Abstract
The type 1 angiotensin II (ANG II) receptor (AT1R) undergoes internalization following stimulation by ANG II. Internalization reduces cell surface AT1Rs, and it is required for AT1R resensitization. In this process AT1R may interact with caveolin-1 (Cav1), the main scaffolding protein of caveolae. We hypothesized that the interaction between Cav1 and AT1R delays AT1R resensitization and thereby prevents sustained ANG II-induced resistance artery (RA) constriction under normal conditions and in experimental obesity. In rat and mouse skeletal muscle RA (diameter: ∼90–120 μm) ANG II-induced constrictions were reduced upon repeated (30-min apart) administrations. Upon disruption of caveolae with methyl-β-cyclodextrin or in RA of Cav1 knockout mice, repeated ANG II applications resulted in essentially maintained constrictions. In vascular smooth muscle cells, AT1R interacted with Cav1, and the degree of cell surface interactions was reduced by long-term (15-min), but not short-term (2-min), exposure to ANG II. When Cav1 was silenced, the amount of membrane-associated AT1R was significantly reduced by a short-term ANG II exposure. Moreover, Cav1 knockout mice fed a high-fat diet exhibited augmented and sustained RA constriction to ANG II and had elevated systemic blood pressure, when compared with normal or high-fat fed wild-type mice. Thus, Cav1, through a direct interaction, delays internalization and subsequent resensitization of AT1R. We suggest that this mechanism prevents sustained ANG II-induced RA constriction and elevated systemic blood pressure in diet-induced obesity.
Keywords: obesity, arteriole, angiotensin receptor, trafficking, caveolae, hypertension
obesity is accompanied by pathologic activation of both systemic and vascular renin-angiotensin-aldosterone system (RAAS), which eventually leads to the development of elevated systemic blood pressure (17). A sustained activation of type 1 angiotensin II (ANG II) receptor (AT1R) by ANG II, one of the main effectors of RAAS, has been proposed to contribute to increased peripheral vascular resistance in obesity-associated hypertension (33). It is known that unlike type 2 ANG II receptor, AT1R exhibits downregulation by its own ligands (19). In the short term, AT1R undergoes rapid desensitization and consequent internalization upon stimulation by ANG II, thereby decreasing the number of AT1R on the cell surface and the role of this pathway in further signaling (18, 19). This negative feedback regulation of AT1R plays an important role in preventing sustained receptor stimulation by ANG II. As a functional consequence in the vasculature, sequential administration of ANG II elicits diminished vasoconstriction, a well-known phenomenon, called ANG II tachyphylaxis (22, 25, 35, 39). The problem is that under pathological conditions this normal, negative feedback regulation of AT1R signaling can be compromised (4, 6). The underlying mechanism(s) through which AT1R-mediated microvascular signaling becomes augmented and sustained remains poorly understood.
Upon stimulation AT1R is internalized primarily by clathrin-coated vesicles, in a dynamin and β-arrestin dependent manner, although other mechanisms, such as internalization by noncoated vesicles, have been demonstrated (19). It has been long recognized that internalization directs AT1R into the endosome, where it is dephosphorylated by protein phosphatases, resensitized, and recycled to the plasma membrane (2, 24). Interestingly, earlier studies showed that the recycling of AT1R to the cell surface is quite rapid (37). Given that, it is possible that a rapid internalization of AT1R enables a quick recycling, whereas mechanisms that prevent AT1R internalization delay the reactivation of AT1R. In support of this scenario, it has been shown that caveolin-1 (Cav1), the main scaffolding protein of caveolae, interacts with AT1R in vascular smooth muscle cells and that upon ANG II stimulation AT1R moves to caveolae-enriched membrane microdomains (20). There is some evidence that a direct interaction of between AT1R and Cav1 also contributes to the exocytic trafficking of AT1R (43). The role of AT1R-Cav1 interaction in affecting ANG II-induced constriction of resistance arteries and systemic blood pressure is not well understood.
Previously, we demonstrated that Cav1 plays an important role in the maintenance of resistance artery dilator function in a rodent model of high-fat diet (HFD)-induced obesity (14). We also found that in patients with diabetes the normal function of Cav1 is compromised, which leads to an impaired, flow-mediated dilation in coronary resistance arteries (8). In this study we raised the novel hypothesis that the interaction between AT1R and Cav1 is mainly to delay AT1R reactivation and hence prevents sustained ANG II-induced constriction in resistance arteries. This normal, physiological regulatory mechanism can be compromised in obesity and may contribute to the development of obesity-associated hypertension. To test these hypotheses we assessed AT1R-mediated constriction in intact rodent skeletal muscle resistance arteries, in which ANG II was administered in a repeated fashion, before and after interfering with Cav1 and also after HFD-induced metabolic challenge.
METHODS
Pressure myography of isolated skeletal muscle resistance artery.
All protocols were approved by the Institutional Animal Care and Use Committee at Georgia Regents University. Male Wistar rats (weighing about 300 g, n = 12, purchased from Charles River) and male Cav1 knockout mice (weighing about 25 g, n = 20; and respective wild-types, n = 20; purchased from Jackson Laboratories) were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg). Under anesthesia, the gracilis muscle was excised and placed in ice-cold, oxygenated Krebs solution. Euthanasia was then performed by additional intraperitoneal injection of pentobarbital sodium (150 mg/kg). With the use of microsurgical instruments and an operating microscope, a resistance artery (90 and 150 μm in internal diameter, in mice and rats, respectively) running intramuscularly was isolated and cannulated in the pressure myograph chamber. The cannulated artery was connected with silicone tubing to a pressure servo control system (Living Systems Instrumentation) to adjust the intraluminal pressure to 80 mmHg. Vessels were observed with videomicroscopy, and the diameter was measured with a microangiometer (7, 13).
Protocols for assessment of AT1R-mediated, ANG II-induced resistance artery constriction.
In the first series of experiments cumulative concentrations of angiotensin II (ANG II, 10 pM-10 nM; Sigma) were administered to the rat gracilis muscle artery and change in diameter was measured. After washout and 30 min of time period ANG II administration was repeated. Repeated norepinephrine (NE)-induced (1 nM - 0.1 μM; Sigma) vasomotor responses were also assessed in these arteries.
In other set of experiments, arteries were exposed to methyl β-cyclodextrin (mβCD, 3 mM for 90 min), an agent known to disrupt caveolae (1, 15), and repeated ANG II- and NE-induced vasomotor responses were assessed. In separate experiments gracilis muscle arteries were isolated from wild-type and Cav1 knockout mice and ANG II- and NE-induced arteriolar responses were measured in similar protocols.
In situ proximity ligation assay.
Rat vascular smooth muscle cells (VSMC; SV40LT-transfected; American Cell Type Culture Collection) were grown on Ibidi Slide IV0.4 to reach 20–30% confluence, similar as previously described (6). VSMCs were exposed to ANG II (0.1 μM) for 2 or 15 min, washed, and fixed with 4% paraformaldehyde. To detect AT1R-Cav1 interaction proximity ligation assay (PLA; Duolink in situ from Olink Bioscience) was performed according to the manufacturer's instructions. Briefly, fixed, permeabilized VSMCs were incubated with primary antibodies (anti-Cav-1, 1:100, Abcam ab17052, and anti-AT1R, 1:100, Enzo, BML-SA608) 1 h at room temperature, followed by the administration of oligonucleotide-labeled secondary antibodies (Orange PLA probes). For negative controls either the anti-AT1R or anti-Cav1 primary antibody was omitted while both secondary antibodies were present. DAPI was used for staining the nuclei. Fluorescence images (excitation: BP545/25; emission: BP605/70) were captured with a microscope (Zeiss AxioimagerM2, 63× oil objective, numerical aperture: 1.4). The positive PLA signals were automatically counted (ImageJ) and presented as normalized to the number of nuclei, similar as previously described (8). PLA interactions observed in the close proximity (less than 500 nm) of the cell boundaries were also counted and were considered as cell surface localized interactions.
Cell fractionation of VSMC.
The amount of membrane-associated and cytosolic AT1R was determined in cultured VSMCs before and after administration to ANG II (0.1 μM, for 2 or 15 min) by using a cell fractionation kit and following the manufacturer's instructions (Cell Signaling). Briefly, vehicle or ANG II-exposed VSMCs were rinsed with ice-cold PBS, harvested, homogenized in CIB buffer, and centrifuged. Supernatant was collected as cytosolic fraction, and pellet contained the membrane fraction. Protein concentration was measured by BCA protein assay, and equal amounts of total lysate, cytosolic, and membrane fractions were loaded for gel electrophoresis and Western blotting. After blocking, the membranes were incubated with anti-AT1R antibody (dilution 1:1,000, ab124505; Abcam) and were also reprobed with anti-Cav1 antibody (dilution 1:1,000, 3267; Cell Signaling) and for loading control, β-actin (dilution 1:2,000, 4970; Cell Signaling). In separate experiments, HEK293 cells were used with or without overexpression of AT1R to demonstrate the level of specificity of the used antibody (ab124505; Abcam). Cells were grown to reach ∼70% confluence and were transfected according to the manufacturer's protocol with AT1R-DDK (OriGene) plasmid using X-tremeGENE HP transfection reagent (Roche). At 48 h post-transfection, cells were harvested and probed for AT1R protein expression and for tubulin for loading control. Chemiluminescence was visualized with ChemiDoc MP system (Bio-Rad) by using the corresponding horseradish peroxidase-labeled secondary antibodies.
Depletion of Cav1 in VSMCs.
VSMCs were treated with a pool of two target-specific 20–25 nt short-interfering RNAs (siRNAs) designed to knock down Cav1 gene expression (Santa Cruz Biotechnology). Nonspecific siRNAs were used as a control. VSMCs were transfected at 70–80% confluence with 75 nM final concentration of siRNA using siPORT Amine transfection reagent (Ambion; Life Technologies) and used for protein detection experiments at 48–72 h posttransfection.
High-fat feeding of wild-type and Cav1 knockout mice.
In a separate set of experiments 8-wk-old male wild-type (n = 10) and Cav1 knockout mice (n = 10) were fed an HFD (60% of saturated fat, 58Y1, TestDiet; PMI Nutrition) for 12 wk. Systolic and diastolic blood pressures were measured by the tail-cuff method in awake mice. Isolated and pressurized skeletal muscle resistance artery responses to ANG II and NE were obtained in similar experimental conditions and protocols as described above. Protein expression of Cav1 was measured in skeletal muscle arteries of wild-type and HFD mice.
Data analysis.
Agonist-induced resistance artery constrictions were expressed as changes in diameter as a percentage of the initial diameter. Statistical analysis was performed by repeated-measures ANOVA (agonist induced responses) or one-way (comparing 2 or more experimental groups) followed by Tukey's post hoc test. P < 0.05 was considered statistically significant. Data are expressed as means ± SE.
RESULTS
Cav1 prevents sustained ANG II-induced constriction in the rodent resistance artery.
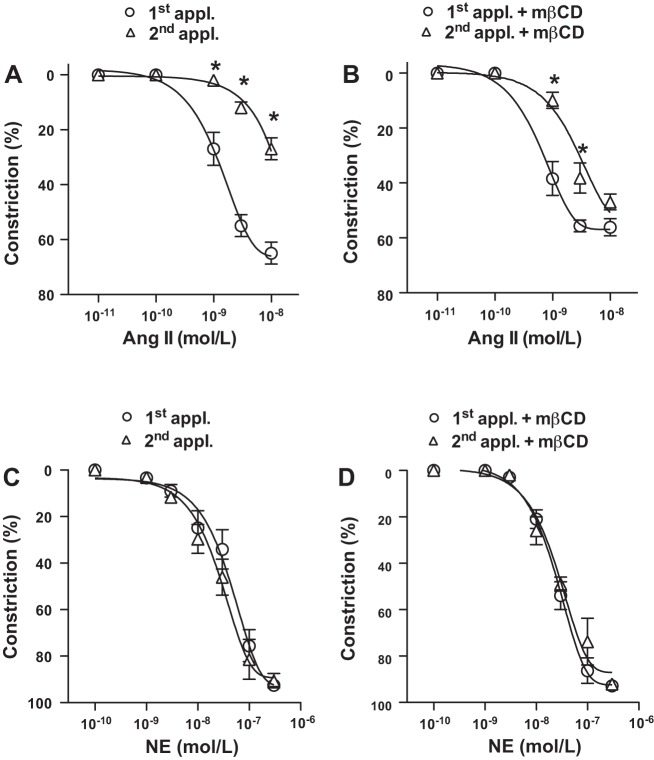
It has been long recognized that sequential activation of AT1R by ANG II results in a subsequently diminished vasoconstriction, also known as ANG II tachyphylaxis (22, 25, 35, 39). In the present study we first confirmed this phenomenon by showing that in the skeletal muscle resistance arteries (∼150 μm in internal diameter) isolated from the normal Wistar rat repeated administration of ANG II (30 min apart) resulted in reduced constrictions (Fig. 1A), whereas constrictions to α1-adrenergic agonist norepinephrine (NE) were essentially maintained upon the repeated administrations (Fig. 1C).
Fig. 1.
Disruption of caveolae by methyl-β-cyclodextrin leads to a sustained vasoconstriction to ANG II. Summary data show rat skeletal muscle resistance artery constrictions to repeated [1st and 2nd applications (appl.)] ANG II (10 pM–10 nM, n = 7; A) and to norepinephrine (NE; 0.1–300 nM, n = 5; C) in the presence of methyl-β-cyclodextrin (mβCD; n = 5–7; B and D). Data are means ± SE. Asterisk indicates significant differences (P < 0.05).
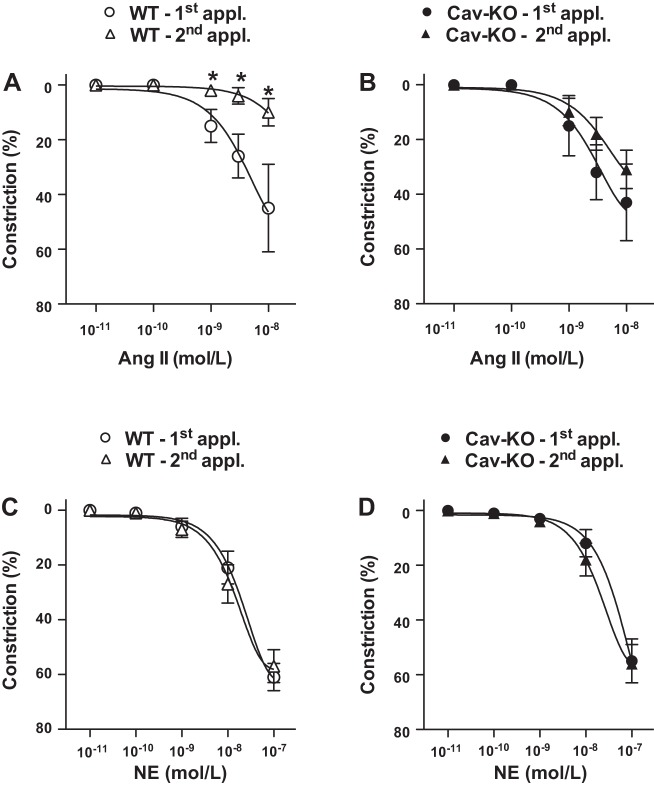
To examine the role of membrane caveolae and Cav1 in AT1R-mediated vasomotor responses, ANG II-induced constrictions were assessed in the presence of methyl-β-cyclodextrin (mβCD) and also in resistance arteries (∼90 μm) of Cav1 knockout mice (CavKO), similar to our earlier studies (8, 14). In the presence of mβCD, ANG II elicited constriction upon the first application with a similar magnitude as seen without mβCD treatment (Fig. 1B). We found that the magnitude of constrictor response, however, remained substantial to the repeated ANG II administration in the presence of mβCD (Fig. 1B). A similar observation was made when resistance artery responses were assessed in CavKO mice, in which ANG II-induced constrictions were essentially maintained upon repeated ANG II administrations (Fig. 2B), compared with responses of wild-type controls mice exhibiting the normal ANG II tachyphylaxis (Fig. 2A). No significant effects of mβCD treatment or the lack of Cav1 were found on arterial constrictions to repeated NE administrations, which remained the same under these conditions (Fig. 1D and Fig. 2D). These findings indicated a significant regulatory role for Cav1 in preventing a sustained AT1R activation and ANG II-induced constriction in the rodent resistance artery.
Fig. 2.
Cav1 knockout mice exhibit a sustained vasoconstriction to ANG II. Summary data of wild-type mice (WT; A and C) and Cav1 knockout mice (KO; B and D) skeletal muscle resistance artery constrictions to repeated [1st and 2nd applications (appl.)] ANG II (10 pM–10 nM, n = 10) and to NE (10 pM–100 nM, n = 10) are shown. Data are means ± SE. Asterisks indicate significant differences (P < 0.05).
Direct interaction between Cav1 and AT1R in VSMCs.
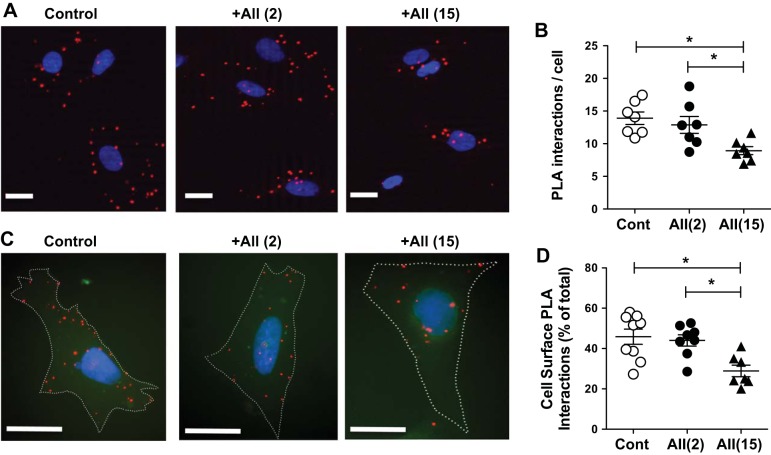
According to our hypothesis upon ANG II stimulation a direct interaction between Cav1 and AT1R prevents the internalization of AT1R and therefore reduces the number of active receptors availability for the second, repeated agonist stimulation. To test this hypothesis and examine the role of the AT1R-Cav1 interaction in regulating the initial phase of AT1R trafficking, we used cultured VSMCs. To follow and dynamically assess the interaction between AT1R and Cav1, a highly sensitive PLA was used (8). In brief, in this assay PLA probes create a positive and amplified signal (shown in red dots in Fig. 3, A and C) only when the epitopes of the targeted proteins are in close proximity (<40 nm). Using this technique we quantified the level of AT1R-Cav1 interaction under basal condition and also after stimulating VSMCs with ANG II for a short (2 min) and a longer (15 min) periods of time. We found that AT1R interacted with Cav1 even in unstimulated VSMCs and observed that the level of interaction was unaltered by short-term (2-min) exposure to ANG II, which was significantly reduced by a longer-term (15-min) ANG II administration (Fig. 3, A and B). In situ PLA also allowed us to assess subcellular, i.e., cell surface versus cytosol, distribution of AT1R-Cav1 interactions. We found that the percentage of cell surface PLA signals significantly reduced after 15-min ANG II exposure (Fig. 3, C and D). We interpreted these findings to mean that upon stimulation with ANG II AT1R interacts with Cav1 within the membrane compartments, which is then followed by a delayed, subsequent internalization of AT1R.
Fig. 3.
Interaction between type 1 ANG II receptor (AT1R) and caveolin-1 (Cav1) is reduced after a prolonged ANG II stimulation. Representative immunocytochemistry images (A and C) and summary data of triplicate experiments (B and D) of proximity ligation assay (PLA) show colocalization of Cav1 and AT1R in vehicle- (Cont) or ANG II-exposed (+AII, for 2 or 15 min) VSMCs. PLA interactions, shown as red blobs around the nuclei (4′,6-diamidino-2-phenylindole in blue) of each cells, and also PLA signals in the close proximity of the cell boundary (white dotted lines were manually drawn to visualize cell boundary) were automatically counted (ImageJ) and are shown in absolute numbers or expressed in percentages as total per cell, respectively. Scale bar, 10 μm. Asterisks indicate significant differences (P < 0.05).
Cav1 directs AT1R to membrane compartment in ANG II-stimulated VSMCs.
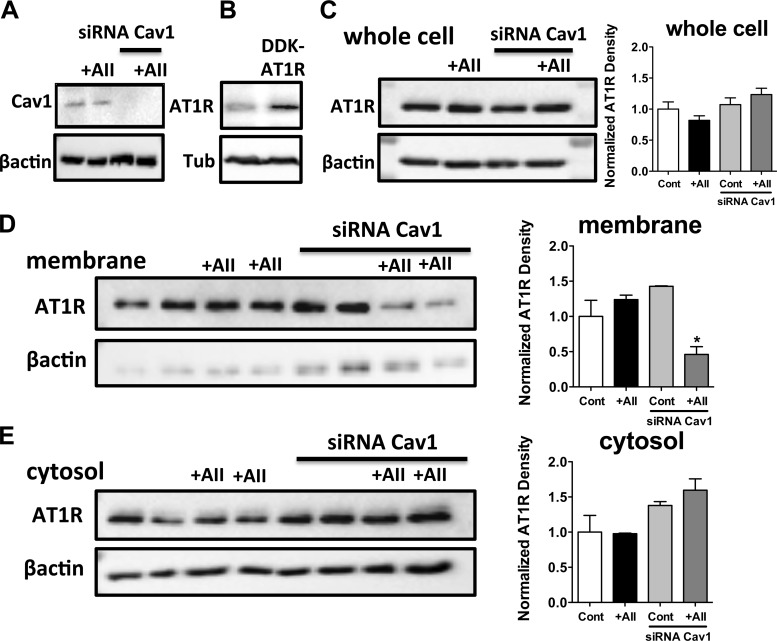
To detect changes in ANG II-induced subcellular distribution of AT1R, cell fractionation approach was also used in cultured VSMCs. We have found a trend toward an increased membrane associated AT1Rs in ANG II-exposed VSMCs (Fig. 4D). More importantly, the membrane associated AT1R was markedly reduced after siRNA knockdown of Cav1 (Fig. 4D). Correspondingly, we observed an increase in the cytosolic AT1R content after Cav1 deletion, both in control and ANG II-exposed VSMCs, a trend, which did not reach statistical significance (1-way ANOVA, Fig. 4E). Neither Cav1 knockdown nor the ANG II-exposure changed significantly the AT1R content in the whole cell lysates (Fig. 4C). Similar observation was made after 2 min and after 15 min exposure to ANG II. We interpreted these findings that Cav1 play a role in the maintained membrane localization of AT1R after ANG II stimulation.
Fig. 4.
Cav1 deletion reduces membrane-associated AT1R in ANG II-stimulated vascular smooth muscle cells (VSMCs). Representative Western blots demonstrate Cav1 expression after Cav1 short-interfering RNA (siRNA) transfection in VSMCs (A), and blots also show AT1R expression in DDK-AT1R transfected HEK293 cells (B). Representative Western blot (C–E) and summary of densitometry data (n = 3) showing Cav1 and AT1R expression in whole cell lysate, membrane, and cytosolic fraction in vehicle- (Cont) or ANG II-exposed (+AII) VSMCs before and after Cav1 silencing are shown. Data are means ± SE. Asterisks indicate significant differences (P < 0.05).
HFD is accompanied by elevated blood pressure and sustained resistance artery constriction to ANG II only in the Cav1 knockout mice.
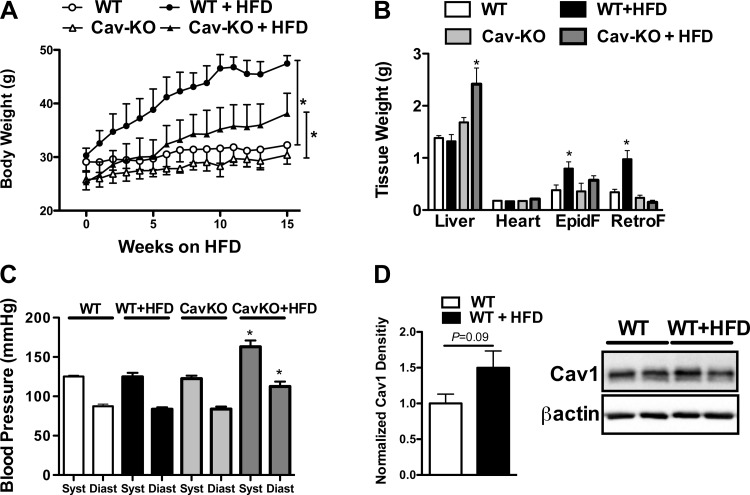
Based on our results we assigned an important regulatory role for Cav1 in preventing sustained AT1R activation in skeletal muscle resistance artery under normal condition. It is possible, however, when this normal regulatory function of Cav1 is compromised in disease, such as we found earlier in obesity and diabetes (8, 14), it may affect ANG II responsiveness of resistance arteries and also alter systemic blood pressure. To test this hypothesis in this study wild-type and Cav1 knockout mice were fed a HFD for 3 mo and vascular reactivity of ex vivo skeletal muscle resistance arteries and systemic blood pressure were measured and compared, in similar protocols as we did in our previous studies (5, 14, 21). When fed a HFD, both wild-type and Cav1 knockout mice gained weight compared with those mice on normal chow diet. We found that the weight gain was significantly greater in wild-type than in Cav1 knockout mice (Fig. 5A). There was significantly increased weight of epididymal and retroperitoneal fat pads in HFD fed wild-type mice than in Cav1 knockout mice (Fig. 5B). These findings correspond to the data in the literature (34) and suggest that Cav1 knockout mice are somewhat resistant to weight gain when challenged with a HFD. It is known that Cav1 knockout mice on HFD have normal fasting glucose levels (10), but the mice are characterized by a marked increase in serum triglyceride, free fatty acid, and cholesterol levels, which is due to the altered binding, transport, and/or storage of fatty acids and cholesterol (34). In this study we found that Cav1 knockout mice had significantly higher serum total cholesterol levels, either with or without HFD (Cav1KO: 166 ± 10 vs. Cav1KO + HFD: 169 ± 29 mg/dL), than wild-type mice (WT: 78 ± 12 vs. WT + HFD: 99 ± 8 mg/dL).
Fig. 5.
High-fat diet (HFD) elevates blood pressure in Cav1 knockout mice. Effect of 15-wk HFD on body weight (A), on the weight of various organs [liver, heart, epididymal fat (EpidF), and retroperitoneal fat (RertoF); B], and on systolic (Syst) and diastolic (Diast) blood pressure (C) in normal wild-type (WT) and Cav1 knockout (Cav-KO) mice is shown. Representative Western blot and summary of densitometry data (D; n = 4 in each group) showing Cav1 expression in skeletal muscle artery of WT mice with our without HFD are shown. Data are means ± SE. Asterisks indicate significant differences (P < 0.05).
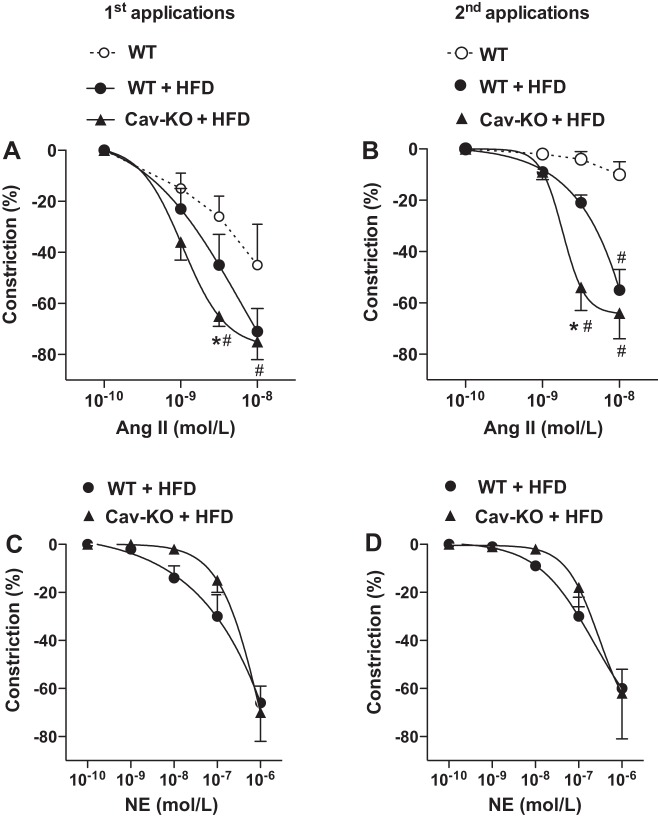
It is of particular interest that only HFD-fed Cav1 knockout mice exhibited a significantly increased systolic and diastolic blood pressure than wild-type animals either on normal chow diet or HFD (Fig. 5C). In similar protocols as described above resistance artery responses to ANG II and NE were obtained in isolated, pressurized skeletal muscle resistance arteries of HFD-fed wild-type and Cav1 knockout mice. We found a clear trend toward an increase in the vascular protein expression of Cav1 between normal, wild-type, and HFD-fed mice (Fig. 5D). When compared with those of wild-type, either on normal chow or HFD, arteries of Cav1 knockout mice exhibited an augmented constriction to ANG II (Fig. 6A), which remained at the same, augmented level even after repeated ANG II administration (Fig. 6B). Arteries of wild-type mice fed a HFD exhibited only a trend toward augmented constrictions to repeated ANG II administrations, when compared with those mice on normal chow diet (Fig. 6B). No significant changes were found in resistance artery constrictions to repeated NE administrations between the experimental groups (Fig. 6, C and D).
Fig. 6.
Cav1 knockout mice fed a HFD exhibit an augmented and sustained vasoconstriction to ANG II. Summary data of skeletal muscle resistance artery constrictions to repeated (1st and 2nd applications) ANG II (0.1–10 nM; n = 5 to 6 in each group; A and B) and to NE [0.1 nM–1 µM, n = 5 to 6 in each groups (C and D) in wild-type (WT) and Cav1 knockout mice (Cav-KO)]. Data are means ± SE. *Significant differences, Cav-KO HFD vs. WT HFD; #significant differences from response of WT (P < 0.05).
DISCUSSION
The present study indicates that a direct interaction between AT1R and Cav1 delays AT1R reactivation after ANG II stimulation and thereby prevents sustained ANG II-induced constriction in skeletal muscle resistance arteries. We also show that this interaction may protect against the development of obesity-associated increased resistance artery constriction and elevated systemic blood pressure. These conclusions are supported by our results showing that Cav1 interacts with AT1R in VSMCs and the interaction is reduced only after a prolonged exposure to ANG II. After genetic deletion of Cav1 ANG II-induced AT1R translocation from membrane to cytosol fraction is augmented. In addition, in Cav1 knockout mice, but not in wild-type, repeated ANG II applications result in maintained resistance artery constriction and Cav1 knockout mice are predisposed to develop elevated systolic and diastolic blood pressure when fed a HFD.
It has been proposed earlier that upon stimulation by ANG II, AT1R interacts with Cav1 in the caveolae membrane microdomains (20, 38, 43). In the present study we raised the possibility that this interaction serves mainly to prevent internalization of the AT1R, hence delaying its resensitization and recycling to the cell surface. In this context, an earlier study by Linder et al. has shown that disruption of caveolae by mβCD reduced the tachyphylactic response to ANG II in the thoracic aorta of normal rats (25). Confirming this observation and extending it to include the rat skeletal muscle resistance artery, herein we show that mβCD augments ANG II-induced constrictions to repeated administration of ANG II, but not to the first application. In addition, we show that skeletal muscle resistance arteries from mice with genetic deletion of Cav1 exhibit an essentially maintained constriction to repeated administration of ANG II. Thus it seems that Cav1 plays a fundamental role in preventing sustained ANG II-induced constrictions and contributes to normal ANG II-induced tachyphylaxis both in conduit vessels and, as we demonstrate here, in resistance arteries.
AT1R trafficking is composed of ANG II-induced AT1R internalization, receptor resensitization, and recycling to the plasma membrane (19). Ishizaka et al. (20) has studied of the initial phase of AT1R trafficking in VSMCs and showed that AT1R translocates to caveolin-rich membrane fractions after 1 to 10 min stimulation with ANG II and also that Cav1 co-immunoprecipitates with AT1R. In our present study, using in situ proximity ligation assay, we detected a direct interaction between AT1R and Cav1 in VSMCs, which remained unchanged after short, 2-min ANG II exposure, but was significantly reduced after a prolonged, 15-min treatment with ANG II. We found that the level of cell surface localized AT1R-Cav1 interactions decreased significantly after 15 min of ANG II exposure. Collectively, these data suggested that after the initial interaction between AT1R and Cav1 ANG II stimulation promotes AT1R internalization and its dissociation from Cav1. We also found that the amount of membrane-associated AT1R was significantly reduced after genetic silencing of Cav1 in ANG II-stimulated VSMCs. Taken together, based on these observations we propose that upon stimulation by ANG II, AT1R moves and physically interacts with Cav1 in the plasma membrane of VSMCs and this interaction delays the internalization and subsequent reactivation of AT1R. As an important functional consequence, our data indicate that the interaction between AT1R and Cav1 is likely to contribute to normal ANG II-induced tachyphylaxis (22, 25, 35, 39) and prevents sustained ANG II-mediated signaling in the rodent skeletal muscle resistance artery.
Our present findings corresponds well with the literature data describing the adverse cardiovascular phenotype of Cav1 knockout mice, which comprises cardiac hypertrophy (9), impaired systolic and diastolic myocardial contractile function, and the development of pulmonary arterial hypertension (12, 42). Many of these pathological changes are believed to be mediated by overactivated RAAS and consequently enhanced ANG II signaling. Interestingly, studies assessing alteration in the systemic blood pressure, which is also affected by the activated RAAS, are controversial in the Cav1 knockout mice. For example, detailed assessment of systolic and diastolic blood pressure and variability found no differences between Cav1 knockout and wild-type mice (12). Similarly, Pojoga et al. (31, 32) showed no significant changes in systolic blood pressure of Cav1 knockout at baseline, whereas the authors reported recently a significantly elevated systolic blood pressure in these animals. In contrast, mean arterial pressure of Cav1 knockout mice was found to be significantly reduced, an alteration, which was normalized after genetically reconstituting the vascular endothelial Cav-1 (27). The possible mechanisms of these controversial observations are still debated.
In this study we also examined the role of Cav1-AT1R interaction in modulating ANG II-induced resistance artery constriction and its potential systemic consequence(s) on blood pressure in an experimental model of obesity. Previously, we found that normal function of Cav1 is required for maintenance of resistance artery dilator function in obesity (14), but when the regulatory function of the Cav1 is compromised this led to an impaired vasodilation of coronary arteries (8). Also, it is known that in obese and diabetic patients AT1R blockers not only reduce systemic blood pressure but also prevent the development of the functional and morphological alterations of resistance arteries (11, 29). Given that, it is possible that obesity is accompanied by an augmented ANG II-mediated vascular signaling. Studies in animal models of obesity are in line with this and found an increased contraction to ANG II in aortae from obese Zucker rats (28), db/db mice (16), and in rats fed a HFD (40). In these studies a key role for enhanced Ca2+ sensitivity of contractile apparatus (28), increased cyclooxygenase-2-derived constrictor prostaglandins (16), or enhanced production of reactive oxygen species has been proposed to account for augmented AT1R-mediated vasoconstriction (40). There is some evidence that augmented AT1R signaling occurs without any change in vascular AT1R expression (28). In support of this, our earlier study demonstrated that skeletal muscle resistance arteries exposed to a short-term (1-h) high glucose concentration resulted in an augmented and sustained constriction to ANG II (6). Previously, we also found that Wistar rats fed a HFD exhibit an impaired dilation of skeletal muscle resistance arteries, which was associated with elevated mean arterial blood pressure of obese rats (13). Given that observation, in this study we raised the hypothesis that in obese mice ANG II-induced resistance artery constriction is augmented and becomes sustained, which contributes to the development of elevated systemic blood pressure. Contrary to our assumption, results from this study show that normal wild-type mice fed a HFD exhibited no significant increase in systolic and diastolic blood pressure. We found only a marginally augmented ANG II-induced constriction in skeletal muscle resistance arteries in the HFD-induced obese mice. Similar to this observation HFD-induced obesity in mice was found to be associated with only slight (5–8 mmHg) increases in mean arterial blood pressure (41). The reason why mice are resistant to develop high blood pressure in response to HFD is not clear and may be due to experimental conditions, duration of diet, and/or age of animals as well as strain differences. According to our hypothesis we raised another possibility, namely that in obesity there are mechanisms that could compensate for the impaired vasomotor regulation. Indeed, adaptation of mechanisms intrinsic to vascular wall has been documented to contribute to the maintained dilator function of coronary resistance arteries in obesity (3). We hypothesized that Cav1, through its interaction with AT1R may serve such adaptive mechanism and thereby prevent a sustained ANG II-induced vascular signaling and the development of obesity-associated hypertension. In support of this scenario we found a clear trend toward increased Cav1 protein expression in the skeletal muscle arteries of HFD-fed wild-type mice, which suggested potential adaptive role of Cav1 in this model. To examine the adaptive role of Cav1, Cav1 knockout mice were fed a HFD and vascular reactivity of ex vivo skeletal muscle resistance arteries and systemic blood pressure were measured. Interestingly, we found that Cav1 knockout mice fed a HFD, but not those on normal chow diet, exhibited a significantly increased systolic and diastolic blood pressure, when compared with other experimental groups. Of note, the HFD-fed Cav1 knockout mice showed a markedly augmented constriction to ANG II, which remained at the same augmented level even after repeated ANG II administrations. Collectively, we interpret these findings to indicate that normal mice are protected against the ability of a HFD to increase AT1R-mediated constriction in the resistance arteries and to elevate systemic blood pressure. We suggest that Cav1 through a direct interaction with AT1R prevents sustained ANG II signaling in this model of experimental obesity. In line with our present observation, we found that Cav1 plays a pivotal role in the maintenance of endothelium-dependent hyperpolarizing factor-mediated coronary artery dilation in obesity (14). It should be noted that there are also recent reports suggesting that ANG II, when administered exogenously in an excess amount, induces pathological cardiovascular signaling through Cav1. For example, an earlier study demonstrated that while exhibiting a cardiac damage at baseline, ANG II-induced cardiac pathology is less prominent in the Cav1 knockout mice (30). In a mouse model of ANG II-induced abdominal aortic aneurism formation, Cav1 knockout mice were remarkable protected against the abdominal aortic aneurism development and rupture, but, interestingly, not against the development of increased blood pressure (36). Moreover, Cav1 deletion seemed to be protective against ANG II-induced inactivation of large conductance calcium-activated potassium (BK) channels in smooth muscle cells (26). Although these studies argued for the pathological role of Cav1 in mediating the adverse ANG II effects, the AT1R antagonist, telmisartan, treatment effectively prevented vascular and left ventricular hypertrophy and also improved cardiac pump function in the Cav1 knockout mice (23). This latter study corresponds to our present findings and suggests an augmented and sustained AT1R-mediated signaling in the absence of Cav1. Clearly, further studies are needed to solve this apparent controversy and the nature of mechanisms through which Cav1 may initially protect but at a later stage may contribute to ANG II-mediated cardiovascular pathology.
In summary, the present study demonstrates that an interaction between AT1R and Cav1 contributes to ANG II-mediated tachyphylaxis in the rodent skeletal muscle resistance artery via delaying AT1R internalization and subsequent reactivation. We propose a model, in which binding of AT1R and Cav1 delays AT1R reactivation and thereby prevents augmented vasoconstriction and elevations in systemic blood pressure in diet-induced obesity.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01 HL-104126 (to Z. Bagi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.C., A.F., R.L., D.J.F., and Z.B. conception and design of research; I.C., A.F., and Z.B. performed experiments; I.C., A.F., and Z.B. analyzed data; I.C., A.F., and Z.B. interpreted results of experiments; I.C., A.F., and Z.B. prepared figures; I.C., A.F., and Z.B. drafted manuscript; I.C., A.F., R.L., D.J.F., and Z.B. edited and revised manuscript; I.C., A.F., R.L., D.J.F., and Z.B. approved final version of manuscript.
REFERENCES
- 1.Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SKCa channels and caveolin-rich domains. Br J Pharmacol 151: 332–340, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anborgh PH, Seachrist JL, Dale LB, Ferguson SS. Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol Endocrinol 14: 2040–2053, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bagi Z. Mechanisms of coronary microvascular adaptation to obesity. Am J Physiol Regul Integr Comp Physiol 297: R556–R567, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Bagi Z, Erdei N, Koller A. High intraluminal pressure via H2O2 upregulates arteriolar constrictions to angiotensin II by increasing the functional availability of AT1 receptors. Am J Physiol Heart Circ Physiol 295: H835–H841, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A, Kaley G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25: 1610–1616, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bagi Z, Feher A, Cassuto J, Akula K, Labinskyy N, Kaley G, Koller A. Increased availability of angiotensin AT1 receptors leads to sustained arterial constriction to angiotensin II in diabetes—role for Rho-kinase activation. Br J Pharmacol 163: 1059–1068, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagi Z, Koller A. Lack of NO-mediation of flow-dependent arteriolar dilation in diabetes is restored by sepiapterin. J Vasc Res 40: 47–57, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, Belin de Chantemele E, Feher A, Romero MJ, Bagi Z. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes 63: 1381–1393, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, Pereira de Souza A, Kitsis RN, Russell RG, Weiss LM, Tang B, Jelicks LA, Factor SM, Shtutin V, Tanowitz HB, Lisanti MP. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol 284: C457–C474, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol 285: C222–C235, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Cooper ME. The role of the renin-angiotensin-aldosterone system in diabetes and its vascular complications. Am J Hypertens 17: 16S–20S, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Desjardins F, Lobysheva I, Pelat M, Gallez B, Feron O, Dessy C, Balligand JL. Control of blood pressure variability in caveolin-1-deficient mice: role of nitric oxide identified in vivo through spectral analysis. Cardiovasc Res 79: 527–536, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Erdei N, Toth A, Pasztor ET, Papp Z, Edes I, Koller A, Bagi Z. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: role of xanthine oxidase-derived superoxide anion. Am J Physiol Heart Circ Physiol 291: H2107–H2115, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Feher A, Rutkai I, Beleznai T, Ungvari Z, Csiszar A, Edes I, Bagi Z. Caveolin-1 limits the contribution of BKCa channel to EDHF-mediated arteriolar dilation: implications in diet-induced obesity. Cardiovasc Res 87: 732–739, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graziani A, Bricko V, Carmignani M, Graier WF, Groschner K. Cholesterol- and caveolin-rich membrane domains are essential for phospholipase A2-dependent EDHF formation. Cardiovasc Res 64: 234–242, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E, Gong MC. COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res 67: 723–735, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE. Pathophysiology of obesity hypertension. Curr Hypertens Rep 2: 139–147, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol 11: 1266–1277, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Hunyady L, Catt KJ, Clark AJ, Gaborik Z. Mechanisms and functions of AT1 angiotensin receptor internalization. Regul Pept 91: 29–44, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Ishizaka N, Griendling KK, Lassegue B, Alexander RW. Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension 32: 459–466, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Jebelovszki E, Kiraly C, Erdei N, Feher A, Pasztor ET, Rutkai I, Forster T, Edes I, Koller A, Bagi Z. High-fat diet-induced obesity leads to increased NO sensitivity of rat coronary arterioles: role of soluble guanylate cyclase activation. Am J Physiol Heart Circ Physiol 294: H2558–H2564, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Juul B, Aalkjaer C, Mulvany MJ. Responses of femoral resistance vessels to angiotensin in vitro. Eur J Pharmacol 135: 61–68, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Krieger MH, Di Lorenzo A, Teutsch C, Kauser K, Sessa WC. Telmisartan regresses left ventricular hypertrophy in caveolin-1-deficient mice. Lab Invest 90: 1573–1581, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem 273: 18677–18680, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Linder AE, Thakali KM, Thompson JM, Watts SW, Webb RC, Leite R. Methyl-beta-cyclodextrin prevents angiotensin II-induced tachyphylactic contractile responses in rat aorta. J Pharmacol Exp Ther 323: 78–84, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Lu T, Zhang DM, Wang XL, He T, Wang RX, Chai Q, Katusic ZS, Lee HC. Regulation of coronary arterial BK channels by caveolae-mediated angiotensin II signaling in diabetes mellitus. Circ Res 106: 1164–1173, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 204: 2373–2382, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimatsu H, Suzuki E, Satonaka H, Takeda R, Omata M, Fujita T, Nagai R, Kitamura T, Hirata Y. Endothelial dysfunction and hypercontractility of vascular myocytes are ameliorated by fluvastatin in obese Zucker rats. Am J Physiol Heart Circ Physiol 288: H1770–H1776, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Pahor M, Psaty BM, Alderman MH, Applegate WB, Williamson JD, Furberg CD. Therapeutic benefits of ACE inhibitors and other antihypertensive drugs in patients with type 2 diabetes. Diabetes Care 23: 888–892, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-l-arginine methyl ester and angiotensin II. Endocrinology 151: 1236–1246, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pojoga LH, Yao TM, Opsasnick LA, Garza AE, Reslan OM, Adler GK, Williams GH, Khalil RA. Dissociation of hyperglycemia from altered vascular contraction and relaxation mechanisms in caveolin-1 null mice. J Pharmacol Exp Ther 348: 260–270, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, Williams GH, Khalil RA. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol 294: H1258–H1265, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension 45: 9–14, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem 277: 8635–8647, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Sorokin G, Grant NM, Egan BM, Lombard JH. Cyclooxygenase products do not modulate angiotensin II-induced contractions of human chorionic plate arteries. Am J Obstet Gynecol 167: 110–114, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Takayanagi T, Crawford KJ, Kobayashi T, Obama T, Tsuji T, Elliott KJ, Hashimoto T, Rizzo V, Eguchi S. Caveolin 1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clin Sci (Lond) 126: 785–794, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ullian ME, Linas SL. Role of receptor cycling in the regulation of angiotensin II surface receptor number and angiotensin II uptake in rat vascular smooth muscle cells. J Clin Invest 84: 840–846, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ushio-Fukai M, Alexander RW. Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension 48: 797–803, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Vicaut E, Montalescot G, Hou X, Stucker O, Teisseire B. Arteriolar vasoconstriction and tachyphylaxis with intraarterial angiotensin II. Microvasc Res 37: 28–41, 1989. [DOI] [PubMed] [Google Scholar]
- 40.Viswanad B, Srinivasan K, Kaul CL, Ramarao P. Effect of tempol on altered angiotensin II and acetylcholine-mediated vascular responses in thoracic aorta isolated from rats with insulin resistance. Pharmacol Res 53: 209–215, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol 30: 769–778, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Wunderlich C, Schober K, Schmeisser A, Heerwagen C, Tausche AK, Steinbronn N, Brandt A, Kasper M, Schwencke C, Braun-Dullaeus RC, Strasser RH. The adverse cardiopulmonary phenotype of caveolin-1 deficient mice is mediated by a dysfunctional endothelium. J Mol Cell Cardiol 44: 938–947, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Wyse BD, Prior IA, Qian H, Morrow IC, Nixon S, Muncke C, Kurzchalia TV, Thomas WG, Parton RG, Hancock JF. Caveolin interacts with the angiotensin II type 1 receptor during exocytic transport but not at the plasma membrane. J Biol Chem 278: 23738–23746, 2003. [DOI] [PubMed] [Google Scholar]