Abstract
Despite considerable progress in identifying health risks to crewmembers related to exposure to galactic/cosmic rays and solar particle events (SPE) during space travel, its long-term effects on the pulmonary system are unknown. We used a murine risk projection model to investigate the impact of exposure to space-relevant radiation (SR) on the lung. C3H mice were exposed to 137Cs gamma rays, protons (acute, low-dose exposure mimicking the 1972 SPE), 600 MeV/u 56Fe ions, or 350 MeV/u 28Si ions at the NASA Space Radiation Laboratory at Brookhaven National Laboratory. Animals were irradiated at the age of 2.5 mo and evaluated 23.5 mo postirradiation, at 26 mo of age. Compared with age-matched nonirradiated mice, SR exposures led to significant air space enlargement and dose-dependent decreased systemic oxygenation levels. These were associated with late mild lung inflammation and prominent cellular injury, with significant oxidative stress and apoptosis (caspase-3 activation) in the lung parenchyma. SR, especially high-energy 56Fe or 28Si ions markedly decreased sphingosine-1-phosphate levels and Akt- and p38 MAPK phosphorylation, depleted anti-senescence sirtuin-1 and increased biochemical markers of autophagy. Exposure to SR caused dose-dependent, pronounced late lung pathological sequelae consistent with alveolar simplification and cellular signaling of increased injury and decreased repair. The associated systemic hypoxemia suggested that this previously uncharacterized space radiation-associated lung injury was functionally significant, indicating that further studies are needed to define the risk and to develop appropriate lung-protective countermeasures for manned deep space missions.
Keywords: 56Fe, 28Si, protons, gamma radiation, emphysema, oxidative stress, senescence, lung injury, inflammation, hypoxemia
space travel has been associated with increased risk of developing cancer and central nervous system damage attributed to unique exposures to galactic cosmic rays (GCR) and solar particle events (SPE) (22, 57). Despite considerable progress to identify health risks to crewmembers during space travel, particularly from exposure to space radiation (SR), effects on the pulmonary system remain unexplored.
Although the incidence and mortality due to cancers such as leukemia and solid tumors are known to be increased in astronauts (15), nontumor death was recognized recently as the most common cause of mortality in this group (56). This finding was similar to the observed increase in noncancer, cardiovascular mortality in A-bomb survivors (27, 40) and was replicated in mice irradiated with 56Fe ions, a particularly damaging component of GCR (54). Given the importance of the pulmonary system to cardiovascular health as well as the impact of endothelial dysfunction to lung pathophysiology, we investigated whether SR exposure is associated with significant chronic pulmonary pathology.
In space, radiation risk stems from exposure to GCR and SPE, which contain high-energy nuclei (HZE) with an electric charge higher than +2 (such as 56Fe and 28Si ions) and high- and medium-energy protons (H+). Little is known about the lung's response to GCR and SPE exposures whose high linear energy transfer (LET) components penetrate deeply into exposed tissues and produce secondary radiations. The few previous reports of high-LET radiation effect on the lung have focused on potential mutagenic effects of radiation-induced oxidative stress or DNA damage on lung epithelial cells (32). In contrast, there are extensive studies on the effect of high-dose terrestrial, low-LET radiation such as gamma and X-rays on various tissues, including the lung. Given their distinct physical properties, the pathobiology of lung damage induced by low-dose space-relevant radiation exposure cannot be extrapolated from that induced by high-dose terrestrial radiation.
As part of a NASA-sponsored study to investigate space radiation-induced carcinogenesis, C3H/HeNCrl mice were exposed to 137Cs gamma rays, protons (acute, low-dose exposure mimicking the 1972 SPE), 56Fe ions (600 MeV/u), or 28Si ions (350 MeV/u) at the NASA Space Radiation Laboratory at Brookhaven National Laboratory and then followed for over 2 years prior to assessment. Of note, although this mouse strain is relatively fibrosis resistant, compared with C57L/J (31) for example, C3H/He mice have been routinely used in studies evaluating radiation effects on the lungs, which typically consist of alveolitis/pneumonitis (36). Since no previous reports exist of long-term pulmonary effects of space radiation, we are reporting here for the first time findings in a mouse model of total body exposure to relatively low doses of protons or of HZE (56Fe, 28Si ions), collectively referred to as SR, or similar doses of gamma radiation (found both in space and terrestrial). The doses chosen for this study reflect the relevant dose ranges of space radiations that astronauts are likely to be exposed to and enable detections of potential dose thresholds and/or dose-rate effects (21). Although exposure of the lung parenchyma to high-dose terrestrial radiation in the context of radiotherapy or following a radiological accident is typically associated with the development of fibrosis (42), exposure to low-dose SR in this fibrosis-resistant yet radiosensitive mouse strain resulted in previously unappreciated radiation-induced pathology, characterized by enlargement of air spaces and biochemical and cellular changes indicative of chronic lung injury with decreased repair. This study identifies for the first time Space Radiation-Associated Lung Injury (SPRALI) as a new potential risk associated with space flight or similar exposures on Earth.
MATERIALS AND METHODS
Animals and irradiation procedures.
Eight- to 10-wk-old male C3H/HeNCrl mice, an inbred mouse strain selected for its susceptibility to radiation-induced malignancies (66, 67), were obtained from Charles River (Wilmington, MA) and shipped to Brookhaven National Laboratory (BNL) for irradiation. Animals were acclimated for at least 5 days prior to being irradiated with 137Cs gamma rays at doses of 1, 2, or 3 Gy (terrestrial or space radiation) or with −50–2,000 MeV/nucleon protons (1, 2, or 3 Gy); 600 MeV/nucleon 56Fe ions (0.1 to 1 Gy); or 350 MeV/nucleon of 28Si ions (0.1 to 1 Gy). The irradiation was performed at the NASA Space Radiation Laboratory at BNL. Because of the large numbers of animals, each dose group consisted of two cohorts irradiated ∼12 mo apart. The doses chosen for this study reflect relevant exposure ranges of space radiations for astronauts (21) and were decided based on discussions with NASA. Details of the irradiation procedure have been described previously (66). Control, age-matched mice were sham-irradiated at BNL under the same conditions as the irradiated groups. Three to 5 days postirradiation, mice were shipped to the University of Texas Medical Branch (UTMB), where they were housed in the Animal Resource Center in ventilated cage racks throughout the study. The total number of animals in each respective radiation source group and dose are as follows: n = 18 control; n = 24, 20, and 3 for 1, 2, and 3 Gy gamma, respectively; n = 25, 25, and 11 at 1, 2, and 3 Gy proton, respectively; n = 27, 24, 18, and 11 at 0.1, 0.2, 0.4, and 1 Gy 56Fe, respectively; n = 13, 12, 11, and 7 at 0.1, 0.2, 0.4, and 1 Gy 28Si, respectively. All animal work was approved by the Institutional Animal Care and Use Committees at UTMB, BNL, the Children's Hospital of Philadelphia, and NASA-Ames Research Center. All animal facilities are AAALAC accredited.
Oxygen saturation measurements.
As a readout of respiratory function, the levels of systemic oxygenation were measured with a noninvasive method, using a mouse-adapted pulse-oximeter (Starr Life Sciences, Oakmont, PA) (64). Mouse irradiation was staggered, resulting in groups of mice reaching end of experimentation at variable times. Thus mice were received in three separate shipments to be evaluated in a blinded fashion for lung function after an equilibration time of 3–4 days. For this, a pulse oximeter clip was placed on the shaved neck, above the carotid artery (50). Mice were allowed to walk freely in a small chamber covered by a light blocking fabric supplied by the manufacturer. Mice were preadapted with a mock collar the day prior to evaluation. A 3-min reading of arterial oxygen saturation (SpO2) was taken from each mouse and the average of these readings was calculated, and measurements containing error codes were excluded. All mice, including age-matched sham-irradiated controls, were processed for SpO2 measurements (n > 10 mice per group) prior to assignment to separate evaluation paths such as bronchoalveolar lavage (BAL), histology, etc.
BALF analysis.
BAL was performed through a 20-gauge angiocatheter (BD Pharmingen, San Diego, CA), with the intratracheal instillation of 1 ml PBS containing anti-protease cocktail (Sigma-Aldrich, St. Louis, MO) and 5 mM EDTA, in 0.5-ml increments. An aliquot was immediately separated to measure total leukocyte cell counts [cells/ml BAL fluid (BALF)] by using a Coulter Cell and Particle Counter (Beckman Coulter, Miami, FL). The remaining BALF was centrifuged (1,200 rpm; 10 min) and the cell-free supernatant was analyzed for total protein in the BALF (BCA Protein Assay Kit; Pierce, Rockford, IL) as per manufacturer's instructions. Absorbance was read at 560 nm (MRX Microplate Reader, Dynatech Laboratories, Chantilly, VA) and protein levels (mg/ml of BALF) were calculated.
Evaluation of oxidative lung injury.
The measurement of thiobarbituric acid-reactive substances (TBARS) is a well-established method for screening and monitoring lipid peroxidation, a measure of excess oxidative stress that damages cellular lipids (4). Malondialdehyde (MDA), a product of lipid peroxidation and an indicator of oxidative stress (23), was measured in BALF by use of a commercially available kit (Cayman, Ann Arbor, MI). The results were recorded as micromoles of MDA.
Tissue harvesting and histopathological evaluation.
Since this mouse model of SR exposure was originally designed to study leukemia, the duration of the protocol included monitoring for enlarged spleens (as a sign of leukemia) until up to 800 days of age. Our lung study was performed on mice that survived to 800 days of age, or 730 days postirradiation (n = 18 control mice; 24 gamma-, 25 proton-, 53 56Fe-, and 20 28Si-irradiated mice). For histological studies, the lungs were instilled in situ with buffered formalin through a 20-gauge angiocatheter placed in the trachea. The lungs of two subgroups of mice (56Fe- and 28Si-irradiated mice) were inflated gravitationally, under 22 cmH2O pressure. After ligating the trachea to entrap the intrapulmonary fixative, lungs were excised and immersed in buffered formalin overnight and processed for conventional paraffin histology. Sections were stained with hematoxylin and eosin (H&E) and examined by light microscopy. The glass slides contained cross sections from all five lobes of the lungs. The pathological changes investigated included the presence of malignancy, interstitial pneumonitis (expansion of alveolar walls with increased inflammatory infiltrate, edema, and/or increased interstitial collagen), bronchus-associated lymphoid tissue (BALT) hyperplasia, and emphysema-like air space enlargement.
Quantitative and semiquantitative assessment of air space size was performed by two approaches.
H&E-stained sections from all lungs were evaluated by unbiased automated lung morphometry, performed as previously described (13). Mean linear intercepts (MLI) were then analyzed by frequency distribution using Metamorph (Molecular Devices, Sunnyvale, CA) macros developed by Dr. Rubin Tuder (University of Colorado). Ideally, all lungs should have been harvested in a similar manner to exclude the possibility that differences in morphometry induced by the inflation procedures (i.e., manual inflation vs. gravitational inflation) may exist. We identified two groups of mice that were exposed to similar conditions (1 Gy 56Fe) but that underwent a different inflation procedure. Automated, blinded morphometry on these groups identified minor, statistically insignificant differences between the two inflation groups (means ± SE of 41.31 ± 0.8 vs. 43.5 ± 1.2 for manual vs. gravitational inflation, respectively; P = 0.13). This led us to group mice irrespective of the inflation method used.
Semiquantitative assessment of fibrosis.
Although the mouse strain used for this study is not known to develop prominent lung fibrosis in response to radiation (36), we evaluated lungs for fibrosis. Semiquantitative evaluation of fibrosis was done histologically by determining a radiation fibrotic index (FI) on histological lung sections stained with Masson's trichrome blue (39).
Determination of apoptosis using lung tissue IHC and staining.
Active caspase-3 immunohistochemistry (IHC) was performed as previously described (13) with blinded automated image analysis of captured random images of stained lung parenchyma, by using Metamorph (13). TUNEL staining, using ApopTag Plus-Peroxidase (Millipore, Billerica, MA), was performed as described, by the suggested manufacturers' protocol (26).
Sphingolipid measurements.
Measurement of ceramides and sphingosine-1-phosphate (S1P) were performed on frozen lung tissue, using liquid chromatography tandem mass spectrometry (LC-MS/MS) and normalization by lipid phosphorus essentially as previously described (5, 6) with the following exceptions: the LC-MS/MS analysis was performed on AB Sciex 5500 QTRAP triple quadrupole ion trap mass spectrometer (AB Sciex, Foster City, CA) interfaced with Agilent 1260 LC system and using Ascentis Express C8 (75 × 2.1 mm, 2.7 μm) column for lipid separation. Standard curves for sphingoid base-1-phosphates and ceramide molecular species were constructed via the addition of increasing concentrations of the individual analytes to 40 pmol of the structural analogs of the sphingolipid classes used as the internal standards. Linearity and the correlation coefficients of the standard curves were obtained via linear regression analysis.
Western blotting.
Levels of proteins of interest were detected by Western blotting of lung tissue lysates as previously described (55). Briefly, protein lysates were prepared with RIPA buffer and resolved by SDS-PAGE. Unless otherwise stated, antibodies were from Cell Signaling Technology (Beverly, MA). The following primary antibodies were used for immunoblotting: p21 (1:500; Millipore), p16 (1:1,000; LifeSpan Biosciences, Seattle, WA), LC3B (1:20,000; Sigma-Aldrich), vinculin (1:10,000; Abcam, Cambridge, MA), phospho-Akt (1:500), Akt (1:1,000), mouse-specific SIRT1 (1:500), phosphor (Thr180/Tyr182)-p38 (1:500), total p38 (1:1,000). Respective horseradish peroxidase-conjugated secondary antibodies (1:5,000–1:10,000) were from Amersham and proteins were detected by using ECL Plus or ECL Prime.
Statistical analysis.
Results are expressed as means ± SE. Statistical differences among groups were determined by one-way ANOVA. When statistically significant differences were found (P < 0.05) comparisons vs. control were made by Dunnett's multiple-comparison test (Statview 4.0 and Prism 6, GraphPad Software). Statistical analysis of the results from the histopathological evaluation was performed by Fisher's exact test, comparing individual exposure groups (by radiation source and dose) to nonirradiated unexposed controls.
RESULTS
Hypoxemia.
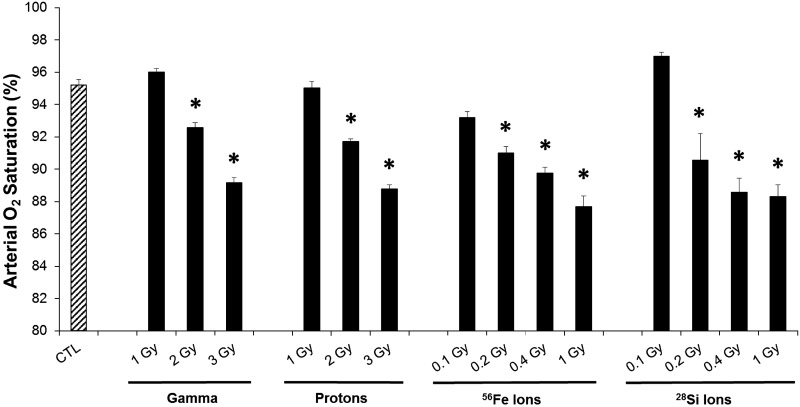
We investigated lung sequelae of total body GCR exposure in the C3H/HeNCrl mouse strain developed to study GCR-induced carcinogenesis (7), such as acute myeloid leukemia. Mice were exposed to either low-LET 137Cs gamma rays, or high-LET proton, 56Fe, 28Si, relevant to GCR and SPE (66). At the time of euthanasia, 730 days postirradiation at 800 days of age, mean SpO2 was determined prior to necropsy. Whereas in sham-irradiated control mice, SpO2 was >95%, reflecting normal oxygenation, significant decreases in oxygen saturation to ∼88% were noted in all mouse cohorts that were exposed to radiation (Fig. 1). Interestingly, the decreases in oxygen saturation were dose dependent following radiation exposures, and similar doses (1 Gy) of 56Fe ion or 28Si ion radiation led to significantly lower oxygen saturation than gamma or proton-ray exposures.
Fig. 1.
Impact of space-relevant radiation (SR) on blood oxygenation. Arterial blood oxygen saturation was measured in 26-mo-old mice, at 23.5 mo (730 days) postexposure to gamma (1, 2, 3 Gy), proton (1, 2, 3 Gy), 56Fe (0.1, 0.2, 0.4, 1 Gy), and 28Si (0.1, 0.2, 0.4, 1 Gy) radiation via a mouse-adapted pulse oximeter. Mean ± SE; median of N = 10/group. ANOVA *P < 0.05 vs. control (CTL) (Dunnett's multiple-comparison tests).
Given that irradiated mice exhibited significant hypoxemia, we next evaluated whether their lungs exhibited pathological changes that could explain this abnormal respiratory function.
Histopathology.
Light microscopic evaluation of lung sections from all irradiated cohorts and nonirradiated controls revealed several histopathological abnormalities. A notable finding was the presence of emphysema-like morphology, manifested as air space enlargement in lungs exposed to all radiation types (Fig. 2A). These changes were confirmed by an experienced pathologist, blinded to the identity of the lung sections. In the sham-irradiated control cohort, the majority (∼60%) of lungs displayed minimal, emphysema-like air space enlargement (involving <10% of the lung section), consistent with mild air space enlargement that occurs with age (10). In contrast, lungs from mice exposed to both gamma radiation or SR had markedly increased presence of severe, air space enlargement. Notably, no lung fibrosis was observed in any lung sections, as determined following Masson's trichrome staining (data not shown), which may be explained by the strain's relative resistance to developing lung fibrosis (36). In addition to air space enlargement, lung malignant tumors were observed in several animals exposed to all types of radiation, but not in the age-matched control, nonirradiated group (Table 1). Similarly, BALT hyperplasia was noted in lungs exposed to all radiation types, with the exception of 28Si. We defined these late histopathological changes associated with low levels of systemic oxygenation as SPRALI.
Fig. 2.
Long-term histopathological changes induced by SR. A: representative photomicrographs of hematoxylin and eosin (H&E)-stained lung parenchyma sections from mice irradiated with the respective sources (1 Gy each) compared with nonirradiated controls (CTL). Size bar = 50 μm. B: mean linear intercepts (MLI), measured by automated morphometry of H&E-stained lung sections. Means ± SE; N = 3–5/group. P < 0.001 1-way ANOVA (*Dunnett's multiple-comparison tests). C: histogram of cumulative frequency (%) of alveoli of indicated size (measured by MLI), showing a right shift (enlargement) in the sizes of alveoli in irradiated lungs at the indicated doses compared with control lungs (N = 3–5/group).
Table 1.
Histopathological evaluation of lung sections
| Interstitial Pneumonitis, n (%) | Tumor-Positive Lungs, n (%) | BALT Hyperplasia Positivity, n (%) | |
|---|---|---|---|
| Untreated | |||
| CTL (n = 18) | 4 (22) | 0 (0) | 0 (0) |
| Gamma | |||
| 1 Gy (n = 14) | 2 (14) | 4 (29)* | 2 (14) |
| 2 Gy (n = 10) | 3 (30) | 1 (10) | 2 (20) |
| Proton | |||
| 1 Gy (n = 15) | 3 (20) | 5 (33)* | 2 (13) |
| 2 Gy (n = 10) | 1 (10) | 3 (30)* | 2 (20) |
| 56Fe Ions | |||
| 0.1 Gy (n = 22) | 4 (18) | 3 (14) | 2 (9) |
| 0.2 Gy (n = 21) | 3 (14) | 6 (29)* | 4 (19) |
| 0.4 Gy (n = 5) | 0 (0) | 1 (20) | 0 (0) |
| 1.0 Gy (n = 5) | 1 (20) | 0 (0) | 0 (0) |
| 28Si Ions | |||
| 0.1 Gy (n = 5) | 1 (20) | 1 (20) | 0 (0) |
| 0.2 Gy (n = 5) | 1 (20) | 1 (20) | 0 (0) |
| 0.4 Gy (n = 5) | 1 (20) | 0 (0) | 0 (0) |
| 1.0 Gy (n = 5) | 0 (0) | 2 (40)* | 0 (0) |
Hematoxylin and eosin (H&E)-stained lung sections from control and irradiated animals were evaluated in a blinded fashion by a pathologist. Sections were evaluated for interstitial pneumonitis, defined as expansion of alveolar walls with increased inflammatory infiltrate, edema, and/or increased interstitial collagen. In addition, lungs were evaluated for presence of tumor nodules and bronchus-associated lympohid tissue (BALT) hyperplasia. Number of animals per group (N) ranged from 5 to 22. Significant differences (*P < 0.05) were detected by Fisher's exact test comparing individual exposure groups (by radiation source and dose) with nonirradiated unexposed controls. Significant differences (*P < 0.05) tested using Fisher's exact test comparing individual exposure groups (by radiation source and dose) to nonirradiated unexposed controls. Data reflect number (percent) of lungs that were histologically evaluated.
To further quantify the histopathology induced by space radiation, we performed automated morphometry on lung parenchyma only, as described (13), and obtained measurements of MLI of peripheral air spaces. Compared with nonirradiated mice, gamma ray-, proton-, and 28Si ion-irradiated lungs demonstrated statistically significant increases in MLI (ANOVA with post hoc Dunnett's test, P < 0.05 vs. control). Although statistically insignificant, the MLI of 56Fe ion-exposed irradiated lungs tended to be higher than those of controls (P = 0.054) (Fig. 2B). This effect was further evaluated by the cumulative frequency of alveolar size distribution, showing that SR-exposed lungs showed a right shift in the sizes of air spaces (Fig. 2C), consistent with the pathologist's scoring of lung histology.
Inflammation.
To further elucidate possible mechanisms associated with SPRALI development, we focused on cellular and tissue processes typically involved in air space enlargement in emphysema. Lung inflammation was evaluated by measuring protein levels, total inflammatory cells, and neutrophil (polymorphonuclear cells) predominance in the BALF.
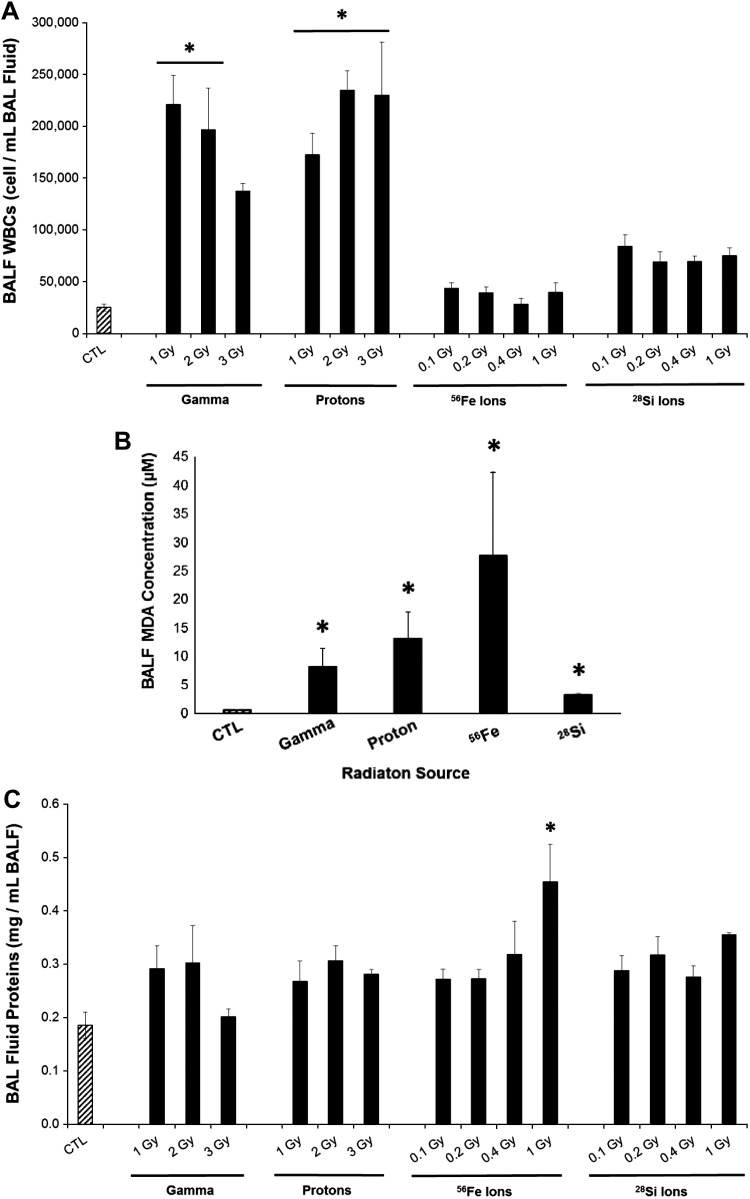
Dependent on the source, space radiation exposure induced distinct inflammatory cell responses in the BALF. Gamma and proton radiation significantly increased white blood cells (WBCs) in the BALF (up to 8.8-fold and 9.5-fold vs. control, respectively, Fig. 3A), unlike exposure to 56Fe, and 28Si, which did not elevate WBCs. Cell differentials (Table 2) indicated that this late stage of SPRALI was associated with predominance of macrophages and neutrophils in the BALF in all groups. Neither eosinophils nor lymphocytes were detected in the BALF of these mice (Table 2). Interestingly, the lungs exposed to 56Fe or 28Si radiation had higher percentages of neutrophils in the BALF compared with other radiation types, analogous to findings in BALF of cigarette smoke-exposed mice (14). Following remote exposure to radiation, with the exception of high-dose 56Fe, lungs had minor, nonsignificant increases in BALF protein levels, irrespective of radiation source (Fig. 3C). These results are indicative of minimal endothelial barrier dysfunction (or effective repair following initial injury) and absence of lung edema.
Fig. 3.
Lung inflammation and oxidative stress following SR. White blood cells (WBC) (A), malondialdehyde (MDA) concentration (μM) (B), and protein levels (mg/ml) (C) in the bronchoalveolar lavage fluid (BALF) following exposure to the indicated sources of radiation (1 Gy) compared with age-matched nonirradiated controls (CTL). Mean ± SE; median of N = 10/group. ANOVA *P < 0.05 vs. control (Dunnett's multiple-comparison tests).
Table 2.
Evaluation of BALF cell differentials
| BALF Differential Cell Counts (% of Total Cells in BALF) |
||
|---|---|---|
| Alveolar macrophages | Neutrophils | |
| Untreated | ||
| CTL | 81.2 ± 6.0 | 15.2 ± 5.4 |
| Gamma | ||
| 1 Gy | 98.0 ± 1.0* | 2.0 ± 1.0* |
| 2 Gy | 94.5 ± 1.8 | 5.5 ± 1.8 |
| 3 Gy | 96.0 ± 1.0 | 4.0 ± 1.0 |
| Proton | ||
| 1 Gy | 90.2 ± 4.6 | 9.8 ± 5.4 |
| 2 Gy | 96.5 ± 1.2* | 3.5 ± 5.4* |
| 3 Gy | 94.0 ± 1.8 | 6.0 ± 5.4 |
| 56Fe Ions | ||
| 0.1 Gy | 82.4 ± 3.6 | 17.6 ± 3.6 |
| 0.2 Gy | 85.3 ± 3.4 | 14.7 ± 3.4 |
| 0.4 Gy | 91.4 ± 3.5 | 8.6 ± 3.5 |
| 1.0 Gy | 87.9 ± 7.4 | 12.1 ± 7.4 |
| 28Si Ions | ||
| 0.1 Gy | 94.6 ± 0.9 | 5.4 ± 0.9 |
| 0.2 Gy | 73.1 ± 6.4† | 26.9 ± 6.4† |
| 0.4 Gy | 88.2 ± 5.3 | 11.8 ± 5.3 |
| 1.0 Gy | 76.0 ± 4.0† | 24.0 ± 4.0† |
Abundance (percent of total cells) of alveolar macrophages and neutrophils in BALF from mice following exposure to the indicated sources and doses of radiation compared with controls (CTL). Total cells ± SE; N = 10/group.
Significant difference from untreated CTL;
significant difference from respective 0.1 Gy dose.
Oxidative stress.
Oxidative tissue damage relevant to spaceflight has been identified and confirmed recently in both lipids and DNA (45). Specifically, lipid peroxidation plays a major role in mediating oxidative damage in tissues and is a quantitative indicator of oxidative stress within tissues and cells. In particular, the amount of MDA, a product of lipid peroxidation in lung tissues, has been a well-accepted measurement of levels of oxidative stress (38, 39). SR-irradiated lungs had evidence of increased oxidative stress, as measured by MDA levels in the BALF (Fig. 3B). Indeed, all SR types (1 Gy) that were evaluated exhibited a severalfold increase in oxidatively modified lipids ranging from 3.2 to 27.6 μM MDA reflecting 14.5-, 23.4-, 49.2-, and 5.7-fold increase over nonirradiated age-matched control mice (about 730 days postirradiation) for gamma, proton, 56Fe, and 28Si, respectively.
Lung apoptosis.
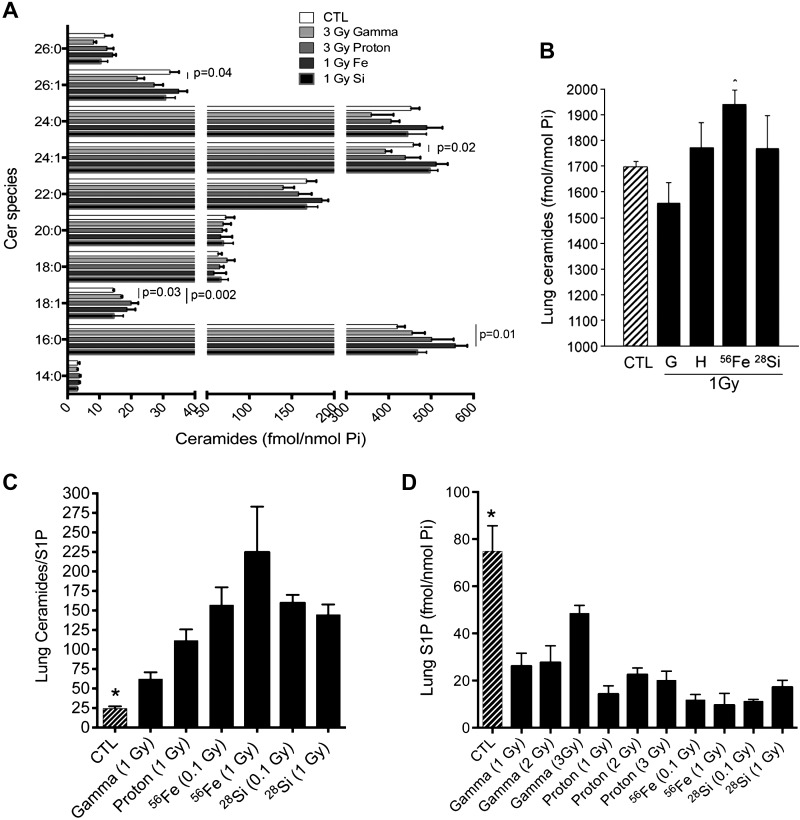
To focus on lung parenchyma cell apoptosis rather than large airways, we performed IHC on fixed lung sections using an antibody against active caspase-3, followed by blinded quantification of the signal in peripheral air spaces and alveoli using image analysis software (55). SR-exposed lung parenchyma exhibited a significant increase in active caspase-3 expression compared with nonirradiated lung, and even compared with lungs exposed to similar doses of reference radiation (1 Gy) (Fig. 4, A and B). Interestingly, even higher exposures to gamma radiation (2 Gy) had no significant effect on caspase-3 activation in the lung at this time point, or on the number of TUNEL-positive cells in the lung parenchyma (data not shown). Since we have previously shown that the ratio of the proapoptotic ceramide to that of antiapoptotic S1P closely correlates with lung apoptosis and air space enlargement (18), we next measured lung levels of these two bioactive sphingolipids using tandem mass spectrometry. At this (late) time point following irradiation, even the highest doses of radiation were not associated with ceramide species accumulations markedly different from control lungs (Fig. 5A). Only 56Fe radiation was associated with significantly elevated total lung ceramide levels (Fig. 5B), primarily on the basis of palmitoyl fatty acid (C16:0)-containing ceramide species (Fig. 5A). Compared with nonirradiated lungs, ceramide/S1P ratios were significantly higher especially in the SR-exposed group (proton, 56Fe, and 28Si), compared with sham-irradiated controls (Fig. 5C). These effects were primarily due to significant depletion of the prosurvival, proproliferative sphingolipid S1P in the lungs of all irradiated animals (Fig. 5D).
Fig. 4.

Lung cell proapoptotic signaling induced by SR. Lung cell proapoptotic signaling measured postexposure to the indicated radiation sources (1 Gy each) compared with age-matched nonirradiated controls. A: abundance of active caspase-3-positive cells in parenchyma detected by immunohistochemistry (IHC); means + SE; *P < 0.05 vs. age-matched nonirradiated controls. N = 3/group. B: representative micrograph of lung parenchyma immunostaining for active caspase-3 (dark brown, arrow) in proton-exposed mice.
Fig. 5.
SR-exposed lungs display increased lung ceramide/S1P ratios. A: lung ceramide (Cer) species profile; means + SE; N = 3–5/group. B: total lung ceramide levels in 1 Gy-irradiated mice; means + SE; P < 0.01 ANOVA *P < 0.01 vs. control (Dunnett's). N = 3–5/group. C: ceramide/S1P; means + SE; P < 0.01 ANOVA *P < 0.01 vs. control (Dunnett's multiple-comparison tests). N = 3–5/group. D: lung S1P levels; means + SE; P < 0.01 ANOVA *P < 0.01 vs. control (Dunnett's multiple-comparison tests). N = 3–5/group. All measurements performed with LC-MS/MS.
Lung cell signaling in vivo.
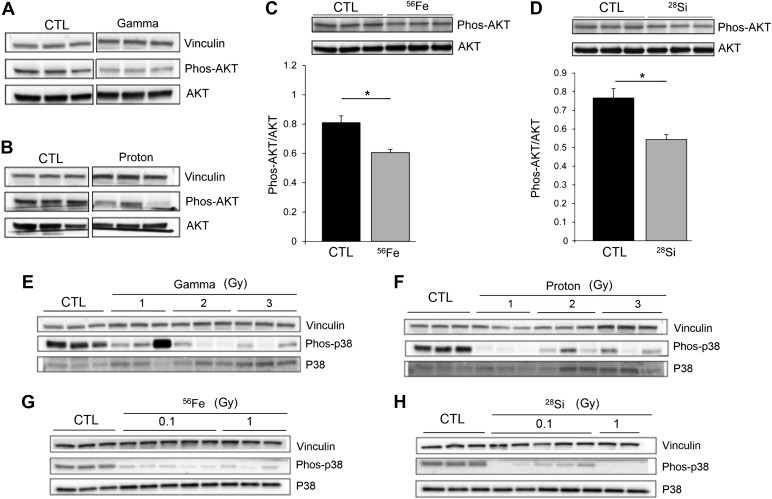
The structural cell integrity in lung alveoli may be determined by the balance between cell death and proliferation/repair. Given the marked decrease in (proproliferative) S1P levels in SR-exposed lungs, we expected to identify other markers of reduced cell proliferative signaling in lungs with enlarged air spaces. We therefore measured the activation of the Akt signaling pathway, typically engaged by proproliferative and angiogenic actions of S1P (8, 30). Lungs exposed to both reference radiation and GCR radiation displayed decreased phosphorylation of Akt (Fig. 6, A and D), consistent with decreased activation of Akt pathway. This is in contrast to exposures to high doses of radiation in the setting of tumor treatment, which are reported to induce prosurvival Akt phosphorylation, via p38 MAPK activation (33). Concordant with the status of decreased Akt phosphorylation in SR-exposed lungs, we measured markedly lower levels of p38 MAPK phosphorylation, especially in 56Fe- and 28Si-irradiated lungs (Fig. 6, E–H). Interestingly, in addition to the lack of Akt activation, the downregulation of p38 MAPK signaling may be associated with the observed elevation in lung oxidative stress (48).
Fig. 6.
Decreased proproliferative signaling in the lungs exposed to SR. A–D: phosphorylated Akt compared with total Akt as marker of Akt pathway activity in control lungs compared with lungs exposed to gamma rays (3 Gy; A), protons (3 Gy; B), 56Fe (0.1 Gy; C), or 28Si (0.1 Gy; D) ions. Vinculin and total Akt used as loading controls. Akt phosphorylation in the 56Fe (C)- and 28Si (D)-irradiated lungs was quantified as densitometric ratio to total Akt (means + SE; n = 3; *P < 0.01). E–H: phosphorylated p38 MAPK compared with total p38 in lung homogenates from mice exposed to gamma (E), proton (F), 56Fe (G), or 28Si (H). Vinculin was loading control. Each lane represents the lung protein extract from a distinct mouse.
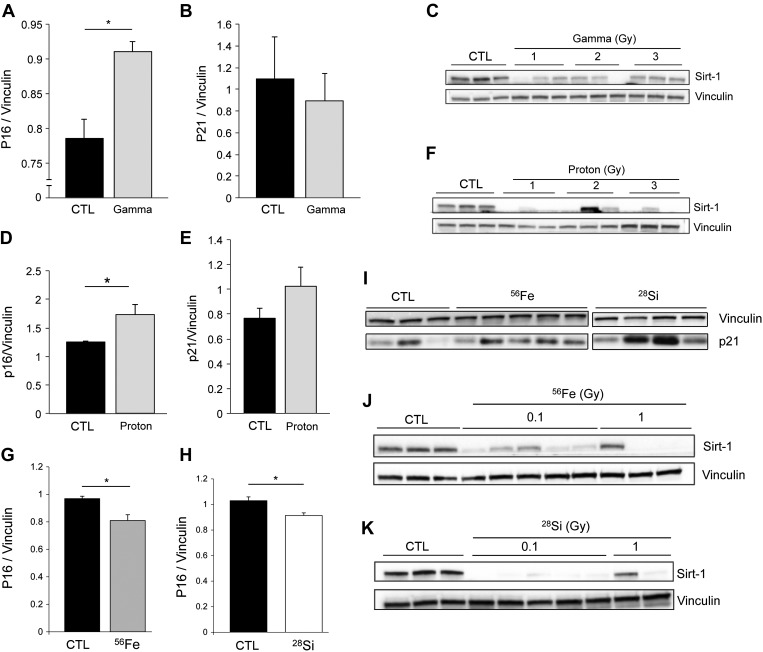
Decreased cell proliferation may indicate not only reduced repair via repopulation but also could result from stress-induced autophagy or onset of cellular senescence. Recently, both autophagy and senescence were linked to the development of air space enlargement in emphysema (11, 72). Identified by the presence of the lipidated form of autophagy protein microtubule-associated protein 1 light chain-3b (LC3B), the initiation of autophagy may indicate cellular stress and an effort to maintain cell survival during necessary repair or recovery from injury. One of the critical molecules involved in autophagy is LC3B. Both LC3B (I) and its lipidated form LC3B (II), identified as a lower molecular-weight band on Western blotting, were markedly elevated in the lungs exposed to radiation, regardless of source or dose (Fig. 7, A–D). GCR-exposed lungs had decreased p62 protein expression, which typically indicates an active autophagy flux (Fig. 7E). Senescence-associated markers included p16 and p21 (CIP1/WAF1), elevated in senescent cells, as well as the anti-aging deacetylase protein sirtuin-1 (Sirt-1), which decreases in emphysema-associated lung senescence (72). Long-term response to gamma radiation increased p16 and decreased Sirt-1, without significantly affecting p21 (Fig. 8, A–C). Proton-irradiated lungs exhibited all markers of accelerated senescence compared with age-matched mice (Fig. 8, D–F). Although displaying reduced levels of p16, and variable abundance of p21, lungs of 56Fe or 28Si-irradiated mice displayed profound depletion of Sirt-1 (Fig. 8, G–K). These data indicated that, in addition to increased apoptosis and autophagy, SPRALI may also be characterized by accelerated lung senescence.
Fig. 7.
Lung cell autophagy markers induced by SR. A–D: immunoblots for LC3I and its lipidated form LC3II in lungs followed indicated radiation sources (unless otherwise noted, doses of 1 Gy). Vinculin was loading control. Each lane represents a distinct mouse lung. E: levels of p62 in the lung lysates of mice exposed to 56Fe compared with control normalized to loading control. Means + SE; *P < 0.05. N = 3–5/group.
Fig. 8.
Markers of senescence in the lung tissue exposed to SR. Immunoblots for senescence markers following irradiation with the sources indicated (unless otherwise noted, doses of 1 Gy). p16 (A, D, G, H), p21 (B, E, I), and sirtuin-1 (Sirt-1; C, F, J, K) protein expression normalized by vinculin loading controls (means + SE; n = 3; *P < 0.05; each lane represents a different animal).
DISCUSSION
Our results indicate a novel risk associated with space travel, space radiation-induced lung injury (SPRALI), characterized by loss of lung alveolar structures and functional changes resulting in air space enlargement and impaired respiration. These sustained histopathological changes in the lungs of mice persist even at almost 2 years following a single exposure to SR and are associated with aberrant cell signaling.
Radiation-induced lung injury has been well characterized as a serious clinical repercussion in patients receiving unfractionated high-dose radiotherapy for lung cancer (42, 46). Acute responses to high-dose radiotherapy may include acute pneumonitis, in which a lung pathology of exuberant lung inflammation manifests as early as 2 wk after radiation. Chronically, high-dose radiotherapy can lead to radiation-induced lung fibrosis, characterized by collagen deposition and scarring that manifest several months after exposure. Radiation pneumonopathy has been previously modeled in C57Bl/6 mice, which are highly susceptible to develop radiation-induced lung fibrosis (24, 28, 37, 43, 47). Unlike these lung risks of high-dose low-LET radiation associated with radiotherapy, space travel-associated radiation risk is the result of low-dose high-LET effects on the lung tissue. Although the acute effects of SR on the lung are largely unknown, our results indicate that long-term pulmonary effects are pathologically distinct from radiotherapy, with no noticeable lung fibrosis, but with evidence of air space enlargement. Previous work using exposure to SR such as heavy ions showed additional effects, including mutagenesis of the lung epithelium, directly from DNA damage or indirectly via production of reactive oxygen species (32, 65), and epithelial-mesenchymal transition (3, 68–70). Although our study did not focus on the development of lung cancer, the lung histopathology of mice following remote SR exposure exhibited occasional tumor development along with marked remodeling manifested predominantly as air space enlargement. Although pulmonary function tests were not performed in these experiments, the marked degree of air space destruction coupled with impaired respiration manifested as systemic hypoxemia reflects that SPRALI may have significant functional consequences.
The findings of air space enlargement, mild neutrophilic inflammation, associated with impairment of prosurvival, cell repair signaling pathways indicate the mechanisms of SPRALI may share certain pathogenic features with the development of emphysema. Pulmonary emphysema is a major component of chronic obstructive pulmonary disease (COPD), a condition typically induced by chronic exposure to cigarette smoking. The key mechanisms responsible for COPD development include oxidative stress, cell death by apoptosis, adaptation via autophagy, and senescence with decreased repair (63). Similar to models of apoptosis-dependent emphysema (18, 49) or following high-dose ionizing radiation exposures (34, 47), we measured increased ceramide/S1P ratio in the lungs of SR-exposed mice. Ceramide is a metabolic precursor to S1P (25, 29, 71), which is involved in cell survival and proliferation, in addition to modulation of immune cell development, differentiation, activation, and migration (44, 51).
A major pathway of S1P signaling occurs by binding to a family of five G protein-coupled receptors (S1P1–S1P5) (41, 53). We have previously shown that the lung ratio of ceramide/S1P modulates cell fate and lung remodeling (18). In particular, high ceramide/S1P causes cell death and emphysema-like pathology. In the present study, we observed that at 2 years following SR exposure, ceramide/S1P was increased in irradiated lungs, primarily due to decreased S1P expression. This could lead to decreased prosurvival signaling via S1P1, a receptor expressed on lung structural cells that, when stimulated by S1P, signals via the Akt pathway (59). Consistent with decreased S1P levels, Akt phosphorylation was reduced in SR-exposed animals, supporting the notion that SR-injured lungs could not engage necessary cellular repair of enlarged air spaces. Typically, p38 is activated upstream of Akt by radiation exposure (33). In addition, p38 is a kinase typically engaged by S1P to cause cell migration (9).
In concordance with decreased Akt activation and low S1P levels in SR-exposed lungs, we noted profound inhibition of p38 MAPK activity. Although we cannot exclude that p38 MAPK may be activated early in the time course of SR exposure, the mechanism by which p38 activity decreased below that of control animals is intriguing. In principle, it could be related to Cdk5rap3 (LZAP) (2), a putative tumor suppressor gene involved in DNA damage responses, or it could be related to upregulation of phosphatases such as PP2Cδ (WIP1), which directly dephosphorylates p38 (60). Although the elucidation of the signaling pathways engaged by SR awaits future investigations, the functional effects of the profound, chronic p38 inhibition cannot be extrapolated from the traditional proapoptotic role of p38 activation triggered by acute stress. There are several less appreciated functions of p38α (61), which may be specifically induced by radiation exposure. In this context of DNA damage, the functional significance of lower p38 phosphorylation levels may be related to inhibited cell mobility and repair, decreased cell proliferation via decreased cell cycle checkpoint progression, increased apoptosis, and/or increased oxidative stress (48). Indeed, several of these features were identified in SPRALI.
Oxidative and nitrosative damage resulting from GCR and SPE exposure has been identified in several studies. Oxidatively injured organs such as the eyes (45), brain (17, 58), and bones (1, 35) were identified as primary targets of SR exposure. An important finding of the present study is the identification of lung susceptibility to oxidative stress associated with SR. Prefaced by our reports of models lung sensitivity to therapeutic gamma ionizing radiation (50), this study is the first report of long-term oxidative lung damage from SR.
In addition to oxidative stress, we detected elevated markers of cell autophagy and senescence, cellular states associated with decreased cell proliferation. Recent studies demonstrated that oxidative injury, including that caused by cigarette smoking, trigger autophagy, and accelerate lung aging characterized by cellular senescence and macromolecular DNA damage (52, 62). The findings in SPRALI suggest that a similar mechanism of accelerated lung aging occurs in response to SR.
The doses of gamma radiation used in our studies (1–3 Gy) were significantly lower (less than 10-fold) than those of radiotherapy, but 10-fold higher than lifetime exposures to gamma radiation on Earth. Although not all end points indicated a dose-response, in general SR-specific proton, 56Fe, and 28Si exposures had similar pulmonary effects as gamma radiation (which can be encountered both on Earth and in space, for example on Mars) (73), with several exceptions. We noted that 56Fe and 28Si ion exposure had marked effects on the lung structure and cellular function, dose independently in the range tested in this study. Compared with gamma or proton radiation, exposure to 56Fe or 28Si induced the lowest inflammatory response in BALF of mice. Compared with gamma exposures, all SR types (proton, 56Fe, and 28Si) induced more prominent apoptosis, higher ceramide/S1P ratios, and more pronounced Sirt-1 depletion. The most dramatic effect on air space enlargement was induced by proton exposure, which was characterized by a combination of a more robust lung inflammatory response (compared to other exposures) and a potent proapoptotic and prosenescence effect. However, our study had several limitations, in particular the lack of lung function testing and the exploration of a single late time point in the course of SPRALI, in a single mouse strain, which may not respond with exuberant fibrosis compared with other more typical strains. These considerations should spur future mechanistic studies of GCR types, timing, and doses, as well as efforts to evaluate human lung function even long term, following space travel to determine whether these findings can be extrapolated from mouse to human. This is the first study to determine whether lung effects could be observed following exposure to a low total SR dose. The next logical step is to determine the lung effects of repeated low-dose exposures, which may more accurately model exposures of space crew to SR. However, such studies are technically and logistically challenging, owing to the paucity of heavy ion beams available in the world, which require several years of planning and financing. Nevertheless, the presence of significant pulmonary effects even following a single low total dose of SR suggests that repeated exposure might pose even greater risk and will have to be further investigated.
Several already-recognized adverse effects of space radiation exposure include damage to the immune system, the central nervous system (27), and the eye lens (12), and the development of cancers (16). Risk estimates have been developed for incidence and mortality of cancers such as leukemia and solid tumors (15). However, when considering the lifetime total radiation risk (TRR) of astronauts for space flights, TRR in senior-age astronaut groups appears more likely to result in nontumor death (56). This mortality may be related to the chronic effect of SR on the vascular system, including that of the lung.
In conclusion, the present study shows the first indication of SPRALI and identifies a novel risk relevant to space travel. Understanding the risks to space radiation exposures will inform the design of future approaches to mitigate lung risks to crewmembers' health during deep space exploration flights.
GRANTS
This work was funded by NIH-R01 CA133470 (M. Christofidou-Solomidou), NIH-RC1AI081251 (M. Christofidou-Solomidou), NASA-NNJ11ZSA002NA (M. Christofidou-Solomidou), NIH-RO1HL077328 (I. Petrache), and NASA NNX09AM08G (R. L. Ullrich) and DOE-NASA Interagency Award no. DE-SC0001507, supported by the Office of Science (Biological and Environmental Research), US Department of Energy (R. K. Globus).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.C.-S., R.A.P., Y.Y., R.K.G., R.L.U., and I.P. conception and design of research; M.C.-S., R.A.P., E.A., K.S.S., E.V.B., M.M., A.C., J.S.A., Y.Y., R.K.G., C.C.S., and I.P. performed experiments; M.C.-S., R.A.P., K.S.S., E.V.B., Y.Y., R.K.G., C.C.S., and I.P. analyzed data; M.C.-S., R.A.P., E.V.B., Y.Y., R.K.G., C.C.S., and I.P. interpreted results of experiments; M.C.-S., R.A.P., C.C.S., and I.P. prepared figures; M.C.-S., R.A.P., and I.P. drafted manuscript; M.C.-S., R.A.P., E.V.B., J.S.A., Y.Y., R.K.G., C.C.S., R.L.U., and I.P. edited and revised manuscript; M.C.-S., R.A.P., E.A., K.S.S., E.V.B., M.M., A.C., J.S.A., Y.Y., R.K.G., C.C.S., R.L.U., and I.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Marjorie Albrecht and Mary Van Demark for technical assistance.
REFERENCES
- 1.Alwood JS, Yumoto K, Mojarrab R, Limoli CL, Almeida EA, Searby ND, Globus RK. Heavy ion irradiation and unloading effects on mouse lumbar vertebral microarchitecture, mechanical properties and tissue stresses. Bone 47: 248–255, 2010. [DOI] [PubMed] [Google Scholar]
- 2.An H, Lu X, Liu D, Yarbrough WG. LZAP inhibits p38 MAPK (p38) phosphorylation and activity by facilitating p38 association with the wild-type p53 induced phosphatase 1 (WIP1). PLoS One 6: e16427, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andarawewa KL, Paupert J, Pal A, Barcellos-Hoff M. New rationales for using TGFbeta inhibitors in radiotherapy. Int J Radiat Biol 83: 803–811, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol 366: 43–58, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Berdyshev EV, Gorshkova IA, Garcia JG, Natarajan V, Hubbard WC. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal Biochem 339: 129–136, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Berdyshev EV, Gorshkova IA, Usatyuk P, Zhao Y, Saatian B, Hubbard W, Natarajan V. De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cell Signal 18: 1779–1792, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bielefeldt-Ohmann H, Genik PC, Fallgren CM, Ullrich RL, Weil MM. Animal studies of charged particle-induced carcinogenesis. Health Phys 103: 568–576, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Bonnaud S, Niaudet C, Legoux F, Corre I, Delpon G, Saulquin X, Fuks Z, Gaugler MH, Kolesnick R, Paris F. Sphingosine-1-phosphate activates the AKT pathway to protect small intestines from radiation-induced endothelial apoptosis. Cancer Res 70: 9905–9915, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta 1823: 439–450, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Calvi CL, Podowski M, D'Alessio FR, Metzger SL, Misono K, Poonyagariyagorn H, Lopez-Mercado A, Ku T, Lauer T, Cheadle C, Talbot CC Jr, Jie C, McGrath-Morrow S, King LS, Walston J, Neptune ER. Critical transition in tissue homeostasis accompanies murine lung senescence. PLoS One 6: e20712, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci USA 107: 18880–18885, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chylack LT Jr, Peterson LE, Feiveson AH, Wear ML, Manuel FK, Tung WH, Hardy DS, Marak LJ, Cucinotta FA. NASA study of cataract in astronauts (NASCA) report 1: cross-sectional study of the relationship of exposure to space radiation and risk of lens opacity. Radiat Res 172: 10–20, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Clauss M, Voswinckel R, Rajashekhar G, Sigua NL, Fehrenbach H, Rush N, Schweitzer K, Yildirim AÖ, Kamocki K, Fisher AJ, Gu Y, Safadi B, Nikam S, Hubbard WC, Tuder RM, Twigg HL 3rd, Presson RG Jr, Sethi S, Petrache I. Lung endothelial monocyte-activating protein 2 is a mediator of cigarette smoke-induced emphysema in mice. J Clin Invest 121: 2470–2479, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clauss M, Voswinckel R, Rajashekhar G, Sigua NL, Fehrenbach H, Rush NI, Schweitzer KS, Yildirim AO, Kamocki K, Fisher AJ, Gu Y, Safadi B, Nikam S, Hubbard WC, Tuder RM, Twigg HL 3rd, Presson RG, Sethi S, Petrache I. Lung endothelial monocyte-activating protein 2 is a mediator of cigarette smoke-induced emphysema in mice. J Clin Invest 121: 2470–2479, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cucinotta FA, Chappell LJ. Updates to astronaut radiation limits: radiation risks for never-smokers. Radiat Res 176: 102–114, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Cucinotta FA, Plante I, Ponomarev AL, Kim MH. Nuclear interactions in heavy ion transport and event-based risk models. Radiat Prot Dosimetry 143: 384–390, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Denisova NA, Shukitt-Hale B, Rabin BM, Joseph JA. Brain signaling and behavioral responses induced by exposure to 56Fe-particle radiation. Radiat Res 158: 725–734, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, Schweitzer KS, Skobeleva A, Rajashekhar G, Hubbard WC, Berdyshev EV, Petrache I. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med 181: 344–352, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietze G, Bartlett DT, Cool DA, Cucinotta FA, Jia X, McAulay IR, Pelliccioni M, Petrov V, Reitz G, Sato T. ICRP, 123. Assessment of radiation exposure of astronauts in space. ICRP Publication 123. Ann ICRP 42: 1–339, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nat Rev Cancer 8: 465–472, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Franko AJ, Sharplin J. Development of fibrosis after lung irradiation in relation to inflammation and lung function in a mouse strain prone to fibrosis. Radiat Res 140: 347–355, 1994. [PubMed] [Google Scholar]
- 25.Galadari S, Wu BX, Mao C, Roddy P, El Bawab S, Hannun YA. Identification of a novel amidase motif in neutral ceramidase. Biochem J 393: 687–695, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood 102: 169–179, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Gauger GE, Tobias CA, Yang T, Whitney M. The effect of space radiation of the nervous system. Adv Space Res 6: 243–249, 1986. [DOI] [PubMed] [Google Scholar]
- 28.Gorshkova I, Zhou T, Mathew B, Jacobson JR, Takekoshi D, Bhattacharya P, Smith B, Aydogan B, Weichselbaum RR, Natarajan V, Garcia JG, Berdyshev EV. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J Lipid Res 53: 1553–1568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758: 2016–2026, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. Differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem 276: 12420–12426, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Jackson IL, Xu PT, Nguyen G, Down JD, Johnson CS, Katz BP, Hadley CC, Vujaskovic Z. Characterization of the dose response relationship for lung injury following acute radiation exposure in three well-established murine strains: developing an interspecies bridge to link animal models with human lung. Health Phys 106: 48–55, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Jones JA, Riggs PK, Yang TC, Pedemonte CH, Clarke MS, Feeback DL, Au WW. Ionizing radiation-induced bioeffects in space and strategies to reduce cellular injury and carcinogenesis. Aviat Space Environ Med 78: A67–A78, 2007. [PubMed] [Google Scholar]
- 33.Kim MJ, Byun JY, Yun CH, Park IC, Lee KH, Lee SJ. c-Src-p38 mitogen-activated protein kinase signaling is required for Akt activation in response to ionizing radiation. Mol Cancer Res 6: 1872–1880, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 22: 5897–5906, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Kondo H, Yumoto K, Alwood JS, Mojarrab R, Wang A, Almeida EA, Searby ND, Limoli CL, Globus RK. Oxidative stress and gamma radiation-induced cancellous bone loss with musculoskeletal disuse. J Appl Physiol 108: 152–161, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunwar A, Jain VK, Priyadarsini KI, Haston CK. A selenocysteine derivative therapy affects radiation-induced pneumonitis in the mouse. Am J Respir Cell Mol Biol 49: 654–661, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Lee JC, Bhora F, Sun J, Cheng G, Arguiri E, Solomides CC, Chatterjee S, Christofidou-Solomidou M. Dietary flaxseed enhances antioxidant defenses and is protective in a mouse model of lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 294: L255–L265, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, Solomides CC, Javvadi P, Koumenis C, Cengel KA, Christofidou-Solomidou M. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res 173: 590–601, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, Vachani A, Solomides CC, Cengel KA, Christofidou-Solomidou M. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther 8: 47–53, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot 29: A43–A59, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22: 50–60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machtay M. Pulmonary complications of anticancer treatment. In: Abeloff’s Clinical Oncology (4th ed.), edited by Abeloff M. Philadelphia, PA: Churchill Livingston, 2008, chapt. 62, p. 969–982. [Google Scholar]
- 43.Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, Arguiri E, Shuvaev VV, Sun J, Cengel K, Solomides CC, Christofidou-Solomidou M. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol 81: 196–205, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancuso P, Peters-Golden M. Modulation of alveolar macrophage phagocytosis by leukotrienes is Fc receptor-mediated and protein kinase C-dependent. Am J Respir Cell Mol Biol 23: 727–733, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Mao XW, Pecaut MJ, Stodieck LS, Ferguson VL, Bateman TA, Bouxsein M, Jones TA, Moldovan M, Cunningham CE, Chieu J, Gridley DS. Spaceflight environment induces mitochondrial oxidative damage in ocular tissue. Radiat Res 180: 340–350, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Marks LB, Yu X, Vujaskovic Z, Small W Jr, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol 13: 333–345, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Mathew B, Jacobson JR, Berdyshev E, Huang Y, Sun X, Zhao Y, Gerhold LM, Siegler J, Evenoski C, Wang T, Zhou T, Zaidi R, Moreno-Vinasco L, Bittman R, Chen CT, LaRiviere PJ, Sammani S, Lussier YA, Dudek SM, Natarajan V, Weichselbaum RR, Garcia JG. Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. FASEB J 25: 3388–3400, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naidu S, Vijayan V, Santoso S, Kietzmann T, Immenschuh S. Inhibition and genetic deficiency of p38 MAPK up-regulates heme oxygenase-1 gene expression via Nrf2. J Immunol 182: 7048–7057, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 11: 491–498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietrofesa RA, Turowski JB, Arguiri E, Milovanova TN, Solomides CC, Thom SR, Christofidou-Solomidou M. Oxidative lung damage resulting from repeated exposure to radiation and hyperoxia associated with space exploration. J Pulm Respir Med 3: 1000158, 2013. [PMC free article] [PubMed] [Google Scholar]
- 51.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J 378: 281–292, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Provinciali M, Cardelli M, Marchegiani F. Inflammation, chronic obstructive pulmonary disease and aging. Curr Opin Pulm Med 17, Suppl 1: S3–S10, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 8: 753–763, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rola R, Sarkissian V, Obenaus A, Nelson GA, Otsuka S, Limoli CL, Fike JR. High-LET radiation induces inflammation and persistent changes in markers of hippocampal neurogenesis. Radiat Res 164: 556–560, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Schweitzer KS, Johnstone BH, Garrison J, Rush NI, Cooper S, Traktuev DO, Feng D, Adamowicz JJ, Van Demark M, Fisher AJ, Kamocki K, Brown MB, Presson RG Jr, Broxmeyer HE, March KL, Petrache I. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med 183: 215–225, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shafirkin AV, Petrov VM, Kolomensky AV, Shurshakov VA. Lifetime total radiation risk of cosmonauts for orbital and interplanetary flights. Adv Space Res 30: 999–1003, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Shay JW, Cucinotta FA, Sulzman FM, Coleman CN, Minna JD. From mice and men to earth and space: joint NASA-NCI workshop on lung cancer risk resulting from space and terrestrial radiation. Cancer Res 71: 6926–6929, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suman S, Rodriguez OC, Winters TA, Fornace AJ Jr, Albanese C, Datta K. Therapeutic and space radiation exposure of mouse brain causes impaired DNA repair response and premature senescence by chronic oxidant production. Aging (Albany NY) 5: 607–622, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60: 181–195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H, Taya Y, Imai K. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J 19: 6517–6526, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci 5: 44–51, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med 174: 886–893, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest 122: 2749–2755, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verhoeven D, Teijaro JR, Farber DL. Pulse-oximetry accurately predicts lung pathology and the immune response during influenza infection. Virology 390: 151–156, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang M, Hada M, Huff J, Pluth JM, Anderson J, O'Neill P, Cucinotta FA. Heavy ions can enhance TGFbeta mediated epithelial to mesenchymal transition. J Radiat Res 53: 51–57, 2012. [DOI] [PubMed] [Google Scholar]
- 66.Weil MM, Bedford JS, Bielefeldt-Ohmann H, Ray FA, Genik PC, Ehrhart EJ, Fallgren CM, Hailu F, Battaglia CL, Charles B, Callan MA, Ullrich RL. Incidence of acute myeloid leukemia and hepatocellular carcinoma in mice irradiated with 1 GeV/nucleon 56Fe ions. Radiat Res 172: 213–219, 2009. [DOI] [PubMed] [Google Scholar]
- 67.Whitmore AC, Whitmore SP. Subline divergence within L. C. Strong's C3H and CBA inbred mouse strains. A review. Immunogenetics 21: 407–428, 1985. [DOI] [PubMed] [Google Scholar]
- 68.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2: 103–121, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu R, Jin J, Hu W, Sun W, Bielawski J, Szulc Z, Taha T, Obeid LM, Mao C. Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. FASEB J 20: 1813–1825, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Ronty M, Kinnula VL, Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 122: 2032–2045, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeitlin C, Hassler DM, Cucinotta FA, Ehresmann B, Wimmer-Schweingruber RF, Brinza DE, Kang S, Weigle G, Bottcher S, Bohm E, Burmeister S, Guo J, Kohler J, Martin C, Posner A, Rafkin S, Reitz G. Measurements of energetic particle radiation in transit to Mars on the Mars Science Laboratory. Science 340: 1080–1084, 2013. [DOI] [PubMed] [Google Scholar]