Abstract
Admission hyperglycemia (HG) amplifies vascular injury and neurological deficits in acute ischemic stroke, but the mechanisms remain controversial. We recently reported that ischemia-reperfusion (I/R) injury impairs the myogenic response in both hemispheres via increased nitration. However, whether HG amplifies contralateral myogenic dysfunction and whether loss of tone in the contralateral hemisphere contributes to stroke outcomes remain to be determined. Our hypothesis was that contralateral myogenic dysfunction worsens stroke outcomes after acute hyperglycemic stroke in an oxidative stress-dependent manner. Male wild-type or SOD1 transgenic rats were injected with saline or 40% glucose solution 10 min before surgery and then subjected to 30 min of ischemia/45 min or 24 h of reperfusion. In another set of animals (n = 5), SOD1 was overexpressed only in the contralateral hemisphere by stereotaxic adenovirus injection 2–3 wk before I/R. Myogenic tone and neurovascular outcomes were determined. HG exacerbated myogenic dysfunction in contralateral side only, which was associated with infarct size expansion, increased edema, and more pronounced neurological deficit. Global and selective SOD1 overexpression restored myogenic reactivity in ipsilateral and contralateral sides, respectively, and enhanced neurovascular outcomes. In conclusion, our results show that SOD1 overexpression nullified the detrimental effects of HG on myogenic tone and stroke outcomes and that the contralateral hemisphere may be a novel target for the management of acute hyperglycemic stroke.
Keywords: ischemia-reperfusion injury, admission hyperglycemia, contralateral hemisphere, superoxide dismutase, myogenic tone, stroke outcomes
more than one-third of acute ischemic stroke patients have acute hyperglycemia upon admission (1, 42), which leads to poor clinical outcomes and a higher risk of mortality (1, 7, 32, 43). Intriguingly, patients who have hyperglycemia but no history of diabetes suffer the poorest outcomes (24). We previously showed that a modest acute elevation in blood glucose at the time of stroke amplifies vascular injury and neurological deficits (15). In addition, previous studies have reported a reduction in regional cerebral blood flow (CBF) during acute hyperglycemia (14, 21), which could accentuate brain dysfunction after ischemia (13, 22). Together, all these findings emphasize the detrimental impact of acute hyperglycemia on the cerebrovasculature and the importance of an intact vascular system in functional outcomes after stroke.
Myogenic reactivity is an intrinsic property of smooth muscle cells to constrict in response to pressure by which the brain can maintain adequate blood flow during changes in perfusion pressure (12). We recently showed that ischemia-reperfusion (I/R) injury has a short-term global effect, impairing cerebrovascular myogenic reactivity and lowering perfusion in both ischemic and contralateral hemispheres (11). However, the role of contralateral myogenic dysfunction on stroke outcomes and especially under conditions that amplify I/R injury remain unknown. Thus, our first goal in the present study was to test the hypothesis that hyperglycemia worsens contralateral myogenic dysfunction and that enhancement of contralateral myogenic tone improves stroke outcomes.
It is well established that increased generation of ROS could alter myogenic function after I/R with or without hyperglycemia (11, 35, 38). We have previously shown that 1) I/R injury has a short-term global effect impairing myogenic reactivity in both hemispheres, 2) impaired myogenic tone was due to excess peroxynitrite generation and actin nitration leading to actin depolymerization, and 3) middle cerebral arteries (MCAs) isolated from diabetic rats exposed to oxygen-glucose deprivation ex vivo experience loss of tone due to excess peroxynitrite generation and nitration (11, 26). Therefore, antioxidant agents are a promising therapeutic intervention for acute hyperglycemic stroke. Cu/ZnSOD (SOD1) is one of the antioxidant agents that catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide. Previous studies using SOD1 transgenic rats have supported the beneficial role of SOD1 in improving stroke outcomes after I/R injury (25, 30). Therefore, the second goal of the present study was to test the hypothesis that improvement of myogenic dysfunction by local SOD1 overexpression limits stroke injury and improves functional outcomes.
MATERIALS AND METHODS
Animals.
Experiments were performed on weight-matched (250–350 g) male Wistar rats, SOD1 transgenic rats, and Sprague-Dawley (SD) rats (Harlan, Indianapolis, IN), which served as the control group for experiments involving transgenic animals. Animals were subjected to MCA occlusion (MCAO) with and without acute hyperglycemia. Animals were housed at the Georgia Regents University animal care facility, which has been approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were fed standard rat chow and tap water ad libitum. All animals were euthanized by decapitation after being anesthetized with pentobarbital sodium (Fatal-Plus, Vortech Pharmaceuticals, Dearborn, MI).
Model of ischemia.
Focal cerebral ischemia was achieved in a blinded manner for the various groups using the monofilament suture MCAO model previously described by our group (15). The skin on the cervical region was incised to access the common carotid artery. The external carotid artery was separated, ligated, and severed. Nylon suture with a rounded tip was inserted into the internal carotid artery to approach the origin of the MCA. The nylon suture occluding the MCA was secured along the external carotid artery at its base, and the incision was closed. Rats were subjected to sham operation (sham) or 30 min of MCAO followed by 45 min or 24 h of reperfusion. Acute hyperglycemia was achieved by 2 ml ip of 40% glucose injection 10 min before MCAO and was maintained during 24 h of reperfusion by another injection at the end of the MCAO. Blood glucose levels were measured from a tail vein using a glucometer (Freestyle, Alameda, CA) and reported at baseline, MCAO, and reperfusion. In sham groups, animals were dissected in the neck region, and the common carotid artery was ligated as in stroke surgery but was not subjected to MCAO. At the end of 30 min, the suture was released, and animals were euthanized similarly to the MCAO group.
CBF measurement.
Cerebral perfusion was measured by a scanning laser Doppler imaging system (PeriScan PIM 3 System). In brief, the top of the skull was exposed by a median incision of the skin after the animal was anesthetized with 2% isoflurane inhalation. It was programmed to scan an area covering somatosensory cortex, which is supplied by the MCA. The laser beam was directed at the skull surface (2 mm posterior and 5 mm lateral to the bregma) by a moving-mirror system in the scanner without tissue contact. In this system, a built-in photo detector identifies the reflected light from moving blood cells within 0.5 cm of the cortical surface, and a color-coded image is acquired based on the concentration and mean velocity of these blood cells using LDPIwin software (Perimed, North Royalton, OH).
SOD1 transgenic rats.
Heterozygous SOD1 transgenic rats on the SD background, with increased SOD1 activity, were generously provided by Dr. Pak H. Chan. SOD1 transgenic rats were genotyped by PCR using a mixture of primer sequences (5′-CCATCTCCCTTTTGAGGACA-3′ and 5′-AGGCATGAGGATCAATGGAG-3′, IDT, San Diego, CA), which yielded a 505-bp band.
Stereotaxic injections.
Wistar rats were anesthetized with isoflurane and immobilized on a stereotaxic device 2–3 wk before MCAO. SOD1 adenovirus [3 μl of 1.85 × 1012 viral particles/ml, Ad-r-SOD1/enhanced green fluorescent protein (GFP), Vector Biolabs, Philadelphia, PA] or an empty vector was injected in the contralateral hemisphere over 6 min via a 30-gauge needle adjacent to the MCA at stereotaxic coordinates +0.9 mm anterior, −5.2 mm lateral, and −8.7 mm ventral relative to the bregma (4). SOD1 overexpression was confirmed by Western blot analysis and GFP expression.
SOD assay.
SOD activity was determined in brain homogenates using a Sigma SOD assay kit (Sigma, St. Louis, MO) following the manufacturer's instructions.
Western blot analysis.
SOD1 expression in brain homogenates close to the injection site was analyzed by Western blot analysis. In brief, equal volumes of homogenized tissues were separated by 15% SDS-PAGE and transferred to nitrocellulose membranes. SOD1 was determined using anti-SOD1 antibody (1:500, Sigma). Primary antibodies were detected using horseradish peroxidase-conjugated antibody and enhanced chemiluminescence. Band intensity was quantified by densitometry software (Alpha Innotech, Santa Clara, CA).
Tissue markers of nitrosative stress.
Total nitrotyrosine levels were determined in brain homogenates via slot-blot analysis. In brief, equal amounts of protein were immobilized onto nitrocellulose membranes, and nitrotyrosine was detected by an anti-nitrotyrosine monoclonal antibody (Millipore, Lake Placid, NY). Relative levels of nitrotyrosine were quantified by densitometry software (Alpha Innotech).
Pressurized arteriograph system.
MCA segments from ischemic and contralateral hemispheres were quickly excised and pressurized in an arteriograph chamber (Living Systems, Burlington, VT) at 15 mmHg for 1 h within 45 min of isolation to ensure vessel viability (11). Pressure-diameter curves were obtained first in the presence of Ca2+ (active condition) and then in Ca2+-free buffer (passive condition) with the addition of 0.2 mM papaverine hydrochloride. A video dimension analyzer connected to the arteriograph system was used to measure wall thickness and lumen diameter at pressures ranging from 0 to 180 mmHg in 20-mmHg increments. Using the wall thickness and lumen diameter measurements, percent myogenic tone [percent myogenic tone = 1 − (active outer diameter/passive outer diameter) × 100] was determined.
Neurovascular injury assessment.
All animals were anesthetized with pentobarbital sodium (Fatal-Plus, Vortech Pharmaceuticals) and underwent intracardiac perfusion of ice-cold saline to flush blood out of the vessels at the end of 24 h of reperfusion. Brains were extracted, sliced into 2-mm slices, and then stained with 2% solution of 2,3,5-triphenyltetrazolium chloride (Sigma) to evaluate tissue viability and delineate the infarcted area. Images were captured using a digital scanner, and SPOT Advanced 3.4 software (Diagnostic Instruments, Sterling Heights, MI) was used to quantify grossly visible infarction zones. The infarct volume was determined as a percentage of the ischemic hemisphere. Edema is reported as the percent increase in ischemic hemisphere size to the contralateral hemisphere.
Neurological outcomes assessment.
Beam walk and grip strength tests, which assess sensorimotor function, were performed at baseline and at the end of 24 h of reperfusion. Forelimb grip strength was determined using a digital grip strength meter (Columbus Instruments, Columbus, OH) (46). Beam walk evaluation was done based on the seven-point scale method previously described by Feeney et al. (18).
Statistics.
The area under the curve (AUC) was calculated across intraluminal pressure for vessels from each animal for myogenic tone (40 to 180 mmHg) using NCSS 2007 (NCSS, Kaysville, UT) and was used in the analyses for these variables. Myogenic tone AUC was analyzed using two stroke (sham vs. ischemia or nonischemia) by two hyperglycemia (no vs. yes) ANOVA with interactions where the ischemic and nonischemic sides of the brain were analyzed separately. Data from Wistar rats were analyzed using two stroke (sham vs. MCAO) by two hyperglycemia (no vs. yes) ANOVA to determine the effect of stroke and hyperglycemia on blood glucose at baseline, MCAO, and reperfusion. One-way ANOVA (sham, MCAO, MCAO + hyperglycemia) was used to determine the effect of MCAO and hyperglycemia on myogenic tone AUC for SD and SOD1 transgenic rats. A two-sample t-test was used to establish differences in SOD activity and expression (SD vs. SOD1). A series of two SOD1 (no vs. yes) by two hyperglycemia (no vs. yes) ANOVAs with interactions were used to determine the effect of hyperglycemia and SOD1 on blood glucose at baseline, MCAO, and reperfusion as well as infarct size, edema, beam walk, grip strength, and nitrotyrosine levels. Two stroke (sham vs. MCAO) by two SOD1 (no vs. yes) ANOVA was used to determine the effect of MCAO and SOD1 on nitrotyrosine levels. One-way ANOVA using Wistar rats (MCAO, MCAO + hyperglycemia, and SOD1 adenovirus MCAO + hyperglycemia) was used to determine the effect of hyperglycemia and SOD1 on blood glucose at baseline, MCAO, and reperfusion as well as myogenic tone for both ischemic and nonischemic vessels and infarct size, edema, beam walk, and grip strength. One-way ANOVA using Wistar rats (MCAO, MCAO + hyperglycemia, and SOD1 adenovirus MCAO + hyperglycemia) was used to determine the effect of stroke, hyperglycemia, and SOD1 adenovirus on blood glucose at baseline, MCAO, and reperfusion as well as myogenic tone for both ischemic and nonischemic vessels. SAS 9.3 (SAS, Cary, NC) was used for all analyses. Statistical significance was determined at α < 0.05, and a Tukey's post hoc test was used to compare means from significant ANOVAs.
RESULTS
Effect of hyperglycemia on myogenic tone.
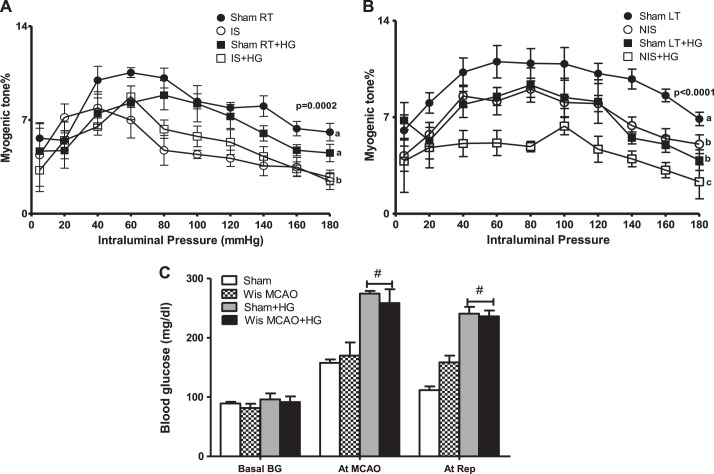
I/R impaired myogenic tone of MCAs isolated from both ischemic and nonischemic hemispheres compared with sham right and left hemispheres, respectively. When I/R injury was superimposed with acute hyperglycemia, myogenic tone impairment was exacerbated in the nonischemic side only. Interestingly, acute hyperglycemia alone reduced myogenic tone in MCAs isolated from the left side of sham hyperglycemic rats but had no effect on the right hemisphere (Fig. 1, A and B). In all groups, baseline blood glucose levels were similar. We achieved an acute elevation in blood glucose levels ranging between 200 and 250 mg/dl in the hyperglycemic group during MCAO and reperfusion (Fig. 1C).
Fig. 1.
Acute hyperglycemia (HG) at the time of stroke exacerbated contralateral myogenic dysfunction. A: ischemia-reperfusion (I/R) with or without HG led to complete loss of the myogenic response in middle cerebral arteries (MCAs) isolated from the ischemic (IS) hemisphere compared with sham-operated (sham) right (RT) and sham RT with HG sides. B: the myogenic response was significantly reduced in the left (LT) hemisphere after I/R injury [nonischemic (NIS) side] or HG alone (sham LT + HG) compared with the sham LT side. Contralateral myogenic dysfunction was aggravated when I/R injury was superimposed with acute HG. a,b,cDifferent letters for pairs of means are significantly different (P < 0.05 by Tukey's test, n = 5–8). C: blood glucose levels at baseline, MCA occlusion (MCAO), and reperfusion in sham, sham + HG, stroked Wistar (Wis MCAO), and Wis MCAO + HG rats. #P < 0.001 vs. sham and Wis MCAO rats (n = 4).
Effect of global SOD1 overexpression on myogenic tone after acute hyperglycemic stroke.
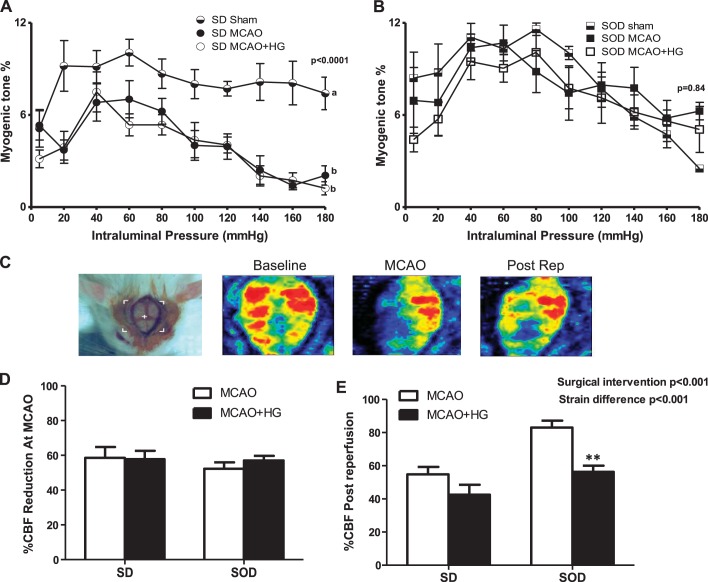
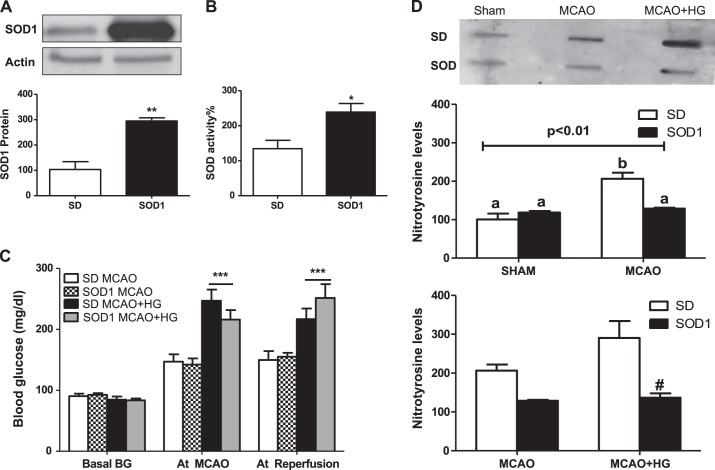
To determine the role of oxidative stress on decreased myogenic tone in hyperglycemic I/R injury, pressurized arteriography experiments were repeated in SOD1 transgenic rats. Since this model has a SD background, SD rats were used as proper controls. Short-term I/R impaired myogenic tone of MCAs obtained from normoglycemic SD rats compared with sham SD rats. MCAs isolated after I/R from normoglycemic and hyperglycemic SD rats displayed similar myogenic tone (Fig. 2A). SOD1 transgenic rats maintained a well-developed myogenic tone, similar to sham SOD1 rats after I/R with or without hyperglycemia (Fig. 2B). We achieved a similar reduction in CBF during MCAO in both SD and SOD1 rats. Percent CBF after reperfusion was improved in SOD transgenic rats exposed to MCAO, which was significantly reduced after acute hyperglycemic stroke (Fig. 2, D and E). SOD1 overexpression was confirmed by measuring SOD1 expression and activity in brain homogenates, which was significantly greater in brain homogenates of transgenic rats compared with wild-type SD rats (Fig. 3, A and B). In all groups, baseline blood glucose levels were similar and elevated at MCAO and reperfusion due to anesthesia. Hyperglycemic SD and SOD1 rats had higher blood glucose levels compared with normoglycemic groups at both time points (Fig. 3C). Nitrotyrosine levels, a marker of increased oxidative stress and peroxynitrite-mediated nitration, were significantly increased in SD rats after 30 min of ishemia/24 h of reperfusion, whereas SOD1 overexpression prevented the elevation of nitrotyrosine levels after I/R with or without hyperglycemia (Fig. 3D).
Fig. 2.
SOD1 transgenic rats maintained well-developed vascular reactivity after acute hyperglycemic stroke. Sprague-Dawley (SD) and SOD1 rats were subjected to 30 min of MCAO/45 min of reperfusion with or without HG. A: I/R injury with or without HG led to loss of the myogenic response in ipsilateral MCAs isolated from SD rats compared with sham SD rats. a,bDifferent letters for pairs of means are significantly different (P < 0.05 by Tukey's test, n = 5–6). B: SOD1 transgenic rats maintained a well-developed myogenic response after I/R injury with or without HG (n = 4–6). C: representative images acquired by the Pim3 laser Doppler at baseline, MCAO, and after reperfusion (Post Rep). D: percent cerebral blood flow (CBF) reduction during MCAO was similar among all groups. E: percent CBF after reperfusion was improved in SOD transgenic rats exposed to MCAO, which was significantly reduced after acute hyperglycemic stroke. **P < 0.01 vs. SOD1 MCAO (n = 4–6).
Fig. 3.
Physiological parameters of SOD1 transgenic rats. A: increased total SOD1 expression in brain homogenates of SOD1 transgenic rats compared with SD rats. **P < 0.01 vs. SD rats (n = 4). B: increased total SOD activity in brain homogenates of SOD1 transgenic rats compared with SD rats. *P < 0.05 vs. SD rats (n = 7). C: blood glucose levels at baseline, MCAO, and reperfusion in SD, SD + HG, SOD1, and SOD1 + HG groups. ***P < 0.001 vs. SD MCAO and SOD1 MCAO (n = 3–8). D: nitration was significantly upregulated after I/R injury with and without HG in SD rats, whereas global SOD1 overexpression blunted that increase. a,bDifferent letters for pairs of means are significantly different (P < 0.05 by Tukey's test, n = 4).
Effect of global SOD1 overexpression on neurovascular outcomes after acute hyperglycemic stroke.
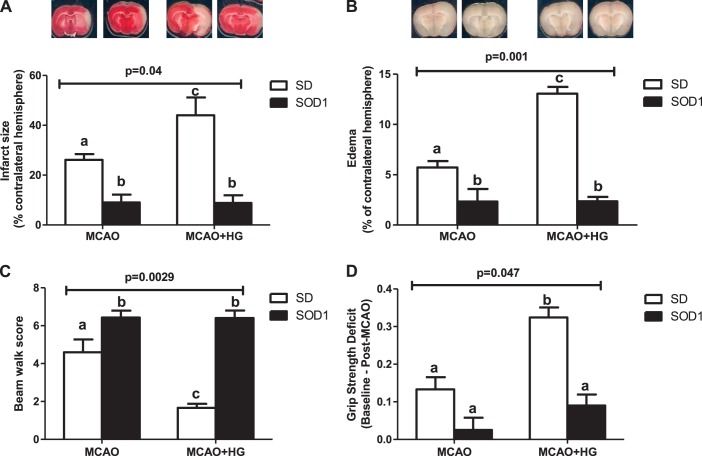
Acute hyperglycemia led to infarct size and edema expansion in SD rats after 30 min of ischemi/24 h of reperfusion. Global SOD1 overexpression significantly reduced infarct size and edema compared with wild-type SD rats and prevented hyperglycemia-mediated increases in infarct size and edema (Fig. 4, A and B).
Fig. 4.
Global SOD1 overexpression improved stroke outcomes after acute hyperglycemic stroke. SD and SOD1 rats were subjected to 30 min of MCAO/24 h of reperfusion with or without HG. A and B: acute HG at time of stroke led to infarct size and edema expansion in SD rats, whereas normoglycemic and hyperglycemic SOD1 transgenic rats showed smaller infarct size and edema compared with SD rats exposed to I/R injury with or without HG. C: hyperglycemic SD rats showed poor beam walk performance compared with normoglycemic SD rats after I/R injury, whereas normoglycemic and hyperglycemic SOD1 transgenic rats experienced better performance compared with stroked SD rats with or without HG. D: acute HG at the time of stroke exacerbated grip strength deficits in SD rats but had no additional effect on SOD1 rats. P values indicate an interaction such that HG affects infarct size, edema, and neurological tests differently in SD vs. SOD rats. a,b,cDifferent letters on pairs of means are significantly different by Tukey's post hoc pairwise comparison test (n = 5–8).
Effect of global SOD1 overexpression on behavioral outcomes after acute hyperglycemic stroke.
Acute hyperglycemia worsened beam walk performance and induced grip strength deficits in SD rats after 30 min of ischemia/24 h of reperfusion. Hyperglycemic SOD1 transgenic rats displayed better beam walk performance and reduced grip strength deficits compared with hyperglycemic SD rats (Fig. 4, C and D).
Effect of focal contralateral SOD1 overexpression on myogenic tone after acute hyperglycemic stroke.
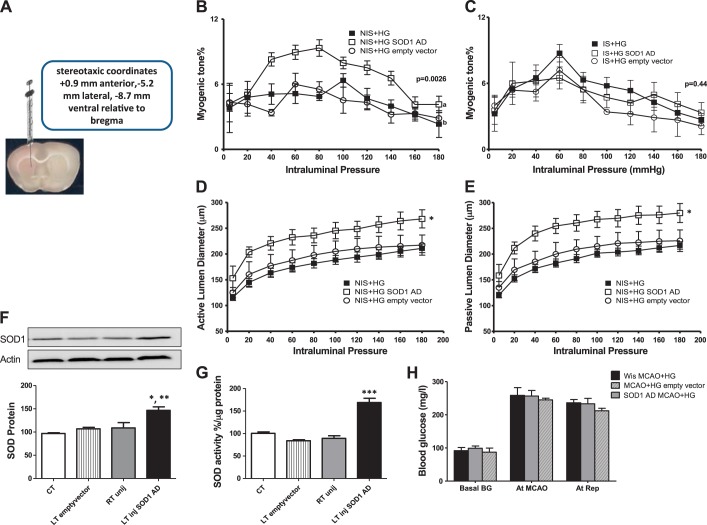
Focal SOD1 overexpression in the MCA territory of the contralateral hemisphere significantly improved the myogenic response of vessels isolated from the nonischemic hemisphere but had no effect on the ischemic side compared with the effect of an empty vector injection and with MCAs isolated from rats exposed to acute hyperglycemic stroke (Fig. 5, B and C). Myogenic reactivity curves across pressure range under active and passive conditions for the nonischemic side showed a well-maintained autoregulatory response after focal SOD1 overexpression only (Fig. 5D). Passive vasodilation was significantly improved after contralateral SOD1 overexpression compared with the effect of an empty vector injection and with MCAs isolated from rats exposed to acute hyperglycemic stroke (Fig. 5E). To confirm contralateral SOD1 overexpression, SOD1 levels and activity were measured in brain homogenates. We found that SOD1 expression (Fig. 5F) and activity (Fig. 5G) were significantly upregulated in brain homogenates of the left injected hemisphere by nearly 50% compared with the right uninjected side, the left side injected with an empty vector, and control Wistar rats. In all groups, baseline blood glucose levels were similar. We achieved an acute elevation in blood glucose levels ranging between 200 and 250 mg/dl in hyperglycemic groups during MCAO and reperfusion (Fig. 5H).
Fig. 5.
Contralateral SOD1 overexpression in control Wistar rats. Wistar rats were subjected to a stereotaxic injection of SOD1 adenovirus (AD) or an empty vector in the LT hemisphere 2–3 wk before acute hyperglycemic stroke. A: representative image of the stereotaxic injection of SOD1 AD in the contralateral hemisphere. B: focal contralateral SOD1 overexpression significantly improved contralateral myogenic dysfunction compared with NIS + HG and with acutely hyperglycemic stroked rats injected with an empty vector. C: contralateral SOD1 overexpression did not improve the myogenic response of vessels obtained from IS hemisphere (IS + HG SOD1 AD) after acute hyperglycemic stroke compared with IS + HG and with rats injected with an empty vector. a,bDifferent letters on pairs of means are significantly different (P < 0.05 by Tukey's test, n = 3–6). D: focal contralateral SOD1 overexpression increased lumen diameter under active conditions while maintaining a well-developed autoregulatory response in the contralateral hemisphere compared with acute hyperglycemic stroke with and without the empty vector injection. E: focal contralateral SOD1 overexpression improved passive vasodilation in the contralateral hemisphere compared with the effect of an empty vector injection and with MCAs isolated from rats exposed to acute hyperglycemic stroke. *P < 0.001 vs. NIS + HG and NIS + HG empty vector (n = 3–6). F: SOD1 expression was significantly upregulated after stereotaxic injection of SOD1 AD in the LT hemisphere compared with the RT uninjected (unij) side, LT side injected with an empty vector, and control Wistar rats. *P < 0.05 vs. RT uninjected side; **P < 0.01 vs. control and LT empty vector (n = 4–5). G: SOD activity was significantly upregulated after stereotaxic injection of SOD1 AD in the LT hemisphere compared with the RT uninjected side, LT side injected with an empty vector, and control Wistar rats. ***P < 0.001 vs. control, RT uninjected side, and LT empty vector (n = 3–6). H: blood glucose levels at baseline, MCAO, and reperfusion in hyperglycemic stroked Wistar rats (Wis MCAO + HG), Wistar rats injected with SOD1 AD (SOD1 AD MCAO + HG), or Wistar rats injected with empty vector before hyperglycemic stroke (MCAO + HG empty vector) (n = 3–6).
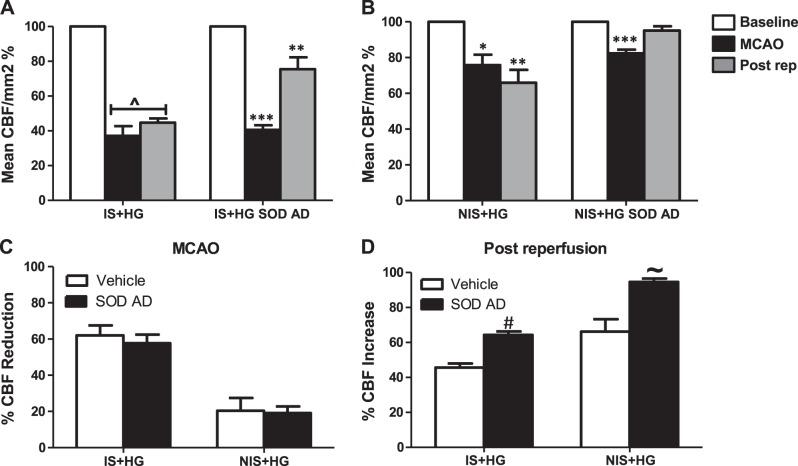
Effect of focal contralateral SOD1 overexpression on CBF after acute hyperglycemic stroke.
Mean CBF was significantly reduced in ischemic and nonischemic hemispheres, albeit to a different degree during MCAO and after reperfusion after acute hyperglycemic stroke compared with baseline (Fig. 6, A and B). Rats injected with SOD1 adenovirus 2 wk before acute hyperglycemic stroke displayed an improvement in CBF in both hemispheres after reperfusion (Fig. 6, A and B). We achieved a similar reduction in CBF during MCAO in both groups exposed to acute hyperglycemic stroke (Fig. 6C). Contralateral SOD1 overexpression increased CBF after reperfusion in both ischemic and nonischemic hemispheres after acute hyperglycemic stroke (Fig. 6D).
Fig. 6.
Contralateral SOD1 overexpression improved CBF after acute hyperglycemic stroke. Wistar rats were subjected to a stereotaxic injection of SOD1 AD or an empty vector in the LT hemisphere 2–3 wk before acute hyperglycemic stroke. A and B: mean percent CBF was significantly decreased in both hemispheres during MCAO and postreperfusion after acute hyperglycemic stroke compared with baseline. Contralateral SOD1 overexpression improved CBF after reperfusion in both IS and NIS hemispheres. C: percent CBF reduction during MCAO in IS and NIS hemispheres was similar between the two groups. D: contralateral SOD1 overexpression improved postreperfusion CBF in IS and NIS hemispheres after acute hyperglycemic stroke. P^ < 0.001, **P < 0.01, and *P < 0.05 vs. baseline; ***P < 0.001 vs. postreperfusion and baseline; #P < 0.001 vs. IS + HG vehicle; ∼P < 0.01 vs. NIS + HG vehicle (n = 5–6).
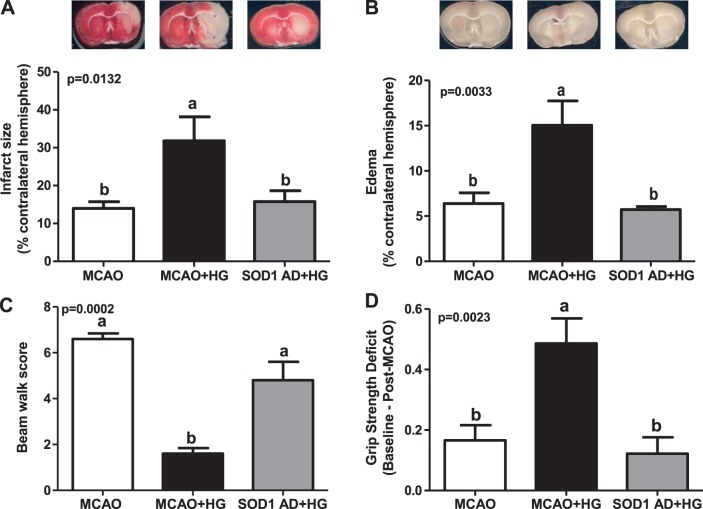
Effect of focal contralateral SOD1 overexpression on neurovascular outcomes after acute hyperglycemic stroke.
Acute hyperglycemia significantly increased the infarct size and edema in Wistar rats after 30 min of ischemia/24 h of reperfusion. Contralateral SOD1 overexpression prevented hyperglycemia-mediated increases in infarct size and edema (Fig. 7, A and B).
Fig. 7.
Contralateral SOD1 overexpression improved stroke outcomes after acute hyperglycemic stroke. Wistar rats were subjected to a stereotaxic injection of SOD1 AD in the Lt hemisphere 2–3 wk before acute hyperglycemic stroke. A and B: acute HG at the time of stroke led to infarct size and edema expansion in Wistar rats (MCAO + HG), which were significantly reduced by contralateral SOD1 overexpression (SOD1 AD + HG). C and D: hyperglycemic Wistar rats showed poor beam walk performance and grip strength deficits compared with normoglycemic SD rats after I/R injury. Contralateral SOD1 overexpression (SOD1 AD + HG) significantly enhanced behavioral outcomes compared with MCAO + HG. a,bDifferent letters on pairs of means are significantly different (P < 0.05 by Tukey's test, n = 5–7).
Effect of focal contralateral SOD1 overexpression on behavioral outcomes after acute hyperglycemic stroke.
Acute hyperglycemia worsened beam walk performance and induced grip strength deficits in Wistar rats after 30 min of ischemia/24 h of reperfusion. SOD1 overexpression in the contralateral hemisphere significantly improved neurological outcomes after acute hyperglycemic stroke (Fig. 7, C and D).
DISCUSSION
In the present study, we revealed a novel association between contralateral myogenic dysfunction and stroke outcomes after acute hyperglycemic stroke. Furthermore, we highlighted the critical role of SOD1 overexpression in improving vascular function and stroke outcomes. We provided evidence that contralateral myogenic dysfunction was exacerbated in hyperglycemia and was associated with poor stroke outcomes. We showed that improving vascular function specifically in the nonischemic hemisphere ameliorated neurovascular outcomes after acute hyperglycemic stroke. These findings are very important because they identify the contralateral hemisphere as a therapeutic target. Once the mechanisms and modulators of cerebrovascular function in both hemispheres are known, it will be possible to develop more effective strategies to deliver neuroprotective therapies to improve stroke outcomes and recovery.
Admission hyperglycemia (>7.8 mmol/l) is very common in ischemic stroke patients due to a history of diabetes or acute elevations in blood glucose (29). Both human and animal studies have reported that elevated blood glucose at stroke onset is associated with a larger infarct size, poor clinical outcomes, and an increased risk of mortality (2, 5, 7, 15). Until now, the only Federal Drug Administration-approved treatment for ischemic stroke is the intravenous administration of recombinant tissue plasminogen activator to restore blood flow (1). However, hyperglycemia at the time of ischemic stroke increases the risk of hemorrhagic transformation and poor clinical outcomes with recombinant tissue plasminogen activator administration (45). Moreover, several animal studies have shown that the exacerbated neurovascular injury in hyperglycemic stroke is more common with transient occlusion, suggesting that reperfusion contributes to increased brain damage by hyperglycemia (28, 39). However, the mechanisms by which acute hyperglycemia and diabetes aggravate vascular injury and neurological outcomes are multifactorial and still controversial (24, 28, 40). Since the only successful therapeutic target identified for the 800,000 annual victims of ischemic stroke is the cerebral vasculature (23), in our study, we focused on the impact of acute hyperglycemic reperfusion on cerebrovascular function. While diabetes also has a detrimental effect of cerebrovascular function and stroke outcomes, as recently reviewed (16, 26, 27), nondiabetic hyperglycemic patients may suffer the most from acute ischemic stroke compared with diabetic or normoglycemic patients (7, 23). As such, we narrowed our study to acute hyperglycemia.
The cerebrovascular myogenic response, discovered >100 yr ago by Bayliss (3), is the change in smooth muscle tone in response to pressure fluctuation. The myogenic response is an inherent property of smooth muscle cells that is crucial for maintaining vascular resistance and constant blood flow (31). Several experimental studies have shown a detrimental effect of I/R on the myogenic response of cerebral vessels isolated from ischemic hemispheres (9, 10). We recently showed in an animal model of transient MCAO, that I/R has a short-term global effect on cerebrovascular function. We reported that 30 min of MCAO/45 min of reperfusion reduced cerebral perfusion in both hemispheres and led to myogenic tone impairment in ischemic and contralateral hemispheres via increased peroxynitrite generation and nitration (11). The aim of the present study was to expand our previous findings by determining 1) the impact of admission hyperglycemia, a condition associated with poor outcomes, on myogenic tone in both hemispheres; 2) the role of oxidative stress on myogenic tone regulation in hyperglycemic stroke; and 3) the role of contralateral myogenic dysfunction reactivity on hyperglycemic stroke outcomes. We found that acute elevation of blood glucose at the time of stroke exacerbated myogenic dysfunction in MCAs isolated from the contralateral hemisphere, which was associated with infarct size expansion, increased edema, and poor neurological outcomes. However, acute hyperglycemia did not display any further effect on the myogenic reactivity of vessels isolated from the ischemic hemisphere compared with I/R alone. These results suggest that augmented contralateral myogenic dysfunction could contribute to poor outcomes after acute hyperglycemic stroke. Understanding this phenomenon is essential for the development of rational therapies that reduce hyperglycemic reperfusion injury in both hemispheres and thus improve clinical outcomes in patients.
Growing evidence indicates the involvement of oxidative stress in the pathogenesis of stroke through different mechanisms at neuronal and vascular levels (8, 36). Superoxide anion, a ROS, has emerged as an important mediator of vascular dysfunction (17). Besides its direct effect on blood vessels, superoxide is also the precursor for other ROS and some reactive nitrogen species. Considerable attention has been dedicated to the interaction of superoxide and nitric oxide, which reduces nitric oxide bioavailability, leading to peroxynitrite generation (44), which profoundly influences vascular function at multiple levels (33, 35). Our laboratory has previously reported the detrimental effect of excess peroxynitrite generation on myogenic reactivity in control and diabetic rats after I/R (11) or short periods of oxygen-glucose deprivation (26). Another study (38) showed that MCAs perfused intraluminally with plasma of acutely hyperglycemic rats that underwent 2 h of MCAO/2 h of reperfusion experienced increased myogenic tone and that this was reversed by peroxynitrite decomposition. It is well established that ROS play a pivotal role in altering cerebrovascular function after I/R with or without hyperglycemia. Therefore, to test if depleting peroxynitrite parent radical (superoxide) could reverse myogenic dysfunction and hence improve stroke outcomes in our model of acute hyperglycemic stroke, we used SOD1 transgenic rats. We found that global SOD1 overexpression decreased brain nitrotyrosine levels (a marker for peroxynitrite generation), reserved myogenic behavior, and improved neurovascular injury after I/R with and without hyperglycemia compared with wild-type animals. These findings were in agreement with previous studies (25, 30, 37) that revealed the protective effect of SOD1 after cerebral ischemia. We then investigated whether improving myogenic tone only in the contralateral side could ameliorate poor neurovascular outcomes after acute hyperglycemic stroke. SOD1 expression was upregulated in the contralateral hemisphere using a stereotaxic injection of SOD1 adenovirus 2–3 wk before I/R. Our results showed that increased SOD1 activity in the contralateral hemisphere improved contralateral myogenic dysfunction after acute hyperglycemic stroke, which was associated with reduced infarct size and edema and better neurological performance. Several studies (19, 24, 25) have shown the protective role of SOD1 in improving stroke outcomes via modifying matrix metalloproteinase-9 activity, Akt activation, and others. Since we did not measure the effect of SOD1 overexpression on any of the previously proposed mechanisms in both hemispheres, we cannot conclude that the enhanced stroke outcomes are solely dependent on myogenic tone. However, the improvements in stroke outcomes after contralateral SOD1 overexpression seem to be due, in part, to the maintenance of a well-functioning myogenic response, as loss of contralateral myogenic tone was associated with poor neurovascular outcomes. These findings suggest that the nonischemic hemisphere may be a novel target for the management of stroke. The findings of the present study are limited by the fact that isoflurane was used as a method for anesthesia during stroke surgery, which can reduce myogenic reactivity. To normalize this effect, vessels were isolated from sham rats exposed to similar levels of isoflurane. However, these limitations do not outweigh the significant findings of the present study.
Stroke is the fourth leading cause of death and one of the major causes of permanent disability worldwide. The economic burden of stroke is significant, with the expected increase for stroke-related medical costs and disability from $71.6 to $184.1 billion between 2012 and 2030. Therefore, more detailed understanding of factors contributing to poor neurovascular outcomes and functional recovery will decrease the health and economic burdens of stroke. Several clinical studies have reported a reduction in CBF after stroke not only in the ischemic region but also in the contralateral hemisphere, known as cerebral diaschisis. Moreover, the persistent reduction of CBF in the hemisphere contralateral to the infracted region proved to be involved in poor outcomes and recovery (6, 41). In addition, various experimental and clinical studies have found major pathological changes in the contralateral hemisphere, including perivascular edema, blood-brain barrier damage, astrogliosis, and increased apoptosis after acute stroke (20, 34). Both clinical and experimental studies have suggested that pathological neurovascular changes in the contralateral hemisphere may contribute to stroke pathology and impede recovery processes. In agreement with the aforementioned studies, our findings showed that CBF was reduced in both hemispheres after acute hyperglycemic stroke, which was associated with poor neurovascular outcomes. Interestingly, we demonstrated that improving contralateral myogenic dysfunction in the contralateral hemisphere only was accompanied with an increase in CBF after reperfusion in both hemispheres, which led to better stroke outcomes. Although these results indicate the importance of a well-developed contralateral myogenic tone in improving functional outcomes after acute hyperglycemic stroke, this study was limited to the acute ischemic phase. Further studies are needed to demonstrate the impact of contralateral myogenic dysfunction at late ischemic stages. The role of the contralateral hemisphere in stroke outcomes and recovery is an intriguing but challenging new field of research. In our study, we highlight the importance of establishing effective clinical treatments that target both hemispheres to repair the injured vasculature close and distal to the site of ischemic injury to achieve better patient quality of life.
GRANTS
A. Ergul is a Research Career Scientist at the Charlie Norwood Veterans Affairs (VA) Medical Center in Augusta, Georgia. This work was supported in part by VA Merit Award BX000347, a VA Research Career Scientists Award, and National Institutes of Health (NIH) Grant R01-NS-083559 (to A. Ergul); VA Merit Award BX000891 and NIH Grant NS-063965 (to S. C. Fagan); and American Heart Association Predoctoral Fellowship 12PRE11300001 (to M. Coucha).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C. and A.E. conception and design of research; M.C., W.L., S.H., and M.A. performed experiments; M.C. and M.H.J. analyzed data; M.C. interpreted results of experiments; M.C. prepared figures; M.C. drafted manuscript; M.C., S.C.F., and A.E. edited and revised manuscript; M.C., W.L., S.H., M.A., M.H.J., S.C.F., and A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Pak H. Chan for providing SOD1 transgenic rats.
REFERENCES
- 1.Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38: 1655–1711, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Baker L, Juneja R, Bruno A. Management of hyperglycemia in acute ischemic stroke. Curr Treat Options Neurol 13: 616–628, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28: 220–231, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biernaskie J, Corbett D, Peeling J, Wells J, Lei H. A serial MR study of cerebral blood flow changes and lesion development following endothelin-1-induced ischemia in rats. Magn Reson Med 46: 827–830, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Bruno A, Biller J, Adams HP Jr, Clarke WR, Woolson RF, Williams LS, Hansen MD. Acute blood glucose level and outcome from ischemic stroke Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology 52: 280–284, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, D'Olhaberriague L, Vikingstad EM, Levine SR, Welch KM. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke 29: 112–122, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32: 2426–2432, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21: 2–14, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: the threshold duration of reperfusion for myogenic activity. Stroke 33: 2094–2099, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Cipolla MJ, Lessov N, Hammer ES, Curry AB. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke 32: 1658–1664, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Coucha M, Li W, Johnson M, Fagan SC, Ergul A. Protein nitration impairs myogenic tone of rat middle cerebral arteries in both ischemic and nonischemic hemispheres after ischemic stroke. Am J Physiol Heart Circ Physiol 305: H1726–H1735, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich WD, Alonso O, Busto R. Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke 24: 111–116, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Duckrow RB, Beard DC, Brennan RW. Regional cerebral blood flow decreases during hyperglycemia. Ann Neurol 17: 267–272, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Elgebaly MM, Ogbi S, Li W, Mezzetti EM, Prakash R, Johnson MH, Bruno A, Fagan SC, Ergul A. Neurovascular injury in acute hyperglycemia and diabetes: a comparative analysis in experimental stroke. Transl Stroke Res 2: 391–398, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Endocr Metab Immune Disord Drug Targets 12: 148–158, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol (1985) 100: 739–743, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217: 855–857, 1982. [DOI] [PubMed] [Google Scholar]
- 19.Fujimura M, Morita-Fujimura Y, Narasimhan P, Copin JC, Kawase M, Chan PH. Copper-zinc superoxide dismutase prevents the early decrease of apurinic/apyrimidinic endonuclease and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke 30: 2408–2415, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Garbuzova-Davis S, Rodrigues MC, Hernandez-Ontiveros DG, Tajiri N, Frisina-Deyo A, Boffeli SM, Abraham JV, Pabon M, Wagner A, Ishikawa H, Shinozuka K, Haller E, Sanberg PR, Kaneko Y, Borlongan CV. Blood-brain barrier alterations provide evidence of subacute diaschisis in an ischemic stroke rat model. PLos One 8: e63553, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginsberg MD, Welsh FA, Budd WW. Deleterious effect of glucose pretreatment on recovery from diffuse cerebral ischemia in the cat. I. Local cerebral blood flow and glucose utilization. Stroke 11: 347–354, 1980. [DOI] [PubMed] [Google Scholar]
- 22.Gisselsson L, Smith ML, Siesjo BK. Hyperglycemia and focal brain ischemia. J Cereb Blood Flow Metab 19: 288–297, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129: e28–e292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res 5: 442–453, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke 38: 1044–1049, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, Johnson M, Ergul A. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther 342: 407–415, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly-Cobbs AI, Prakash R, Li W, Pillai B, Hafez S, Coucha M, Johnson MH, Ogbi SN, Fagan SC, Ergul A. Targets of vascular protection in acute ischemic stroke differ in type 2 diabetes. Am J Physiol Heart Circ Physiol 304: H806–H815, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent TA, Soukup VM, Fabian RH. Heterogeneity affecting outcome from acute stroke therapy: making reperfusion worse. Stroke 32: 2318–2327, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Kiers L, Davis SM, Larkins R, Hopper J, Tress B, Rossiter SC, Carlin J, Ratnaike S. Stroke topography and outcome in relation to hyperglycaemia and diabetes. J Neurol Neurosurg Psychiatry 55: 263–270, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA 88: 11158–11162, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res 49: 375–389, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol 6: 145–155, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Li W, Altura BT, Altura BM. Peroxynitrite-induced relaxation in isolated canine cerebral arteries and mechanisms of action. Toxicol Appl Pharmacol 196: 176–182, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Lorberboym M, Blankenberg FG, Sadeh M, Lampl Y. In vivo imaging of apoptosis in patients with acute stroke: correlation with blood-brain barrier permeability. Brain Res 1103: 13–19, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Maneen MJ, Cipolla MJ. Peroxynitrite diminishes myogenic tone in cerebral arteries: role of nitrotyrosine and F-actin. Am J Physiol Heart Circ Physiol 292: H1042–H1050, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 67: 181–198, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noshita N, Sugawara T, Lewen A, Hayashi T, Chan PH. Copper-zinc superoxide dismutase affects Akt activation after transient focal cerebral ischemia in mice. Stroke 34: 1513–1518, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Palomares SM, Gardner-Morse I, Sweet JG, Cipolla MJ. Peroxynitrite decomposition with FeTMPyP improves plasma-induced vascular dysfunction and infarction during mild but not severe hyperglycemic stroke. J Cereb Blood Flow Metab 32: 1035–1045, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quast MJ, Wei J, Huang NC, Brunder DG, Sell SL, Gonzalez JM, Hillman GR, Kent TA. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab 17: 553–559, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Quinn TJ, Lees KR. Hyperglycaemia in acute stroke–to treat or not to treat. Cerebrovasc Dis 27, Suppl 1: 148–155, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Riecker A, Groschel K, Ackermann H, Schnaudigel S, Kassubek J, Kastrup A. The role of the unaffected hemisphere in motor recovery after stroke. Hum Brain Mapp 31: 1017–1029, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet 353: 376–377, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Stead LG, Gilmore RM, Bellolio MF, Mishra S, Bhagra A, Vaidyanathan L, Decker WW, Brown RD Jr.. Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care 10: 181–186, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, Levine SR. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation 105: 1679–1685, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Terry AV Jr, Gearhart DA, Beck WD Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther 322: 1117–1128, 2007. [DOI] [PubMed] [Google Scholar]