with the ER stress inhibitor TUDCA.
DISCUSSION
It has been well established that the brain RAS plays critical roles in the central regulation of BP and in the pathogenesis of neurogenic hypertension (17). Many brain regions are involved in this process. The SFO is a site where ANG II stimulates water intake, sodium intake, and pressor response (25). All of these contribute to the increase in BP. High density of AT1R expression has been found in this region (11). Because of the lack of blood-brain barrier, SFO has access to both systemic and intraventricular ANG II. Elevated AT1R has been found in the SFO in several hypertension animal models, such as chronic ANG II infusion (29), spontaneously hypertensive rats (SHR) (19), and DOCA-salt (21). SFO also communicates with hypothalamic nuclei, including the PVN. The PVN is a central site for the integration of sympathetic nerve activity and receives input from ANG II-containing neurons in the forebrain (1). Neurons in the PVN also project to the RVLM, a critical region in the maintenance of sympathetic nerve activity (23). In addition, some neurons in the PVN have projections to the spinal cord where sympathetic preganglionic neurons are located (6, 23). Therefore, these connections enable the PVN to indirectly or directly influence sympathetic nerve activity. All of the components of the RAS have been found in the PVN, including ANG II, AT1R, and ACE (17). ANG II excites neurons in the PVN to increase sympathetic activity (5), thus increasing BP. Therefore, SFO and PVN are two of the major regions where RAS plays its role in BP regulation and development of hypertension. The cellular mechanisms involved include oxidative stress (38, 39), inflammation (26), altered NO signaling (3), and, as recently suggested, ER stress (7, 37).
ACE2, a pivotal component of the RAS, counterbalances the effects of the classic RAS through conversion of the vasoconstrictor ANG II to the vasodilator ANG-(1–7). Numerous studies have shown the ability of ACE2 in preventing ANG II-mediated cardiovascular dysfunction (4). We previously identified the presence of ACE2 in many brain regions involved in BP regulation, including the SFO, PVN, NTS, and RVLM (8), suggesting that ACE2 is involved in these regulatory mechanisms. Indeed, reduced ACE2 expression and/or enzyme activity have been found in various brain regions in hypertension models (30, 31, 35). Using transgenic mice overexpressing ACE2 in the brain, we previously showed that ACE2 overexpression blunts both ANG II infusion-mediated hypertension (9) and DOCA-salt hypertension (31). However, it is unclear which brain regions could be more important for ACE2 beneficial effects. Using an adenovirus coding for ACE2, we reported that overexpression of ACE2 in the SFO or PVN inhibits the acute ANG II intracerebroventricular injection-mediated increase in BP in mice (10) or chronic ANG II infusion-mediated hypertension in rats (26), respectively. These findings suggest that SFO and PVN are important brain regions for ACE2 prevention of ANG II-mediated hypertension. In this study, we assess the role of ACE2 in both SFO and PVN in DOCA-salt hypertension. It has been shown that increased RAS components, including angiotensinogen, ANG II, and AT1R levels, were observed in the brain but not the periphery in this model (21), therefore an excellent model to study neurogenic hypertension. Immunofluorescence data show that knockdown of exogenous ACE2 from the SFO, or the PVN, partially blunted the brain ACE2 overexpression-mediated reduction of hypertension in DOCA-salt-treated SL mice. Whereas MAP was higher than that in AAV-GFP-transfected SL mice, it did not reach the same levels as observed in DOCA-salt-treated NT mice. Our data suggest that ACE2 activity in the SFO and PVN is important; however, knockdown of ACE2 in one of these regions of SL mice is not a limiting factor for the beneficial effect on BP. This could be because of the angiotensinergic projections between neurons in different regions (15, 17). For example, in SFO AAV-Cre-infected SL mice, although hACE2 was knocked down from this region, the PVN still overexpress ACE2 and therefore could inhibit the deleterious effects of ANG II originating from the SFO and promote inhibition of oxidative stress and inflammation. On the other hand, in AAV-Cre-infected SL mice, where ACE2 expression was knocked down selectively in the PVN, the SFO and other surrounding regions still exhibit overexpressed ACE2 and therefore could potentially reduce ANG II levels, thus inhibiting neuronal activation of the PVN. Furthermore, previous studies have also shown that overexpression of ACE2 in the RVLM or NTS improves baroreflex sensitivity and decreases high BP in SHR (34, 35), suggesting that RVLM and NTS are other regions critical for ACE2 prevention of hypertension. Despite deletion of hACE2 in the SFO or PVN, the presence of ACE2 in the RVLM or NTS of SL mice could also contribute to the reduction of DOCA-salt hypertension. Although complex to perform, removal of hACE2 from multiple brain regions could be a way to better understand the critical role of ACE2 in these brain regions. Also, we acknowledge that immunofluorescence only provides semiquantitative data. Although the images show a clear reduction of ACE2 in the SFO or PVN of SL mice following AAV-Cre treatment, we cannot determine precisely how much ACE2 loss was achieved. It is possible that hACE2 was not completely removed from these regions in AAV-Cre-treated SL mice. This could also contribute to the partial blunting of ACE2 beneficial effects on hypertension. Western blot or enzyme activity assays could be used to demonstrate the amount of reduction of hACE2. However, the size of the studied regions also limits the use of these assays, and, in our case, we did not have enough tissue to perform these experiments.
In vitro (18) and in vivo (27, 33) studies have shown that ANG II stimulates ER stress. Accumulating evidence implicates the involvement of ER stress in various diseases, including neurodegenerative diseases, metabolic diseases, cancer, and some cardiovascular diseases (13, 16). However, the role of ER stress in the development of hypertension has been suggested only recently (7, 37). Young et al. reported that ER stress is induced in the brain, particularly in the SFO, in an ANG II infusion-mediated hypertension model (37). Chao et al. showed that ER stress is induced in the RVLM in SHR (7). Both studies showed that Bip (also known as GRP78), the ER stress chaperone, and other ER stress markers were increased in these regions, and inhibition of ER stress in the brain, or specific brain regions, prevented hypertension in these models. In this study, we assessed ER stress in the SFO and PVN in DOCA-salt hypertension. Because ANG II induces ER stress, we expected that ER stress would be present in these regions in our DOCA-salt hypertensive mice and could possibly be responsible for the development and maintenance of hypertension. Surprisingly, although mRNA levels of the ATF4, s-XBP, CHOP, and Bip were increased in DOCA-salt mice in both SFO and PVN, and ACE2 overexpression in the brain showed inhibition effects at some levels, these changes on mRNA levels were not translated to protein levels or physiological function. We failed to detect CHOP protein expression in any of the groups tested although we could easily detect it in the hypothalamus of obese mice (data not shown). In addition, we did not see significant elevation on the protein expression of ATF4 or Bip in either SFO or PVN of DOCA-salt-treated NT mice, despite a significant increase in BP in these animals. Moreover, the protein levels in these regions were not affected by ACE2 overexpression in the brain, despite a significant reduction in BP in DOCA-salt-treated SL mice. These data indicate that ER stress might not be induced in the SFO and PVN in DOCA-salt hypertension. To confirm this, we analyzed autophagy in these regions as well, since ER stress induces autophagy (14). During autophagy, LC3 I is converted to LC3 II (2); therefore, the ratio of LC3 II over I has been used as a marker for autophagy. LAMP-2 has been shown to be critical for autophagy (28) and used as another marker for this condition (7). A previous study showed increased expression of LAMP-2 and the ratio of LC3 II/I in the RVLM of SHR compared with Wistar-Kyoto rats, where ER stress is induced (7). In our DOCA-salt mice, we did not see increased LC3 II or LAMP-2 protein expression in either SFO or PVN, and the ratio of LC3 II over I was not significantly different among the groups. Thus, our data suggest that ER stress was not induced in these regions in DOCA-salt mice. However, because we only tested SFO and PVN, our results cannot exclude the presence of ER stress in other brain regions. Finally, in contrast to the observations made in chronic ANG II infusion (37) and SHR (7) hypertension models, BP recording in DOCA-salt mice showed that infusion of the ER stress inhibitor TUDCA to the brain did not reduce BP in DOCA-salt hypertensive mice, confirming that ER stress is not involved in this model or does not contribute to the development of DOCA-salt hypertension.
Perspectives and Significance
Our study suggests that SFO and PVN contribute to ACE2 compensatory properties in neurogenic hypertension but are not limiting factors for this process. Moreover, brain ER stress and autophagy do not appear to contribute to the development of DOCA-salt hypertension. Therefore, our data suggest that brain ACE2 overexpression reduces DOCA-salt hypertension independently of ER stress. In light of recent reports showing a role for microglia in hypertension, future studies should also take into account the different cell populations that might contribute to the beneficial effects of ACE2 in neurogenic hypertension.
GRANTS
This work was supported by an Established Investigator Award from the American Heart Association (12EIA8030004) and National Institutes of Health (NIH) Grants HL-093178 and GM-103514 to E. Lazartigues and HL-094709 to I. N. Mungrue. Transgenic mice were generated at the University of Iowa Transgenic and Genome Manipulation Facility directed by Dr. Curt D. Sigmund and supported in part by grants from the NIH and from the Roy J. and Lucille A. Carver College of Medicine. AAV viruses were purchased from the University of Iowa Gene Transfer Vector Core.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.X., I.N.M., and E.L. conception and design of research; H.X., T.M.d.Q., S.S., Y.F., T.J., and E.L. performed experiments; H.X., T.M.d.Q., and T.J. analyzed data; H.X., I.N.M., and E.L. interpreted results of experiments; H.X. prepared figures; H.X. drafted manuscript; H.X. and E.L. edited and revised manuscript; H.X., T.M.d.Q., S.S., Y.F., T.J., I.N.M., and E.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Maria Scheel for technical expertise.
REFERENCES
- 1.Bains J, Ferguson A. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol 268: R625–R633, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Bross P, Gregersen N, Tanida I, Waguri S. Measurement of autophagy in cells and tissues. In: Protein Misfolding and Cellular Stress in Disease and Aging. Clifton, NJ: Humana, 2010, p. 193–214. [DOI] [PubMed] [Google Scholar]
- 3.Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthase and interleukin-1β mediates angiotensin ii-dependent stimulation of sympathetic nerve activity. Hypertension 39: 519–524, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Chaves P, Cerqueira R, Pintalhao M, Leite-Moreira AF. New pathways of the renin-angiotensin system: the role of ACE2 in cardiovascular pathophysiology and therapy. Exp Opinion Ther Targets 14: 485–496, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Cato M, Toney G. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cechetto D, Saper C. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neural 272: 579–604, 1988. [DOI] [PubMed] [Google Scholar]
- 7.Chao YM, Lai MD, Chan JYH. Redox-sensitive endoplasmic reticulum stress and autophagy at rostral ventrolateral medulla contribute to hypertension in spontaneously hypertensive rats. Hypertension 61: 1270–1280, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Doobay M, Talman L, Obr T, Tian X, Davisson R, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 292: R373–R381, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RAS, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res 106: 373–382, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin II-mediated pressor and drinking responses and is associated with angiotensin II type 1 receptor downregulation. Circ Res 102: 729–736, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grob M, Trottier JF, Mouginot D. Heterogeneous co-localization of AT1A receptor and Fos protein in forebrain neuronal populations responding to acute hydromineral deficit. Brain Res 996: 81–88, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, Sigmund CD. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 57: 600–607, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosoi T, Ozawa K. Endoplasmic reticulum stress in disease: mechanisms and therapeutic opportunities. Clin Sci 118: 19–29, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14: 1576–1582, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Meehan J, Collister J. Lesion of the subfornical organ attenuates neuronal activation of the paraventricular nucleus in response to angiotensin II in normal rats. Open J Neurosci 1: 1–14, 2011. [PMC free article] [PubMed] [Google Scholar]
- 16.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat M, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116: 1226–1233, 2007. [DOI] [PubMed] [Google Scholar]
- 17.O'Callaghan EL, Choong YT, Jancovski N, Allen AM. Central angiotensinergic mechanisms associated with hypertension. Auton Neurosci 175: 85–92, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike K, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aorticconstriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110: 705–712, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Saavedra J, Correa F, Kurihara M, Shigematsu K. Increased number of angiotensin II receptors in the subfornical organ of spontaneously hypertensive rats. J Hypertens Suppl 4: S27–S30, 1986. [PubMed] [Google Scholar]
- 20.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and mas: new players of the renin angiotensin system. J Endocrinol 216: R1–R17, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Schenk J, McNeill J. The pathogenesis of DOCA-salt hypertension. J Pharmacol Toxicol Methods 27: 161–170, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Schroder M, Kaufman R. ER stress and the unfolded protein response. Mutat Res 569: 29–63, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Shafton A, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Shen X, Zhang K, Kaufman R. The unfolded protein response-a stress signaling pathway of the endoplasmic reticulum. J Chem Neuroanat 28: 79–92, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Simpson J. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology 32: 248–256, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Sriramula S, Cardinale J, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res 92: 401–408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukumaran V, Watanabe K, Veeraveedu P, Gurusamy N, Ma M, Thandavarayan R, Lakshmanan A, Yamaguchi K, Suzuki K, Kodama M. Olmesartan, an AT1 antagonist, attenuates oxidative stress, endoplasmic reticulum stress and cardiac inflammatory mediators in rats with heart failure induced by experimental autoimmune myocarditis. Int J Biol Sci 7: 154–167, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PML, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 406: 902–906, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Wei S, Yu Y, zhang Z, Felder R. Angiotensin II upregulates hypothalamic AT1 receptor expression in rats via the mitogen-activated protein kinase pathway. Am J Physiol Heart Circ Physiol 296: H1425–H1433, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension 53: 210–216, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia H, Sriramula S, KChhabra K, Lazartigues E. Brain ACE2 shedding contributes to the development of neurogenic hypertension. Circ Res 113: 1087–1096, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu C, Bailly-Maitre B, Reed J. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115: 2656–2664, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L. Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med 13: 1499–1512, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazato M, Ferreira AJ, Yamazato Y, Diez-Freire C, Yuan L, Gillies R, Raizada MK. Gene transfer of angiotensin-converting enzyme 2 in the nucleus tractus solitarius improves baroreceptor heart rate reflex in spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst 12: 456–461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49: 926–931, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida H. ER stress and diseases. FEBS J 274: 630–658, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Young C, Cao X, Guruju M, Pierce J, Morgan D, Wang G, Iadecola C, Mark A, Davisson R. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004. [DOI] [PubMed] [Google Scholar]