Abstract
Over the past few decades, two-dimensional (2D) and layered materials have emerged as new fields. Due to the zero-band-gap nature of graphene and the low photocatalytic performance of MoS2, more advanced semiconducting 2D materials have been prompted. As a result, semiconductor black phosphorus (BP) is a derived cutting-edge post-graphene contender for nanoelectrical application, because of its direct-band-gap nature. For the first time, we report on robust BP@TiO2 hybrid photocatalysts offering enhanced photocatalytic performance under light irradiation in environmental and biomedical fields, with negligible affected on temperature and pH conditions, as compared with MoS2@TiO2 prepared by the identical synthesis method. Remarkably, in contrast to pure few layered BP, which, due to its intrinsic sensitivity to oxygen and humidity was readily dissolved after just several uses, the BP@TiO2 hybrid photocatalysts showed a ~92% photocatalytic activity after 15 runs. Thus, metal-oxide-stabilized BP photocatalysts can be practically applied as a promising alternative to graphene and MoS2.
Applications of nanoelectronics and optoelectronics as well as energy-related batteries and cells of two-dimensional (2D) and layered materials such as graphene with zero band gap1,2,3,4,5, not to mention the transition-metal dichalcogenide (TMDC) family (e.g., MoS2 with 1.23 eV–1.69 eV of indirect band-gap energy), are highly attractive research topics3,4. Although graphene has been widely and intensively developed for electronic and optical device applications6,7,8, limitations in its semi-metallic characteristics are emerging. These days, black phosphorus (BP) is a cutting-edge material due to its direct band-gap nature (i.e., ~2.0 eV for several layers and ~0.3 eV for the single layer, mainly depending on exfoliation of BP layers)2,3. Comparing the three main allotropes (white, red, and black) of phosphorus, the BP has merits including thermodynamic stability and insolubility in most solvents and lesser chemical reactivity and non-flammability9,10. As for crystalline structures, BP is present in three types, orthorhombic, rhombohedral, and cubic, along with the amorphous state11.
Several-layer BP crystals have been reported to demonstrate single-sheet-BP (i.e., phosphorene) practical feasibility1,2, showing, compared with the graphene- and MoS2-based alternatives3,4, higher carrier mobility and both p- and n-type configurations in field-effect transistor (FET) sensors12. Moreover, BP is used in lithium-ion batteries9,13 and thin-film solar cells14 that demonstrate large specific capacity and excellent cyclic performance, respectively which characteristics result in ~18% higher power-conversion efficiency than has been reported for trilayer graphene@ TMDCs solar cells.
Notwithstanding BP's superior optoelectronics, its photocatalytic performances in environmental and biomedical applications remain largely unexplored. Herein, taking into consideration semiconducting few layered BP and intercalation of TiO2 into a BP-layer system (BP@TiO2 hybrid) by one-pot reaction at room temperature, and comparing that system with an MoS2@TiO2 hybrid system fabricated by the identical preparation method, intriguingly novel photocatalytic performances over those of the traditional graphene-TiO2 hybrid photocatalytic composites15,16,17,18,19 were demonstrated.
Results
Morphological structures and elemental analysis
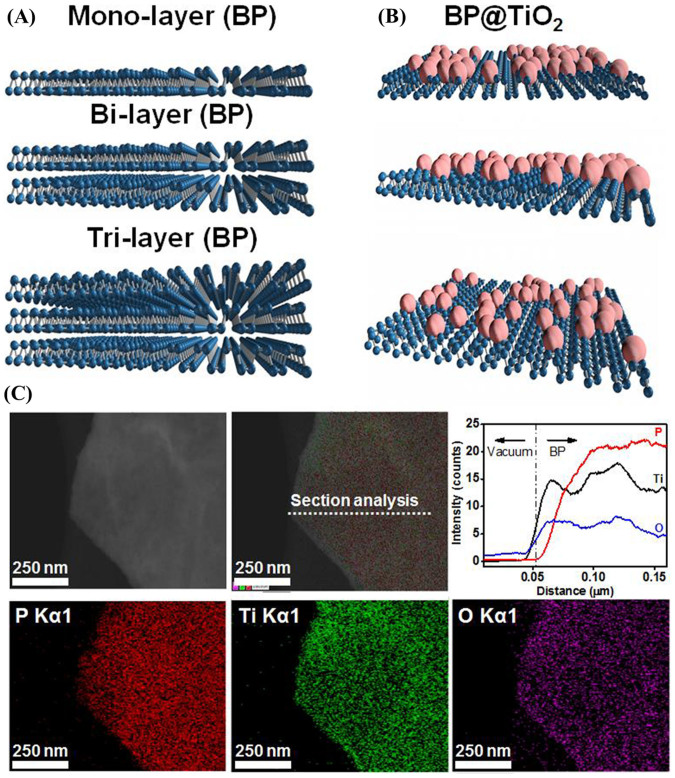
BP is generally known to have a puckered honeycomb structure with out-of-plane ridges in which each phosphorus atom is covalently linked to three neighboring single-layer phosphorus atoms while individual layer sheets are stacked vertically by van der Waals interaction11,20,21, as like the usual graphite pattern. The orthorhombic BP can be consisted of monolayer, bilayer and trilayer model structures (Fig. 1A). The well-defined crystalline morphology of few layered BP, which is prepared by an ultrasound-assisted delaminating process, displays crystal lattice fringes of ~2.5 Å (Supplementary Fig. 1) towards the (111) plane. A high-resolution transmission electron microscopy (HR-TEM) image of TiO2 substituted onto the BP surface reveals clearly distinct defects (Supplementary Fig. 1, yellow arrows)22, exactly corresponding to the modelling of the BP@TiO2 hybrid system (Fig. 1B), as looking like irregularly contained eggs (TiO2) in the tray. Reaction equations 1 and 2 show a possible BP@TiO2 formation mechanism. Ti(O4C4H9)4, when dropped into water, begins to rapidly hydrolyze, forming Ti(OH)4 on the few layered BP surface.
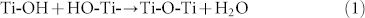 |
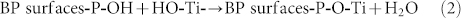 |
Then, Ti-OH was aggregated, forming Ti-O-Ti or Ti-O(H) bonds. Finally, an ultrasound irradiation process, the resultant ultrasonic cavitations creating a unique environment, induces a BP@TiO2 hybrid system with a better crystal structure23,24,25,26.
Figure 1. Modelled structures and elemental mapping analysis.
Modelled mono-, bi-layer, and tri-layered BP structures (A), TiO2 substitution on BP structure (B), and line profile (top panel) and its elemental mapping (bottom panel) of P, Ti, and O elements in BP@TiO2 hybrid photocatalyst (C).
Elemental analyses of the BP@TiO2 hybrid photocatalysts entailed energy-dispersive X-ray (EDX) mapping of P, Ti, and O, where P is emitted from few layered BP, Ti indicates the presence of TiO2-x (related titania compounds), and O originates from both few layered BP and TiO2 (Fig. 1C). Notably, Ti is uniformly distributed through the entire phosphorene surface. In the literature, TiO2 on graphene and MoS2, is deposited predominantly on the edges, due to the abundant functional groups of several-layer matrixes there27,28, leading to poor photocatalytic activity. As a result, it is needed additional carbon-coating steps to improve the uniformity of TiO2 nanoparticle distribution on the basal plane of MoS229,30 or reduction in TiO2 band-gap energy18.
X-ray diffraction (XRD) patterns and X-ray photoelectron spectra (XPS) analysis
In order to confirm the crystalline structures and impurities of the novel photocatalysts, the X-ray diffraction (XRD) patterns (Fig. 2A) and X-ray photoelectron spectra (XPS) (Fig. 2B, Supplementary Fig. 2 and Table 1) were examined. The phases of as-synthesized TiO2 are composed of anatase/brookite at room temperature by sol-gel processing and ultrasound irradiation31. In particular, the synthesis of TiO2 nanoparticles with conjugation or substitution of other nanomaterials, the brookite phase in as-synthesized TiO2 was disappeared (data not shown)32. The XRD pattern of few layered BP was matched to the orthorhombic phase, with representative peaks of d020 = 5.243 Å, d040 = 2.6216 Å, and d060 = 1.7472 Å at 2θ = 16.897, 34.174, and 52.32, respectively (JCPDS no. 76–1957)33; the BP@TiO2 hybrid, meanwhile, formed anatase-structure peaks of d020 = 5.243Å, d040 = 2.6216 Å, d040 = 2.6216 Å, and d060 = 1.7472 Å at 2θ = 25.148, 37.671, 47.934, and 53.713, respectively (JCPDS no. 71–1168) but still retained the distinctive few layered BP peaks. A surface-elemental binding analysis of the XPS spectra of the BP@TiO2 hybrid photocatalyst confirmed, via general scanning, the presence of Ti2p, O1s, P2p, Au4f as a substrate, Sn3d (catalyst for BP preparation) and I3d (catalyst for BP preparation); in the pure few layered BP XPS spectra34 by contrast, Ti was not detected (Fig. 2B and Supplementary Table 1). XPS fitting of P2p and O1s was plotted to obtain specific information on the Ti-P and phosphated titania peaks at 128.6 and 134.4 eV, respectively (Supplementary Fig. 2). The phosphate titania exhibited a binding energy for P2p at 134.4 eV, indicating that the phosphorus in the sample exists in the pentavalent-oxidation state and apparently as P-O bonded species. The XPS profiles revealed, moreover, that the phosphorus ions at the surface of the BP@TiO2 exist in the P5+ and P3+ states.
Figure 2. Crystalline structures and binding energy studies.
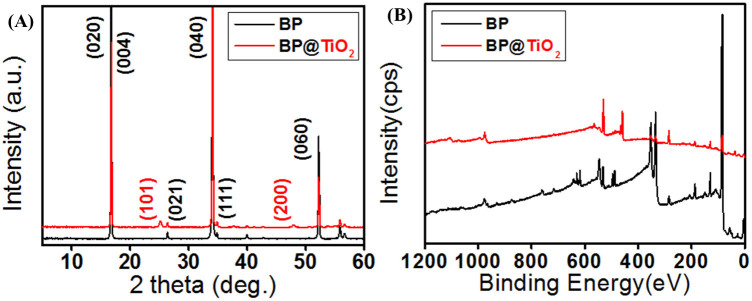
X-ray diffraction (XRD) patterns (A) and scanning X-ray photoelectron spectra (XPS) results (B) for few layered BP and BP@TiO2. hybrid photocatalysts.
Optical properties of photocatalysts
In a further optical investigation of the few layered BP and BP@TiO2 hybrid photocatalysts, Ultraviolet-visible-near infrared (UV-Vis-NIR) reflection (%) and the absorbance spectra in few layered BP and BP@TiO2 hybrid photocatalysts showed most of the solar spectral regimes to be between the 250 nm and 1200 nm wavelengths, whereas the reference naked TiO2 was found mainly in the ultraviolet (UV)-absorbable region (Supplementary Fig. 3). These results indicated that few layered BP and BP@TiO2 hybrid photocatalysts are strongly responsive in visible-light regions, unlike naked TiO2, which is activated under UV-light irradiation. Photoluminescence (PL) spectra (Supplementary Fig. 4) at ~600 nm, for the few layered BP and BP@TiO2 hybrid photocatalysts at 350 nm excitation, showed mostly quenching phenomena, which was reflected in the fact that the BP@TiO2 hybrid photocatalyst was blue-shifted 40 nm, by which inhibition of electron-hole recombination and persistent production of powerful ·OH free radicals would be expected.
Photocatalytic mechanism
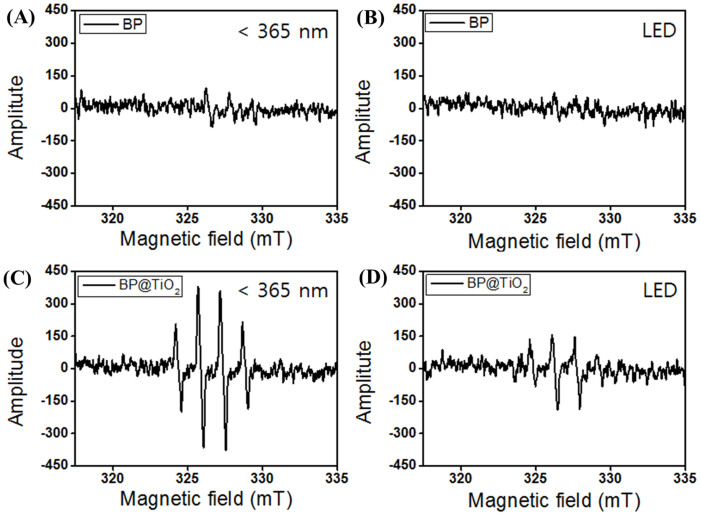
Also, the electron spin resonance (ESR) spectra derived in the present study suggest spin-trapping method of photocatalysis for the few layered BP and BP@TiO2 hybrid photocatalysts (Fig. 3). After 5 mins' 365 nm irradiation of BP, a weak 1:2:2:1 pattern of ·OH free radicals was shown, but under light emitting diode (LED) irradiation, weaker and more negligible peaks were shown. Contrastingly, the BP@TiO2 hybrid photocatalyst exhibited strong ·OH free radical peaks under 365 nm UV-light irradiation, and even under LED irradiation, produced half-intensity ·OH free radical peaks23. According to the order in the ·OH free radical peak intensity for the few layered BP and BP@TiO2 hybrid photocatalysts, the order of the photocatalytic activity under visible-light irradiation was BP@TiO2 hybrid > few layered BP > TiO2. On this basis, the mechanism of the photocatalytic performance of the BP@TiO2 hybrid was derived (Supplementary Fig. 4). One fundamental assumption on the use the BP@TiO2 hybrid structure under visible-light irradiation is that the heterojunction between few layered BP and TiO2 will enhance the photo-generated electron-hole pair separation with electrons from the conduction band (CB) of the TiO2 injected into the few layered BP, while the hole trapped from valence band (VB) in the few layered BP and/or TiO2 will have longer lifetimes (Supplementary Fig. 5).
Figure 3. Detection of generated radicals.
Electron spin resonance (ESR) spectra of few layered BP (A, B) and BP@TiO2 hybrid photocatalysts (C, D) at 365 nm and light-emitting diode (LED) irradiation, respectively.
Photocatalytic performances under UV- and visible light
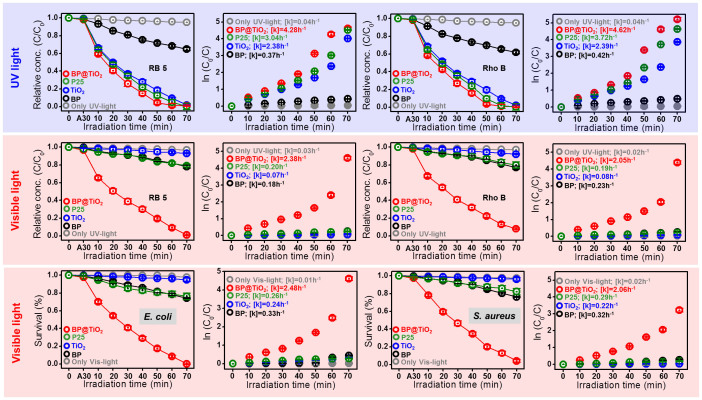
Based on the measured intensities of the ·OH free radicals in the few layered BP and BP@TiO2 hybrid photocatalysts, the degradation of RB 5 (an anionic dye model) and Roh B (a cationic dye model) were tested. As plotted in Fig. 4A, during 70 min UV irradiation, the only-UV-light, few layered BP photocatalyst, naked TiO2 photocatalyst, P25, and BP@TiO2 hybrid photocatalysts showed apparent rate constants of 0.04, 0.37, 2.38, 3.04, 4.28 h−1 and 0.04, 0.42, 2.39, 3.72, 4.62 h−1 for RB 5 and Roh B, respectively. The UV-light condition of the BP@TiO2 hybrid photocatalyst afforded an apparent rate constant double that of naked TiO2 photocatalyst. By contrast, visible-light irradiation for the only-visible-light, few layered BP photocatalyst, naked TiO2 photocatalyst, P25, and BP@TiO2 hybrid photocatalyst showed 0.03, 0.18, 0.07, 0.20, 2.38 h−1 and 0.02, 0.23, 0.08, 0.19, 2.05 for RB 5 and Roh B, respectively (Fig. 4B), highlighting an almost ten-times-higher apparent rate constant for the BP@TiO2 hybrid system than for few layered BP photocatalyst. Few layered BP photocatalyst, meanwhile, showed an approximately three-fold-higher rate constant relative to that of naked TiO2 photocatalyst. As the reference experiments, in dark conditions, the photocatalytic activities in BP@TiO2, TiO2, P25, and few layered BP photocatalysts showed negligible performances (Supplementary Fig. 6). Next, the intermediate by-products concentration including dyes was measured by a total organic carbon (TOC) analyzer (Supplementary Fig. 7). After 70 min treatment, most of TOC values had reached ~92% with detoxified CO2 and H2O formation. As regards the recovery of the BP@TiO2 hybrid photocatalysts (Supplementary Fig. 8), significantly, even after the 15th run, ~92.04% activity was maintained. Pure few layered BP photocatalyst, gradually decreasing after repeated runs, showed only ~30% photocatalytic activity: by the 8th run, all of it had dissolved into the liquid state, thereby illustrating the difficult BP recovery. The robust stability of the BP@TiO2 hybrid system possibly is related to the substitution of Ti atoms into BP atomic lattices, which system effects resistance to humid and oxygen conditions12. Furthermore, similarly to the visible-light photocatalytic dye-degradation performance, the apparent rate constants of the antibacterial activities under the only-visible-light, few layered BP, TiO2, P25, and BP@TiO2 hybrid photocatalysts were 0.01, 0.33, 0.24, 0.26, and 2.48 h−1 for Escherichia coli (E. coli, as a gram negative species) and 0.02, 0.32, 0.22, 0.29, and 2.06 h−1 for Staphylococcus aureus (S. aureus, as a gram-positive species) (Fig. 4C). The colloidal behavior of the few layered BP and BP@TiO2 hybrid photocatalysts showed ~+12.73 mV and ~+3.18 mV zeta potentials in ethanol and ~−40.47 mV and ~−24.87 mV in distilled water, respectively. Clearly, in aqueous solution, few layered BP was hydrolyzed to form PO42− with a negatively charged surface, which resulted in a significantly diminished mechanistic stability. The BP@TiO2 hybrid system, though, was less hydrolyzed, and evidenced BP stabilization by displacement of TiO2 nanoparticles. In detail, at the empty sites of P atoms in few layered BP, Ti atoms were displaced at those positions in few layered BP <5 nm-sized TiO2 particles were substituted and formed onto the BP surface, uniformly and with negligible aggregations, finally producing BP@TiO2 hybrid photocatalysts and improving mechanical and photocatalytic stabilities in practical environmental and biomedical applications. According to zeta potential of BP@TiO2 hybrid photocatalysts, it exhibited negatively charged surface, but the adsorption amount of two different dyes displayed no marked difference. Thus, organic dye Roh B as a cationic dye resulted in slightly enhanced apparent rate of 4.60 h−1, compared to that of organic dye RB 5 (an anionic dye model).
Figure 4. Photocatalytic performances.
Relative concentrations and apparent reaction rate constants of RB 5 and Rho B of BP@TiO2 hybrid, P25, and few layered BP photocatalysts under UV- (A) and visible-light (B) irradiation, and antibacterial activities (C) and apparent reaction rate constants of E. coli and S. aureus.
Discussion
In the diverse literature on graphene-based TiO2 hybrid photocatalytic activity enhancement15,16,17,18,19, graphene has played a multi-functional role. First of all, graphene enhances the visible-light absorbance region and the inhibition of electron-hole recombination by electron transfer from TiO2 to the graphene matrix as well as by reduction of TiO2 nanoparticle aggregation. Secondly, adsorption of targeting pollutants onto graphene@TiO2 hybrid photocatalysts accelerates degradation by TiO2 nanoparticles in graphene (oxide). Correspondingly, Lee et al. found that the wrapping of graphene sheets onto TiO2 nanoparticles decreased the band-gap energy in TiO2 for activation even under visible light18. For comparative purposes, in the present study, we investigated the photocatalytic activities of MoS2@TiO2 hybrid photocatalysts prepared according to the identical protocol. In the results, relatively very low apparent rate constants were obtained (Supplementary Fig. 9), resulting in approximately 20% dye degradation activity and antibacterial activity under visible-light irradiation. These poor photocatalytic activities can be attributed to the difficulty of uniformly decorating TiO2 nanoparticles on the MoS2 surface, despite the semiconducting action of the several-layer MoS2 sheets.
In summary, in order to effectively utilize unstable few layered BP in various applications, it is suggested that TiO2 nanoparticles can be substituted in the P atomic positions of BP with Ti by a simple method. The photocatalytic performances of BP@TiO2 hybrid photocatalysts in comparison with those of the semiconducting MoS2@TiO2 hybrid photocatalysts system were remarkable. Unlike MoS2, few layered BP can be mass-produced by phase transformation of red phosphorus35,36,37 or via a fast low-pressure transport route38. In the near future, studies on promising practical alternatives to graphene and MoS2, namely other unique metal oxides such as magnetic iron oxide (Fe3O4) and cerium oxide (CeO2), as deposited on few layered BP as a drug-delivery platform or therapeutic agent, currently are being planned, with particular emphasis on cellular targeting and smart retention time in the body.
Methods
Synthesis of BP@TiO2 hybrid photocatalysts
All of the reagents including commercial TiO2-P25 were of analytical grade (Sigma Aldrich, MO, USA) and were used without further purification. In the typical synthesis, black phosphorous (BP, 0.2 g, 6.4 mmol) was dispersed in a solution of anhydrous ethyl alcohol (400 mL) by high-intensity ultrasound irradiation for 2 h, to form few layered BP. 0.025 mol titanium isopropoxide dissolved ethyl alcohol solution (40 mL) was mixed with the BP-dispersed solution, 2 mL of which was then added drop-wise to deionized (DI) water with vigorous stirring for 10 min and finally treated by high-intensity ultrasound for 30 min. The dark-brown-colored BP@TiO2 hybrid solution was poured into the petri-dishes and dried on a plate at 60°C. The obtained product was dried under vacuum at room temperature (RT). Dispersion of BP and crystallization of TiO2 nanoparticles were performed under high-intensity ultrasound of 20 kHz frequency applied from the top of a polypropylene bottle reactor (~40 mL) using a Sonics and Materials VC750 ultrasonic generator. The electrical energy input was maintained at 100 W.
Characterization
The crystalline structures of the BP@TiO2 hybrid samples were investigated with reference to X-ray diffraction (XRD; Rigaku RDA-cA X-ray diffractometer, Japan) patterns obtained by passing Cu Kα radiation through a nickel filter. The morphology of the BP@TiO2 particles was recorded by high-resolution transmission electron microscopy (HR-TEM; JEOL, JEM 2200, Japan). The samples prior to their analysis were placed on carbon coated copper grids and dried under ambient conditions. High-resolution X-ray photoelectron spectroscopy (HR-XPS) carried out using monochromatic Al Kα X-ray radiation (hν = 1486.6 eV) with a power of 120 W (Kratos Analytical, AXIS Nova, UK) was used to investigate the surface properties of the samples. The shift in the binding energy due to the relative surface charging was corrected according to the C1s level at 284.6 eV as an internal standard. Zeta potential measurements also were carried out, using a dynamic laser light scattering (DLS, Malvern Zetasizer NanoZS, USA) unit. A He-Cd laser (Kimmon, 1K, Japan) of 325 nm wavelength and 50 mW power was employed as an excitation source for photoluminescence (PL) measurements carried out using a spectrograph (f = 0.5 m, Acton Research Co., Spectrograph 500i, USA) with an intensified charge-coupled device (CCD, PI-MAX3, Princeton Instruments, IRY1024, USA). For free-radical detection by 5,5-dimethyl-1-pyrroline N-oxide (DMPO, 0.3 M in PBS buffer at pH 7.2, Sigma-Aldrich, USA) as a spin trap agent, an aliquot of sample (100 μL of 5 mg BP@TiO2 hybrid sample mixed with 300 μL DMPO solution) was filled into a capillary tube and directly irradiated with a UV (λ = 365 nm) or LED light (>400 nm) source for 5 min39,40, and the spectra were recorded by electron spin resonance (ESR) spectrometry (JES-FA200, JEOL, Japan). The specific ESR conditions were as follows: center field: 327 mT; powder: 1 mW; amplitude: 5.0 × 100; modulation width: 0.4 × 1; sweep width: 1 × 10; sweep time: 30 s.
Measurement of photocatalytic and antibacterial activities
The photocatalytic degradations of reactive black 5 (RB 5; 3 mg/L, Sigma-Aldrich, USA) and rhodamine B (Rho B; 3 mg/L, pH 5.5, Sigma-Aldrich, USA) solutions by photocatalyst samples (1 g/L) were carried out under UV (source: 4 W, <365 nm, VSLAB VL-4CL, Korea) and visible-light (source: 150 W Xe lamp, λ > 420 nm, SCHOTT, USA) irradiation, and the absorbance of the solutions was measured using a UV-Vis-NIR spectrophotometer (Varian, Cary 5000, Australia) in the 200–800 nm wavelength region41,42. Before photocatalytic performances in all samples, adsorption-desorption equilibriums for 30 min were conducted. The concentrations of RB 5 and Rho B in the solutions after photoirradiation were measured from the absorbance peak intensities of the solutions at 598 and 555 nm, respectively. The changes in the concentration [ln(C0/C) = kt, where k is the apparent reaction rate constant, and C0 and C are the initial and reaction concentrations, respectively, of Rho B] of the dye solution with reaction time for the samples were also investigated41,42. To demonstrate the stability of the photocatalysts, the BP@TiO2 hybrid was reused for the testing of other photocatalytic activities. The recycling tests for evaluation of the photocatalytic activity of BP@TiO2 hybrid photocatalysts were performed after washing the samples three times with DI water at 8500 rpm centrifugation and drying them in an oven for 6 h after each cycle. Additionally, the total organic carbon (TOC) of the solution was determined by a Shimadzu TOC-V analyzer (ELEMENTAR, vario TOC cub, Japan).
The antibacterial activities of the samples were evaluated according to the inhibition of gram-negative Escherichia coli (E. coli) and gram-positive Staphylococcus aureus (S. aureus) under visible-light irradiation41,42. Before these tests, all glassware and samples were sterilized by autoclaving at 120°C for 15 min. Bacterial cultures were grown in Luria-Bertain (LB) media overnight at 37°C with continuous shaking at ~200 rpm. The treated bacterial cells were diluted with DI water to a cell suspension of ~2 × 105 colony-forming units (CFU/mL). Also, before photocatalytic performances in all samples, adsorption-desorption equilibriums for 30 min were conducted. The mass of the photocatalyst was adjusted to 25 μg/mL. The suspensions were stirred with a magnetic stirrer to prevent the samples from settling, and were then exposed to visible light for various irradiation times (0–70 min). Then, 1 mL of the suspension was sampled, added to the LB plate, and incubated overnight at 37°C. After incubation, the bacterial colonies were observed and quantified. As the reference experiments, dark experiments for all samples were conducted.
In measuring the photocatalytic and antimicrobial test performances, the data were averaged and expressed as mean ± standard deviations (SE). Each test was repeated up to five times. An analysis of variance (ANOVA) statistical analysis was performed, wherein p-values < 0.05 were considered significant.
Author Contributions
H.U.L., Y.-C.L. and J.L. designed the project, organized the entire research. H.U.L., S.C.L., Y.-C.L., S.C., H.-S.K. and J.L. wrote the manuscript. H.U.L., S.C.L., Y.-C.L., J.W., Y.K. and S.Y.P. carried out the sample preparation and characterization. B.-S. performed the XPS analysis. H.U.L. and S.C. performed the photocatalytic performances. All authors discussed the results and commented on the manuscript.
Supplementary Material
Supplementary information
Acknowledgments
This research was supported by the KBSI research Grant No. E35800.
References
- Reich E. S. Phosphorene excites materials scientists. Nature 506, 19 (2014). [DOI] [PubMed] [Google Scholar]
- Churchill H. O. H. & Jarillo-Herrero P. Phosphorus joins the family. Nat. Nanotechnol. 9, 330–331 (2014). [DOI] [PubMed] [Google Scholar]
- Li L. et al. Black phosphorus field-effect transistors. Nat. Nanotechnol. 9, 372–377 (2014). [DOI] [PubMed] [Google Scholar]
- Xia F., Wang H. & Jia Y. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 5, 4458 (1–6) (2014). [DOI] [PubMed] [Google Scholar]
- Qiao J., Kong X., Hu Z.-X., Yang F. & Ji W. High-mobility transport anisotropy and linear dichroism in few-layer black phosphorus. Nat. Commun. 5, 4475 (1–7) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei R. & Yang L. Strain-engineering the anisotropic electrical conductance of few-layer black phosphorus. Nano Lett. 14, 2884–2889 (2014). [DOI] [PubMed] [Google Scholar]
- Buscema M. et al. Fast and broadband photoresponse of few-layer black phosphorus. Nano Lett. 14, 3347–3352 (2014). [DOI] [PubMed] [Google Scholar]
- Deng Y. et al. Black phosphorus-monolayer MoS2 van der Waals heterojunction p-n diode. ACS Nano 8, 8292–8299 (2014). [DOI] [PubMed] [Google Scholar]
- Park C.-M. & Sohn H.-J. Black phosphorus and its composite for lithium rechargeable batteries. Adv. Mater. 19, 2465–2468 (2007). [Google Scholar]
- Qian J. et al. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angew. Chem.-Int. Edit. 52, 4633–4636 (2013). [DOI] [PubMed] [Google Scholar]
- Appalakondaiah S., Vaitheeswaran G., Lebègue S., Christensen N. E. & Svane A. Effect of van der Waals interactions on the structural and elastic properties of black phosphorus. Phys. Rev. B 86, 035105 (1–9) (2012). [Google Scholar]
- Koenig S. P., Doganov R. A., Schmidt H., Neto A. H. C. & Özyilmaz B. Electric field effect in ultrathin black phosphorus. Appl. Phys. Lett. 104, 103106 (1–4) (2014). [Google Scholar]
- Sun L.-Q. et al. Electrochemical activity of black phosphorus as an anode material for lithium-ion batteries. J. Phys. Chem. C 116, 14772–14779 (2012). [Google Scholar]
- Dai J. & Zeng X. C. Bilayer phosphorence: effect of stacking order on bandgap and its potential applications in thin-film solar cells. J. Phys. Chem. Lett. 5, 1289–1293 (2014). [DOI] [PubMed] [Google Scholar]
- Williams G., Seger B. & Kamat P. V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2, 1487–1491 (2008). [DOI] [PubMed] [Google Scholar]
- Štengl V., Popelková D. & Vláčil P. TiO2-graphene nanocomposite as high performance photocatalysts. J. Phys. Chem. C 115, 25209–25218 (2011). [Google Scholar]
- Pan X. et al. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces 4, 3944–3950 (2012). [DOI] [PubMed] [Google Scholar]
- Lee J. S., You K. H. & Park C. B. Highly photoactive, low bandgap TiO2 nanoparticles wrapped graphene. Adv. Mater. 24, 1084–1088 (2012). [DOI] [PubMed] [Google Scholar]
- Huang Q. et al. Enhanced photocatalytic activity of chemically bonded TiO2/graphene composites based on the effective interfacial charge transfer through the C-Ti bond. ACS Catal. 3, 1477–1485 (2013). [Google Scholar]
- Zhu Z. & Tománek D. Semiconducting layered blue phosphorus: a computational study. Phys. Rev. Lett. 112, 176802 (1–5) (2014). [DOI] [PubMed] [Google Scholar]
- Tran V., Soklaski R., Ling Y. & Yang L. Layer-controlled band gap and anisotropic excitons in few-layer black phosphorus. Phys. Rev. B 89, 235319 (1–6) (2014). [Google Scholar]
- Zhang C. D. et al. Surface structures of black phosphorus investigated with scanning tunneling microcopy. J. Phys. Chem. C 113, 18823–18826 (2009). [Google Scholar]
- Lee H. U. et al. Innovative three-dimensional (3D) eco-TiO2 photocatalysts for practical environmental and bio-medical applications. Sci. Rep. 4, 6740 (1–8) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. U. et al. Highly visible-light active nanoporous TiO2 photocatalysts for efficient solar photocatalytic applications. Appl. Catal. B-Environ. 129, 106–113 (2013). [Google Scholar]
- Lee H. U. et al. Influence of visible-light irradiation on physicochemical and photocatalytic properties of nitrogen-doped three-dimensional (3D) titanium dioxide. J. Hazard. Mater. 258–259, 10–18 (2013). [DOI] [PubMed] [Google Scholar]
- Lee H. U. et al. Room temperature synthesis of nanoporous anatase and anatase/brookite TiO2 photocatalysts with high photocatalytic performance. Chem. Eng. J. 223, 209–215 (2013). [Google Scholar]
- Thurston T. R. & Wilcoxon J. P. Photooxidation of organic chemicals catalyzed by nanoscale MoS2. J. Phys. Chem. B 103, 11–17 (1999). [Google Scholar]
- Pourabbas B. & Jamshidi B. Preparation of MoS2 nanoparticles by a modified hydrothermal method and the photo-catalytic activity of MoS2/TiO2 hybrids in photo-oxidation of phenol. Chem. Eng. J. 138, 55–62 (2008). [Google Scholar]
- Liu M. et al. Noble-metal-free photocatalysts MoS2-graphene/CdS mixed nanoparticles/nanorods morphology with high visible light efficiency for H2 evolution. Chem. Commun. 50, 11004–11007 (2014). [DOI] [PubMed] [Google Scholar]
- Xiang Q., Yu J. & Jaroniec M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 134, 6575–6578 (2012). [DOI] [PubMed] [Google Scholar]
- Lee H. U. et al. Room temperature synthesis of nanoporous anatase and anatase/brookite TiO2 photocatalysts with high photocatalytic performance. Chem. Eng. J. 223, 209–215 (2013). [Google Scholar]
- Lee Y.-C. et al. Aminoclay-conjugated TiO2 synthesis for simultaneous harvesting and wet-disruption of oleaginous Chlorella sp. Chem. Eng. J. 245, 143–149 (2014). [Google Scholar]
- Brown A. & Rundqvist S. Refinement of the crystal structure of black phosphorus. Acta Cryst. 19, 684–685 (1965). [Google Scholar]
- Goodman N. B., Ley L. & Bullett D. W. Valence-band structures of phosphorus allotropes. Phys. Rev. B 27, 7440–7450 (1983). [Google Scholar]
- Winchester R. A. L., Whitby M. & Shaffer M. S. P. Synthesis of pure phosphorus nanostructures. Angew. Chem.-Int. Edit. 48, 3616–3621 (2009). [DOI] [PubMed] [Google Scholar]
- Lange S., Schmidt P. & Nilges T. Au3SnP7@black phosphorus: an easy access to black phosphorus. Inorg. Chem. 46, 4028–4035 (2007). [DOI] [PubMed] [Google Scholar]
- Köpf M. et al. Access and in situ growth of phosphorene-precursor black phosphorus. J. Cryst. Growth 405, 6–10 (2014). [Google Scholar]
- Nilges T., Kersting M. & Pfeifer T. A fast low-pressure transport route to large black phosphorus single crystals. J. Solid State Chem. 181, 1707–1711 (2008). [Google Scholar]
- Lee Y.-C. et al. Self-assembled graphene oxide with organo-building blocks of Fe-aminoclay for heterogeneous Fenton-like reaction at near-neutral pH: a batch experiment. Appl. Catal. B-Environ. 142–143, 494–503 (2013). [Google Scholar]
- Lee Y.-C. et al. Oil extraction by aminoparticle-based H2O2 activation via wet microalgae harvesting. RSC Adv. 3, 12802–12809 (2013). [Google Scholar]
- Lee H. U. et al. Efficient visible-light responsive TiO2 nanoparticles incorporated magnetic carbon photocatalysts. Chem. Eng. J. 240, 91–98 (2014). [Google Scholar]
- Lee H. U. et al. Improved photocatalytic and antibacterial activities of three-dimensional polycrystalline anatase TiO2 photocatalysts. App. Catal. A-Gen. 467, 394–399 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information