Abstract
Changes in oxidative capacities and phospholipid remodeling accompany temperature acclimation in ectothermic animals. Both responses may alter redox status and membrane susceptibility to lipid peroxidation (LPO). We tested the hypothesis that phospholipid remodeling is sufficient to offset temperature-driven rates of LPO and, thus, membrane susceptibility to LPO is conserved. We also predicted that the content of LPO products is maintained over a range of physiological temperatures. To assess LPO susceptibility, rates of LPO were quantified with the fluorescent probe C11-BODIPY in mitochondria and sarcoplasmic reticulum from oxidative and glycolytic muscle of striped bass (Morone saxatilis) acclimated to 7°C and 25°C. We also measured phospholipid compositions, contents of LPO products [i.e., individual classes of phospholipid hydroperoxides (PLOOH)], and two membrane antioxidants. Despite phospholipid headgroup and acyl chain remodeling, these alterations do not counter the effect of temperature on LPO rates (i.e., LPO rates are generally not different among acclimation groups when normalized to phospholipid content and compared at a common temperature). Although absolute levels of PLOOH are higher in muscles from cold- than warm-acclimated fish, this difference is lost when PLOOH levels are normalized to total phospholipid. Contents of vitamin E and two homologs of ubiquinone are more than four times higher in mitochondria prepared from oxidative muscle of warm- than cold-acclimated fish. Collectively, our data demonstrate that although phospholipid remodeling does not provide a means for offsetting thermal effects on rates of LPO, differences in phospholipid quantity ensure a constant proportion of LPO products with temperature variation.
Keywords: oxidative stress, lipid peroxidation, phospholipid hydroperoxides, vitamin E, temperature acclimation
lipid peroxidation (LPO) is unique among the various types of oxidative damage that may be inflicted on biological molecules. Unless chain-breaking antioxidants (e.g., vitamin E) and/or antioxidant enzymes [e.g., phospholipid hydroperoxide (PLOOH) glutathione peroxidase] terminate the process, the reactions of LPO can self-propagate within biological membranes when oxidized lipids damage other membrane lipids (10, 21, 31, 32, 44). LPO can disrupt the structure of biological membranes, because LPO involves the addition of polar hydroperoxy groups to both phospholipids and cholesterol, positioning a polar element within the otherwise hydrophobic core of the membrane. These LPO-induced changes in biological membranes may alter lipid-lipid and lipid-protein interactions, ultimately placing membrane integrity and function at risk (23, 26, 31).
Body temperatures of ectothermic animals are determined by environmental temperatures and vary widely for many temperate species (16). Ectotherms maintain cellular function during temperature acclimation/adaptation, in part, by remodeling biological membranes (15) and altering capacities for oxidative metabolism (9). Biological membranes from cold-acclimated/adapted fishes contain highly unsaturated phospholipids (12) and also possess increased ratios of phosphatidylethanolamine (PE) to phosphatidylcholine (PC) relative to their warm-acclimated counterparts (13). While these changes in lipid composition confer some degree of constancy in the physical properties of membranes, both of these compositional modifications may place membranes from cold-acclimated/adapted ectotherms at a greater risk of LPO-induced damage than membranes from warm-acclimated/adapted animals (3, 17, 46). An increased susceptibility of LPO associated with lipid remodeling in cold-acclimated/adapted fishes may be further exacerbated by a heightened potential for generating reactive oxygen species (ROS), because cold-bodied fishes often possess elevated mitochondrial densities (6, 22, 33) and increased oxidative capacities (9) compared with animals at warm body temperatures.
While phospholipid remodeling and metabolic changes may increase the overall apparent risk of LPO at cold body temperatures (3, 17, 46), it is also possible that these alterations are beneficial, ensuring a steady rate (and/or amount) of LPO products and ROS production with temperature variation (4). Our major objective was to evaluate whether the physiological changes associated with lipid remodeling are sufficient to alter rates of LPO in a manner that partially or completely offsets the effects of temperature. We utilized an ectothermic model, the striped bass (Morone saxatilis), with a focus on the response of the skeletal muscles to temperature acclimation, because these (this) tissues (organism) have (has) been previously characterized (5, 6, 36). Furthermore, because skeletal muscle has a more limited source of ROS than other tissues (e.g., liver), it is a more defined system for assessment of pro- and antioxidant components of the redox system.
Striped bass were acclimated to 7°C or 25°C, and membranes from mitochondria and sarcoplasmic reticulum (SR) were prepared from red (oxidative) and white (glycolytic) skeletal muscle. Rates of LPO were quantified in vitro using a free radical-generating system, while the extent of LPO was monitored with the fluorometric probe C11-BODIPY, in both sets of membranes at common and physiological (acclimation) temperatures. Total tissue contents of PLOOH were quantified and also analyzed as discrete phospholipid classes using high-performance thin-layer chromatography to assess the impact of acclimation temperature on endogenous LPO products. Phospholipid compositions (analyzed by phospholipid class and acyl chain composition) and contents of neutral lipids involved in antioxidant defense (vitamin E and homologs of ubiquinone) were also characterized. This study utilizes a comprehensive approach to examine how, and to what extent, phospholipid and neutral lipid remodeling with temperature acclimation affect rates of LPO and levels of LPO products. This study tests the hypothesis that membrane restructuring during temperature acclimation contributes to preservation of membrane susceptibility to LPO and levels of LPO products.
MATERIALS AND METHODS
Fish maintenance and acclimation.
Juvenile striped bass (M. saxatilis) were purchased from Delmarva Aquatics (Smyrna, DE) and housed at the Laboratory Animal Resources facility on the campus of Ohio University in two 1,200-liter recirculating brackish water tanks (∼5 ppt) equipped with biological, chemical, and UV filtration. Water quality parameters were monitored daily and maintained with weekly partial water changes. Fish were fed daily to satiation with Zeigler's Finfish Gold floating pellets throughout the periods prior to and during acclimations.
Fish were held under ambient conditions (20 ± 1°C, 12:12-h light-dark cycle) for 12 mo before they were used in the acclimation experiments. After the initial growth period, the temperature of each tank was decreased by 1°C/day to a final temperature of 17°C. This intermediate temperature set point (intermediate to final acclimation temperatures of 7°C and 25°C) was maintained for a period of 2 wk to ensure a common thermal history of all experimental animals. After 2 wk, the temperature of each tank was changed by ±1°C/day until the final acclimation temperatures (7°C or 25°C) were reached. A 6-wk acclimation period commenced once animals had been exposed to the final acclimation temperatures for 24 h and showed no obvious signs of stress. All protocols were approved by the Ohio University Institutional Animal Care and Use Committee (IACUC 13-L-011).
Tissue sampling and membrane preparation.
Fish (mean mass = 19 ± 0.7 and 28 ± 1 g for 7°C- and 25°C-acclimated animals, respectively) were stunned with a cranial blow and euthanized by cervical transection. The entire axial muscle mass was removed from each side of the animal, and red (oxidative) and white (glycolytic) muscles were separated by dissection. Between 1.5 and 5 g (wet weight) each of oxidative and glycolytic muscles were pooled from approximately four to five individuals and homogenized [10% (wt/vol)] in medium containing 140 mM KCl, 20 mM HEPES, 10 mM EDTA, 0.1 mM EGTA, 5 mM MgCl2, and 0.5% BSA (pH 7.1) with a BioSpec tissuemizer (three 5-s bursts at low speed) and then by five passes of a Potter-Elvehjem homogenizer. A 1-ml aliquot of crude homogenate was saved for enzymatic marker analyses (see below), and the remaining volume was used to prepare mitochondrial membranes and SR following modifications (29, 30, 45) described elsewhere (7). Final membrane preparations were divided into six aliquots that were snap-frozen in liquid nitrogen and stored at −70°C for further analysis. All dissection, homogenization, and centrifugation steps were performed at 4°C to minimize sample degradation.
Activity of cytochrome-c oxidase (CCO) in mitochondria (47) measured as described elsewhere (11, 39) and activity of SR Ca2+-ATPase (SERCA) (40) measured as described elsewhere (36) were used to calculate enrichment factors to ensure comparable purities of membrane preparations between acclimation groups. Mitochondria and SR were enriched by an average of 6.3 and 8.3 times, respectively, between acclimation groups. All enzymatic assays were conducted at 20°C using a Beckman 640 UV/VIS spectrophotometer equipped with a circulating water bath.
Susceptibility to LPO.
Susceptibility of biological membranes to LPO was quantified in eight individual membrane preparations per temperature treatment by following the rate of oxidation of the fluorescent probe C11-BODIPY 581/591 [4,4,-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoic acid], as described previously (7). Briefly, a C11-BODIPY solution (10 μM) was diluted to a final concentration of 148 nM with a 0.05 mg/ml membrane solution, and the probe-membrane mixture was stirred for 60 min in darkness at 4°C. Subsequently, LPO was induced in mitochondria and SR using hydroxyl radicals produced by the Fenton reaction between Cu2+ (as CuSO4) and cumene hydroperoxide in a 1:4 ratio (14) to final concentrations of 13 and 52 μM, respectively. Probe oxidation was monitored for all membranes at 7, 16, and 25°C with excitation and emission wavelengths of 568 and 590 nm, respectively, and linear portions of the decay slope were recorded as the rate of LPO (Δfluorescence intensity/Δmin). Autoxidation was detectable for both membrane types at all assay temperatures and, therefore, was subtracted from rates in the presence of the LPO induction system in all final calculations. All rates of LPO were normalized to phospholipid and protein content by measurement of hydrolyzable phosphate (37) and total protein (41).
Phospholipid compositional analysis.
Following the methods of Bligh and Dyer (1), lipids were extracted from aliquots of the same eight preparations of intracellular membranes used for quantification of LPO susceptibility. Lipid extracts were analyzed by triple-quadrupole mass spectrometry at the Kansas State University Lipidomics Research Center to quantify the relative abundance of phospholipid headgroups and molecular species of phospholipids. Membrane unsaturation index (UI) was calculated following the modification of Hulbert et al. (20), described by Grim et al. (7), to account for the potential 12 double bonds present in diacyl phospholipids, relative to 6 double bonds in individual fatty acyl chains.
Quantification of tocopherol and ubiquinones.
To prevent the oxidation of tocopherol (vitamin E) and ubiquinone (CoQ), butylated hydroxytoluene was added to each of 10 mitochondrial membrane samples from red muscle (2 μl of 1 mM butylated hydroxytoluene in ethanol/100 μl). Samples were deprotonized with 100% ethanol [1:1 (vol/vol)], and lipid-soluble compounds were extracted with n-hexane [2:1 (vol/vol)]. After centrifugation at 8,000 g for 5 min at 4°C, the organic supernatant was collected and the hexane was evaporated under nitrogen. Dried samples were immediately dissolved in 100 μl of ethanol, and 50 μl of sample were injected onto an unmodified Kromasil C18 (25 cm × 0.46 cm, 0.5-μm particle size) HPLC column. Tocopherol and ubiquinone were eluted with hexane-methanol (15:85) at a flow rate of 1 ml/min and detected at 275 nm. Quantification was performed on the basis of detector response of known concentrations and retention times [5.4 min for tocopherol, 7.8 min for an unidentified homolog of ubiquinone (CoQ?), and 16 min for CoQ9] of authentic standard compounds. Tocopherol and ubiquinone amounts were normalized to phospholipid content.
Quantification of endogenous PLOOH.
Lipids were extracted from previously collected axial red and white muscle (see above for dissection methods) using modifications of Bligh and Dyer (1). If not used immediately, samples were stored at −20°C under a nitrogen atmosphere in Teflon-capped glass vials.
Phospholipids were precipitated from total lipids (dissolved in hexane) in the presence of five volumes of ice-cold acetone under a nitrogen atmosphere at −20°C for 30 min. The decanted acetone was allowed to stand for an additional 30 min at −20°C, and the resulting precipitate was collected. Both precipitates were dried completely under nitrogen gas, resuspended in CHCl3 (for quantification of total LPO) or hexane with 3% isopropyl alcohol (for high-performance thin-layer chromatography analyses to compare PLOOH among phospholipid classes), and finally combined. Samples not immediately used were stored at −20°C under a nitrogen atmosphere in Teflon-capped glass vials. This extraction protocol resulted in a high efficacy of phospholipid recovery (95–99%) from total lipid extracts.
Partitioning of PLOOH among phospholipid classes was modified from Kriska and Girotti (25). Dried phospholipid samples were resuspended in 50–200 μl of hexane-isopropyl alcohol (3% isopropyl alcohol), and samples were spotted under high pressure onto glass silica gel chromatography plates (Si60, EMD) using a Linomat5 semiautomatic sample applicator (Camag Scientific, Wilmington, NC). Separation was carried out with a mobile-phase solvent consisting of 100:75:7:4 CHCl3-methanol-acetic acid-water, and developed plates were removed from the chamber and dried under argon gas. Dried plates were sprayed with a N,N,N′,N′-tetramethyl-p-phenylenediamine solution (250 mg N,N,N′,N′-tetramethyl-p-phenylenediamine in 12.5 ml each of methanol and H2O and 0.25 ml of acetic acid) until faint purple bands were visible. Bands continued to develop under argon gas until their maximum intensity was reached. Plates were photographed on a covered light box using a 12-megapixel camera, and band intensities were quantified using Gel Analyzer (freeware by Dr. Istvan Lazar) against a standard curve of the PLOOH 13-hydroperoxy-9,11E-octadecadienoic acid (Cayman Chemical, Ann Arbor, MI).
Total PLOOH (LPO) amounts from phospholipid isolates were measured using a commercial kit (Cayman Chemical). The manufacturer's protocol was followed with the exception of sample preparation as described above. The absorbance of assay reactions was measured at 500 nm with a Beckman DU640 spectrophotometer. All samples were compared with a PLOOH standard curve of 13-hydroperoxy-9,11E-octadecadienoic acid.
Statistical analyses.
LPO susceptibility (as slope of fluorescence decay), total partitioned levels of PLOOH, levels of tocopherol (vitamin E) and ubiquinone, and all phospholipid data were compared between temperature acclimation groups using parametric unpaired t-tests or Mann-Whitney tests when assumptions of normality and/or homogeneity of variance were violated (as determined by D'Agostino-Pearson omnibus test and Bartlett's test, respectively; GraphPad Prism version 6.0, GraphPad Software, La Jolla, CA). All statistical conclusions were based on α = 0.05. Unless otherwise noted, values are means ± SE.
RESULTS
Susceptibility to LPO.
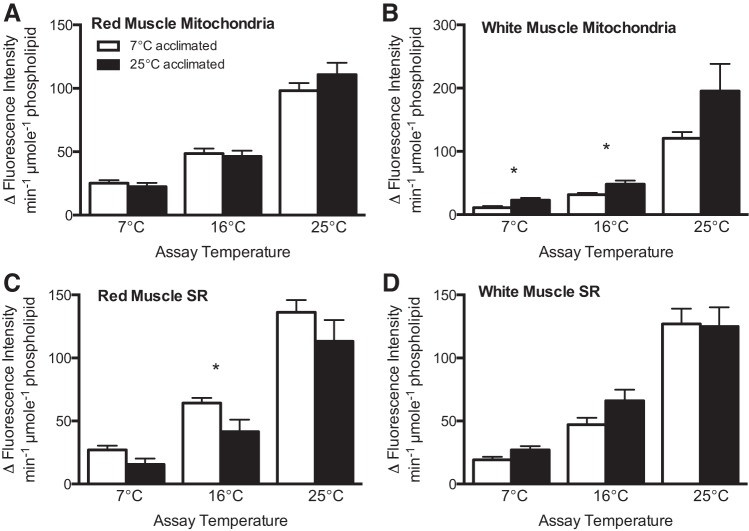
When compared at common temperatures, rates of membrane LPO normalized to phospholipid content are generally similar between acclimation groups, regardless of assay temperature or membrane type (Fig. 1). Exceptions are mitochondrial membranes prepared from white muscle, in which rates of LPO in membranes from warm-acclimated individuals are, on average, 1.8 times higher at low (7°C) and intermediate (16°C) assay temperatures than in mitochondrial membranes from cold-acclimated fish (Fig. 1B; P < 0.05, by t-test).
Fig. 1.
Rate of lipid peroxidation (LPO) normalized to phospholipid content in mitochondria (A and B) and sarcoplasmic reticulum (SR; C and D) of red (oxidative; A and C) and white (glycolytic; B and D) muscle from cold (7°C) and warm (25°C) temperature-acclimated striped bass Morone saxatilis. LPO was induced at all assay temperatures (7, 16, and 25°C), with hydroxyl radicals produced by Fenton chemistry. *Significant differences between temperature groups at a given assay temperature (P < 0.05). Data are presented as absolute value of the mean ± SE; n = 7–8.
When normalized to membrane protein, rates of LPO in membrane fractions prepared from red (oxidative) and white (glycolytic) muscle are affected by acclimation temperature (Fig. 2). Rates of LPO in membranes from red muscle of cold-acclimated fish are, on average, 1.4 and 2.1 times higher in mitochondria and SR, respectively, at all assay temperatures (7°C, 16°C, and 25°C) than in membranes from warm-acclimated fish (Figs. 2, A and C; P < 0.05, by t-test). In contrast, rates of LPO in membrane fractions prepared from white muscle in warm-acclimated animals are, on average, 1.8 and 1.9 times higher in mitochondria and SR at all assay temperatures, respectively, than in membranes prepared from cold-acclimated individuals (Fig. 2, B and D; P < 0.05, by t-test).
Fig. 2.
Rate of LPO normalized to protein content in mitochondria (A and B) and SR (C and D) of red (oxidative; A and C) and white (glycolytic; B and D) muscle from cold (7°C) and warm (25°C) temperature-acclimated striped bass M. saxatilis. LPO was induced at all assay temperatures (7, 16, and 25°C), with hydroxyl radicals produced by Fenton chemistry. *Significant differences between temperature groups at a given assay temperature (P < 0.05). Data are presented as absolute value of the mean ± SE; n = 7–8.
Quantification of endogenous PLOOH.
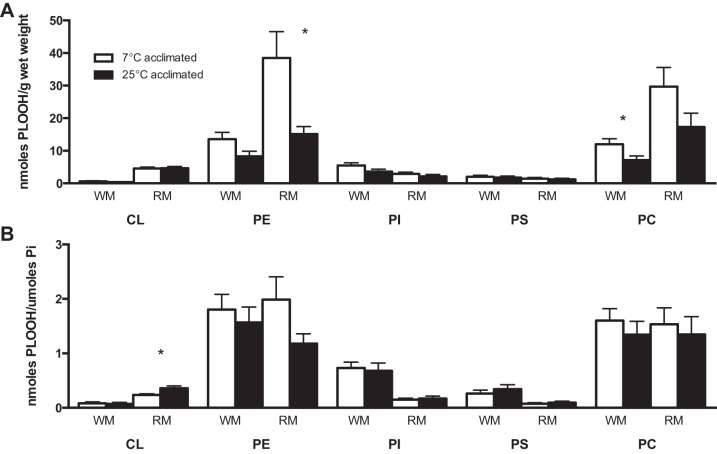
The total content of PLOOH, normalized to tissue weight, is 1.7 times higher in both red and white muscle from animals at an acclimation temperature of 7°C than 25°C (Fig. 3A; P < 0.05, by t-test). However, when the content of PLOOH is normalized to the content of phospholipid, there are no significant differences for comparisons between acclimation temperatures (7°C vs. 25°C; Fig. 3B) or muscle types (red vs. white). The higher absolute levels of PLOOH (i.e., on a per gram basis) for muscular tissues of cold-acclimated animals are driven, in large part, by the increase (2.5 times) in PLOOH containing the ethanolamine headgroup (i.e., PEOOH; Fig. 4A; P < 0.05, by Mann-Whitney test) and the rise (1.7 times) in PLOOH containing the choline headgroup (i.e., PCOOH; Fig. 4A; P < 0.05, by t-test) for red and white muscle, respectively.
Fig. 3.
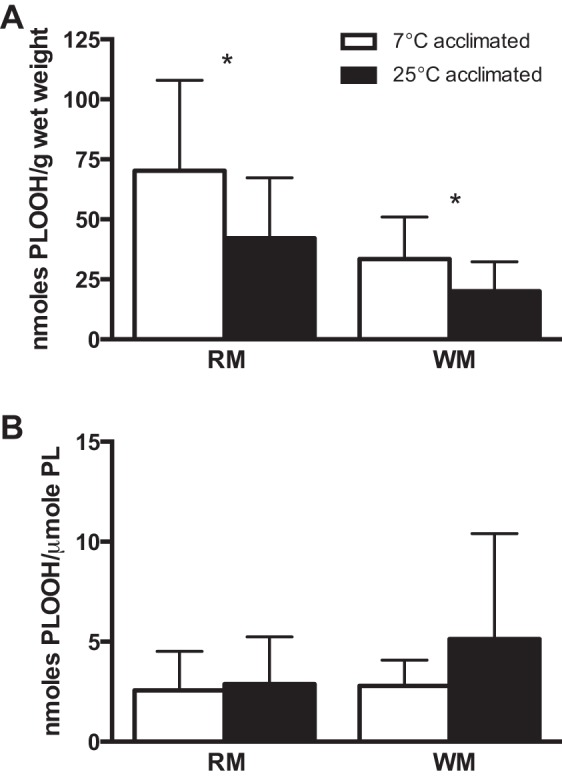
Total LPO as indicated by content of phospholipid hydroperoxides (PLOOH) in whole red (oxidative; RM) and white (glycolytic; WM) muscle from cold (7°C) and warm (25°C) temperature-acclimated striped bass M. saxatilis normalized to tissue weight (A) or phospholipid content (B). *Significant differences between temperature groups within a tissue (P < 0.05). Values are means ± SE; n = 9–12.
Fig. 4.
PLOOH of white (glycolytic - WM) and red (oxidative - RM) muscle from cold (7°C) and warm (25°C) temperature-acclimated striped bass M. saxatilis partitioned among phospholipid classes [cardiolipin (CL), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidylcholine (PC)]. Data are expressed relative to tissue weight (A) or phospholipid content (B). *Significant differences between temperature groups within a phospholipid class (P < 0.05). Values are means ± SE; n = 11–12.
PLOOH partition among five major phospholipid classes: cardiolipin (CL), PE, PC, phosphatidylinositol (PI), and phosphatidylserine (PS). Similar to the trend for total PLOOH, there are no significant differences among temperature treatments for individual PLOOH classes relative to total phospholipid content. One exception, however, is the increase (1.5 times) in oxidation of CL in red muscle of warm-acclimated fish compared with cold-acclimated fish (Fig. 4B).
The rank order of PLOOH by class is different among acclimation groups within a muscle type and also between red and white muscles. The rank order of oxidized phospholipids for white muscle from 7°C- and 25°C-acclimated animals is PE > PC > PI > PS > CL. In contrast, the rank order of PLOOH for red muscle varies between acclimation groups (PE > PC > CL > PI > PS at 7°C and PC > PE > CL > PI > PS at 25°C). These results demonstrate an increased oxidation of CL in red muscle compared with white muscle, which is likely explained by higher contents of mitochondria in oxidative (red) fibers.
Levels of vitamin E and ubiquinone.
Levels of vitamin E and ubiquinone (CoQ) in mitochondrial membranes are altered by temperature acclimation. Vitamin E, CoQ9, and an unidentified ubiquinone homolog (CoQ?) relative to phospholipid contents, are 5 to 3 times higher in mitochondrial membranes of warm-acclimated animals than their cold-acclimated counterparts (Fig. 5; P < 0.05, by t-test).
Fig. 5.
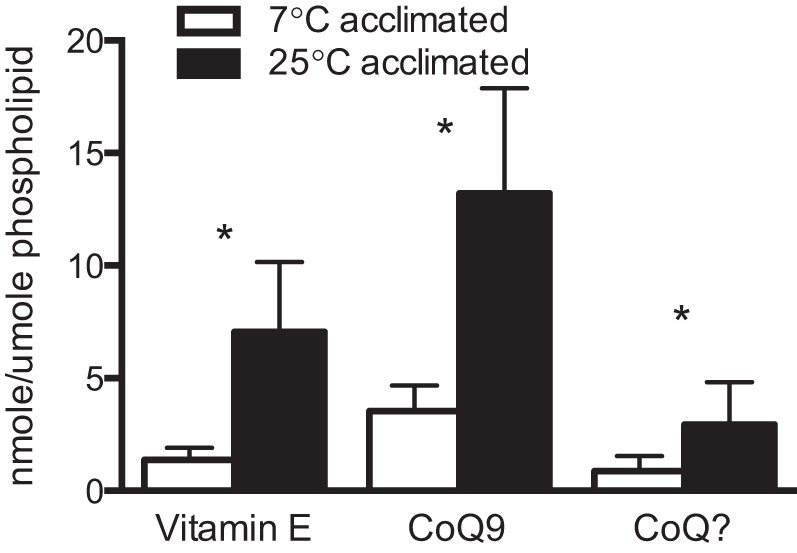
Vitamin E and ubiquinone (CoQ9 and CoQ?) levels in mitochondria from red (oxidative) muscle of cold (7°C) and warm (25°C) temperature-acclimated striped bass M. saxatilis. *Significant differences between temperature groups (P < 0.05). Values are means ± SE; n = 10.
Phospholipid composition.
Temperature acclimation induces significant remodeling of phospholipid headgroups in membranes from red and white muscle (Tables 1 and 2). Although mitochondria and SR are dominated by phospholipids with PE and PC headgroups, nine other phospholipid classes are identifiable in both membranes, and the majority of these vary in response to temperature acclimation (Tables 1 and 2). As expected, membranes from cold-acclimated fish are enriched in PE. PE-to-PC ratios are, on average, 1.8 times higher in mitochondrial membranes from cold- than warm-acclimated animals, while SR membranes from the cold-acclimated group show a more modest increase of 1.4 times, on average, in this ratio (Fig. 6, A and D; P < 0.05, by t-test).
Table 1.
Relative amounts of phospholipid classes in mitochondria and sarcoplasmic reticulum from red (oxidative) muscle of temperature-acclimated striped bass M. saxatilis
| Mitochondria |
Sarcoplasmic Reticulum |
|||
|---|---|---|---|---|
| 7°C | 25°C | 7°C | 25°C | |
| Lyso PC | 0.48 ± 0.05 | 0.31 ± 0.03* | 0.10 ± 0.00 | 0.12 ± 0.01* |
| SM/DSM | 0.92 ± 0.03 | 1.64 ± 0.06* | 1.48 ± 0.05 | 3.00 ± 0.13* |
| ePC | 3.76 ± 0.08 | 5.25 ± 0.05* | 4.50 ± 0.07 | 5.68 ± 0.18* |
| PC | 44.51 ± 0.82 | 54.12 ± 0.31* | 58.24 ± 0.65 | 59.93 ± 0.69 |
| Lyso PE | 0.63 ± 0.05 | 0.28 ± 0.01* | 0.16 ± 0.00 | 0.14 ± 0.00* |
| ePE | 3.00 ± 0.06 | 2.29 ± 0.02* | 2.36 ± 0.05 | 1.98 ± 0.05* |
| PE | 43.05 ± 0.49 | 31.98 ± 0.28* | 27.13 ± 0.65 | 21.46 ± 0.79* |
| PI | 2.84 ± 0.13 | 2.90 ± 0.07 | 4.71 ± 0.07 | 5.65 ± 0.11* |
| PS | 0.23 ± 0.05 | 0.73 ± 0.04* | 0.96 ± 0.03 | 1.53 ± 0.05* |
| PA | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.25 ± 0.02 | 0.30 ± 0.02* |
| PG | 0.31 ± 0.02 | 0.35 ± 0.01* | 0.13 ± 0.00 | 0.16 ± 0.01* |
| Total | 100 | 100 | 100 | 100 |
Values (means ± SE) are presented as mol% (n ≥ 7).
PC, phosphatidylcholine; PC, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; PA, phosphatidic acid; PG, phosphatidylglycerol; SM/DSM, sphingomyelin/dihydrosphingomyelin; ePC, ether-linked PC; ePE, ether-linked PE.
Significant difference between temperature groups within a membrane type (P < 0.01).
Table 2.
Relative amounts of phospholipid classes in mitochondria and sarcoplasmic reticulum of white (glycolytic) muscle from temperature-acclimated striped bass M. saxatilis
| Mitochondria |
Sarcoplasmic Reticulum |
|||
|---|---|---|---|---|
| 7°C | 25°C | 7°C | 25°C | |
| Lyso PC | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.08 ± 0.00 | 0.09 ± 0.00 |
| SM/DSM | 2.76 ± 0.12 | 4.46 ± 0.13* | 1.69 ± 0.10 | 3.11 ± 0.11* |
| ePC | 4.93 ± 0.14 | 6.17 ± 0.11* | 4.91 ± 0.10 | 6.07 ± 0.04* |
| PC | 51.47 ± 0.56 | 61.73 ± 0.79* | 62.31 ± 0.40 | 66.96 ± 0.38 |
| Lyso PE | 0.17 ± 0.00 | 0.08 ± 0.01* | 0.08 ± 0.00 | 0.04 ± 0.00* |
| ePE | 2.51 ± 0.06 | 1.32 ± 0.07* | 2.31 ± 0.07 | 1.14 ± 0.04* |
| PE | 32.97 ± 0.59 | 20.83 ± 0.81* | 21.27 ± 0.50 | 14.42 ± 0.09* |
| PI | 3.57 ± 0.16 | 3.87 ± 0.14 | 5.84 ± 0.21 | 5.45 ± 0.07 |
| PS | 1.11 ± 0.07 | 1.13 ± 0.10 | 1.21 ± 0.15 | 2.18 ± 0.16* |
| PA | 0.07 ± 0.01 | 0.10 ± 0.01* | 0.11 ± 0.01 | 0.15 ± 0.01* |
| PG | 0.19 ± 0.01 | 0.15 ± 0.01* | 0.02 ± 0.00 | 0.06 ± 0.01* |
| Total | 100 | 100 | 100 | 100 |
Values (means ± SE) are presented as mol% (n ≥ 7). See Table 1 footnote for abbreviations.
Significant difference between temperature groups within a membrane type (P < 0.01).
Fig. 6.
PE/PC and unsaturation indexes for mitochondria and SR of red (oxidative; A–C) and white (glycolytic; D and E) muscle from cold (7°C) and warm (25°C) temperature-acclimated striped bass M. saxatilis. *Significant differences between temperature groups within a phospholipid class (P < 0.05). Values are means ± SE; n = 7–8.
Phospholipids varying in contents of saturated and unsaturated fatty acids are also altered by temperature acclimation in mitochondria and SR (Tables 3 and 4). Higher amounts of polyunsaturated fatty acids (PUFA) following cold acclimation significantly raise the unsaturation index (UI) of some phospholipid classes, including PC (SR only) and PE (both SR and mitochondria), while the UI in PS is higher in both membranes from warm- than cold-acclimated fish (Tables 5 and 6). More specifically, mitochondrial membranes from red muscle following cold acclimation have a 1.4-times higher UI of PE phospholipids, leading to a total increase in UI of 1.2 times in mitochondria prepared from cold-acclimated animals (Fig. 6B; P < 0.05, by t-test), while SR has 1.2- and 1.3-times higher UI for PC and PE, respectively (Fig. 6C; P < 0.0001, by t-test). While the magnitude of response is less than in red muscle, total UI of mitochondria and SR membranes from white muscle of cold-acclimated animals increased by a modest 10% relative to animals at warm body temperature (Fig. 6, E and F; P < 0.05, by t-test).
Table 3.
Relative amounts of phospholipid species in mitochondria and sarcoplasmic reticulum from red (oxidative) muscle of temperature-acclimated striped bass M. saxatilis
| Mitochondria |
Sarcoplasmic Reticulum |
|||
|---|---|---|---|---|
| 7°C | 25°C | 7°C | 25°C | |
| PC 32:1 | 1.21 ± 0.07 | 1.47 ± 0.03* | 2.10 ± 0.07 | 2.30 ± 0.08 |
| PC 34:3 | 1.89 ± 0.02 | 2.31 ± 0.06* | 2.84 ± 0.11 | 2.35 ± 0.05* |
| PC 34:2 | 9.34 ± 0.23 | 12.68 ± 0.20* | 12.93 ± 0.16 | 13.96 ± 0.31* |
| PC 34:1 | 2.43 ± 0.04 | 8.53 ± 0.20* | 3.75 ± 0.14 | 9.95 ± 0.19* |
| PC 36:5 | 6.89 ± 0.23 | 5.20 ± 0.11* | 8.66 ± 0.12 | 5.42 ± 0.20* |
| PC 36:4 | 2.20 ± 0.07 | 3.05 ± 0.03* | 2.70 ± 0.04 | 2.86 ± 0.08 |
| PC 36:3 | 3.90 ± 0.12 | 5.01 ± 0.09* | 5.15 ± 0.10 | 4.90 ± 0.09* |
| PC 36:2 | 1.29 ± 0.03 | 2.37 ± 0.05* | 1.79 ± 0.04 | 2.81 ± 0.06* |
| PC 38:6 | 6.26 ± 0.14 | 4.67 ± 0.14* | 7.57 ± 0.11 | 5.09 ± 0.24* |
| PC 38:5 | 1.57 ± 0.03 | 1.59 ± 0.04 | 2.00 ± 0.03 | 1.65 ± 0.06* |
| PC 40:7 | 2.08 ± 0.06 | 1.24 ± 0.05* | 2.26 ± 0.04 | 1.22 ± 0.05* |
| SM 16:0 | 0.38 ± 0.02 | 1.00 ± 0.07* | ||
| SM 24:1 | 0.65 ± 0.03 | 1.19 ± 0.02* | 1.00 ± 0.02 | 1.91 ± 0.05* |
| ePC 38:0 | 1.02 ± 0.03 | 0.92 ± 0.02* | 1.16 ± 0.02 | 0.86 ± 0.02* |
| PE 34:2 | 2.00 ± 0.08 | 1.75 ± 0.02* | 1.29 ± 0.05 | 1.37 ± 0.05 |
| PE 36:5 | 2.52 ± 0.08 | 1.29 ± 0.01* | 1.79 ± 0.07 | 1.09 ± 0.05* |
| PE 36:4 | 1.31 ± 0.04 | 1.04 ± 0.01* | 1.04 ± 0.04 | 0.91 ± 0.03* |
| PE 36:3 | 1.49 ± 0.08 | 1.00 ± 0.02* | 1.62 ± 0.07 | 1.00 ± 0.04* |
| PE 36:2 | 0.61 ± 0.03 | 1.06 ± 0.02* | ||
| PE 38:6 | 11.74 ± 0.14 | 6.92 ± 0.06* | 6.47 ± 0.18 | 4.15 ± 0.22* |
| PE 38:5 | 3.88 ± 0.06 | 2.85 ± 0.02* | 2.24 ± 0.07 | 1.87 ± 0.06* |
| PE 38:4 | 0.89 ± 0.02 | 1.03 ± 0.01* | ||
| PE 40:8 | 1.64 ± 0.02 | 1.25 ± 0.02* | 1.12 ± 0.01 | 0.89 ± 0.02* |
| PE 40:7 | 5.16 ± 0.07 | 2.95 ± 0.04* | 3.07 ± 0.05 | 1.98 ± 0.05* |
| PE 40:6 | 3.97 ± 0.07 | 4.31 ± 0.08* | 2.21 ± 0.06 | 2.29 ± 0.06 |
| PE 40:5 | 1.02 ± 0.02 | 1.09 ± 0.01* | ||
| PE 42:7 | 1.35 ± 0.03 | 0.76 ± 0.02* | ||
| ePE 38:0 | 0.98 ± 0.03 | 0.55 ± 0.00* | ||
| PI 38:5 | 1.17 ± 0.02 | 1.38 ± 0.03* | ||
| PI 40:6 | 1.11 ± 0.02 | 1.31 ± 0.03* | ||
Values (means ± SE) are presented as mol% (n ≥ 7); data for phospholipids less abundant than 1 mol% are not shown. XX:Y = sum chain length of the 2 acyl chains:total number of double bonds present between the 2 acyl chains. See Table 1 footnote for abbreviations.
Significant difference between temperature groups within a membrane type (P < 0.01).
Table 4.
Relative amounts of phospholipid species in mitochondria and sarcoplasmic reticulum from white (glycolytic) muscle of temperature-acclimated striped bass, M. saxatilis
| Mitochondria |
Sarcoplasmic Reticulum |
|||
|---|---|---|---|---|
| 7°C | 25°C | 7°C | 25°C | |
| PC 32:1 | 1.00 ± 0.02 | 0.73 ± 0.03* | 1.20 ± 0.05 | 0.72 ± 0.02* |
| PC 34:3 | 1.50 ± 0.05 | 1.13 ± 0.02* | 2.07 ± 0.10 | 1.37 ± 0.04* |
| PC 34:2 | 9.63 ± 0.26 | 9.76 ± 0.15 | 13.12 ± 0.59 | 11.82 ± 0.25 |
| PC 34:1 | 2.52 ± 0.05 | 5.88 ± 0.17* | 3.75 ± 0.14 | 9.95 ± 0.19* |
| PC 36:6 | 1.12 ± 0.02 | 0.83 ± 0.02* | 1.34 ± 0.03 | 0.92 ± 0.02* |
| PC 36:5 | 9.71 ± 0.26 | 7.49 ± 0.16* | 11.58 ± 0.26 | 8.23 ± 0.18* |
| PC 36:4 | 2.06 ± 0.05 | 3.16 ± 0.08* | 2.44 ± 0.03 | 3.71 ± 0.03* |
| PC 36:3 | 1.81 ± 0.03 | 2.33 ± 0.06* | 2.17 ± 0.03 | 2.59 ± 0.06* |
| PC 36:2 | 0.86 ± 0.01 | 1.56 ± 0.05* | 0.87 ± 0.02 | 1.75 ± 0.06* |
| PC 36:1 | 0.39 ± 0.01 | 0.87 ± 0.04* | ||
| PC 38:6 | 9.36 ± 0.50 | 12.32 ± 0.28* | 11.17 ± 0.22 | 12.39 ± 0.45* |
| PC 38:5 | 2.22 ± 0.10 | 2.27 ± 0.06 | 2.38 ± 0.04 | 2.38 ± 0.09 |
| PC 40:8 | 1.16 ± 0.05 | 1.96 ± 0.06* | 1.23 ± 0.04 | 1.98 ± 0.07* |
| PC 40:7 | 1.60 ± 0.06 | 2.22 ± 0.04* | 1.86 ± 0.07 | 2.24 ± 0.07* |
| PC 40:6 | 0.86 ± 0.03 | 1.04 ± 0.02* | 0.91 ± 0.04 | 1.05 ± 0.03* |
| PC 44:11 | 0.53 ± 0.03 | 1.19 ± 0.06* | 0.57 ± 0.02 | 1.21 ± 0.07* |
| PC 44:12 | 0.33 ± 0.03 | 0.95 ± 0.06* | 0.40 ± 0.02 | 1.03 ± 0.08* |
| SM 24:1 | 1.81 ± 0.08 | 3.28 ± 0.13* | 1.15 ± 0.06 | 2.22 ± 0.10* |
| ePC 38:0 | 1.33 ± 0.04 | 1.19 ± 0.03* | 1.48 ± 0.05 | 1.98 ± 0.05* |
| PE 36:5 | 1.62 ± 0.05 | 0.45 ± 0.07* | 1.57 ± 0.06 | 0.29 ± 0.01* |
| PE 36:3 | 0.84 ± 0.04 | 0.30 ± 0.02* | ||
| PE 38:6 | 6.57 ± 0.20 | 2.49 ± 0.20* | 3.20 ± 0.09 | 2.00 ± 0.03* |
| PE 38:5 | 2.22 ± 0.06 | 0.96 ± 0.07* | 1.31 ± 0.03 | 0.57 ± 0.02* |
| PE 40:8 | 1.95 ± 0.05 | 1.27 ± 0.06* | 2.04 ± 0.12 | 1.38 ± 0.02* |
| PE 40:7 | 3.33 ± 0.10 | 1.82 ± 0.08* | 2.42 ± 0.10 | 1.44 ± 0.02* |
| PE 40:6 | 4.81 ± 0.11 | 5.20 ± 0.20 | 1.54 ± 0.07 | 1.89 ± 0.05* |
| PE 42:7 | 1.89 ± 0.03 | 1.01 ± 0.06* | 1.25 ± 0.03 | 0.73 ± 0.01* |
| PE 44:12 | 2.50 ± 0.04 | 1.89 ± 0.04* | 1.84 ± 0.07 | 1.79 ± 0.07 |
| ePE 38:0 | 0.94 ± 0.04 | 0.31 ± 0.01 | ||
| PI 38:5 | 0.99 ± 0.04 | 0.88 ± 0.01* | ||
| PI 38:4 | 0.65 ± 0.02 | 0.81 ± 0.03* | 1.05 ± 0.04 | 1.13 ± 0.03 |
| PI 40:6 | 1.15 ± 0.06 | 1.26 ± 0.05 | 1.97 ± 0.07 | 1.92 ± 0.06 |
Values (means ± SE) are presented as mol% (n ≥ 7); data for phospholipids less abundant than 1 mol% are not shown. See Table 1 footnote for abbreviations.
Significant difference between temperature groups within a membrane type (P < 0.01).
Table 5.
Unsaturation indexes of individual phospholipid classes in mitochondria and sarcoplasmic reticulum from red (oxidative) muscle of temperature-acclimated striped bass M. saxatilis
| Mitochondria |
Sarcoplasmic Reticulum |
|||
|---|---|---|---|---|
| 7°C | 25°C | 7°C | 25°C | |
| Lyso PC | 1.04 ± 0.12 | 0.43 ± 0.03* | 0.15 ± 0.01 | 0.14 ± 0.01 |
| SM/DSM | 0.69 ± 0.04 | 1.37 ± 0.06* | 1.03 ± 0.02 | 1.97 ± 0.06* |
| ePC | 10.03 ± 0.26 | 14.45 ± 0.15* | 11.96 ± 0.21 | 14.08 ± 0.14* |
| PC | 171.80 ± 3.81 | 168.5 ± 1.63 | 214.50 ± 2.06 | 178.34 ± 2.75* |
| Lyso PE | 2.11 ± 0.17 | 0.83 ± 0.05* | 0.37 ± 0.02 | 0.34 ± 0.01 |
| ePE | 7.46 ± 0.12 | 6.66 ± 0.07* | 4.91 ± 0.11 | 5.17 ± 0.13 |
| PE-cer | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| PE | 246.06 ± 2.48 | 176.05 ± 1.78* | 149.19 ± 3.39 | 116.73 ± 2.85* |
| PI | 14.55 ± 0.67 | 13.61 ± 0.37 | 24.42 ± 0.37 | 27.20 ± 0.52* |
| ePS | 0.03 ± 0.01 | 0.10 ± 0.01* | 0.90 ± 0.01 | 0.16 ± 0.01* |
| PS | 1.24 ± 0.24 | 3.48 ± 0.17* | 4.77 ± 0.13 | 6.40 ± 0.24* |
| PA | 0.16 ± 0.03 | 0.14 ± 0.02 | 0.94 ± 0.06 | 0.89 ± 0.05 |
| PG | 0.48 ± 0.03 | 0.68 ± 0.02* | 0.21 ± 0.01 | 0.31 ± 0.02* |
| Total | 456.39 ± 1.27 | 386.56 ± 2.45* | 414.73 ± 2.42 | 355.95 ± 2.44* |
Unsaturation indexes [means ± SE (n ≥ 7)] were calculated following the modifications of Hulbert et al. (20) by Grim et al. (17).
PE-cer, ceramide phosphorylethanolamine; see Table 1 footnote for other abbreviations.
Significant difference between temperature groups within a membrane type (P < 0.01).
Table 6.
Unsaturation indexes of individual phospholipid classes in mitochondria and sarcoplasmic reticulum from white (glycolytic) muscle of temperature-acclimated striped bass M. saxatilis
| Mitochondria |
Sarcoplasmic Reticulum |
|||
|---|---|---|---|---|
| 7°C | 25°C | 7°C | 25°C | |
| Lyso PC | 0.32 ± 0.04 | 0.43 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 |
| SM/DSM | 2.45 ± 0.11 | 4.16 ± 0.13* | 1.52 ± 0.10 | 2.83 ± 0.11* |
| ePC | 13.14 ± 0.24 | 14.61 ± 0.24* | 12.83 ± 0.28 | 14.13 ± 0.12* |
| PC | 215.23 ± 1.85 | 264.45 ± 4.72* | 256.14 ± 2.58 | 279.53 ± 6.02* |
| Lyso PE | 0.50 ± 0.02 | 0.24 ± 0.02* | 0.19 ± 0.01 | 0.10 ± 0.00* |
| ePE | 6.01 ± 0.11 | 3.59 ± 0.17* | 4.06 ± 0.11 | 2.59 ± 0.11* |
| PE | 208.68 ± 3.17 | 131.98 ± 5.14* | 135.70 ± 2.97 | 95.81 ± 0.48* |
| PI | 19.12 ± 0.90 | 20.08 ± 0.73 | 31.53 ± 1.15 | 29.23 ± 0.72 |
| ePS | 0.10 ± 0.01 | 0.10 ± 0.02 | 0.11 ± 0.01 | 0.16 ± 0.01* |
| PS | 6.00 ± 0.36 | 5.57 ± 0.45 | 6.69 ± 0.74 | 10.84 ± 0.69* |
| PA | 0.32 ± 0.02 | 0.40 ± 0.02 | 0.50 ± 0.04 | 0.64 ± 0.06 |
| PG | 0.25 ± 0.01 | 0.20 ± 0.02* | 0.03 ± 0.00 | 0.09 ± 0.01* |
| Total | 476.10 ± 1.78 | 446.28 ± 3.45* | 459.38 ± 4.65 | 436.11 ± 5.70* |
DISCUSSION
Our results demonstrate that membrane restructuring among phospholipid classes and levels of acyl chain unsaturation during thermal acclimation are not sufficient to offset the effects of temperature on rates of LPO and, as such, the susceptibility of intracellular membranes to LPO is not conserved with temperature variation. In fact, patterns of phospholipid remodeling do not predict overall susceptibility to LPO in skeletal muscle of striped bass (M. saxatilis). Even with elevated levels of unsaturated fatty acyl chains and PE in membranes from animals acclimated to cold temperature (7°C), rates of LPO normalized to phospholipid content are generally similar to (red muscle) or even lower than (white muscle) those in membranes prepared from M. saxatilis acclimated to 25°C. However, use of a different normalization criterion (i.e., membrane protein) indicates that modest changes in the relationship between rates of LPO and levels of membrane protein do occur with temperature acclimation. These different outcomes stem from the accumulation of phospholipid during cold acclimation (6) (Table 7) and indicate that the choice of normalization criteria can influence interpretations of results concerning LPO.
Table 7.
Relative amounts of protein and phospholipid, and ratio of phospholipid to protein content in mitochondria and sarcoplasmic reticulum from red (oxidative) and white (oxidative) muscle of temperature-acclimated striped bass M. saxatilis
| Mitochondria |
Sarcoplasmic Reticulum |
|||
|---|---|---|---|---|
| 7°C | 25°C | 7°C | 25°C | |
| White (glycolytic) muscle | ||||
| Protein, mg/ml | 10.1 ± 0.5 | 7.9 ± 1.0 | 18.1 ± 1.1 | 16.8 ± 1.8 |
| Phospholipid, mM | 2.5 ± 0.2 | 2.2 ± 0.3 | 5.1 ± 0.2 | 5.7 ± 0.6 |
| Phospholipid/protein, μmol/mg | 0.28 ± 0.03 | 0.31 ± 0.07 | 0.29 ± 0.02 | 0.36 ± 0.04 |
| Red (oxidative) muscle | ||||
| Protein, mg/ml | 30.9 ± 2.3 | 24.4 ± 2.3 | 22.1 ± 1.8 | 20.2 ± 1.0 |
| Phospholipid, mM | 22.0 ± 0.8* | 13.4 ± 1.6 | 15.1 ± 1.4* | 10.2 ± 0.7 |
| Phospholipid/protein, μmol/mg | 0.72 ± 0.03* | 0.55 ± 0.03 | 0.68 ± 0.03* | 0.51 ± 0.03 |
Values are means ± SE (n ≥ 7).
Significant difference between temperature groups within a membrane type (P < 0.05).
A constant proportion of PLOOH relative to total phospholipid is maintained in skeletal muscle of cold- and warm-acclimated striped bass, reflecting a requirement for LPO products in cellular processes (reviewed in Ref. 4). This is particularly noteworthy, given that rates of LPO are temperature-dependent and amounts of red muscle phospholipid vary significantly with acclimation temperature (Table 7). Capacities for antioxidant defense and thermal sensitivities of turnover rates of LPO end products are likely to be responsible for establishing a set point for PLOOH content in the face of temperature variation. Our results further demonstrate that mitochondrial membranes of warm-acclimated animals are fortified with low-molecular-weight antioxidants (vitamin E and homologs of ubiquinone), which may provide protection against increased rates of LPO at warm temperatures.
Thermal compensation of LPO rates.
It is well known (and demonstrated here) that phospholipid composition of biological membranes is modified in response to temperature variation; with this in mind, the major objective of the current study was to evaluate whether, and to what extent, these phospholipid modifications facilitate compensation in rates of LPO across a range of body temperatures. Comparison of rates of LPO in membranes prepared from cold- and warm-acclimated fish measured at physiological temperatures makes it possible to assess the degree of thermal compensation. Thermal compensation of LPO in cold-acclimated animals can be calculated as a percentage using the following equation with terms of “Rate of LPOx y,” where x = temperature acclimation group and y = assay temperature (e.g., Rate of LPOColdAcc7C represents rates of LPO in membranes prepared from cold-acclimated animals measured at 7°C).
When rates of LPO are considered in the context of physiological adjustments to temperature described by Precht (35), there is an absence of thermal compensation on the basis of phospholipid content in membranes from SR and mitochondria prepared from red muscle, yet a partial compensation of LPO when rates are normalized to protein (Table 8). In contrast, an inverse thermal compensation is apparent in membranes prepared from white muscle for both normalization criteria (Table 8). Inverse compensation is indicated by our observation that LPO rates are generally higher in membranes prepared from warm- than cold-acclimated fish when compared at a common temperature. Collectively, these data indicate that the modest compensation of LPO rates relative to membrane protein in red (axial) muscle may be physiologically relevant in the primary muscle used for sustained locomotion in these animals.
Table 8.
Percent compensation in rates of LPO at cold physiological temperatures following temperature acclimation
| %Compensation in Rates of LPO |
||
|---|---|---|
| Rate of LPO/mg protein | Rate of LPO/mmol phospholipid | |
| Red (oxidative) muscle | ||
| Mitochondria | 12.1 | 3.1 |
| Sarcoplasmic reticulum | 21.8 | 11.9 |
| White (glycolytic) muscle | ||
| Mitochondria | −10.4 | −6.8 |
| Sarcoplasmic reticulum | −17.8 | −8.2 |
Values represent compensation in lipid peroxidation (LPO) rates in cold-bodied animals following cold acclimation. See discussion for calculations.
When rates of LPO are compared in membranes at their respective acclimation temperatures (7°C and 25°C), rates of LPO (normalized to either membrane protein or phospholipid) are significantly higher in membranes of warm-acclimated individuals. These results imply a greater requirement for antioxidant defenses at warm temperatures to defend against an imbalance in LPO end products. Our previous work indicates that the glutathione system of antioxidant defense lacks thermal compensation and, as a result, capacities for antioxidant defense are significantly higher in muscle tissues from warm- than cold-acclimated animals when compared at their respective physiological temperatures (8). We have also reported a similar result for central antioxidant enzymes (superoxide dismutase and catalase) (7).
One might predict elevated levels of vitamin E in membranes from cold-acclimated animals with increased unsaturation, because this neutral lipid has been shown to associate preferentially with phospholipids containing the PUFA docosahexaenoic acid (22:6) (43). Yet mitochondria from red muscle of warm-acclimated animals, with lower levels of PUFA, are enriched in vitamin E and homologs of ubiquinone relative to mitochondria from cold-acclimated animals (Fig. 5). This result is consistent with our previous work, which demonstrated higher levels of the water-soluble antioxidant ascorbate (vitamin C) in skeletal muscle of warm- than cold-acclimated striped bass (8). While in both cases elevated levels of ascorbate and vitamin E are likely to reflect increased feeding rates of warm-acclimated animals, fortification of low-molecular-weight antioxidants in membranes at warm temperatures may ensure controlled rates of LPO in warm-bodied animals, which might otherwise experience relatively high rates of LPO. It is also possible that reduced levels of low-molecular-weight antioxidants at cold body temperatures may promote the maintenance of PLOOH levels, even with reduced rates of LPO at low temperatures.
Membranes from red and white muscles differ in response to temperature acclimation.
While we originally predicted that phospholipid (15) and metabolic (9) remodeling would be responsible for the maintenance of LPO rates following temperature acclimation, we also expected that the magnitude of these responses would be greater in red than white muscle, because red muscle contains larger quantities of intracellular lipid (6) and is used for sustained locomotion. This latter prediction appears to be the case. At a common assay temperature, rates of LPO in mitochondria and SR prepared from red muscle of cold-acclimated animals are generally similar to (normalized to phospholipid) or higher than (normalized to protein) those in membranes prepared from warm-acclimated animals. In contrast, a different pattern was observed in membranes prepared from white muscle, in which rates of LPO are generally higher in warm- than cold-acclimated fish (i.e., inverse compensation of rates of LPO).
While phospholipid quantity varies between tissue types, several aspects of phospholipid remodeling are also distinct among red and white muscle and may, therefore, contribute to the LPO trends observed. The degree of membrane remodeling associated with temperature acclimation among phospholipid classes is greater for red than white muscle. Our results demonstrate that a greater number of phospholipid classes are affected by acclimation temperature in red than white muscle. In addition to changes in the major phospholipid classes (PC and PE), there are also changes in red muscle of some of the less abundant phospholipid classes that were not observed in white muscle (e.g., PI in SR and PS in mitochondria). The magnitude of the change in unsaturation is also greatest in red muscle membranes, although the dominant phospholipid class (PC) has a greater degree of unsaturation at warm than cold acclimation temperatures in white muscle. Not surprisingly, total UIs are highest in both membrane types from cold-acclimated animals (Fig. 6), yet the average difference between UIs in membranes from cold- and warm-acclimated animals is more than 10% greater in red than white muscle.
While we cannot explain with certainty our observations that a modest, positive thermal compensation of LPO, relative to membrane protein, exists in red, but not white muscle, the greater abundance of phospholipid in red muscle from cold- than warm-acclimated fish is likely to be an important factor (Table 7). Because of the contribution of red muscle to sustained subcarangiform locomotion, a functional consequence of these results is also plausible. Elevated SERCA activity has been linked with modest levels of LPO, and this enzyme activation can be mimicked by the addition of exogenous PE hydroperoxides (23). While acclimation of striped bass to 5°C and 25°C did not change SERCA activity of white muscle crude homogenates (36), we found that activities of SERCA are 1.3 times higher in crude homogenates of red muscle from cold-acclimated striped bass than warm-bodied counterparts measured at a common assay temperature (P < 0.05 by t-test; data not shown). We also observed that while total levels of PLOOH are unchanged by temperature acclimation (Fig. 3B), a trend for elevated levels of PE PLOOH is found in cold-bodied animals relative to warm-acclimated animals (Fig. 4B). We propose that even a small degree of compensation in rates of LPO relative to membrane protein and the defense of a set point for PLOOH content (driven by small targeted changes in the amount and unsaturation of PE phospholipids) may stimulate SERCA activity via lipid hydroperoxides in red muscle. Targeted enhancement of SERCA activity could provide a means to maintain locomotory activity at reduced body temperatures.
Perspectives and significance.
The ways in which modifications in quality and quantity of biological membranes influence LPO have received relatively little attention in ectothermic model systems. Previous work has characterized the impacts of phospholipid composition on physical properties of membranes (14, 15, 18, 27) and the function of membrane-associated proteins (28, 34, 42, 48). Increasing the amount of intracellular membranes (e.g., mitochondrial densities) affects oxygen flux and delivery by shortening diffusion distances and also by enhancing oxygen solubility within the lipid “highways” of intracellular membranes (5, 38). Because LPO and its products are necessary for a variety of cellular events (2, 24, 26, 31), this has led others (and ourselves) to hypothesize that cells (ectotherms) “defend” a steady level of LPO and/or levels of lipid hydroperoxides (i.e., “peroxide tone”) (2, 4, 10). We originally proposed that biological membranes from ectotherms would exhibit some degree of thermal compensation in their propensity to undergo LPO, since modulation of oxidative capacities and phospholipid restructuring are likely to affect significantly the rates of LPO in a manner that should counter the direct effects of temperature. In fact, peroxidation indexes (a measure of likelihood of LPO) of mammal and insect tissues are relatively constant, even when the peroxidation index of the diet varies (19). Our data demonstrate that while thermal compensation of membrane susceptibility to LPO, on strictly a phospholipid basis, is absent among intracellular membranes from temperature-acclimated striped bass, the relative quantity of LPO products is preserved. Constancy in peroxide tone is likely to accompany a compensatory response in the physical properties of membranes (e.g., fluidity and dynamic phase behavior) and a reorganization of mitochondrial density to maintain oxygen delivery. We suggest that preservation of levels of LPO, and not rates of LPO per se, may be most critical in the face of temperature variation. Our data are also consistent with the hypothesis that lipid availability (i.e., quantity), rather than lipid quality, is the major factor ensuring LPO-dependent cellular processes in ectothermic animals.
GRANTS
This work was supported, in large part, by National Science Foundation Award IOS 0842624. Instrument acquisition and lipidomics method development were supported by National Science Foundation Grants EPS 0236913, MCB 0920663, DBI 0521587, and DBI1228622, Kansas Technology Enterprise Corporation, National Institutes of Health K-IDeA Networks of Biomedical Research Excellence Grant P20 GM-103418, and Kansas State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.G. and E.L.C. developed the concept and designed the research; J.M.G., M.C.S., D.E.K., T.K., and A.K. performed the experiments; J.M.G., M.C.S., D.E.K., T.K., and A.K. analyzed the data; J.M.G., M.C.S., D.E.K., and E.L.C. interpreted the results of the experiments; J.M.G. prepared the figures; J.M.G. and E.L.C. drafted the manuscript; J.M.G., M.C.S., D.E.K., T.K., A.K., and E.L.C. edited and revised the manuscript; J.M.G., M.C.S., D.E.K., T.K., A.K., and E.L.C. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Tammy Mace (Ohio University Laboratory Animal Resources) for careful work and invaluable efforts with fish maintenance. We appreciate the assistance of Elizabeth Simonik during fish dissection and membrane preparation. Lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory.
REFERENCES
- 1.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 2.Brigelius-Flohe R. Tissue-specific functions of individual gluathione peroxidases. Free Radic Biol Med 27: 951–965, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove JP, Church DF, Pryor WA. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids 22: 299–304, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Crockett EL. The cold but not hard fats in ectotherms: consequences of lipid restructuring on susceptibility of biological membranes to peroxidation, a review. J Comp Physiol B 178: 795–809, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Desaulniers N, Moerland TS, Sidell BD. High lipid content enhances the rate of oxygen diffusion through fish skeletal muscle. Am J Physiol Regul Integr Comp Physiol 271: R42–R47, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Egginton S, Sidell BD. Thermal acclimation induces adaptive changes in subcellular structure of fish skeletal muscle. Am J Physiol Regul Integr Comp Physiol 256: R1–R9, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Grim JM, Miles DR, Crockett EL. Temperature acclimation alters oxidative capacities and composition of membrane lipids without influencing activities of enzymatic antioxidants or susceptibility to lipid peroxidation in fish muscle. J Exp Biol 213: 445–452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grim JM, Simonik EA, Semones MC, Kuhn DE, Crockett EL. The glutathione-dependent system of antioxidant defense is not modulated by temperature acclimation in muscle tissues from striped bass, Morone saxatilis. Comp Biochem Physiol A Mol Integr Physiol 164: 383–390, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Guderley H. Metabolic responses to low temperature in fish muscle. Biol Rev Camb Philos Soc 79: 409–427, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. Oxford, UK: Oxford University Press, 2007. [Google Scholar]
- 11.Hansen CA, Sidell BD. Atlantic hagfish cardiac muscle: metabolic basis of tolerance to anoxia. Am J Physiol Regul Integr Comp Physiol 244: R356–R362, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Hazel JR. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu Rev Physiol 57: 19–42, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Hazel JR, Landrey SR. Time course of thermal adaptation in plasma membranes of trout kidney. 1. Headgroup composition. Am J Physiol Regul Integr Comp Physiol 255: R622–R627, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Hazel JR, McKinley SJ, Gerrits MF. Thermal acclimation of phase behavior in plasma membrane lipids of rainbow trout hepatocytes. Am J Physiol Regul Integr Comp Physiol 275: R861–R869, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Hazel JR, Williams EE. The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29: 167–227, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Hochachka PW, Somero GN. Biochemical Adaption. Oxford, UK: Oxford University Press, 2002. [Google Scholar]
- 17.Holman RT. Autoxidation of fats and related substances. Prog Chem Fats Other Lipids 2: 51–98, 1954. [Google Scholar]
- 18.Hulbert AJ, Else PL. Membranes and the setting of energy demand. J Exp Biol 208: 1593–1599, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hulbert AJ, Kelly M, Abbott S. Polyunsaturated fats, membrane lipids and animal longevity. J Comp Physiol B 184: 149–166, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87: 1175–1213, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34: 145–169, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Johnston I, Maitland B. Temperature acclimation in crucian carp, Carassius carassius L., morphometric analyses of muscle fibre ultrastructure. J Fish Biol 17: 113–125, 1980. [Google Scholar]
- 23.Kagan V. Lipid Peroxidation in Biomembranes. Boca Raton: CRC, 1988, p. 11. [Google Scholar]
- 24.Kagan VE, Bayir HA, Belikova NA, Kapralov O, Tyurina YY, Tyurin VA, Jiang J, Stoyanovsky DA, Wipf P, Kochanek PM, Greenberger JS, Pitt B, Shvedova AA, Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med 46: 1439–1453, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriska T, Girotti AW. Separation and quantitation of peroxidized phospholipids using high-performance thin-layer chromatography with tetramethyl-p-phenylenediamine detection. Anal Biochem 327: 97–106, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn H, Borchert A. Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic Biol Med 33: 154–172, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta 1666: 62–87, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta 1612: 1–40, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Moyes CD, Buck LT, Hochachka PW, Suarez RK. Oxidative properties of carp red and white muscle. J Exp Biol 143: 321–331, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Moyes CD, Mathieu-Costello OA, Brill RW, Hochachka PW. Mitochondrial metabolism of cardiac and skeletal muscles from a fast (Katsuwonus pelamis) and a slow (Cyprinus carpio) fish. Can J Zool 70: 1246–1253, 1992. [Google Scholar]
- 31.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 47: 469–484, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun 338: 668–676, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Orczewska JI, Hartleben G, O'Brien KM. The molecular basis of aerobic metabolic remodeling differs between oxidative muscle and liver of threespine sticklebacks in response to cold acclimation. Am J Physiol Regul Integr Comp Physiol 299: R352–R364, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Phillips R, Ursell T, Wiggins P, Sens P. Emerging roles for lipids in shaping membrane-protein function. Nature 459: 379–385, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Precht H. Concepts of the temperature adaptation of unchanging reaction systems of cold-blooded animals. In: Physioloigical Adaptation, edited by Prosser CL. Washington, DC: Am. Physiol. Soc., 1958, p. 50–78. [Google Scholar]
- 36.Riemenschneider WK, Sidell BD. Cold acclimation induces proliferation of sarcoplasmic reticulum without increase in Ca2+-ATPase activity in white axial muscle of striped bass (Morone saxatilis). J Exp Zool 292: 231–240, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5: 494–496, 1970. [DOI] [PubMed] [Google Scholar]
- 38.Sidell BD. Intracellular oxygen diffusion: the roles of myoglobin and lipid at cold body temperature. J Exp Biol 201: 1119–1128, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Sidell BD, Driedzic WR, Stowe DB, Johnston IA. Biochemical correlations of power development and metabolic fuel preferenda in fish hearts. Physiol Zool 60: 221–232, 1987. [Google Scholar]
- 40.Simonides WS, van Hardeveld C. An assay for sarcoplasmic reticulum Ca2+-ATPase activity in muscle homogenates. Anal Biochem 191: 321–331, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85, 1985. [DOI] [PubMed] [Google Scholar]
- 42.Starke-Peterkovic T, Turner N, Else PL, Clarke RJ. Electric field strength of membrane lipids from vertebrate species: membrane lipid composition and Na+-K+-ATPase molecular activity. Am J Physiol Regul Integr Comp Physiol 288: R663–R670, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Stillwell W, Dallman T, Dumaual AC, Crump FT, Jenski LJ. Cholesterol versus α-tocopherol: effects on properties of bilayers made from heteroacid phosphatidylcholines. Biochemistry 35: 13353–13362, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med 43: 4–15, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vornanen M, Tiitu V, Kakela R, Aho E. Effects of thermal acclimation on the relaxation system of crucian carp white myotomal muscle. J Exp Zool 284: 241–251, 1999. [PubMed] [Google Scholar]
- 46.Wang JY, Wang ZY, Kouyama T, Shibata T, Ueki T. Significance of amino acid groups of phosphatidylethanolamine in phospholipid peroxidation of mixed liposomes. Chem Phys Lipids 71: 197–203, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Wharton DC, Tzagoloff A. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol 10: 245–260, 1967. [Google Scholar]
- 48.Wu BJ, Else PL, Storlien LH, Hulbert AJ. Molecular activity of Na+/K+-ATPase from different sources is related to the packing of membrane lipids. J Exp Biol 204: 4271–4280, 2001. [DOI] [PubMed] [Google Scholar]