Abstract
Pulmonary oxygen toxicity is a major clinical problem for patients undergoing supplemental oxygen therapy. Thioredoxin (Trx) is an endogenous antioxidant protein that regenerates oxidatively inactivated proteins. We examined how Trx contributes to oxygen tolerance by creating transgenic mice with decreased levels of functional thioredoxin (dnTrx-Tg) using a dominant-negative approach. These mice showed decreased Trx activity in the lung although the expression of mutant protein is three times higher than the wild-type mice. Additionally, we found that these mice showed increased oxidation of endogenous Trx in room air. When exposed to hyperoxia (>90% O2) for 4 days, they failed to recover and showed significant mortality. Even in normal oxygen levels, these mice displayed a significant decrease in aconitase and NADH dehydrogenase activities, decreased mitochondrial energy metabolism, increased p53 and Gadd45α expression, and increased synthesis of proinflammatory cytokines. These effects were further increased by hyperoxia. We also generated mice overexpressing Trx (Trx-Tg) and found they maintained lung redox balance during exposure to high oxygen and thus were resistant to hyperoxia-induced lung injury. These mice had increased levels of reduced Trx in the lung in normoxia as well as hyperoxia. Furthermore, the levels of aconitase and NADH dehydrogenase activities were maintained in these mice concomitant with maintenance of mitochondrial energy metabolism. The genotoxic stress markers such as p53 or Gadd45α remained in significantly lower levels in hyperoxia compared with dnTrx-Tg or wild-type mice. These studies establish that mice deficient in functional Trx exhibit a phenotype of sensitivity to ambient air and hypersensitivity to hyperoxia.
Keywords: hyperoxia, lung injury, redox, thioredoxin-deficient mice
although high oxygen therapy is often a clinical requirement [e.g., for patients with respiratory insufficiency and in conjunction with anesthetics (53)], the treatment has significant negative side effects (8, 54, 55). For example, hyperoxia has been implicated in the progression of several pulmonary disorders, including bronchopulmonary dysplasia (2, 56), acute respiratory distress syndrome (7, 17, 32), persistent pulmonary hypertension of the newborn (1, 52), and lung fibrosis (16). Hyperoxia also causes the generation of reactive oxygen species (ROS) that oxidize cellular macromolecules, resulting in lung injury and failure to recover following oxygen therapy. Surprisingly, however, various antioxidant knockout mice are not hypersensitive to hyperoxia-mediated lung injury (21–23). Furthermore, higher levels of antioxidants alone are not sufficient to provide protection against lung injury (24), which indicates that additional causative factors are involved in pulmonary oxygen toxicity.
Cytosolic thioredoxin (Trx, also known as Trx1; Txn, mouse; and TXN1, human) is a multifunctional small (12 kDa) antioxidant protein with ubiquitous distribution. The Trx system, which includes Trx reductase (TrxR) in addition to Trx, is an efficient protein-disulfide reductase involved in regeneration of thiol moieties oxidized due to increased oxidative stress conditions. The active site of this protein contains two cysteine residues C32 and C35 that take part in redox reactions. One of the major functions of Trx is to provide deoxyribonuceotides for DNA replication by providing reducing power to ribonucleotide reductase (46). It also provides reducing equivalents to peroxiredoxins (Prx) for detoxification of peroxides, which constitutes an important antioxidant function of this protein [reviewed by Holmgren (26–28)]. Trx regenerates oxidatively inactivated proteins by restoring the critical −SH groups in enzymes and proteins via TrxR with reducing equivalents provided by NADPH (26, 30). As regards to its direct antioxidant function, it is not a superoxide anion scavenger but scavenges hydroxyl radicals and singlet oxygen (12). The mitochondrial Trx (Trx2) is localized to the mitochondria and is similar to bacterial Trx with two active cysteine residues but lacks the structural cysteines that are present in the mammalian cytosolic Trx. Trx2 along with its reductase (TrxR2) constitutes the mitochondrial Trx system (40). Although potentially important, the role of Trx in hyperoxia-mediated lung injury has not been adequately evaluated due to the lack of a Trx knockout mouse model as Trx knockout mice die in utero (44). Therefore, we used a dominant negative strategy to generate transgenic mice (dnTrx-Tg) that express only low levels of functional Trx. Since mutant Trx competes with wild-type (WT) Trx for reduction by TrxR, dominant-negative strategy is an ideal alternative to knockout mice to determine if Trx deficiency sensitizes mice to hyperoxia. In complementary experiments, we also generated Trx-overexpressing mice to determine if high levels of the protein could rescue the lung from hyperoxia-induced injury. Thus, by simultaneously using mice with loss-of-function (dnTrx-Tg) and gain-of-function (Trx-Tg) Trx genotypes, we were able to determine the specific effects Trx has on the consequences of hyperoxia.
We found that the Trx-deficient mice are hypersensitive to oxygen toxicity and fail to recover from hyperoxia. We show that dnTrx-Tg mice experience oxidative stress and produce genotoxic stress markers, even in ambient air. In addition, we established that lungs from Trx-deficient mice express higher levels of proinflammatory cytokines in normal oxygen, indicating that they are more susceptible to inflammation. Type II alveolar epithelial cells (AECII) isolated from dnTrx-Tg mice had lower mitochondrial oxygen metabolism than controls during normoxia, and this decreased further in cells isolated from hyperoxia-exposed mice. In contrast, significant number of mice were able to survive hyperoxic insult and recovered from exposure. The mice maintained normal lung redox balance and mitochondrial energetics, decreased inflammation, and recovered effectively following hyperoxia. Taken together, our study establishes that enhanced levels of Trx provide significant protection against hyperoxia-mediated lung injury and these mice effectively recover. In contrast, the dnTrx-Tg mouse strain is an oxidant-sensitive and proinflammatory phenotype in ambient air further demonstrating that Trx has important roles in maintaining lung redox balance and antioxidant status and preventing lung inflammation.
MATERIALS AND METHODS
Reagents.
All reagents were purchased from Sigma Chemical (St. Louis, MO) unless otherwise stated. Sources of antibodies are mentioned in the specific materials and methods section. Antimycin A, oligomycin, carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), rotenone, and pyruvate were purchased from Sigma Chemical. Oligomycin, rotenone, antimycin A, and FCCP were prepared in ultrapure DMSO (Sigma Chemical) at 2.5 mM. ADP, succinate, malate, and pyruvate were prepared in ultrapure water, and the pH was adjusted to 7.2 using KOH. Pyruvate was always freshly prepared (11).
Generation of Trx and dnTrx transgenic mice.
The human Trx coding sequence was cloned into pcDNA3, and the redox-active Cys-32 and Cys-35 were mutated to serine by site-directed mutagenesis (10). The chimeric CMV enhancer/chicken β-actin promoter/chicken β-actin intron sequence was excised from pCAGGSnew (a kind gift from Dr. Andy McMahon, Harvard University) by SpeI/EcoRI digestion and cloned into pcDNA3-Trx or pcDNA3-dnTrx vector by replacing its CMV promoter at the same sites to generate pCA-Trx or pCA-dnTrx. The transgene was released from pCA-Trx or pCA-dnTrx by MluI/PvuII digestion, purified, and used to generate transgenic mice. The transgene was injected into the pronuclei of fertilized eggs derived from hyperovulated C57BL/6 mice, and the litters were screened by PCR of their tail DNA.
Mouse model for hyperoxia.
The use of animals for these studies was approved by the Institutional Animal Care and Use Committee of the Texas Tech University Health Sciences Center. Mice were exposed to 21% oxygen (room air) or >90% oxygen in Plexiglass chambers (Biospherix, New York, NY). Oxygen was provided from bottled gas (100% medical grade oxygen). The oxygen concentration in the exposure chamber was continuously monitored and maintained at >90% by a Proox110 oxygen regulator (Biospherix). Ambient humidity was maintained in the chambers. Chamber CO2 was also continuously monitored by a ProOx 110 CO2 regulator (Biosphereix) and was kept <0.2% by soda lime inside the exposure chambers. Mice were provided food and water ad libitum and maintained on a 12-h dark-light cycle in their own cages. The mortality during the 4-day period was recorded. Following 4 days of exposure, surviving mice were removed from oxygen chamber and kept in room air for 72 h, during which the mortality was recorded at every 12-h interval.
Harvesting lung tissue.
After exposure either to room air or hyperoxia, mice were anesthetized with isoflurane. After onset of deep anesthesia, a midline tracheostomy was performed, and the trachea was cannulated for continuous ventilation. Mice were exsanguinated by left ventricular puncture, and the lung was flushed via the pulmonary circulation with sterile PBS introduced through the right ventricle. After blood was removed from the lung, the entire organ was inflated via instillation of 10% neutral formalin through the trachea for 5–10 min. The inflated lung was removed en bloc and immersed in this fixative. For biochemical analyses, the lung tissue was immediately frozen at −80°C after flushing. For the redox state assay, the lung tissue was immediately homogenized in carboxymethylation buffer.
Trx, TrxR, and Prx assays.
The frozen lung tissue was homogenized in 0.05 M potassium phosphate buffer (pH 7.0) containing 1 mM EDTA. Following homogenization in a Waring blender, the homogenate was centrifuged in a microfuge at 14,000 rpm at 4°C for 45 min. The supernatant was transferred to another tube, and the Trx activity assay was performed immediately as described in our previous publications (14, 29, 46). Briefly, the reaction mixture was comprised of NADPH (200 μM) and porcine insulin (80 μM; Sigma) in 0.05 M potassium phosphate buffer (pH 7.0) containing EDTA (1 mM) in a total volume of 0.15 ml. The assay was standardized using Escherichia coli Trx and bovine TrxR (American Diagnostica, Greenwich, CT). The reaction was started by addition of rat TrxR (0.1 μM). Trx activity was calculated as micromoles of NADPH oxidized per minutes per milligrams of protein at 25°C. TrxR activity was determined by a modified method Holmgren et al. as described in our previous publications (14, 29). The specific activity was determined by monitoring the auranofin-inhibitable rate at 412 nm, and the rate was expressed as nanomoles of 5-thio-2-nitro benzene (TNB; εM = 13.6 × 103 M−1·cm−1) formed per milligrams of protein per minute at 25°C. Prx activity was determined by the rate of decrease of NADPH using H2O2 as substrate in the presence of Trx and TrxR. Trx-dependent specific Prx activity is expressed as nanomoles of NADPH oxidized per minutes per milligrams of protein at 30°C. All of the kinetic assays were performed using a Beckman DU800 spectrophotometer with micro-quartz cuvettes (maximal volume of 50–250 μl) with temperature control of a six-cell cuvette chamber.
Aconitase and NADH dehydrogenase assays.
Aconitase activity was measured as described by Gardner (19) and published previously (45), and NADH dehydrogenase activity was measured by modified method of Bergmayer et al. (5) as described in my previous publication (11). All of the kinetic assays were performed using Beckman DU800 spectrophotometer with micro-quartz cuvettes with temperature control of a six-cell cuvette chamber.
Assay for TrxR state in the lungs.
Carboxymethylation of mice lung tissue was performed as described in our previous publications (14, 15, 46, 49). Briefly, lung tissue (5–20 mg) was homogenized in 0.5 ml carboxymethylation buffer (0.1 M Tris·HCl pH 8.8, 6 M guanidine hydrochloride, and 10 mg/ml iodoacetic acid), and after the addition of 5 μl of 10% Triton X-100 the samples were incubated for 1 h at 37°C in the dark. Samples were centrifuged in a tabletop refrigerated centrifuge (Ependorff) for 10 min at 2,500 rpm, and the supernatants (0.5 ml) were transferred to desalting columns to remove guanidine hydrochloride. Protein content was determined by the Bradford method (Bio-Rad, Hercules, CA). Twenty micrograms of carboxymethylated lung homogenate were fractionated on a 15% native polyacrylamide gel (Bio-Rad). The protein was transferred to nitrocellulose (Hybond-ECL; GE Biotechnology) using a Miniprotean transblot apparatus (Bio-Rad). Nitrocellulose was washed and incubated with goat IgG antibodies to human Trx (American Diagnostica, Greenwich, CT). After being washed, the blot was incubated with a horseradish peroxidase conjugate of anti-goat IgG (Pierce, Rockford, IL) for 1 h at room temperature. Binding of the secondary antibody was detected with Amersham's (GE Biotechnology) enhanced chemiluminescence system.
Immunohistochemistry.
Following exposure to normoxia or hyperoxia, the lung was inflation fixed with 10% neutral formalin and then embedded in paraffin. Immunostaining was performed in the immunohistochemistry core facility of University of Arkansas for Medical Sciences (author's previous institution). Endogenous peroxidase was eliminated with a peroxidase quench solution, and sections were treated with serum to block nonspecific binding and then incubated with primary antibody followed by horseradish peroxidase-conjugated secondary antibody.
Western analysis.
Lung tissue homogenates were prepared in a lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl (pH 7.5), 1% Triton X-100, 10% glycerol, 1 mM EDTA, 1 mM Na3PO4, 10 mM NaF, 1 mM PMSF, and 10 μg/ml aprotinin. After homogenization, the lysates were centrifuged at 14,000 g in a microcentrifuge for 45 min at 4°C. The supernatant was transferred to another tube, and protein concentration was estimated by Bio-Rad protein assay kit (Bio-Rad). Equal amounts of protein were loaded onto polyacrylamide gels and transferred either to PVDF or nitrocellulose membrane. Specific proteins were labeled with specific primary and secondary antibodies, and the bands detected by enhanced chemiluminescence (GE Biotechnology). The primary antibodies used in this study were as follows: anti-mouse/human Trx, anti-p53, anti-Gadd45α, and β-actin from Santa Cruz Biotechnology (Santa Cruz, CA) and anti-mouse Trx from Cell Signaling Technology (Beverly, MA).
Isolation of alveolar type II cells.
WT, Trx-Tg, or dnTrx-Tg mice were exposed to normoxia or hyperoxia (90% oxygen) for 3 days. Following exposure type II cells (AECII) were isolated from mice anesthetized with isoflurane using an adaptation of the procedures described by Corti et al. (9) and modified by Lee et al. (38) and Barker et al. (4). The thorax was opened and the lungs perfused with PBS via the right ventricle. Following flushing, tracheostomy was performed, the trachea was cannulated, and 1 ml of neutral protease was instilled into the lungs. Molten agarose (1 ml) was immediately instilled, and then the thorax was covered with ice for 2–4 min to harden the agarose. The lungs were removed and freed from connective tissue and the heart. They were then immersed in 1.5-ml dispase and then rocked gently for 45 min. The lung tissue was teased apart and progressively filtered through 100- and 40-μM nylon filters. The resulting cell suspension was seeded onto a dish coated with CD45 and CD32 antibodies for 1 h. The cell suspension was carefully removed and pelleted, suspended in 10 ml of DMEM, and seeded onto six-well plates or chambered glass slides coated with fibronectin.
Mitochondrial oxygen consumption analysis.
After isolation, AECII were seeded on fibronectin-coated microwell of the XF24 plates (Seahorse Biosciences, Billerica, MA) and allowed to grow for 3 days. Optimization of cell number and injections of inhibitors for the XF24 flux analyzer (Seahorse Biosciences) was performed using MLE-2 cells as described before (11). AECIIs isolated from WT, Trx-Tg, and dnTrx-Tg mice were run in one microwell plate with four to five replicate wells to increase reproducibility of the experiment. Sequential injections of oligomycin, FCCP, and antimycin/rotenone were made through ports A, B, and C, respectively, as per my published protocol (11). The oxygen consumption rate (OCR) was normalized to number of cells in each well, and the data are expressed as picomoles per minute per 104 cells.
RESULTS
The expression and activity of Trx increased in the lungs of Trx-Tg, but the activity of Trx decreased in the lungs of dnTrx-Tg mice.
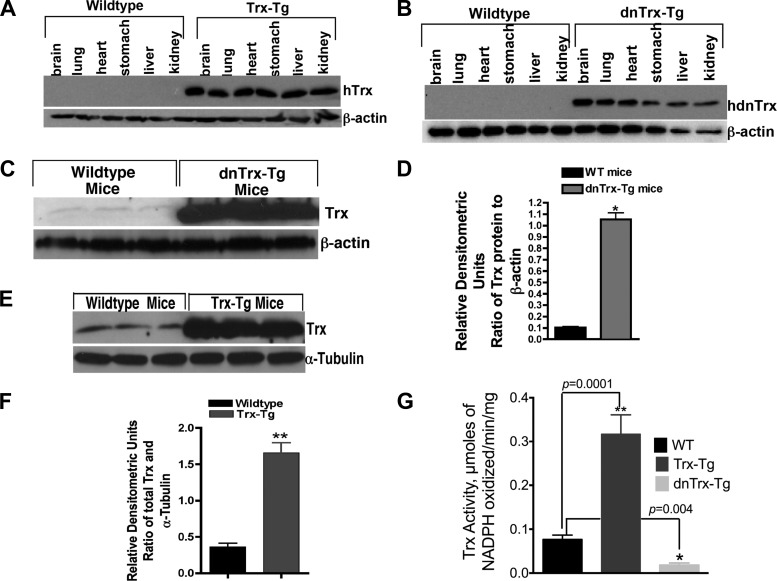
Because oxidant damage, inflammation, loss of energy metabolism, and decline of proliferative potential are underlying mechanisms of hyperoxia-induced lung injury, we reasoned that transgenic mice with higher levels of Trx will be protected from, whereas Trx-deficient mice will be sensitive to, hyperoxia-mediated lung injury. Furthermore, because one of the major functions of Trx is to regenerate oxidized thiols (its substrate) in a nonenzymatic, but stoichiometric manner, we reasoned that recovery from hyperoxia in room air would be more effective in mice expressing higher levels of Trx than WT or dnTrx-Tg mice. We tested this hypothesis by developing Trx-Tg and dnTrx-Tg mice. As indicated above, attempts to generate Trx knockout mice were unsuccessful due to embryonic lethality (44). We therefore utilized a dominant-negative approach to generate mice with decreased levels of Trx (dnTrx-Tg). The catalytic cysteines of Trx, C32, and C35 are necessary for the protein's disulfide reductase activity. Both of these residues are targets for reduction by TrxR. Thus mutation of both of these residues inactivates the disulfide reductase activity of Trx. Mutant Trx (C32S, C35S) has been shown to competitively inhibit TrxR reduction of Trx, with a Ki of 1.8 μM (48). When the mutant Trx is overexpressed, the activity of endogenous Trx is decreased due to the failure of TrxR to regenerate sufficient Trx. Hence, overexpression of mutant Trx in cells is dominant-negative because it suppresses the activity of endogenous Trx (10, 18, 48, 50). As demonstrated in Fig. 1A, total Trx expression is increased in all the organs evaluated in the Trx-Tg mice. Additionally, the mutant Trx protein is also expressed in all of the organs in dnTrx-Tg mice (Fig. 1B). When lung tissue was analyzed for Trx expression, we found 4 times more Trx in the lungs of Trx-Tg mice and 10 times more mutant Trx in the lungs of dnTrx-Tg mice (Fig. 1, C–F). Trx antibody reacts with both WT and mutant Trx; therefore, the Western blot showed increased expression of both Trx and mutant Trx. To determine the level of active Trx, we performed a Trx activity assay that measures the reduction of insulin (28, 41). As shown in Fig. 1G, the pulmonary Trx activity in the lungs of Trx-Tg mice showed three times higher activity compared with nontransgenic (NT/WT) littermates. In contrast, lungs of dnTrx-Tg mice showed threefold less Trx activity compared with WT mice and sixfold less Trx activity compared with lungs of Trx-Tg mice, demonstrating that the mutant Trx is biologically inactive to carryout a reductase reaction.
Fig. 1.
Mice with decreased levels of functional thioredoxin (dnTrx-Tg) and overexpressing Trx (Trx-Tg) show increased levels of immunoreactive Trx in various organs including the lung. F1 mice were killed, and protein expression was analyzed in various organs by immunoblot analysis using an anti-Trx antibody reactive to both human and mouse antigen. Blots were reprobed for β-actin expression to confirm equivalent protein loading. A: Trx-Tg mice. B: dnTrx-Tg mice. C: expression of Trx in the lungs of wild-type (WT) or Trx-Tg mice. D: densitometry of Fig 1C; *P < 0.05, Student's t-test. E: expression of Trx in the lungs of WT or dnTrx-Tg mice. F: densitometry of Fig 1E; **P < 0. 05, Student's t-test. G: Trx activity in the lungs of WT, Trx-Tg, and dnTrx-Tg mice; *P = 0.04; **P = 0.0001.
Mice with higher levels of Trx effectively recover, whereas mice deficient in functional Trx show increased mortality and failure to recover.
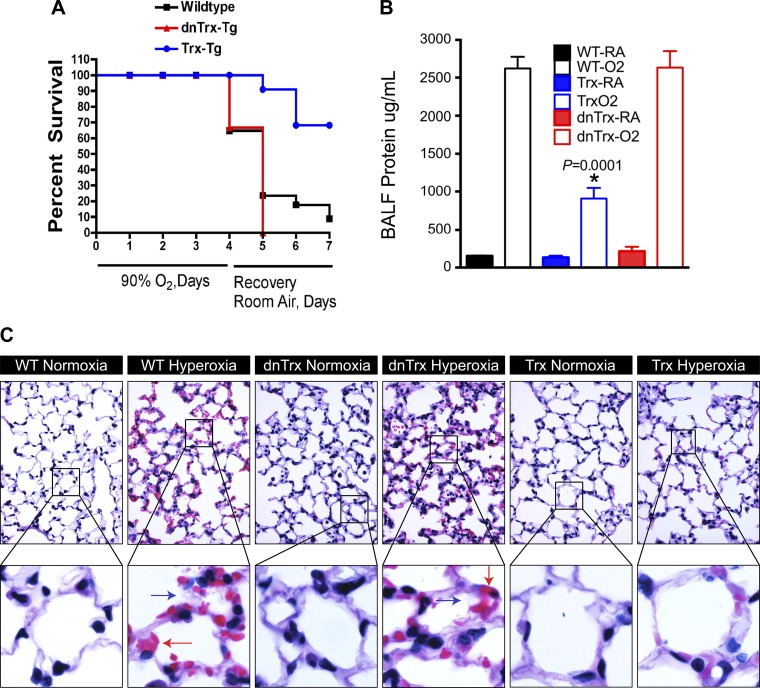
To determine whether oxygen toxicity in hyperoxia is modulated due to high levels of active Trx in vivo, we exposed NT/WT (nontransgenic littermates), Trx-Tg, and dnTrx-Tg mice to >90% oxygen. We exposed Trx-Tg, dnTrx-Tg, and NT/WT littermates to this treatment followed by recovery in ambient air for 24, 48, or 72 h. Recovery of mice in room air following hyperoxia results in significant mortality (38). Therefore, to determine the effect of Trx deficiency or increased expression in a more stringent stress condition, we recovered mice in room air following hyperoxia. As shown in Fig. 2A, before this recovery period 30% of WT mice and 40% of dnTrx-Tg mice had expired, whereas survival of Trx-Tg mice was 100%. At the end of 72-h recovery, survival of WT mice was 15%, survival of dnTrx-Tg mice was 0%, and survival of Trx-Tg mice was 80% (Fig. 2A). Since bronchoalveolar lavage fluid (BALF) protein concentration is a marker of lung injury, we evaluated the total protein content of BALF protein. We observed significant protein leakage (indicating capillary damage) into the lungs of WT and dnTrx-Tg mice during hyperoxia, whereas the lungs of Trx-Tg mice showed significantly lower protein levels in comparable samples (Fig. 2B). However, the protein content in BALF from dnTrx-Tg mice was not different than the WT mice in hyperoxia (P = ns). When we examined lung histopathology, we found increased alveolar damage and focal alveolar hemorrhage and hyaline membrane deposits (Fig. 2C, blue and red arrows, top and bottom) in WT and dnTrx-Tg mice exposed to hyperoxia. Thus the expression of functional Trx strongly correlates with survival during hyperoxic stress and protects against mortality during the recovery period in the Trx-Tg mice. Furthermore, by using both types of mice it is clearly determined that high level of functional Trx expression is a critical factor that determines the toxicity of mice in hyperoxia.
Fig. 2.
Mice deficient in functional Trx show increased mortality in hyperoxia. A: mice were exposed to 90% oxygen for 4 days followed by 3 days of recovery in room air (RA); Kaplan and Meier survival curve, P = 0.0084. B: amount of bronchoalveolar lavage fluid (BALF) protein is increased in WT or dnTrx-Tg mice but not in Trx-Tg mice exposed to hyperoxia. BALF protein is determined by Bradford protein assay. *Significant at P < 0.05, ANOVA. C: increased lung injury in hyperoxia in WT or dnTrx-Tg mice but not in Trx-Tg mice: lung tissue was fixed and processed as mentioned in materials and methods. The sections were processed for hematoxylin and eosin stain for observation of lung histopathology. The slides were observed under Zeiss Axioimager Z2.
Effect of hyperoxia on Trx, TrxR, and Prx activity in WT, Trx-Tg, and dnTrx-Tg mice in hyperoxia and during recovery.
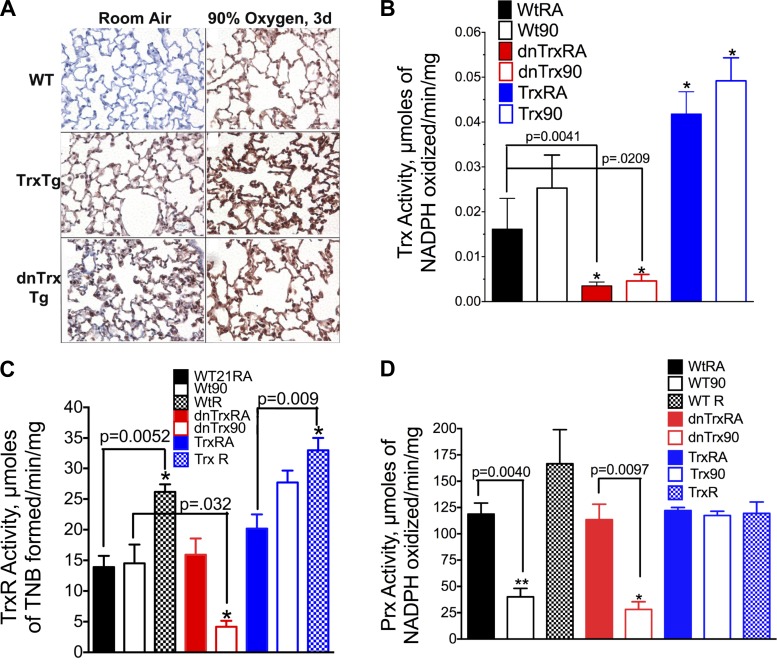
The expression of Trx was increased in the lungs of Trx-Tg and dnTrx-Tg mice in normoxia compared with WT mice (Fig. 3A, left). Exposure to high oxygen further increased the expression of Trx (Fig. 3A, right) in Trx-Tg and dnTrx-Tg mice lungs. The lungs of WT mice also had increased levels of Trx in the lung. Since immunohistochemistry is a qualitative method, we determined the activity of Trx in WT, Trx-Tg, and dnTrx-Tg mice exposed to normoxia or hyperoxia. As shown in Fig. 3B, the activity of Trx was significantly higher in the lung of Trx-Tg mice compared with dnTrx-Tg or WT mice. In contrast, pulmonary Trx activity in dnTrx-Tg mice was significantly lower in normoxia as well as hyperoxia compared with either WT or Trx-Tg mice. TrxR is a critical enzyme that reduces Trx using reducing equivalents from NADPH. Therefore, the activity of TrxR is rate limiting for conversion of oxidized Trx to reduced form as reduced Trx performs the critical protein disulfide reductase function. We determined whether the activity of TrxR in the lungs of Trx-Tg or Trx-deficient mice is modulated during hyperoxia. As shown in Fig. 3C, the activity of TrxR remains unchanged in hyperoxia in the lungs of WT mice but is significantly decreased in the lungs of Trx-deficient mice. In contrast, the TrxR activity was increased in the lungs of Trx-Tg mice in hyperoxia. Furthermore, the activity of TrxR was increased in the recovery period in WT and Trx-Tg mice. These data suggest that loss of TrxR activity in hyperoxia in the lungs of Trx-deficient mice could compromise the protein disulfide reductase activity of Trx, which could result in increased oxidative stress due to loss of enzymatic activities of critical sulfhydryl enzymes with −SH groups in the catalytic center. Furthermore, these data also suggest that Trx in the lungs of Trx-Tg mice remains in reduced state during hyperoxia or recovery due to increased TrxR activity.
Fig. 3.
A: increased expression of Trx in lungs of WT, Trx-Tg, and dnTrx-Tg mice in hyperoxia. Lungs of mice were inflation fixed with 10% neutral formalin, and paraffin sections were processed for immunohistochemistry. Primary anti-Trx antibody used here was obtained from Santa Cruz Biotechnology. This antibody is reactive to both mouse and human protein: Top left: WT mouse lung in room air; top right: lungs of WT mice in hyperoxia. Middle left; lungs of Trx-Tg mice in room air; Middle right: Lungs of Trx-Tg mice in hyperoxia. Bottom left: Lung of dnTrx mice in room air; bottom right: Lungs of dnTrx mice in hyperoxia. Brown stain represents Trx or dnTrx protein in the lung sections of mice. B: activity of Trx is increased in the lungs of Trx-Tg mice in normoxia as well as hyperoxia but decreased in the dnTrx-Tg mice: lung tissue was homogenized and the Trx activity assay was performed as described in materials and methods. The activity was expressed as μmol NADPH oxidized·min−1·mg lung protein−1 at 25°C. The means were compared using Student's t-test. C: expression of TrxR decreases in the lungs of dnTrx-Tg mice in hyperoxia but increases during recovery in WT/NT and Trx-Tg mice. Mice were exposed to 90% oxygen for 4 days followed by 3 days of recovery in room air. TrxR activity was determined by formation thio-2-nitro benzene (TNB) that was monitored at 412 nm as described in materials and methods. *Significantly increased or decreased compared with WT, Trx-Tg, or dnTrx-Tg mice. D: activity of peroxiredoxins (Prx) decreased in WT or dnTrx-Tg mice in hyperoxia, but remains unchanged in Trx-Tg mice. Mice were exposed to 90% oxygen for 4 days followed by 3 days of recovery in room air. Prx activity was determined using H2O2 as a substrate in presence of Trx-TrxR system as described in the methods. *,**Significantly lower than WT, Trx-Tg, or dnTrx-Tg mice in normoxia.
2-Cys Prx proteins are critical antioxidant enzymes that detoxify peroxides using reducing equivalents from the Trx-TrxR system (25). Prx I to V are known as 2-Cys Prx proteins due to the presence of two cysteines (35, 42). The critical Cys47 of Prx is oxidized by H2O2 and reacts with Cys170 in the other subunit to form an intermolecular disulfide, which is reduced by Trx (35, 42). Thus Trx is required to maintain the activity of Prx I–V. Because Trx is the critical electron donor for Prx I–V, we sought to determine how altered Trx levels in our transgenic mice affect lung Prx during hyperoxia and recovery. As shown in Fig. 3D, total Prx activity was decreased in hyperoxia in NT littermates but was significantly increased during the recovery period. In contrast, the activity of Prx in Trx-Tg mice remained unchanged during exposure to hyperoxia or during recovery (Fig. 3D). Significantly, dnTrx-Tg mice (those with decreased Trx levels) had lower Prx activities in ambient air compared with nontransgenic littermates or Trx-Tg mice (Fig. 3D); when these mice were exposed to hyperoxia, the level of Prx was further decreased. There was no difference in the Prx activity in the lungs of WT or dnTrx-Tg mice exposed to hyperoxia (P = ns). However, Prx activity decreased ∼30% (WT90, 40.02 ± 8.09, n = 3 and dnTrx-Tg, 28.18 ± 7.27, n = 3) in the lungs of dnTrx-Tg mice in hyperoxia compared with WT mice exposed to hyperoxia. Because none of the dnTrx-Tg mice survived during recovery, we could not make measurements for this phase. These studies show that functional Trx is required for maintaining Prx in an active state during hyperoxia and in the recovery period.
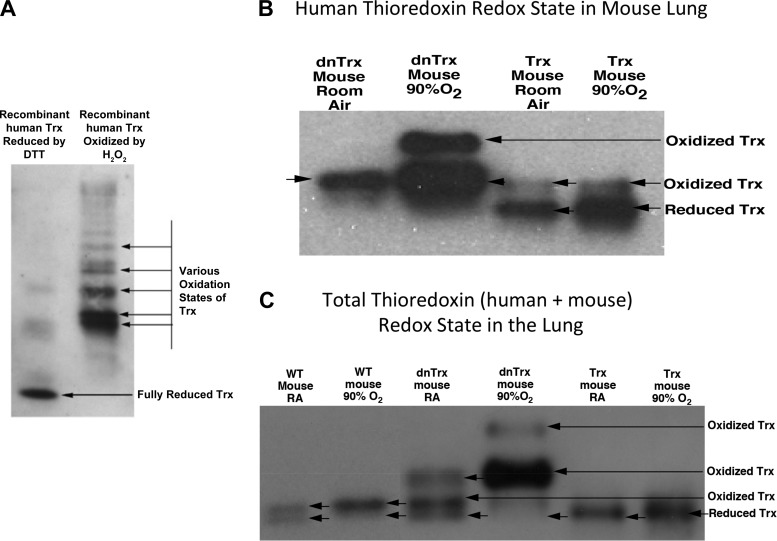
The redox state of Trx is preserved in the lungs of Trx-Tg mice, but increased oxidation of Trx occurs in the lungs of dnTrx-Tg mice in normoxia and hyperoxia.
Our data in Fig. 3C showed increased activity of TrxR in hyperoxia in the lungs of Trx-Tg mice, but the activity decreased in the lungs of dnTrx-Tg mice. Therefore, we speculated that Trx would remain in a reduced state in hyperoxia in the lungs of Trx-Tg mice but significant oxidation of Trx would occur in the lungs of dnTrx-Tg mice in hyperoxia. In a control experiment we determined the redox state of fully reduced and oxidized human (h)Trx using carboxymethylation of purified hTrx. As shown in Fig. 4A, hTrx reduced with DTT showed only one lower band demonstrating its high mobility in native PAGE (14, 46). However, hTrx oxidized with H2O2 (1 mM) showed several bands indicating various states of oxidation among the five cysteine residues present in hTrx (Fig. 4A). We evaluated the redox state of hTrx in the lungs of Trx-Tg or dnTrx-Tg mice exposed to normoxia or hyperoxia. We could not detect any immunoreactive band to hTrx in WT mouse as the antibody used was specific to hTrx (Fig. 4B). As shown in Fig. 4B, human mutant Trx appeared as an oxidized band in dnTrx mouse lung in normoxia (Fig. 4B, lane 1). When it was exposed to hyperoxia, we observed another band of higher oxidizing state in the dnTrx mouse lung (Fig. 4B, lane 2). The redox state of hTrx in vivo in lungs of Trx-Tg mice showed a higher level of reduced hTrx in normoxia. In addition, we observed a significantly higher level of reduced hTrx in these mice exposed to hyperoxia. Taken together, these data show that overexpressed hTrx in the Trx-Tg mouse lung remains in reduced state in hyperoxia. However, overexpressed mutant hTrx in the lungs of dnTrx mice appeared as a strong band of lesser mobility as mutated Trx could not be carboxymethylated and therefore would appear at the same place as the oxidized Trx. Next, we examined the impact of overexpression of hTrx or mutant hTrx expression on the endogenous mouse Trx plus the overexpressed hTrx in normoxia or hyperoxia. WT mice showed almost equal amount of reduced and oxidized Trx in normoxia in the lung (Fig. 4C, lane 1). However, in hyperoxia a higher level of oxidized Trx (mutant + oxidized Trx) was observed in the lungs of these mice (Fig. 4C, lane 2). Interestingly, the endogenous mouse Trx in dnTrx mice showed an increased state of oxidation in normoxia (Fig. 4C, lane 3). When these mice were exposed to hyperoxia, almost all of their pulmonary Trx remained in the oxidized state (Fig. 4C, lane 4). These data show that the dnTrx mice are experiencing a higher state of oxidative environment even in normoxia that is significantly increased in hyperoxia. In contrast to dnTrx-Tg mice, a significant level of total Trx (endogenous mouse Trx + overexpressed human Trx) in Trx-Tg mice remained in the reduced state in normoxia as well as in hyperoxia (Fig. 4, lanes 5 and 6).
Fig. 4.
Increased oxidation of Trx in lungs of dnTrx-Tg mice: the redox state of Trx was determined as described in materials and methods. A. reduction and oxidation of hTrx in vitro using DTT or H2O2. B: redox state of hTrx in Trx-Tg and dnTrx-Tg mice lung in normoxia and hyperoxia. C: total Trx redox (mouse + overexpressed hTrx, both WT and mutant) state in the lungs of WT, Trx-Tg, and dnTrx-Tg mice. The top band is the noncarboxymethylated oxidized/mutated Trx, and the bottom band is the carboxymethylated reduced Trx.
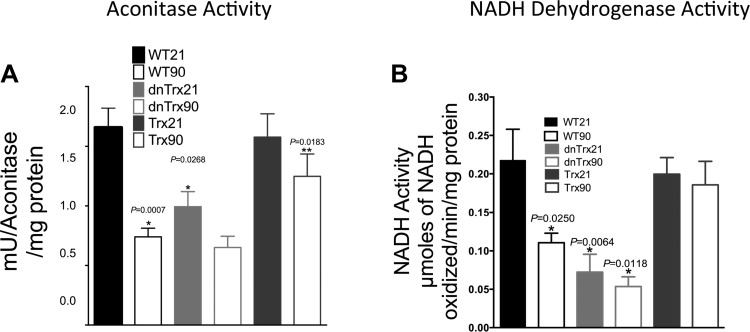
Aconitase and NADH dehydrogenase activities are preserved in the lungs of Trx-Tg mice in hyperoxia but decreased in the lungs of dnTrx-Tg mice in normoxia and hyperoxia.
Aconitase is a mitochondrial iron-sulfur cluster-containing dehydratase that has been implicated in O2 toxicity and is a sensitive target of the superoxide anion (20). We determined whether Trx deficiency could affect the integrity of mitochondrial function by modulating aconitase activity. Our data (Fig. 5A) demonstrate that hyperoxia significantly decreased aconitase activity in WT mice as expected and previously reported (20). A slight decreased in aconitase activity was observed in the lungs of dnTrx-Tg mice during normoxia, but significantly lower levels of activity occurred during hyperoxia, compared with WT mice (Fig. 5A). In contrast, normal aconitase activity was maintained in Trx-Tg mice exposed to hyperoxia, which established that Trx deficiency could contribute to mitochondrial dysfunction.
Fig. 5.
Decreased activities of aconitase and NADH dehydrogenase in the lungs of dnTrx-Tg mice in normoxia and hyperoxia. A: aconitase activity was determined as described in materials and methods. *Significantly decreased in hyperoxia compared with WT, Trx-Tg, or dnTrx-Tg in normoxia; **significantly decreased in normoxia compared with WT or Trx-Tg mice lung, P < 0.05, ANOVA, B: NADH dehydrogenase activity was determined in pulmonary tissue of WT, Trx-Tg, or dnTrx-Tg mice in normoxia and hyperoxia as described in materials and methods. *Significantly decreased compared with WT or Trx Tg mice in normoxia, P < 0.05, ANOVA.
We also measured the effects of Trx on NADH-dehydrogenase, which has an oxidation-sensitive sulfhydryl moiety. We found that its activity was severely decreased in the lungs of WT mice in response to hyperoxia. Compared with controls, the activity in dnTrx-Tg mice was decreased during both normoxia and hyperoxia (Fig. 5B). In contrast, mice with higher levels of Trx showed significant protection against inactivation by hyperoxia (Fig. 5B). These results imply that the critical sulfhydryl enzyme activities of the electron transport chain or the TCA cycle are more sensitive to oxidation in ambient air or hyperoxia in the lungs of dnTrx-Tg mice.
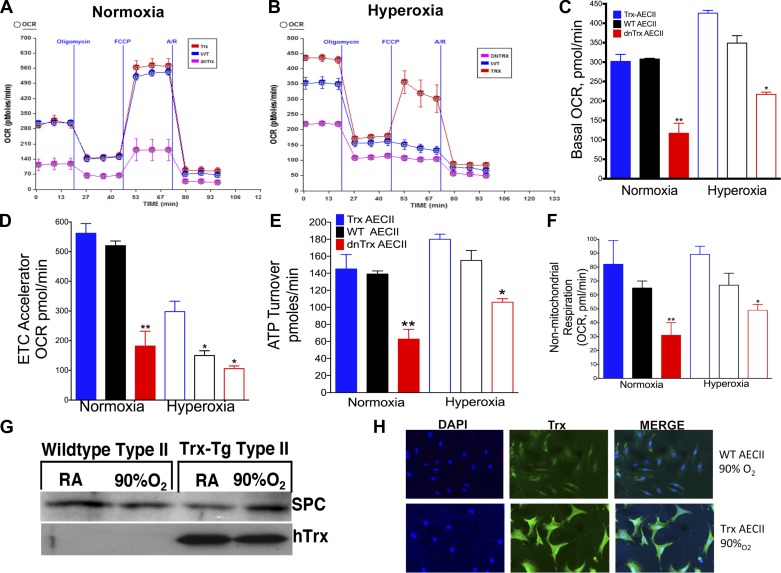
Decreased ATP turnover and decreased maximal respiration in AECII isolated from oxygen exposed dnTrx-Tg mice lung.
During the development or repair of lung injury, type II AEC (AECII) rely on energy from mitochondrial respiration to transform into the type I AEC that make up the respiratory epithelium of adult lung. We examined if the level of Trx affects the mitochondrial bioenergetics of AECII in lungs exposed to hyperoxia, as an efficiently functioning mitochondria would support cell differentiation in the recovery period following exposure. AECII isolated from mice exposed to hyperoxia and cultured for 3 days showed an increased OCR in all strains, but dnTrx-AECII OCR was significantly lower than WT or Trx-Tg mice (Fig. 6, A–C). The maximal respiration in presence of the FCCP was significantly lower in dnTrx-Tg in normoxia as well as hyperoxia (Fig. 6, A, B, and D). Additionally, ATP turnover was significantly decreased in AECII of dnTrx-Tg mice during normoxia or hyperoxia (Fig. 6, A, B, and E). These data indicate that the loss of active Trx in dnTrx-Tg mice compromises mitochondrial energy production in AECII isolated from dnTrx-Tg mice. Nonmitochondrial oxygen consumption was also decreased in dnTrx-Tg mice exposed to either normoxia or hyperoxia (Fig. 6F). Our data in Fig. 6G show that the expression of surfactant protein C (SPC) remains undimished after 3 days of culture of AECII after exposure to normoxia or hyperoxia. Furthermore, we have also confirmed that these cells are positive for Trx and SPC (Fig. 6H). In summary, our data demonstrate that dnTrx-Tg mice have a reduced level of energy utilization during normoxia, which is further decreased during hyperoxia.
Fig. 6.
Decreased mitochondrial oxygen consumption in type II alveolar epithelial cells (AECII) isolated from lungs of dnTrx-Tg mice compared with WT or Trx-Tg mice in hyperoxia: WT, Trx-Tg, and dnTrx-Tg mice were exposed to normoxia or hyperoxia for 24 h. After exposure AECII were isolated and cultured for 3 days followed by oxygen consumption rate (OCR) analysis in a XF24 analyzer. A: OCR of AECII isolated from WT, Trx-Tg, or dnTrx-Tg mice exposed to normoxia and response to oligomycin, carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), and rotenone/antimycin A. B: OCR of AECII isolated from lungs of WT, Trx-Tg, or dnTrx-Tg mice exposed to 24 h 90% oxygen and response to oligomycin, FCCP, and rotenone/antimycin A. C: basal OCR of AEC II in normoxia or hyperoxia. D: maximal respiratory capacity of AECII in normoxia or hyperoxia. E: ATP turnover of AECII in normoxia or hyperoxia. F: nonmitochondrial respiration. Data are presented as means ± SE (minimum number of wells n = 5). *P < 0.05, **P < 0.0001, by ANOVA and Tukey-Kramer posttest. Data was normalized to number of AECII in the micro-well after the assay. OCR measurements taken in 5 replicate wells and data representative of 2 independent experiments using 2 mice for each strain. G: expression of surfactant protein C (SPC) in AECII. H: immunofluorescence localization of Trx in AECII isolated from WT or Trx-Tg mice. ETC, electron transport chain.
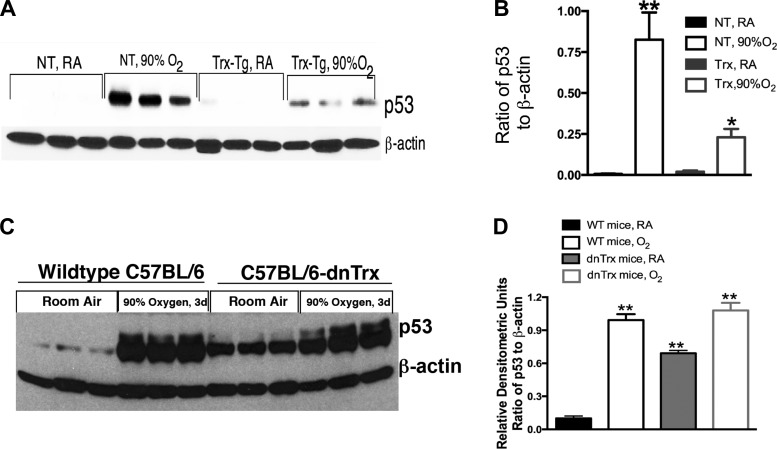
Trx-Tg mice show decreased p53 expression, but dnTrx-Tg mice show increased expression of p53 in normoxia, which is further increased in hyperoxia.
The tumor suppressor protein p53 is a master regulator of genomic stability, and its induction by genotoxic stress results in cell-cycle arrest and apoptotic cell death. Hyperoxia is known to stimulate pulmonary p53 expression (13, 37), so we determined if Trx deficiency increases this response. As expected, we found exposure of WT mice to high oxygen induced p53 expression in the lungs (Fig. 7, A and B) compared with mice in ambient air. Surprisingly, we found that p53 expression in normal oxygen is sixfold higher in the lungs of dnTrx-Tg mice compared with WT mice (Fig. 7, C and D) and this level increased even further during hyperoxia (Fig. 7, C and D). Thus our data further show that normal oxygen poses an acute genotoxic stress to mice deficient in active Trx. On the other hand, hyperoxia-induced p53 expression in Trx-Tg mice was lower compared with WT mice demonstrating that Trx-Tg mice experience significantly decreased levels of genotoxic stress during hyperoxia.
Fig. 7.
p53 expression increases in lungs of dnTrx-Tg mice in normoxia and decreases in lungs of Trx-Tg mice exposed to hyperoxia. A: Western analysis of p53 in WT or Trx-Tg mice. B: densitometry of A. C: Western analysis of p53 in lung tissue of WT or dnTrx-Tg mice. D: densitometry of C. *Significantly decreased in lungs of Trx-Tg mice in hyperoxia (P = 0.0001). **Significantly higher than WT mice exposed to 21% oxygen (ANOVA, Tukey's test, P = 0.0001).
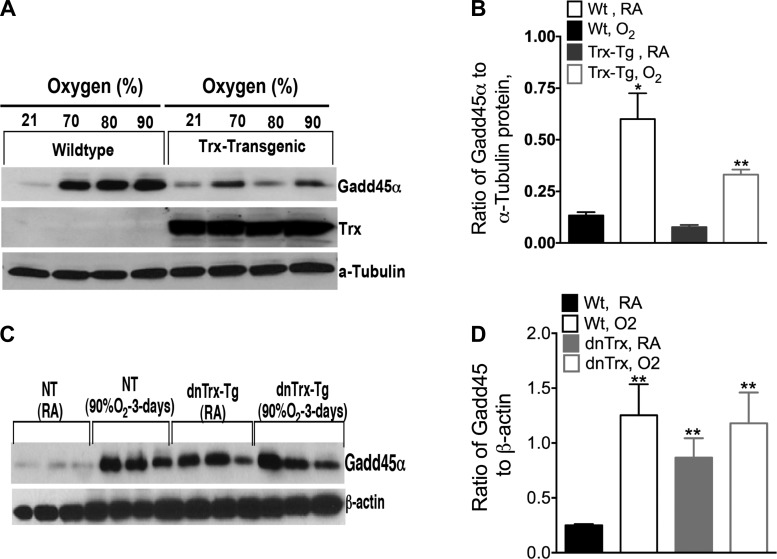
Trx-Tg mice show decreased expression of Gadd45α in hyperoxia, but increased Gadd45a expression occurs in dnTrx-Tg mice in normoxia, which is further increased due to hyperoxia.
The Gadd45α protein is known to be involved in the repair of DNA damage in conjunction with p53 caused by genotoxic stress (47). We sought to determine whether the expression of Gadd45α is affected by modulation of functional Trx level in response to hyperoxia. As shown in Fig. 8A, WT mice exposed to hyperoxia (70, 80, or 90% oxygen) had increased Gadd45α expression, and the expression of protein did not vary among 70, 80, or 90% oxygen exposure. These data suggest that maximal expression is achieved at 70% oxygen exposure and increasing the exposure to 90% oxygen does not further increase the expression of Gadd45α. However, Trx-Tg mice exposed to 90% oxygen had a significantly lower level of Gadd45α compared with WT mice. Surprisingly, the lungs of dnTrx-Tg mice in ambient air had a significantly higher level of Gadd45α than controls (Fig. 8, C and D), which was further increased in hyperoxia, but there was no significant difference between the expression of Gadd45α in dnTrx-Tg mice exposed to normoxia or hyperoxia. Like the results for p53, these data show that normal oxygen levels comprise a genotoxic stress for Trx-deficient mice, causing them to respond with high levels of Gadd45α to repair DNA. By contrast, the expression of Gadd45α in response to hyperoxia was substantially decreased in the lungs of Trx-Tg mice. These data show that Trx plays an important role in maintaining low basal levels of Gadd45α and p53 in ambient air. Our data show that room air poses an acute genotoxic stress to dnTrx-Tg mice demonstrating that a lack of functional Trx correlates with a damaging sensitivity to ambient oxygen levels.
Fig. 8.
Gadd45α expression increases in lungs of dnTrx-Tg mice in normoxia and decreases in lungs of Trx-Tg mice exposed to hyperoxia. A: Western analysis of Gadd45α in WT or Trx-Tg mice exposed to 70, 80, or 90% oxygen for 72 h. B: Western analysis of Gadd45α in mice exposed normoxia or to 90% O2 for 72 h; graph presented shows ratio of Gadd45α to α-tubulin. C: Western analysis of Gadd45α in lung tissue of WT or dnTrx-Tg mice. D: densitometry of C. **Significantly higher than WT mice exposed to 21% oxygen (ANOVA, Tukey's test, P = 0.0001).
Proliferation and apoptosis of lung cells during the posthyperoxia recovery period.
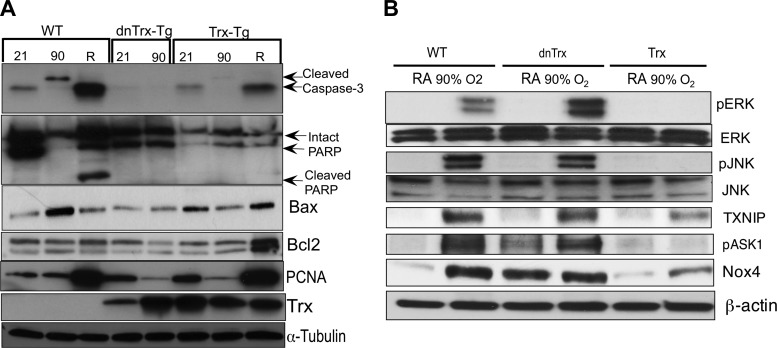
Animal and cell culture studies have already established that hyperoxia causes both necrotic and apoptotic cell death (3, 31, 33, 34). In our hyperoxia survival studies, we observed that 100% mortality occurred in dnTrx-Tg mice 2 days before it occurred in WT mice (Fig. 2A). We suspected that overt necrotic death of lung cells in dnTrx-Tg may have caused this early mortality, so we measured the appearance of apoptosis markers in the lung during hyperoxia. As shown in Fig. 9A, we did not observe cleaved caspase-3 or cleaved poly(ADP-ribose) polymerase (PARP) in the lungs of WT mice during hyperoxia, but both appeared in the postexposure recovery period. By contrast, these markers were not detected in dnTrx-Tg mice at all. However, the WT and Trx-Tg mice only expressed apoptosis markers during recovery from high oxygen exposure (Fig. 9A) but at a lower level. In both WT and Trx-Tg mice, the cell-proliferation marker PCNA was increased during recovery but not during hyperoxia itself. We could not perform comparable experiments with dnTrx-Tg mice because all of them died during the recovery period. Phosphorylation of the upstream apoptosis signal transducers ERK, JNK, and ASK1 was increased during hyperoxia in the lungs of WT and dnTrx-Tg mice but not in Trx-Tg mice (Fig. 9B). Likewise, the expression of thioredoxin-interacting protein (TXNIP) and NADPH oxidase 4 (Nox4) was increased during hyperoxia in WT and dnTrx-Tg mice. Hyperoxia caused increased expression of these markers in Trx-Tg but to a lesser degree. Our data show that, whereas apoptosis does not occur in the lungs of mice during their exposure to hyperoxia, it does occur in the recovery phase, and the survival of the mice depends on shifting the balance in favor of proliferation over apoptosis. Trx appears to promote this shift. Our data also indicate that the death of dnTrx-Tg mice during hyperoxia is likely due to necrosis of lung cells as no apoptotic markers were expressed in the lungs of these mice.
Fig. 9.
Increase apoptosis of lung cells occurs in the recovery period following hyperoxia, but not during the exposure period. Mice were exposed to 21% (room air) or 90% oxygen for 3 days; some were allowed a 3-day recovery. A: expression of cleaved caspase-3, cleaved poly(ADP-ribose) polymerase (PARP), Bax, Bcl2, PCNA, Trx, and α-tubulin was determined using western analysis. B: expression of pERK/ERK, pJNK/JNK, pASK, thioredoxin-interacting protein (TXNIP), and NADPH oxidase 4 (Nox4) during normoxia or hyperoxia in WT, dnTrx-Tg, and Trx-Tg lung in Western analysis.
Synthesis of proinflammatory cytokines is decreased in the lungs of Trx-Tg mice but increased in Trx-deficient mice in hyperoxia.
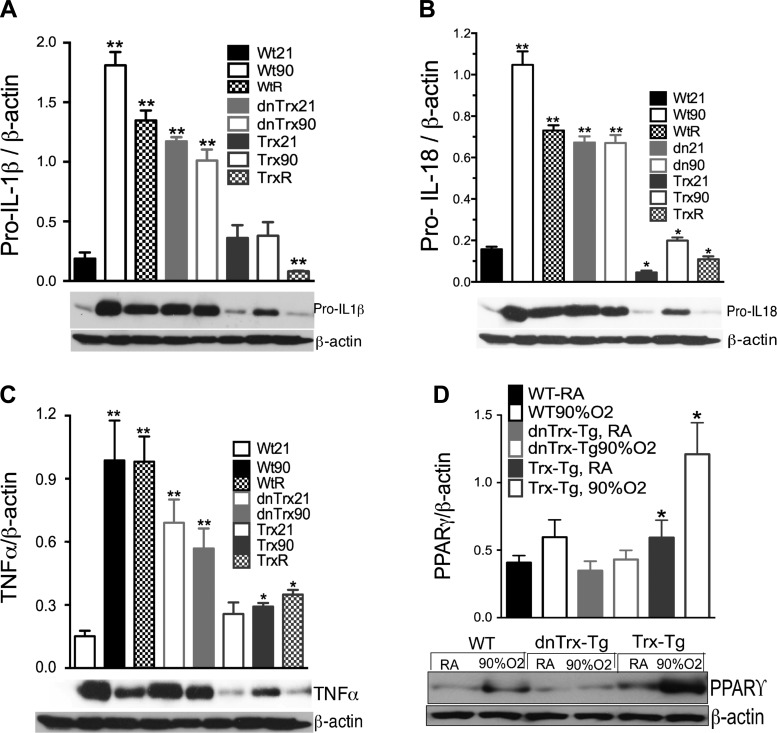
Inflammation of the lung is a major consequence of hyperoxia, so we determined if Trx levels affect the expression of proinflammatory cytokines. We measured an 8- to 10-fold increase in pro-IL18, pro-IL1β, and TNFα in the lungs of WT mice exposed to hyperoxia compared with normoxia (Fig. 10, A–C). Our data show that in dnTrx-Tg mice, high levels of these cytokines occur in the lungs even in normal oxygen. The expression of Pro-IL1β, Pro-IL-18, and TNFα was significantly higher in normoxia in dnTrx-Tg mice compared with WT mice. Thus these mice are more susceptible to inflammation in ambient air, and threshold levels of functional Trx are required to prevent inflammation in ambient conditions. Exposure of dnTrx-Tg mice to hyperoxia did not further increase these already high levels of procytokines, indicating that Trx deficiency has already imposed acute oxidative stress conditions during normoxia. By comparison, pulmonary expression of these cytokines in response to hyperoxia was markedly decreased in Trx-Tg mice. Thus, whereas WT mice experience postexposure lung inflammation, the lungs of Trx-Tg mice are clear of inflammatory mediators during the recovery phase. We also determined if Trx levels modulate peroxisome proliferator-activated receptor-γ (PPARγ) expression, as this protein is known to be induced by hyperoxia and negatively regulates inflammation (6, 51). Our data (Fig. 10D) show that hyperoxia upregulated PPARγ levels in Trx-Tg mice but not in dnTrx-Tg mice.
Fig. 10.
Enhanced expression of proinflammatory cytokines in the lungs of dnTrx-Tg mice in normoxia. Nontransgenic (NT)/WT, Trx-Tg, or dnTrx-Tg mice exposed to 21% oxygen (room air) or 90% O2 for 4 days, followed by 3-day recovery. Pro-IL-18, pro-IL1β, TNFα, PPARγ, and β-actin were determined in the lung lysates by Western analysis (bottom). The ratio of cytokines to β-actin was plotted on graphs (n = 3 for each mouse group). A: expression of pro-IL-18. B: expression of pro-IL-1β. C: expression of TNFα. D: expression of PPARγ. *Significantly higher than WT mice in normoxia (P < 0.05). **Significantly lower than WT or or dnTrx-Tg mice exposed to hyperoxia (P < 0.05).
DISCUSSION
Overexpression of human Trx in lungs of mice protected against hyperoxia-mediated lung injury by maintaining lung redox balance and mitochondrial function and decreasing inflammation of the lung. We also created mice that expresses mutant redox-inactive Trx, because Trx knockout mice die during embryogenesis (44). We therefore used a dominant negative approach to generate mice that are deficient in Trx function and established that they have a pro-oxidant and proinflammatory phenotype in ambient air. Their antioxidant system, mitochondrial bioenergetics, genomic surveillance system, and pulmonary inflammatory response are strongly affected by normal oxygen levels. Because pulmonary Trx activity in these mice is only one-third of WT and one-sixth of the overexpressing Trx-Tg mice, the normal function of this protein in maintaining the lung redox state is compromised. Maintaining the cellular redox state in a reducing condition by proteins like Trx is critical for organisms to survive in ambient air. Thus threshold levels of Trx are required for survival in ambient air, and increased levels of Trx could provide therapeutic protection against hyperoxia-mediated oxidation.
Knockout mice for CuZnSOD, catalase, and glutathione peroxidase all develop normally and do not show an oxidant-sensitive phenotype in ambient air (21–24). In contrast, the Trx-deficient mice studied here developed significant oxidative stress and experienced inactivation of sensitive cytosolic and mitochondrial enzymes in normal oxygen. In fact, our findings indicate that a threshold level of active Trx is a requirement for survival in ambient air. For example, we observed significantly higher pulmonary levels of p53 and Gadd45α expression in dnTrx-Tg mice during normoxia, indicating genotoxic stress. However, these mice do not show morphological manifestations of specific disease types. We believe that continuous DNA damage occurring in the pulmonary tissue of dnTrx-Tg mice in ambient air is constantly being repaired with increased p53 and Gadd45α levels. This process allows the dnTrx-Tg mice to survive in ambient air by preventing the development of diseases associated with genotoxicity. However, exposure to hyperoxia posed an acute oxidative stress to dnTrx-Tg mice, resulting in their accelerated death.
Although the dnTrx-Tg mice showed accelerated death, there was no additional increase in the BALF protein compared with the WT mice. In addition, their Prx activity decreased in hyperoxia, but the decrease was not higher compared with WT mice. In addition, the expression of proinflammatory cytokines was higher in these mice in normoxia, but again these levels did not increase further in response to hyperoxia. Additionally, p53 and gadd45 levels were higher in these mice in normoxia, but these expressions were not significantly higher in hyperoxia. These data suggest that the levels of these lung injury and inflammation markers have reached their maximal level in normoxia. For example, the expression of Gadd45α remained similar at 70, 80, or 90% oxygen exposure. Increasing the oxidant stress by exposing the mice to 90% oxygen did not increase the Gadd45α levels over 70% oxygen in WT mice (Fig. 8A). Therefore, we believe that dnTrx-Tg mice experience the maximal level of oxidant insult in normoxia that could only increase marginally in hyperoxia. However, our histopathology and BALF data show that the lung structure and histology remain undamaged in dnTrx-Tg mice maintained in ambient air in spite of increased expression of genotoxic and proinflammatory markers. This fact suggests that although ambient air induces the genotoxic and inflammatory marker expression the damage to the lung did not reach a irreparable level and as a consequence dnTrx-Tg mice had no difficulty in surviving in ambient conditions. However, a marginal increase in these markers was sufficient to fail the repair mechanism with onset of severe injury leading to death in hyperoxia. Additional studies are required to uncover the exact mechanism of survival of these mice on the face of significant oxidative insult.
We have previously shown that the mutant Trx protein (C32A, C35A, and structural proteins C62A and C69A) is equally effective as a hydroxyl radical scavenger and singlet oxygen quencher (12). Thus in terms of scavenging the hydroxyl radical function overexpressed redox active Trx is no different than the mutant Trx. Given that a significant amount of mutant Trx is produced in the lungs of dnTrx-Tg mice, this mutant Trx could also intercept hydroxyl radicals and decrease hydroxyl radical-mediated lung injury in normal oxygen levels as well as in hyperoxia. However, active Trx could regenerate −SH containing proteins by its thiol-disulfide reductase function in contrast to mutant Trx. Therefore, whereas the thiol-disulfide function is compromised in dnTrx-Tg mice, the hydroxyl radical scavenging function may remain in place. In addition, because Trx is an electron donor for ribonucleotide reductase, and this process is rate limiting for the synthesis of deoxyribonucleotides (46), the DNA replication and DNA repair would be affected in dnTrx-Tg mice compared with Trx-Tg mice. Thus, while recovery from injury could be achieved in Trx-Tg mice due to enhanced cell proliferation, the dnTrx-Tg mice would fail to recover as DNA replication could be halted due to insufficient deoxyribonucleotides and consequently these mice would succumb to death.
Our data further show that, although pulmonary apoptotic markers are not expressed in control mice during the exposure phase of hyperoxia, they do appear in the recovery phase, but to a lesser extent than in the lungs of Trx-Tg mice. Others have shown that apoptosis does not occur in response to hyperoxia in animals or isolated cells (31, 34). However, phosphorylation of ERK, JNK, and ASK1 and expression of TXNIP increase during hyperoxia, and these modifications are associated with apoptosis (36, 39, 43, 57). Although we found these proteins to be phosphorylated in response to hyperoxia (Fig. 9B), we could not detect cleaved caspase-3 or cleaved PARP during the exposure period. Thus we believe that apoptotic signaling events are initiated in the hyperoxic exposure period, ahead of the recovery period when apoptosis actually occurs. We also found that this apoptosis is accompanied by significantly higher cell proliferation during the recovery phase of WT or Trx-Tg mice as demonstrated by increased PCNA expression in the recovery phase.
Hyperoxia induces increased levels of mature cytokines such as IL-18, IL-1β, and TNFα. These factors are derived from procytokines, which are processed by caspase-1 to form the mature molecules responsible for signaling. Our data show that hyperoxia induced the expression of proinflammatory cytokines establishing that the response includes transcriptional upregulation of procytokines in addition to their conversion. We also found the synthesis of pulmonary procytokines to be increased in dnTrx-Tg mice breathing ambient air, indicating that higher levels of oxygen per se are not the inducing factor for procytokine expression. Instead, it appears loss of redox balance in the absence of functional Trx induces the de novo synthesis of proinflammatory cytokines. This interpretation is supported by the fact that cytokine levels do not increase further upon exposure of dnTrx-Tg mice to hyperoxia. Alternatively, it is likely that increased oxidative stress due to hydroxyl radical scavenging by mutant Trx in dnTrx-Tg mice could limit the increase in the expression of procytokines in hyperoxia as mentioned above.
We have previously showed that the increased level of human Trx induces mitochondrial superoxide dismutase 2 expression (MnSOD) in lung cells (15). In addition, Trx is induced in the lung in a baboon bronchopulmonary dysplasia model (14). Although we observed increased expression of Trx in the lungs of Trx-Tg mice, we did not note any increase in MnSOD expression in the lung (data not shown). This is consistent with our prior observation that cells of rodent origin do not induce MnSOD in response to Trx (15). This fact shows that how at molecular level gene expression is differentially regulated in rodents and humans. However, Trx-Tg mice showed significant protection against hyperoxia even in the absence of increased MnSOD. Furthermore, we did not observe any increase in the mitochondrial Trx2 expression in Trx-Tg mice (data not shown). Yet, increased Trx expression protected against mitochondrial dysfunction in hyperoxia. At this point we are unclear of the action of Trx, but studies are underway to delineate this mechanism of action of Trx.
In summary, our results establish that Trx could be an effective agent to decrease oxidative lung injury and thus could be a promising therapeutic molecule. This concept is even more exciting considering the possibility that Trx is freely internalized into lung cells and other cell types via an unidentified mechanism. In addition, in humans Trx could be more effective as it would induce the MnSOD and thereby may provide significant protection against mitochondrial dysfunction and other deleterious consequences of hyperoxia. We are currently pursuing additional studies to elucidate the Trx-mediated protective mechanism in the lung.
GRANTS
Research reported in this publication is supported by National Heart, Lung, And Blood Institute Grants R01-HL-071558, R01-HL-107885, and R01-HL-109397. The content is solely the responsibility of the author and does not necessarily represent the official view of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.C.D. conception and design of research; K.C.D. performed experiments; K.C.D. analyzed data; K.C.D. interpreted results of experiments; K.C.D. prepared figures; K.C.D. drafted manuscript; K.C.D. edited and revised manuscript; K.C.D. approved final version of manuscript.
ACKNOWLEDGMENTS
I gratefully acknowledges helpful discussions with Dr. Charles O'Brien, the director of core facility at University of Arkansas for Medical Sciences for generation of transgenic mice. I acknowledge generous contribution of chicken β-actin promoter from Dr. Andy McMahon, Harvard University. The excellent technical assistance of Harish Muniyappa, Kundan Das, and Venkatesh Sridharan is gratefully acknowledged.
REFERENCES
- 1.Abman SH, Warady BA, Lum GM, Koops BL. Systemic hypertension in infants with bronchopulmonary dysplasia. J Pediatr 104: 928–931, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L1073–L1084, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Barazzone C, Horowitz S, Donati YR, Rodriguez I, Piguet PF. Oxygen toxicity in mouse lung: pathways to cell death. Am J Respir Cell Mol Biol 19: 573–581, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Barker GF, Manzo ND, Cotich KL, Shone RK, Waxman AB. DNA damage induced by hyperoxia: quantitation and correlation with lung injury. Am J Respir Cell Mol Biol 35: 277–288, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmeyer HU. New measurement principles in clinical enzymology. Quad Sclavo Diagn 8: 27–44, 1972. [PubMed] [Google Scholar]
- 6.Cho HY, Gladwell W, Wang X, Chorley B, Bell D, Reddy SP, Kleeberger SR. Nrf2-regulated PPARγ expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med 182: 170–182, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med 5: 47–78, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Coalson JJ, Kuehl TJ, Escobedo MB, Hilliard JL, Smith F, Meredith K, Null DM Jr, Walsh W, Johnson D, Robotham JL. A baboon model of bronchopulmonary dysplasia. II. Pathologic features. Exp Mol Pathol 37: 335–350, 1982. [DOI] [PubMed] [Google Scholar]
- 9.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol 14: 309–315, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Das KC. c-Jun NH2-terminal kinase-mediated redox-dependent degradation of IkappaB: role of thioredoxin in NF-kappaB activation. J Biol Chem 276: 4662–4670, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Das KC. Hyperoxia decreases glycolytic capacity, glycolytic reserve and oxidative phosphorylation in MLE-12 cells and inhibits complex I and II function, but not complex IV in isolated mouse lung mitochondria. PLoS One 8: e73358, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das KC, Das CK. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: redox independent functions. Biochem Biophys Res Commun 277: 443–447, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Das KC, Dashnamoorthy R. Hyperoxia activates the ATR-Chk1 pathway and phosphorylates p53 at multiple sites. Am J Physiol Lung Cell Mol Physiol 286: L87–L97, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Das KC, Guo XL, White CW. Induction of thioredoxin and thioredoxin reductase gene expression in lungs of newborn primates by oxygen. Am J Physiol Lung Cell Mol Physiol 276: L530–L539, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Das KC, Lewis-Molock Y, White CW. Elevation of manganese superoxide dismutase gene expression by thioredoxin. Am J Respir Cell Mol Biol 17: 713–726, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Day BJ. Antioxidants as potential therapeutics for lung fibrosis. Antioxid Redox Signal 10: 355–370, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dos Santos CC. Hyperoxic acute lung injury and ventilator-induced/associated lung injury: new insights into intracellular signaling pathways. Crit Care 11: 126, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallegos A, Gasdaska JR, Taylor CW, Paine-Murrieta GD, Goodman D, Gasdaska PY, Berggren M, Briehl MM, Powis G. Transfection with human thioredoxin increases cell proliferation and a dominant-negative mutant thioredoxin reverses the transformed phenotype of human breast cancer cells. Cancer Res 56: 5765–5770, 1996. [PubMed] [Google Scholar]
- 19.Gardner PR. Aconitase: sensitive target and measure of superoxide. Methods Enzymol 349: 9–23, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci USA 91: 12248–12252, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho YS. Transgenic and knockout models for studying the role of lung antioxidant enzymes in defense against hyperoxia. Am J Respir Crit Care Med 166: S51–56, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem 273: 7765–7769, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem 272: 16644–16651, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Ho YS, Vincent R, Dey MS, Slot JW, Crapo JD. Transgenic models for the study of lung antioxidant defense: enhanced manganese-containing superoxide dismutase activity gives partial protection to B6C3 hybrid mice exposed to hyperoxia. Am J Respir Cell Mol Biol 18: 538–547, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann B, Hecht HJ, Flohe L. Peroxiredoxins. Biol Chem 383: 347–364, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2: 811–820, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Holmgren A. Redox regulation by thioredoxin and thioredoxin reductase. Biofactors 11: 63–64, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Holmgren A. Thioredoxin. Annu Rev Biochem 54: 237–271, 1985. [DOI] [PubMed] [Google Scholar]
- 29.Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol 252: 199–208, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Holmgren A, Johansson C, Berndt C, Lonn ME, Hudemann C, Lillig CH. Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans 33: 1375–1377, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Horowitz S. Pathways to cell death in hyperoxia. Chest 116: 64S–67S, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Ingbar DH. Mechanisms of repair and remodeling following acute lung injury. Clin Chest Med 21: 589–616, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Kazzaz JA, Horowitz S, Li Y, Mantell LL. Hyperoxia in cell culture. A non-apoptotic programmed cell death. Ann NY Acad Sci 887: 164–170, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S. Cellular oxygen toxicity. Oxidant injury without apoptosis. J Biol Chem 271: 15182–15186, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Knoops B, Clippe A, Bogard C, Arsalane K, Wattiez R, Hermans C, Duconseille E, Falmagne P, Bernard A. Cloning and characterization of AOEB166, a novel mammalian antioxidant enzyme of the peroxiredoxin family. J Biol Chem 274: 30451–30458, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Kolliputi N, Waxman AB. IL-6 cytoprotection in hyperoxic acute lung injury occurs via suppressor of cytokine signaling-1-induced apoptosis signal-regulating kinase-1 degradation. Am J Respir Cell Mol Biol 40: 314–324, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulkarni A, Das KC. Differential roles of ATR and ATM in p53, Chk1, and histone H2AX phosphorylation in response to hyperoxia: ATR-dependent ATM activation. Am J Physiol Lung Cell Mol Physiol 294: L998–L1006, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Reddy R, Barsky L, Weinberg K, Driscoll B. Contribution of proliferation and DNA damage repair to alveolar epithelial type 2 cell recovery from hyperoxia. Am J Physiol Lung Cell Mol Physiol 290: L685–L694, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA. Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol 29: 779–783, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Holmgren A. Thioredoxin system in cell death progression. Antioxid Redox Signal 17: 1738–1747, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Luthman M, Holmgren A. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry 21: 6628–6633, 1982. [DOI] [PubMed] [Google Scholar]
- 42.Lyu MS, Rhee SG, Chae HZ, Lee TH, Adamson MC, Kang SW, Jin DY, Jeang KT, Kozak CA. Genetic mapping of six mouse peroxiredoxin genes and fourteen peroxiredoxin related sequences. Mamm Genome 10: 1017–1019, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Makena PS, Gorantla VK, Ghosh MC, Bezawada L, Kandasamy K, Balazs L, Luellen CL, Thompson KE, Parthasarathi K, Ichijo H, Waters CM, Sinclair SE. Deletion of apoptosis signal-regulating kinase-1 prevents ventilator-induced lung injury in mice. Am J Respir Cell Mol Biol 46: 461–469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol 178: 179–185, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Morton RL, Ikle D, White CW. Loss of lung mitochondrial aconitase activity due to hyperoxia in bronchopulmonary dysplasia in primates. Am J Physiol Lung Cell Mol Physiol 274: L127–L133, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Muniyappa H, Song S, Mathews CK, Das KC. Reactive oxygen species-independent oxidation of thioredoxin in hypoxia: inactivation of ribonucleotide reductase and redox-mediated checkpoint control. J Biol Chem 284: 17069–17081, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM, Keng PC. p53-independent induction of GADD45 and GADD153 in mouse lungs exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 278: L552–L559, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Oblong JE, Berggren M, Gasdaska PY, Powis G. Site-directed mutagenesis of active site cysteines in human thioredoxin produces competitive inhibitors of human thioredoxin reductase and elimination of mitogenic properties of thioredoxin. J Biol Chem 269: 11714–11720, 1994. [PubMed] [Google Scholar]
- 49.Ravi D, Das KC. Redox-cycling of anthracyclines by thioredoxin system: increased superoxide generation and DNA damage. Cancer Chemother Pharmacol 54: 449–458, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Ravi D, Muniyappa H, Das KC. Endogenous thioredoxin is required for redox cycling of anthracyclines and p53-dependent apoptosis in cancer cells. J Biol Chem 280: 40084–40096, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Rehan VK, Sakurai R, Corral J, Krebs M, Ibe B, Ihida-Stansbury K, Torday JS. Antenatally administered PPAR-gamma agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury. Am J Physiol Lung Cell Mol Physiol 299: L672–L680, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg AA, Murdaugh E, White CW. The role of oxygen free radicals in postasphyxia cerebral hypoperfusion in newborn lambs. Pediatr Res 26: 215–219, 1989. [DOI] [PubMed] [Google Scholar]
- 53.Schober P, Schwarte LA. From system to organ to cell: oxygenation and perfusion measurement in anesthesia and critical care. J Clin Monit Comput 26: 255–265, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 294: L632–L641, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Tateda K, Deng JC, Moore TA, Newstead MW, Paine R, Kobayashi N 3rd, Yamaguchi K, Standiford TJ. Hyperoxia mediates acute lung injury and increased lethality in murine Legionella pneumonia: the role of apoptosis. J Immunol 170: 4209–4216, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Walsh MC, Szefler S, Davis J, Allen M, Van Marter L, Abman S, Blackmon L, Jobe A. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics 117: S52–56, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Shan P, Sasidhar M, Chupp GL, Flavell RA, Choi AM, Lee PJ. Reactive oxygen species and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase mediate hyperoxia-induced cell death in lung epithelium. Am J Respir Cell Mol Biol 28: 305–315, 2003. [DOI] [PubMed] [Google Scholar]