Abstract
We previously demonstrated that inhibitor κB kinase 2 (IKK2) is a myosin light chain kinase (MLCK). In the present study, we assess whether the prototypical activator of IKK2 tumor necrosis factor-α (TNF-α) regulates the MLCK activity of IKK2 and thus MLC phosphorylation in vascular smooth muscle cells (VSMCs). Kinase activity assay revealed that TNF-α downregulated the MLCK activity of IKK2 in human VSMCs (HVSMCs). However, Western blot analysis did not demonstrate a significant effect of TNF-α on MLC phosphorylation in HVSMCs, and myograph analysis did not reveal a significant effect of TNF-α on the contraction of the aorta from Sprague-Dawley rats and C57Bl/6j mice, suggesting a dual regulation of MLC phosphorylation by TNF-α. Confirming this notion, TNF-α significantly increased MLC phosphorylation in IKK2−/− but not wild-type cells. Furthermore, our results show that TNF-α increased GTP-bound RhoA and MLC phosphatase subunit MYPT1 phosphorylation and markedly reduced MLC phosphorylation in the presence of Rho-kinase inhibitor Y-27632, suggesting that downregulation of MLCK activity of IKK2 by TNF-α is antagonized by simultaneous RhoA/Rho-kinase activation. These results indicate that TNF-α dually regulates MLC phosphorylation through both IKK2 and RhoA/Rho-kinase pathways.
Keywords: IKK2, MLC phosphorylation, vasoconstriction, inflammation, blood pressure
tumor necrosis factor-α (TNF-α) is the prototypical proinflammatory cytokine that activates the inhibitor κB kinase 2 (IKK2)/nuclear factor-κB (NF-κB) intracellular signaling pathway and subsequently changes the expression of many pro- and anti-inflammatory genes. Its role in human blood pressure regulation has also been well established, as its plasma level is correlated to blood pressure and anti-TNF-α therapies lower blood pressure (28). In animal studies, TNF-α has been implicated in diverse hypertensions such as high fructose diet-induced hypertension (34), angiotensin II-induced hypertension (10), and hypertension in pregnant rats (18). How TNF-α leads to abnormal blood pressure regulation, however, remains elusive.
Vascular contractile status determines the total peripheral resistance and thus influences blood pressure regulation. Several studies have demonstrated that TNF-α may modulate vascular tone, offering a mechanism linking TNF-α to blood pressure regulation. For example, TNF-α induces vasoconstriction in human forearm resistance arteries (23) and mouse renal arteries (31), probably by increasing basal bioavailability of the vasoconstrictor prostanoids and reducing the basal bioavailability of nitric oxide (NO). TNF-α also induces bronchial vasoconstriction through induction of endothelin-1 (35). Scherer et al. (30) recently showed that TNF-α enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. However, there are some inconsistent results. For example, TNF-α in rat skeletal muscle arterioles does not have any direct vasoconstrictor effect and exerts a vasodilator action in the presence of endotoxin (8).
The contraction of vascular smooth muscle cells is regulated primarily at the level of myosin light chain (MLC) Ser19 phosphorylation, which is essential for the interaction of actin and myosin and activation of actin-activated myosin ATPase and consequent initiation of smooth muscle contraction. The level of MLC Ser19 phosphorylation is determined by the relative activity of MLC kinases (MLCKs) vs. MLC phosphatase (MLCP). In addition to the classic Ca2+-dependent MLCK, several kinases have been shown to phosphorylate MLC Ser19 in vitro, including Rho kinase (2), ILK (13), PAK (3), and ZIPK (17). However, whether these kinases phosphorylate MLC in living cells remains controversial. We recently demonstrated that IKK2, a kinase best known for its essential role in inhibitor κB (IκB) phosphorylation and subsequent NF-κB activation, is a constitutive MLCK in vascular smooth muscle (39).
TNF-α has been shown to increase MLC phosphorylation in diverse models, including endothelial cells (26), airway smooth muscle cells (14), intestinal epithelia (41), pulmonary endothelial cells (25), renal tubular epithelia (16), renal endothelial cells (38), and Caco-2 (20). However, there are few studies investigating the MLC phosphorylation effect of TNF-α in vascular smooth muscle cells. As the prototypical activator of IKK2, TNF-α has been shown to markedly increase IKK activity. In the present study, we therefore assessed if TNF-α regulates the MLCK activity of IKK2 and thus MLC phosphorylation in vascular smooth muscle.
METHODS
Animals.
Sprague-Dawley rats (8 wk old) were purchased from Harland Laboratories (Indianapolis, IN). C57Bl/6j mice (8 wk old) were purchased from The Jackson Laboratory (Bar Harbor, ME). All other mice were generated in house. The experimental protocols for this study were approved by the Institutional Animal Care and Use Committees at the Ohio State University (mouse studies) and the University of Mississippi Medical Center (rat studies) and were carried out according to both the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and the Guidelines of the Animal Welfare Act. All animals were housed at 22°C (12-h light-dark cycle) with free access to food and water.
Cell culture.
Human aortic vascular smooth muscle cells (HVSMCs) were purchased from ATCC (Manassas, VA). IKK2−/− and wild-type aortic smooth muscle cells (MASMCs) were prepared as previously described. (39) All cells were maintained in DMEM supplemented with 10% fetal bovine serum. Treatments were applied to cells ∼90% confluent in 60-mm dishes. Cell lysates were prepared with RIPA buffer supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (Pierce, Rockford, IL) on ice and stored at −80°C until they were used for further analysis.
Western blot analysis.
Standard techniques as previously reported (40) were used. The following primary antibodies were used: anti-phospho-myosin light chain 2 (Ser19; Cell Signaling Technology, Danvers, MA), anti-MLC (Sigma, St. Louis, MO), anti-IkBα (Cell Signaling Technology), anti-β-actin (Sigma), anti-IKK2 (Sigma), anti-Zip kinase (Abcam, Cambridge, MA), anti-integrin linked ILK (Abcam), anti-NF-κB essential modulator (anti-NEMO; Cayman Chemicals, Ann Arbor, MI), anti-phospho-IkBα (Ser32/36; Cell Signaling Technology), anti-MYPT1 (BD Transduction Laboratories, San Jose, CA), anti-phospho-MYPT1 (Thr696; Upstate, Billerica, MA), anti-RhoA (BD Transduction Laboratories, San Jose, CA), and anti-phospho-IKKalpha/beta (Ser176/Ser180; Cell Signaling Technology). Signals were detected by chemiluminescence and analyzed by densitometry.
Vascular reactivity.
Control and NEMO binding (NBD) peptides were obtained from Imgenex (Littleton, CO). All other materials were purchased from Sigma. Reactivity of rat or mouse aortic rings was assessed as previously described (19, 40). Briefly, animals were anesthetized with pentobarbital sodium (intraperitoneal injection, 200 mg/kg) or CO2, and the thoracic aorta was quickly removed and cleaned in physiological salt solution (PSS) containing the following (mM): 130 NaCl, 14.9 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.18 MgSO4·7H2O, 1.56 CaCl2·2H2O, 0.026 EDTA, and 5.5 glucose. The aorta was cut into 2-mm rings, and the endothelium was mechanically removed by gently rubbing the intimal surface with a stainless steel wire. The aortic rings were then mounted in a muscle bath containing PSS at 37°C and bubbled with 95% O2-5% CO2. Isometric force generation was recorded with a Multi Myograph System (Danish Myo Technology). A resting tension of 30 mN (rats) or 4 mN (mice) was imposed on each ring, and the rings were allowed to equilibrate for 1 h. Arterial integrity was assessed first by stimulation of vessels with 80 mM KCl. Endothelium integrity was assessed by measuring the dilatory response to ACh (10 μM) in phenylephrine-contracted vessels (3 μM). The failure of ACh to relax denuded aortic rings was considered proof of endothelium disruption. The numbers of animals were reported in all experiments. At least two rings/animal were analyzed, and the averages were used for statistical analysis.
In vitro phosphorylation.
The assays were established based on HTScan kinase assay kit (Cell Signaling Technology). Precipitated IKK2 in the kit was incubated with 0.75 μM MLC (catalog no. 476125; Calbiochem) or 0.75 μM IκBα (Biomol) at 37°C in a total of 50 μl buffer containing 60 mM HEPES-NaOH (pH 7.5), 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, and 10 μM ATP. MLCK phosphorylated MLC in a total of 50 μl buffer containing 60 mM HEPES-NaOH (pH 7.5), 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, 10 μM ATP, 0.1 mM CaCl2, and 1 μM calmodulin. Reaction was stopped by adding 50 μl SDS-PAGE sample buffer and boiling for 5 min.
RhoA activation assay.
GTP bound RhoA in cell lines were measured with the G-LISA Small G protein Activation Assays Kit (Cytoskeleton) according to the manufacturer's instruction.
Statistical analysis.
All data are expressed as means ± SD unless otherwise mentioned. Statistical tests were performed using one-way ANOVA followed by Mann-Whitney test or unpaired t-test using GraphPad Prism (version 4.1.2). The significance level was set at P < 0.05.
RESULTS
TNF-α downregulates the MLCK activity of IKK2.
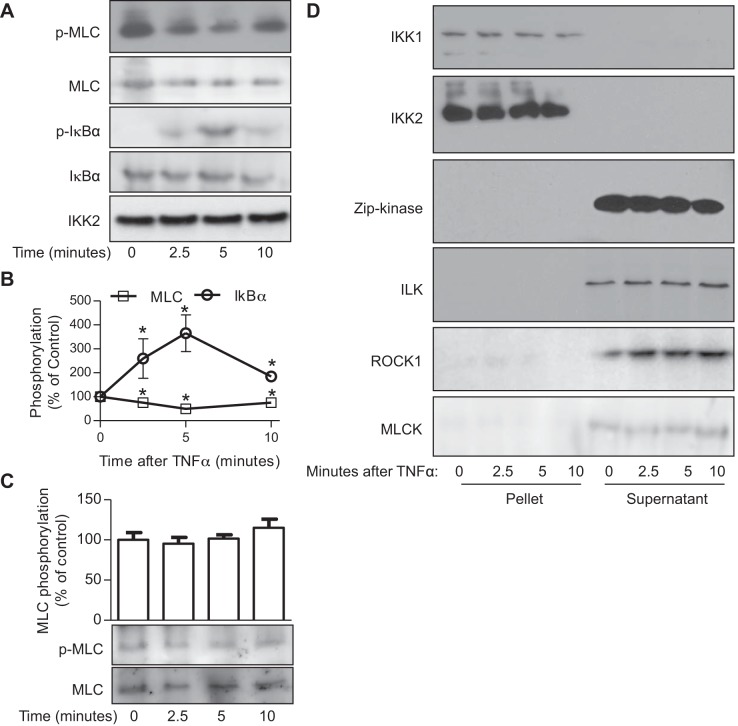
TNF-α is the prototypical regulator of IKK2 activation/phosphorylation, and we recently demonstrated that IKK2 is a constitutive MLCK. To assess if TNF-α regulates the MLCK activity of IKK2, IKK2 was immunoprecipitated from vehicle- or TNF-α-treated HVSMCs and subjected to in vitro kinase assay. Figure 1, A and B, reveals that in contrast to its potent proactive effect on the IKK activity of IKK2, TNF-α treatment unexpectedly decreased the MLCK activity of IKK2. In addition to the classic MLCK, Rho kinase, ILK, and Zip kinase have been shown to phosphorylate MLC in vitro. Figure 1D reveals that TNF-α treatment did not induce any detectable interaction between IKK complex and these MLCKs, suggesting that they are unlikely to mediate the effect of TNF-α on the MLCK activity of the complex precipitated by anti-IKK2.
Fig. 1.
Tumor necrosis factor-α (TNF-α) downregulates the myosin light chain kinase (MLCK) activity of inhibitor κB kinase 2 (IKK2). A and B: human vascular smooth muscle cells (HVSMCs) were treated with TNF-α (10 ng/ml) for the indicated time. IKK2 was precipitated from the cell lysates with anti-IKK2 antibody and used to phosphorylate MLC and IκBα in vitro. Phospho-MLC, phospho-IκBα, total IκBα, and IKK2 in products were analyzed by Western blot. Total MLC was stained with Ponseau S solution. The representative images (A) and the summary (B) of 6 independent experiments are presented. *P < 0.05 vs. 0, one-way ANOVA. C: HVSMCs were treated with TNF-α (10 ng/ml) for the indicated time, and the MLC phosphorylation levels were determined by Western blot. One representative result and the summary of 8 independent experiments are presented. D: HVSMCs were treated with TNF-α (10 ng/ml) for the indicated time, and immunoprecipitation with anti-IKK2 was performed on cell lysates. Both the pellets and supernatants were subjected to Western blot with the indicated antibody.
Dual regulation of MLC phosphorylation by TNF-α.
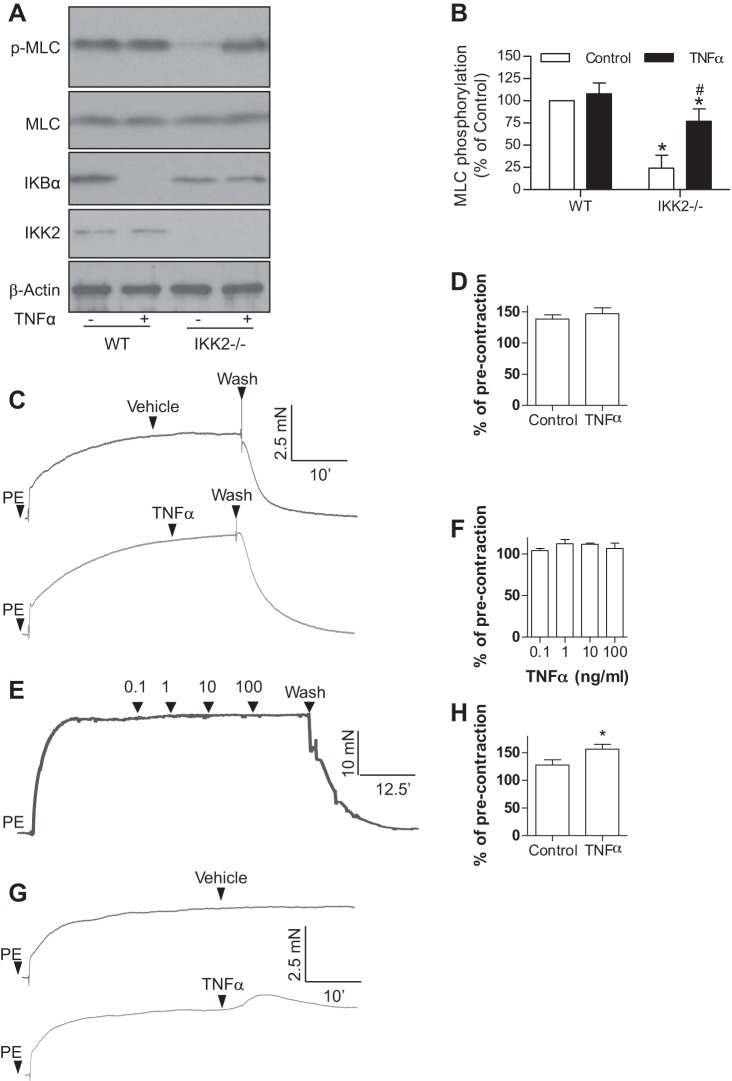
To test if TNF-α regulates cellular MLC phosphorylation levels through its effect on the MLCK activity of IKK2, HVSMCs were treated with TNF-α and subjected to MLC phosphorylation assay. Figure 1C reveals that in contrast to its inhibitory effect on the MLCK activity of IKK2 in vitro, TNF-α treatment did not significantly change the MLC phosphorylation level of HVSMCs. Consistently, Fig. 2, A and B, reveals that TNF-α treatment did not alter the MLC phosphorylation level of wild-type MASMCs. Notably, TNF-α treatment significantly increased the MLC phosphorylation level of IKK2-deficient MASMCs (Fig. 2, A and B), indicating that TNF-α may increase MLC phosphorylation through IKK2-independent pathways. Along with results showing that TNF-α treatment did not change MLC phosphorylation levels in wild-type cells (Figs. 1C and 2, A and B) but decreased the MLCK activity of IKK2 (Fig. 1, A and B), these data further suggest a dual regulation of TNF-α on cellular MLC phosphorylation levels: it reduces MLC phosphorylation through an IKK2-dependent mechanism and increases MLC phosphorylation through other signaling pathway(s).
Fig. 2.
Dual regulation of TNF-α on MLC phosphorylation. A and B: wild-type (WT) and IKK2−/− mouse aortic smooth muscle cells were treated with TNF-α (10 ng/ml) for 5 min, and MLC phosphorylation and IκBα expression were analyzed by Western blot; n = 3. *P < 0.05 vs. WT control; #P < 0.05 vs. IKK2−/− control, two-way ANOVA. C and D: aortic rings were prepared from C57Bl/6j mice and mounted onto myograph. After precontraction by phenylephrine (PE; 1 μM), TNF-α (10 ng/ml) was added. One representative recording (C) and the summary (D) of 4 independent experiments are presented. E and F: endothelium-denuded rat aortic rings were contracted by PE (1 μM), and then the indicated concentration of TNF-α was added in a cumulative manner. A representative recording (E) and the summary (F) from 3 independent experiments is present. G and H: aortic rings were prepared from smooth muscle specific IKK2 knockout (SM22-CreIKK2flox/flox) mice and mounted onto myograph. After precontraction by PE (1 μM), TNF-α (10 ng/ml) was added. One representative recording (C) and the summary (D) of 4 independent experiments are presented.
MLC phosphorylation is central in vascular contractile responses. To test if the dual regulation of MLC phosphorylation by TNF-α is physiologically important, we assessed the effect of TNF-α treatment on vasoconstrictions. Consistent with above MLC phosphorylation assays, Fig. 2C shows that TNF-α treatment did not relax phenylephrine-contracted mouse aortic rings. The lack of a vasodilator action of TNF-α was confirmed in phenylephrine-contracted rat aortic rings (Fig. 2D).
TNF-α increases MLC phosphorylation through activation of the RhoA/Rho-kinase pathway.
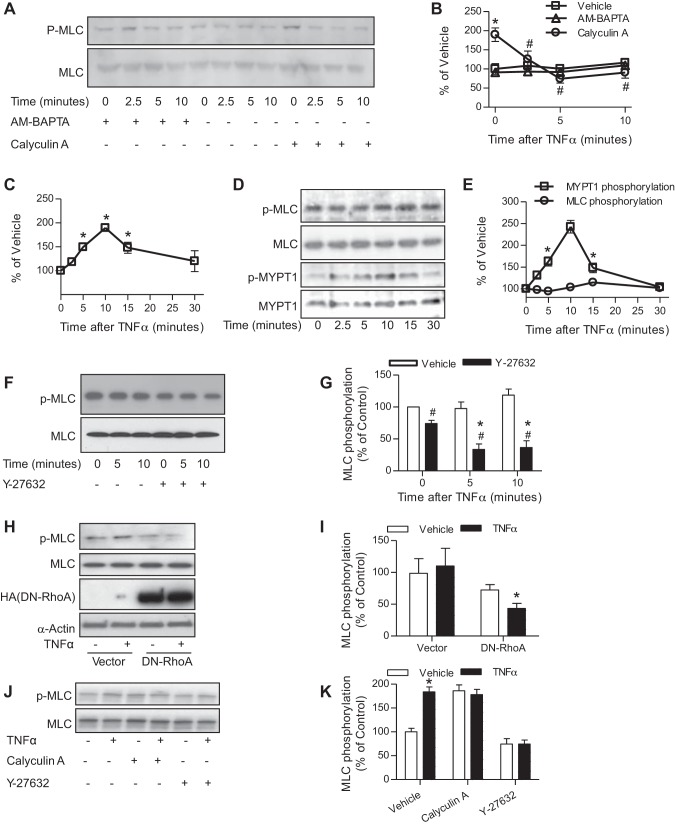
Given that TNF-α treatment did not have a net effect on MLC phosphorylation (Fig. 1C and vehicle controls in Fig. 3, A and B) but downregulated the MLCK activity of IKK2 (Fig. 1A), another signaling pathway that can increase MLC phosphorylation may be simultaneously activated by TNF-α treatment and consequently antagonizes the downregulation of the MLCK activity of IKK2. Calcium-dependent activation of the classic MLCK and RhoA/Rho-kinase-dependent inhibition of MLCP are two primary mechanisms that upregulate MLC phosphorylation. To differentiate their role in antagonizing TNF-α treatment-induced downregulation of the MLCK activity of IKK2, we inhibited the RhoA/Rho-kinase/MLCP pathway with an MLCP inhibitor, calyculin A, or blocked the classic MLCK pathway with an intracellular calcium chelator (thus an inhibitor of the classic MLCK), AM-BAPTA. Figure 3, A and B, shows that TNF-α treatment decreased MLC phosphorylation in the presence of calyculin A but not AM-BAPTA, suggesting that in addition to a IKK2-dependent mechanism TNF-α treatment may regulate MLC phosphorylation through the MLCP-dependent signaling pathway. TNF-α has been shown to activate the RhoA/Rho-kinase pathway in gastric smooth muscle cells (32). Consistently, Fig. 3, C–E, shows that TNF-α treatment time dependently increased activation of RhoA and the inhibitory phosphorylation of MLCP at MYPT1 Thr696. To confirm the counteracting between the activation of RhoA/Rho kinase and the downregulation of the MLCK activity of IKK2, we assessed the effect of TNF-α treatment on MLC phosphorylation in the presence of a Rho-kinase inhibitor Y-27632. Figure 3, F and G, shows that in the presence of Y-27632, TNF-α treatment significantly reduced MLC phosphorylation levels in HVSMCs.
Fig. 3.
TNF-α activates RhoA/Rho-kinase pathway. A and B: HVSMCs were pretreated with AM-BAPTA (10 mM) or calyculin A (0.3 μM) for 15 min, followed by treatment with TNF-α (10 ng/ml) for the indicated time and then subjected to MLC phosphorylation analysis by Western blot. The representative images (A) and summary (B) of 4 independent experiments are presented. *P < 0.05 vs. vehicle of the same time point; #P < 0.05 vs. calyculin A of 0 min, two-way ANOVA. C–E: HVSMCs were treated with TNF-α (10 ng/ml) for the indicated time. GTP-bound RhoA (C; n = 4) was assessed by the RhoA G-LISA activation assay (cytoskeleton); MLC and MYPT1 phosphorylation (D and E; n = 3) was analyzed by Western blot. *P < 0.05 vs. 0, one-way ANOVA. F and G: HVSMCs were pretreated with vehicle or Y-27632 (10 μM) for 30 min and followed by treatment with TNF-α (10 ng/ml) for the indicated time. MLC phosphorylation was then analyzed by Western blot; n = 3. *P < 0.05 vs. 0; #P < 0.05 vs. vehicle, two-way ANOVA. H and I: HVSMCs were transfected with vector or plasmid that expresses HA-tagged dominant negative RhoA (DN-RhoA) and treated with TNF-α (10 ng/ml) for 5 min. MLC phosphorylation was then analyzed by Western blot; n = 3. *P < 0.05 vs. vehicle, two-way ANOVA. J and K: vascular smooth muscle cells were isolated from SM22-CreIKK2flox/flox mice and treated with TNF-α (10 ng/ml) for 5 min in the presence of calyculin A (0.3 μM) or Y-27632 (10 μM); n = 3. *P < 0.05 vs. vehicle, two-way ANOVA.
NEMO is necessary for TNF-α-induced downregulation of the MLCK activity of IKK2.
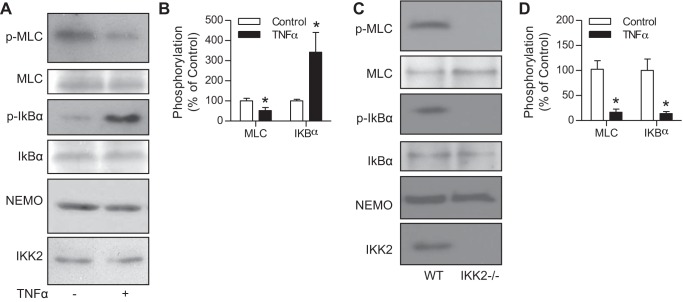
NEMO is essential for TNF-α to upregulate the IKK activity of IKK2. To test the role of NEMO in TNF-α-induced downregulation of the MLCK activity of IKK2, we used anti-NEMO to precipitate IKK complex and analyzed their MLCK and IKK activity. Figure 4, A and B, shows that the NEMO-containing complex had a marked MLCK activity, which was downregulated by TNF-α treatment. Given the essential role of NEMO in the regulation of the IKK activity of IKK2, this result somehow supports that NEMO may also play a role in TNF-α-induced downregulation of the MLCK activity of IKK2. To verify that IKK2 is responsible for this MLCK activity of IKK complex, we compared the MLCK activity precipitated by anti-NEMO from wild-type and IKK2−/− MASMCs. Supporting our concept, Fig. 4, C and D, demonstrates that anti-NEMO precipitated a marked MLCK activity in wild-type but not IKK2−/− MASMCs.
Fig. 4.
The MLCK activity of IKK2 is coprecipitated with NF-κB essential modulator (NEMO). A and B: HVSMCs were treated with TNF-α (10 ng//ml) for 5 min. IKK complex was immunoprecipitated with anti-NEMO and subjected to in vitro phosphorylation reaction. The products were then analyzed by Western blot. The representative result (A) and summary (B) of 3 independent experiments are presented. *P < 0.05 vs. control, one-way ANOVA. C and D: cell lysates were prepared from wild-type and IKK−/− mouse aortic smooth muscle cells. IKK complex was immunoprecipitated with anti-NEMO and subjected to in vitro phosphorylation reaction. The products were then analyzed by Western blot. The representative result (C) and summary (D) of 3 independent experiments are presented. *P < 0.05 vs. control, one-way ANOVA.
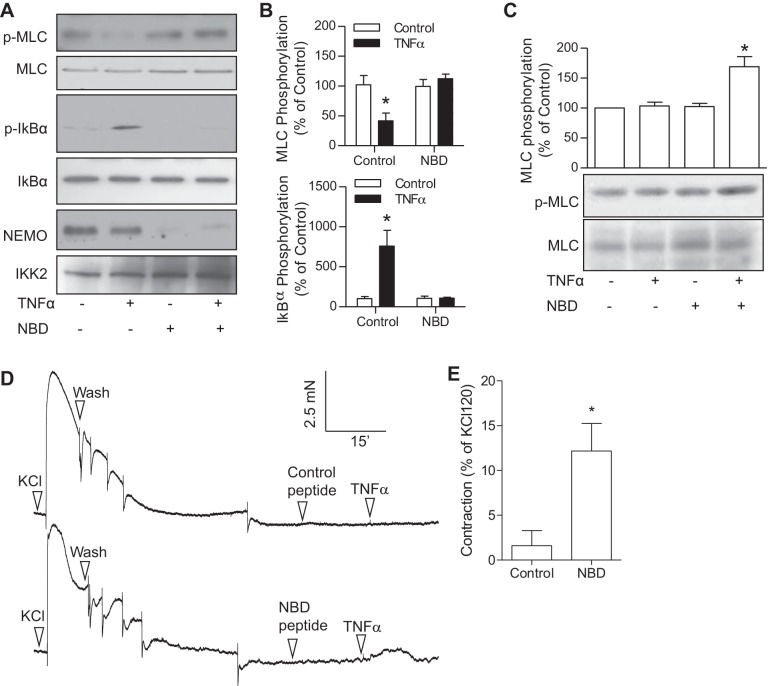
To further document the role of NEMO in TNF-α-induced downregulation of the MLCK activity of IKK2, we used a NBD to disrupt the NEMO-containing complex. Supporting the proposed role of NEMO and consistent with the dual regulation of TNF-α on MLC phosphorylation, TNF-α treatment significantly increased MLC phosphorylation in HVSMCs in the presence of NBD but not control peptide (Fig. 5C). Consistently, NBD almost abolished the effect of TNF-α on the MLCK activity of IKK2 (Fig. 5, A and B). To test the potential (patho)physiological role of the dual regulation of TNF-α on MLC phosphorylation, we investigated the effect of TNF-α on vasoconstriction in the presence of NBD or vehicle. Figure 5, D and E, shows that TNF-α induced aortic contraction in the presence of NBD but not control peptide.
Fig. 5.
NEMO mediates TNF-α-induced downregulation of the MLCK activity of IKK2. A–C: HVSMCs were pretreated with NBD (a cell-permeable cytoplasmic transduction peptide YGRRARRRARR fused to the NEMO binding domain peptide TALDWSWLQTE; Calbiochem) or control peptide [a cell-permeable Antennapedia-NBD mutated (Trp739 and Trp741 replaced with Ala) fusion peptide analog of NEMO-binding domain binding peptide] for 15 min and then stimulated with TNF-α (10 ng/ml) for 5 min. IKK complex was immunoprecipitated from cell lysates with anti-IKK2 and used to perform in vitro phosphorylation reaction. The products were then analyzed by Western blot. The representative result (A) and summary (B) of 5 independent experiments are presented. *P < 0.05 vs. control, one-way ANOVA. The cellular MLC phopshorylation levels (C) were also analyzed by Western blot, and the representative image and summary of 3 independent experiments are presented. *P < 0.05 vs. control peptide, one-way ANOVA. D and E: endothelium-denuded aortic rings from C57Bl/6J mice were mounted to myograph and pretreated with NBD or control peptide for 15 min and then stimulated with TNF-α (10 ng/ml). The maximal contraction was expressed as percentage of contraction induced by KCl (120 mM); n = 3. *P < 0.05 vs. control peptide; Student's t-test.
DISCUSSION
We previously demonstrated that IKK2 has a constitutive MLCK activity (39). In the present study, we show that the best known regulator of IKK2, TNF-α, has dual regulation of MLC phosphorylation in vascular smooth muscle cells. It decreases MLC phosphorylation by downregulating IKK2 MLCK activity and simultaneously increases MLC phosphorylation by activating the RhoA/Rho-kinase pathway.
TNF-α is one of the pivotal mediators in inflammation, and inflammation is clearly implicated in the etiology of vascular dysfunction and abnormal blood pressure regulation. For example, severe inflammation is the cause of septic shock, and chronic inflammation is associated with elevated blood pressure (7). However, it is still unclear why inflammation in septic shock lowers blood pressure, whereas in other disorders, such as in some forms of chronic hypertension, it raises blood pressure. The dual regulation of MLC phosphorylation by TNF-α clearly reflects this complexity of the effects of inflammation on vascular function and blood pressure and provides a potential mechanism to explain the context-dependent differential effects of inflammation on vascular function and blood pressure: context determines the balance between these counteracting effects of inflammation.
The present data show that TNF-α does not significantly change MLC phosphorylation levels (Figs. 1C and 2A) and vascular tone (Fig. 2, C–F). In contrast, studies have shown that TNF-α alters vascular contractile responses (8, 23, 30, 31, 35). This ostensible discrepancy may be due to the difference in used blood vessels. Indeed, these reported effects of TNF-α are inconsistent, varying from increase in contraction to decrease in contraction, and thus already suggest a dependency on the type of blood vessels. In addition, the ostensible discrepancy may also result from the difference of animal models. For example, TNF-α increases blood pressure in pregnant rats but not control rats (1), indicating that the effect of TNF-α on the same blood vessels may also be context dependent. Our results showing that TNF-α downregulates the MLCK activity of IKK2 are consistent with our previous study demonstrating that the MLCK activity of IKK2 is constitutive and primarily contributes to the basal MLC phosphorylation (39). Notably, TNF-α has been shown to upregulate the activity of IKK2 in terms of phosphorylating the majority of substrates, including IκB, insulin substrate 1 (6, 11), 14-3-3β (9), BCL10 (37), and FOXO3a (12). To our knowledge, our study is the first one showing that TNF-α can differentially regulate IKK2 activities. Our results are also consistent with previous studies suggesting a similar differential regulation of IKK2 activities by FcεRI stimulation. Phosphorylation at Ser177 and Ser181 in the activation loop of IKK2 is believed to be important for its kinase activity, which causes conformational changes, resulting in kinase activation (4, 22). FcεRI stimulation markedly increases SNAP-23 phosphorylation by IKK2 (33) but does not significantly induce IKK2 phosphorylation in the activation loop (24).
NEMO was identified as the regulatory subunit of IKK complex essential for TNF-α upregulating the IKK activity of IKK2. The present study suggests that although the MLCK and IKK activities of IKK2 are differentially regulated by TNF-α, NEMO is necessary for both regulations, because anti-NEMO precipitated the MLCK activity and NBD peptide abolished TNF-α-induced downregulation of IKK2 MLCK activity. The latter was further confirmed at the level of vasoconstriction. Interestingly, our data also show that while the deficiency of IKK2 significantly reduces cellular MLC phosphorylation levels (Fig. 2, A and B), the disruption of interaction between IKK2 and NEMO does not alter the cellular MLC phosphorylation levels, suggesting that it is still possible that the constitutive MLCK activity of IKK2 could be independent of the NEMO-containing complex. By far, the mechanism by which NEMO mediates the downregulation of IKK2 MLCK activity by TNF-α remains unknown. Since studies have shown that the activation of IKK2 IKK activity by TNF-α requires NEMO binding to ubiquitinated RIP1 (5), it is important to determine whether the same mechanism underlies TNF-α-induced downregulation of IKK2 MLCK activity in the future.
In the present study, our data reveal that in addition to the downregulation of IKK2 MLCK activity, TNF-α upregulates MLC phosphorylation in vascular smooth muscle cells through activation of RhoA/Rho-kinase pathway, which counteracts the former's effect and leads to no apparent alteration in MLC phosphorylation level upon TNF-α treatment. The activation of RhoA/Rho-kinase pathway by TNF-α in vascular smooth muscle cells is consistent with previous studies showing that TNF-α activates RhoA/Rho-kinase pathway in other cells (15, 21, 27, 36). However, given the recent focused scientific effort on the roles of inflammation and RhoA/Rho-kinase in vascular function and blood pressure regulation, it is surprising that our study is the first one, to our knowledge, showing that TNF-α activates RhoA/Rho-kinase in vascular smooth muscle cells. This may be due to the existence of the dual regulation of MLC phosphorylation by TNF-α in vascular smooth muscle cells. Since both RhoA/Rho-kinase (29) and TNF-α (8, 23, 30, 31, 35) are implicated in the genesis of vascular dysfunction, it is also likely that a TNF-α/RhoA/Rho-kinase pathway in vascular smooth muscle cells may be pathophysiologically important.
Our study, although providing important new findings, has some limitations. These include the fact that we have not definitively delineated how the MLCK activity of IKK2 is downregulated by TNF-α. This will require additional experiments targeting other adaptor proteins of the IKK complex. In addition, we did not directly investigate the role of the proposed mechanism in the context of cardiovascular diseases. Given the availability of diverse genetic mouse models that target the TNF-α signaling pathway, such as those deficient in either IKK2 or RhoA/Rho-kinase pathway, future studies should be conducted to confirm the role of TNF-α in the pathogenesis of cardiovascular diseases and determine the respective role of those two antagonizing pathways in mediating this effect of TNF-α on cardiovascular diseases. In summary, our data provide evidence that TNF-α has a dual regulation on MLC phosphorylation through a decrease in the MLCK activity of IKK2 and an increase in RhoA/Rho-kinase activation.
GRANTS
This work was supported by the National Natural Science Foundation of China Grant 81270342 (to Z. Ying), American Heart Association Grants 11POST7640030 and 13SDG17070131 (to Z. Ying), National Institute of Environmental Health Sciences Grant R01ES024516 (to Z. Ying), and National Heart, Lung and Blood Institute Grant PO1-HL-51971 (to J. E. Hall).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C., L.M., J.E.H., X.L., and Z.Y. conception and design of research; M.C., L.M., and Z.Y. performed experiments; M.C., L.M., and Z.Y. analyzed data; M.C., J.E.H., and Z.Y. interpreted results of experiments; M.C. and Z.Y. prepared figures; M.C., X.L., and Z.Y. drafted manuscript; M.C., L.M., J.E.H., X.L., and Z.Y. approved final version of manuscript; J.E.H., X.L., and Z.Y. edited and revised manuscript.
REFERENCES
- 1.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens 15: 170–175, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J Muscle Res Cell Motil 19: 839–854, 1998. [DOI] [PubMed] [Google Scholar]
- 4.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388: 548–554, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell 22: 245–257, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 277: 48115–48121, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Ghanem FA, Movahed A. Inflammation in high blood pressure: a clinician perspective. J Am Soc Hypertens 1: 113–119, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Glembot TM, Britt LD, Hill MA. Endotoxin interacts with tumor necrosis factor-alpha to induce vasodilation of isolated rat skeletal muscle arterioles. Shock 5: 251–257, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Gringhuis S, Garcia-Vallejo J, Van Dijk W. Convergent action of IkB kinase beta and protein kinase Ctheta modulate mRNA stability through phosphorylation of 14-3-3beta complexed with tristetraprolin. Mol Biol Cell 25: 6454–6463, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Usui I, Ishizuka K, Kanatani Y, Hiratani K, Iwata M, Bukhari A, Haruta T, Sasaoka T, Kobayashi M. Interleukin-1alpha inhibits insulin signaling with phosphorylating insulin receptor substrate-1 on serine residues in 3T3–L1 adipocytes. Mol Endocrinol 20: 114–124, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117: 225–237, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Mahavadi S, Sriwai W, Hu W, Murthy KS. Gi-coupled receptors mediate phosphorylation of CPI-17 and MLC20 via preferential activation of the PI3K/ILK pathway. Biochem J 396: 193–200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter I, Cobban HJ, Vandenabeele P, MacEwan DJ, Nixon GF. Tumor necrosis factor-alpha-induced activation of RhoA in airway smooth muscle cells: role in the Ca2+ sensitization of myosin light chain20 phosphorylation. Mol Pharmacol 63: 714–721, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Hunter I, Nixon GF. Spatial compartmentalization of tumor necrosis factor (TNF) receptor 1-dependent signaling pathways in human airway smooth muscle cells. Lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kappaB and MAPK pathways. J Biol Chem 281: 34705–34715, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakiashvili E, Speight P, Waheed F, Seth R, Lodyga M, Tanimura S, Kohno M, Rotstein OD, Kapus A, Szaszi K. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem 284: 11454–11466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T, Nomura F, Hoshino K, Copeland NG, Gilbert DJ, Jenkins NA, Akira S. Death-associated protein kinase 2 is a new calcium/calmodulin-dependent protein kinase that signals apoptosis through its catalytic activity. Oncogene 18: 3471–3480, 1999. [DOI] [PubMed] [Google Scholar]
- 18.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46: 82–86, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Lash JM, Bohlen HG. Perivascular and tissue Po2 in contracting rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 252: H1192–H1202, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Wang P, Cao M, Li M, Wang F. Protective role of oligomycin against intestinal epithelial barrier dysfunction caused by IFN-gamma and TNF-alpha. Cell Physiol Biochem 29: 799–808, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Matoba K, Kawanami D, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K. Rho-kinase mediates TNF-alpha-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem Biophys Res Commun 402: 725–730, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 278: 860–866, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Yoshida H, Arakawa N, Saitoh S, Satoh M, Hiramori K. Effects of tumor necrosis factor-alpha on basal and stimulated endothelium-dependent vasomotion in human resistance vessel. J Cardiovasc Pharmacol 36: 487–492, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Peng Y, Power MR, Li B, Lin TJ. Inhibition of IKK down-regulates antigen + IgE-induced TNF production by mast cells: a role for the IKK-IkappaB-NF-kappaB pathway in IgE-dependent mast cell activation. J Leukoc Biol 77: 975–983, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun 306: 244–249, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-α-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 280: L1168–L1178, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Ramseyer VD, Hong NJ, Garvin JL. Tumor necrosis factor alpha decreases nitric oxide synthase type 3 expression primarily via Rho/Rho kinase in the thick ascending limb. Hypertension 59: 1145–1150, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandoo A, Panoulas VF, Toms TE, Smith JP, Stavropoulos-Kalinoglou A, Metsios GS, Gasparyan AY, Carroll D, Veldhuijzen van Zanten JJ, Kitas GD. Anti-TNFalpha therapy may lead to blood pressure reductions through improved endothelium-dependent microvascular function in patients with rheumatoid arthritis. J Hum Hypertens 25: 699–702, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Satoh K, Fukumoto Y, Shimokawa H. Rho-kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart Circ Physiol 301: H287–H296, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Scherer EQ, Yang J, Canis M, Reimann K, Ivanov K, Diehl CD, Backx PH, Wier WG, Strieth S, Wangemann P, Voigtlaender-Bolz J, Lidington D, Bolz SS. Tumor necrosis factor-alpha enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke 41: 2618–2624, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahid M, Francis J, Majid DS. Tumor necrosis factor-α induces renal vasoconstriction as well as natriuresis in mice. Am J Physiol Renal Physiol 295: F1836–F1844, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sriwai W, Zhou H, Murthy KS. G(q)-dependent signalling by the lysophosphatidic acid receptor LPA(3) in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by Rho kinase and cAMP-independent PKA. Biochem J 411: 543–551, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki K, Verma IM. Phosphorylation of SNAP-23 by IkappaB kinase 2 regulates mast cell degranulation. Cell 134: 485–495, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran LT, MacLeod KM, McNeill JH. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol Cell Biochem 330: 219–228, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Wagner EM. TNF-α induced bronchial vasoconstriction. Am J Physiol Heart Circ Physiol 279: H946–H951, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Waheed F, Dan Q, Amoozadeh Y, Zhang Y, Tanimura S, Speight P, Kapus A, Szaszi K. Central role of the exchange factor GEF-H1 in TNF-alpha-induced sequential activation of Rac, ADAM17/TACE, and RhoA in tubular epithelial cells. Mol Biol Cell 24: 1068–1082, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wegener E, Oeckinghaus A, Papadopoulou N, Lavitas L, Schmidt-Supprian M, Ferch U, Mak TW, Ruland J, Heissmeyer V, Krappmann D. Essential role for IkappaB kinase beta in remodeling Carma1-Bcl10-Malt1 complexes upon T cell activation. Mol Cell 23: 13–23, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Guo R, Chen P, Wang Q, Cunningham PN. TNF induces caspase-dependent inflammation in renal endothelial cells through a Rho- and myosin light chain kinase-dependent mechanism. Am J Physiol Renal Physiol 297: F316–F326, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Ying Z, do Carmo JM, Xiang L, da Silva AA, Chen M, Ryan MJ, Ostrowski MC, Rajagopalan S, Hall JE. Inhibitor kappaB kinase 2 is a myosin light chain kinase in vascular smooth muscle. Circ Res 113: 562–570, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ying Z, Jin L, Palmer T, Webb RC. Angiotensin II up-regulates the leukemia-associated Rho guanine nucleotide exchange factor (RhoGEF), a regulator of G protein signaling domain-containing RhoGEF, in vascular smooth muscle cells. Mol Pharmacol 69: 932–940, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123: 163–172, 2002. [DOI] [PubMed] [Google Scholar]