Abstract
A known norcembranoidal diterpene, 5-episinuleptolide (1), along with a new analogue, 4α-hydroxy-5-episinuleptolide (2), were isolated from a cultured-type soft coral Sinularia numerosa. The structures of 1 and 2 were elucidated on the basis of spectroscopic methods and by comparison of the data with those of the related metabolites. Cytotoxicity of metabolites 1 and 2 against a panel of tumor cells is also described. Compound 2 exhibited moderate cytotoxicity toward CCRF-CEM cells with an IC50 value 4.21 μg/mL. Preliminary SAR (structure activity relationship) information was obtained from these two compounds.
Keywords: sinuleptolide, cembranoidal diterpene, Sinularia numerosa, cytotoxicity
1. Introduction
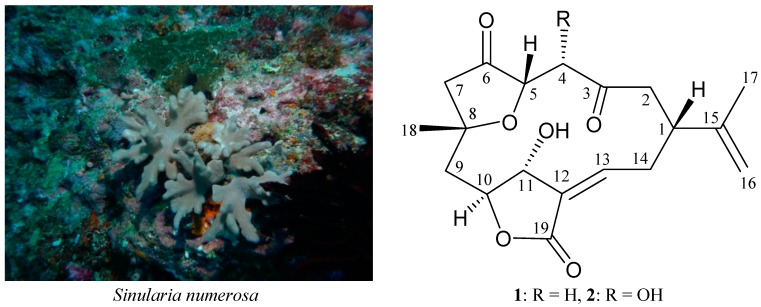
Previous studies on the chemical constituents of octocorals, belonging to the genus Sinularia, have led to the isolation of a number of interesting secondary metabolites and some of these were found to possess interesting bioactivities [1,2]. In previous studies on the chemical constituents of the soft coral Sinularia numerosa (Tixier-Durivault, 1970) (=Sinularia crispa) (phylum Cnidaria, class Anthozoa, order Alcyonacea, family Alcyoniidae) (Scheme 1) [3], many interesting secondary metabolites, including polyhydroxylated sterols [4,5,6], diterpenoids [6,7], sesquiterpenoids [8] and oxylipins [9] were isolated. We undertook further studies on the cultured-type Sinularia numerosa from which a known norcembranoidal diterpene, 5-episinuleptolide (1) [10,11,12,13,14,15], along with a new analogue, 4α-hydroxy-5-episinuleptolide (2) (Scheme 1), were isolated. In this paper, we describe the isolation, structure determination and cytotoxicity of 1 and 2.
Scheme 1.
The soft coral Sinularia numerosa and the structures of 5-episinuleptolide (1) and 4α-hydroxy-5-episinuleptolide (2).
2. Results and Discussion
The known compound 1 was found to be identical with the previously reported 5-episinuleptolide (1) isolated from S. leptoclados [10,13], S. scabra [12], S. lochmodes [14], S. maxima [15] and S. polydactyla [15] by comparison of the physical (mp and ) and NMR data.
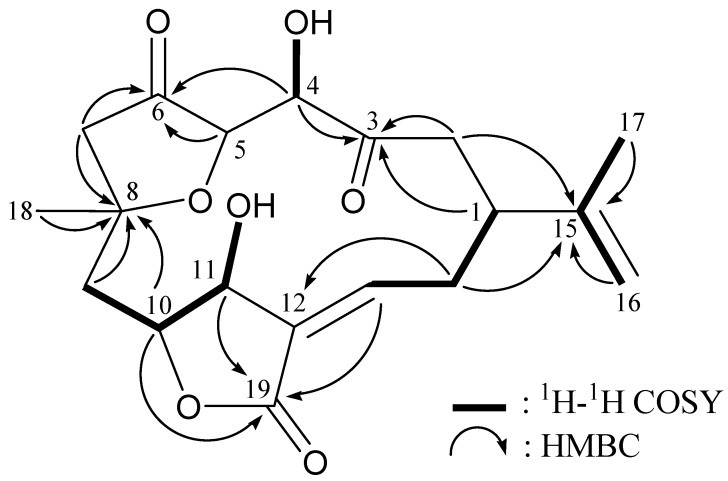
4α-Hydroxy-5-episinuleptolide (2) was obtained as a white powder, − 15 (c 0.9, CHCl3). The molecular formula C19H24O7 was determined (8 unsaturations) based on the HRESIMS (C19H24O7 + Na, m/z 387.14147, calculated 387.14142). From the 13C NMR spectra (Table 1), 2 was found to possess an ester group (δC 169.2, C-19) and two ketone carbonyls (δC 211.5, C-6; 208.7, C-3). Two additional unsaturated functionalities were indicated by 13C resonances at δC 147.2 (CH-13), 147.0 (C-15), 130.9 (C-12) and 110.4 (CH2-16), suggesting the presence of a trisubstituted olefin and an exocyclic carbon-carbon double bond. From the 1H–1H COSY spectrum of 2 (Table 1 and Figure 1), it was possible to differentiate among the separate spin systems of H-9β/H-10/H-11, H-13/H2-14, H-14α/H-1 and H2-16/H3-17 (by allylic coupling). These data, together with the key HMBC correlations between protons and quaternary carbons of 2 (Table 1 and Figure 1), such as H-1, H2-2, H-4/C-3; H-4, H-5, H2-7/C-6; H2-7, H2-9, H-10, H3-18/C-8; H-14α/C-12; H2-2, H-14α, H2-16, H3-17/C-15; and H-10, H-11, H-13/C-19, permitted the elucidation of the carbon skeleton. The hydroxy proton signals at δH 3.66 (1H, d, J = 4.8 Hz, OH-4) and δH 2.01 (1H, br s, OH-11) were revealed by their 1H–1H COSY correlations to δH 4.34 (1H, dd, J = 4.8, 3.6 Hz, H-4) and 4.49 (1H, s, H-11), indicating their attachments to C-4 and C-11, respectively. Thus, compound 2 turned to be a 4-norcembranoidal diterpene possessing a γ-lactone ring, on the basis of the above analysis.
Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, 1H–1H COSY and HMBC correlations for norcembranoidal diterpene 2.
| Position | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 2.80 m | 44.4, CH | H-14α | C-3, -14 |
| 2 | 2.67–2.78 m | 41.7, CH2 | n.o. c | C-1, -3, -14, -15 |
| 3 | 208.7, C | |||
| 4 | 4.34 dd (4.8, 3.6) a | 79.1, CH | OH-4 | C-3, -6 |
| 5 | 4.33 br d (3.6) a | 76.6, CH | n.o. | C-6 |
| 6 | 211.5, C | |||
| 7α | 2.31 d (16.8) | 52.6, CH2 | H-7β | C-6, -8, -9, -18 |
| β | 2.44 d (16.8) | H-7α | C-6, -8, -9, -18 | |
| 8 | 80.2, C | |||
| 9α | 2.10 br d (14.8) | 42.5, CH2 | H-9β | C-7, -8, -18 |
| β | 2.52 dd (14.8, 8.0) | H-9α, H-10 | C-8, -10, -11, -18 | |
| 10 | 4.67 dd (8.0, 0.8) | 83.3, CH | H-9β, H-11 | C-8, -11, -19 |
| 11 | 4.49 s | 76.2, CH | H-10, OH-11 | C-9, -10, -13, -19 |
| 12 | 130.9, C | |||
| 13 | 6.63 ddd (10.4, 6.8, 0.8) | 147.2 CH | H2-14 | C-11, -19 |
| 14α | 2.41 ddd (13.6, 6.8, 6.8) | 31.2, CH2 | H-1, H-13, H-14β | C-1, -2, -12, -13, -15 |
| β | 3.64 ddd (13.6, 10.4, 3.6) b | H-13, H-14α | C-2 | |
| 15 | 147.0, C | |||
| 16a | 4.87 d (0.8) | 110.4, CH2 | H-16b, H3-17 | C-1, -15, -17 |
| b | 4.89 d (0.8) | H-16a, H3-17 | C-1, -15, -17 | |
| 17 | 1.79 s | 20.8, CH3 | H2-16 | C-1, -15, -16 |
| 18 | 1.42 s | 25.6, CH3 | C-7, -8, -9 | |
| 19 | 169.2, C | |||
| OH-4 | 3.66 d (4.8) b | H-4 | n.o. | |
| OH-11 | 2.01 br s | H-11 | n.o. |
a,b Signals overlapping, the coupling constant for OH-4 was deduced from the coupling pattern and correlation observed between H-4 and OH-4; c n.o. = not observed.
Figure 1.
The 1H–1H COSY and key HMBC (protons→quaternary carbons) correlations for 2.
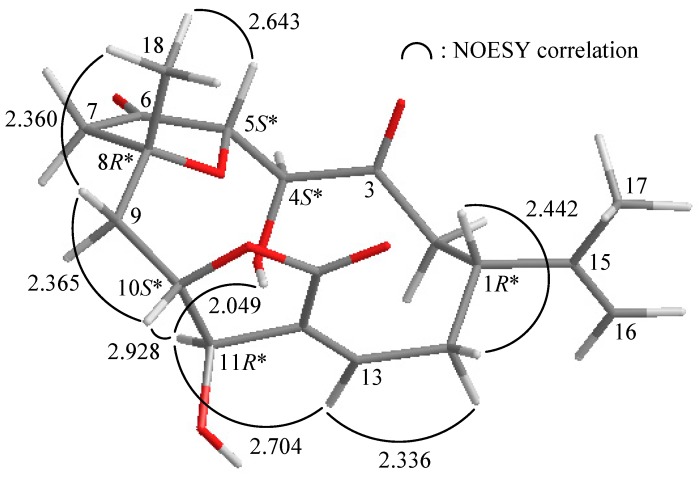
The relative configuration of 2 observed in the NOESY spectrum corroborated the MM2 force field calculations which suggested the most stable conformation as shown in Figure 2 [16]. In the NOESY experiment for 2, it was found that one of the methylene protons at C-14 (δH 3.64) exhibited a correlation with H-1, but not with H-13, and therefore it was assigned as H-14β, and the other C-14 proton (δH 2.41) as H-14α. H-13 showed correlations with H-11 and H-14α, but not with H-1, and H-10 showed a correlation with H-11, as well as the lack of coupling was detected between H-10 and H-11, indicating the dihedral angle between H-10 and H-11 is approximately 90° and the configurations of chiral carbons C-10 and C-11 were assigned as S*- and R*-forms, respectively. One proton of C-9 methylene (δH 2.52) correlated with H-10 and H3-18, but not with H-11, and H3-18 showed a correlation with H-5, indicating that Me-18 and H-5 were β-oriented. A correlation was detected between OH-4 and H-11, indicating that the hydroxy group at C-4 should be α-oriented by modeling analysis. From the above evidences, the relative configurations of the chiral carbons of 2 were assumed to be 1R*, 4S*, 5S*, 8R*, 10S* and 11R*.
Figure 2.
The computer-generated model of 2 using MM2 force field calculations and the calculated distances (Å) between selected protons with key NOESY correlations.
Cytotoxicity of the norcembranoidal diterpenes 1 and 2 toward CCRF-CEM (human acute lymphoblastic leukemia), HL-60 (human acute promyelocytic leukemia), K-562 (human chronic myelogenous leukemia), U-937 (human histiocytic lymphoma), DLD-1 (human colorectal adenocarcinoma), LNCaP (human prostatic carcinoma) and MCF7 (human breast adenocarcinoma) tumor cells were studied, and the results are shown in Table 2. CCRF-CEM cell line was more sensitive to the cytotoxic effects of 2. Compound 2 (4α-hydroxy-5-episinuleptolide) exhibited modest cytotoxicity toward CCRF-CEM cells, and was more potent than 1 (5-episinuleptolide), showing that the presence of a hydroxy substituent at C-4α position would enhance the activity.
Table 2.
Cytotoxic data of norcembranoidal diterpenes 1 and 2.
| Compounds | Cell Lines IC50 (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| CCRF-CEM | HL-60 | K-562 | U-937 | DLD-1 | LNCaP | MCF7 | |
| 1 | 11.07 | 11.11 | NA | NA | NA | NA | NA |
| 2 | 4.21 | 10.38 | 18.07 | 10.08 | NA | 15.33 | NA |
| Doxorubicin a | 0.01 | 0.01 | 0.35 | 0.05 | 0.49 | 0.10 | 0.16 |
a Doxorubicin was used as a positive control and NA = not active at 20 μg/mL for 72 h.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured at a Jasco P-1010 digital polarimeter (Japan Spectroscopic Corporation, Tokyo, Japan). Infrared spectra were recorded on a Jasco FT-4100 FT-IR spectrometer (Japan Spectroscopic Corporation, Tokyo, Japan); peaks are reported in cm–1. NMR spectra were recorded on a Varian Mercury Plus 400 NMR spectrometer (Varian Inc., Palo Alto, CA, USA) using the residual CHCl3 signal (δH 7.26 ppm) as the internal standard for 1H NMR and CDCl3 (δC 77.1 ppm) for 13C NMR. Coupling constants (J) are given in Hz. ESIMS and HRESIMS were recorded using a Bruker 7 Tesla solariX FTMS system (Bruker, Bremen, Germany). Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck); spots were visualized by spraying with 10% H2SO4 solution followed by heating. The normal phase HPLC (NP-HPLC) was performed using a system comprised of a Hitachi L-7110 pump (Hitachi Ltd., Tokyo, Japan) and a Rheodyne 7725 injection port (Rheodyne LLC., Rohnert Park, CA, USA). A normal phase column (Supelco Ascentis® Si Cat #: 581515-U, 25 cm × 21.2 mm, 5 µm, Sigma-Aldrich. Com. St. Louis, MO, USA) was used for NP-HPLC.
3.2. Animal Material
Specimens of the cultured octocoral Sinularia numerosa were collected by hand in a 0.6 ton cultivation tank with a flow-through sea water system located in the National Museum of Marine Biology and Aquarium (NMMBA), Taiwan, in 30 July 2014 and stored in freezer (−20 °C) until extraction. A voucher specimen (NMMBA-TWSC-14009) was deposited in the National Museum of Marine Biology and Aquarium, Taiwan.
3.3. Extraction and Isolation
Specimens of the soft coral Sinularia numerosa (wet weight 487 g, dry weight 69 g) were minced and extracted with ethyl acetate (EtOAc). The EtOAc extract left after removal of the solvent (5.0 g) was chromatographed on silica gel by column chromatography and eluted with acetone in dichloromethane (DCM) (0%–100%, gradient) to yield 22 fractions and compound 1 (5-episinuleptolide) was obtained from fraction 9 (278.0 mg, 8:2). Fraction 13, eluted with DCM/acetone (4:6), was further purified by NP-HPLC using a mixture of DCM and acetone (5:1, flow rate: 2.0 mL/min) to yield 4α-hydroxy-5-episinuleptolide (2) (2.8 mg, tR = 184 min).
5-Episinuleptolide (1): colorless prisms; mp 232–233 °C ([13], 226–227 °C); −169 (c 0.3, CHCl3) (ref. [13], −119 (c 0.16, CHCl3)); IR (neat) νmax 3265, 1748 cm–1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data were in full agreement with those reported previously [13].
4α-Hydroxy-5-episinuleptolide (2): white powder; mp 178–179 °C; −15 (c 0.9, CHCl3); IR (neat) νmax 3421, 1740 cm–1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data, see Table 1, Figures S1 and S2; ESIMS: m/z 387 [M + Na]+; HRESIMS: m/z 387.14147 (calcd. for C19H24O7Na, 387.14142), see Figure S3–S7.
3.4. Molecular Mechasnics Calculations
Implementation of the MM2 force field [16] in CHEM3D PRO software from CambridgeSoft Corporation (ver. 9.0, Cambridge, MA, USA) was used to calculate molecular models.
3.5. MTT Antiproliferative Assay
CCRF-CEM (human acute lymphoblastic leukemia), HL-60 (human acute promyelocytic leukemia), K-562 (human chronic myelogenous leukemia), U-937 (human histiocytic lymphoma), DLD-1 (human colorectal adenocarcinoma), LNCaP (human prostatic carcinoma) and MCF7 (human breast adenocarcinoma) cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM glutamine and antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin) at 37 °C in a humidified atmosphere of 5% CO2. Cells were seeded at 4 × 104 per well in 96-well culture plates before treatment with different concentrations of the tested compounds. The compounds were dissolved in dimethyl sulfoxide (less than 0.02%) and made immediately of 1.25, 2.5, 5, 10 and 20 μg/μL prior to the experiments. After treatment for 72 h, the cytotoxicity of the tested compounds was determined using a MTT cell proliferation assay (thiazolyl blue tetrazolium bromide, Sigma-M2128). The MTT is reduced by the mitochondrial dehydrogenases of viable cells to a purple formazan product. The MTT-formazan product was dissolved in DMSO. Light absorbance values (OD = OD570 − OD620) were recorded at wavelengths of 570 and 620 nm using an ELISA reader (Anthos labtec Instrument, Salzburg, Austria) to calculate the concentration that caused 50% inhibition (IC50), i.e., the cell concentration at which the light absorbance value of the experiment group was half that of the control group. These results were expressed a percentage of the control ± SD established from n = 4 wells per one experiment from three separate experiments [17,18,19].
4. Conclusions
Octocorals have been well recognized as an important source of potential medicinal-use agents. However, because of the octocorals are claimed to be threatened species and most of the compounds from octocorals are difficult to obtain by chemical methods at this stage, bioactive substances from cultured-type marine invertebrates will play an important role in this field. Our further studies on a cultured soft coral Sinularia numerosa for the extraction of additional natural substances, have led to the isolation of a new norcembranoidal diterpene, 4α-hydroxy-5-episinuleptolide (2), and this compound was found to exhibit modest cytotoxicity against CCRF-CEM tumor cells. This study suggested that 4α-hydroxy-5-episinuleptolide (2) is worthy of further biomedical investigation.
Acknowledgments
This research was supported by grants from the National Dong Hwa University; the National Museum of Marine Biology and Aquarium; the Division of Marine Biotechnology, Asia-Pacific Ocean Research Center, National Sun Yat-sen University; the Taiwan Kose Co. Ltd.; the Ministry of Science and Technology (Grant No. NSC 103-2911-I-002-303; MOST 104-2911-I-002-302; MOST 103-2325-B-039-008; MOST 103-2325-B-039-007-CC1; MOST 103-2325-B-291-001; and NSC 101-2320-B-291-001-MY3); the National Health Research Institutes (NHRI-EX103-10241BI), and in part from the grant from Chinese Medicine Research Center, China Medical University (the Ministry of Education, the Aim for the Top University Plan), Taiwan, awarded to Yang-Chang Wu and Ping-Jyun Sung.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/16/02/3298/s1.
Author Contributions
Yang-Chang Wu and Ping-Jyun Sung designed the whole experiment and contributed to manuscript preparation; Wu-Fu Chen and Chen-Ting Yin researched data and wrote the manuscript; Ching-Hsiao Cheng, Mei-Chin Lu, Lee-Shing Fang, Wei-Hsien Wang, Zhi-Hong Wen and Jih-Jung Chen analyzed the data and performed data acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen W.-T., Li Y., Guo Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B. 2012;2:227–237. doi: 10.1016/j.apsb.2012.04.004. [DOI] [Google Scholar]
- 2.Rocha J., Peixe L., Gomes N.C.M., Calado R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs. 2011;9:1860–1886. doi: 10.3390/md9101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai C.-F. Reef environment and coral fauna of Southern Taiwan. Atoll Res. Bull. 1991;354:1–28. doi: 10.5479/si.00775630.354.1. [DOI] [Google Scholar]
- 4.Su J., Yu X., Zeng L., Mak T.C.W. Novel polyhydroxylated sterols from the soft coral Sinularia numerosa. J. Nat. Prod. 1989;52:934–940. doi: 10.1021/np50065a004. [DOI] [Google Scholar]
- 5.Tillekeratne L.M.V., Liyanage G.K., Ratnasooriya W.D., Ksebati M.B., Schmitz F.J. A new spermatostatic glycoside from the soft coral Sinularia crispa. J. Nat. Prod. 1989;52:1143–1145. doi: 10.1021/np50065a038. [DOI] [PubMed] [Google Scholar]
- 6.Tseng Y.-J., Yang Y.-C., Wang S.-K., Duh C.-Y. Numerosol A–D, new cembranoid diterpenes from the soft coral Sinularia numerosa. Mar. Drugs. 2014;12:3371–3380. doi: 10.3390/md12063371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato A., Fenical W., Zheng Q.-T., Clardy J. Norcembrene diterpenoids from Pacific soft-corals of the genus Sinularia (Alcyonacea: Octocorallia) Tetrahedron. 1985;41:4303–4308. doi: 10.1016/S0040-4020(01)97201-1. [DOI] [Google Scholar]
- 8.Qin M.-L., Li X.-M., Wang B.-G. Chemical constituents of sesquiterpenes in soft coral Sinularia numerosa. Oceanol. Limnol. Sin. 2009;40:540–544. [Google Scholar]
- 9.Yamashita T., Nakao Y., Matsunaga S., Oikawa T., Imahara Y., Fusetani N. A new antiangiogenic C24 oxylipin from the soft coral Sinularia numerosa. Bioorg. Med. Chem. 2009;17:2181–2184. doi: 10.1016/j.bmc.2008.10.083. [DOI] [PubMed] [Google Scholar]
- 10.Bowden B.F., Coll J.C., Mitchell S.J., Mulder J., Stokie G.J. Studies of Australian soft corals. IX A novel nor-diterpene from the soft coral Sinularia leptoclados. Aust. J. Chem. 1978;31:2049–2056. [Google Scholar]
- 11.Turner K.E., Bowden B.F., Stokie G.J., Howard C.J. (4R*,8S*,11R*,13S*,14R*)-8,11-epoxy-14-hydroxy-11-methyl-4-(1-methylvinyl)-6,9-dioxocyclotetradec-1-ene-1,13-carbolactone. Acta Cryst. 1979;B35:1283–1284. doi: 10.1107/S056774087900621X. [DOI] [Google Scholar]
- 12.Sheu J.-H., Ahmed A.F., Shiue R.-T., Dai C.-F., Kuo Y.-H. Scabrolides A–D, four new norditerpenoids isolated from the soft coral Sinularia scabra. J. Nat. Prod. 2002;65:1904–1908. doi: 10.1021/np020280r. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed A.F., Shiue R.-T., Wang G.-H., Dai C.-F., Kuo Y.-H., Sheu J.-H. Five novel norcembranoids from Sinularia leptoclados and S. parva. Tetrahedron. 2003;59:7337–7344. doi: 10.1016/S0040-4020(03)01138-4. [DOI] [Google Scholar]
- 14.Tseng Y.-J., Ahmed A.F., Dai C.-F., Chiang M.Y., Sheu J.-H. Sinulochmodins A–C, three novel terpenoids from the soft coral Sinularia lochmodes. Org. Lett. 2005;7:3813–3816. doi: 10.1021/ol051513j. [DOI] [PubMed] [Google Scholar]
- 15.Kamel H.N., Fronczek F.R., Khalifa S.I., Slattery M. Microbial transformation of 5-episinuleptolide. Chem. Pharm. Bull. 2007;55:537–540. doi: 10.1248/cpb.55.537. [DOI] [PubMed] [Google Scholar]
- 16.Allinger N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977;99:8127–8134. [Google Scholar]
- 17.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 18.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 19.Lu M.-C., Hwang S.-L., Chang F.-R., Chen Y.-H., Chang T.-T., Hung C.-S., Wang C.-L., Chu Y.-H., Pan S.-H., Wu Y.-C. Immunostimulatory effect of Antrodia camphorata extract on functional maturation of dendritic cells. Food Chem. 2009;113:1049–1057. doi: 10.1016/j.foodchem.2008.08.089. [DOI] [Google Scholar]