Abstract
The cerebral cortical expansion index refers to the ratio between left and right cortex width and is recognized as an indicator for cortical hyperplasia. Cerebral ischemia was established in CB-17 mice in the present study, and the mice were subsequently treated with recombinant human erythropoietin via subcutaneous injection. Results demonstrated that cerebral cortical width index significantly increased. Immunofluorescence detection showed that the number of nuclear antigen antibody/5-bromodeoxyuridine-positive cells at the infarction edge significantly increased. Correlation analysis revealed a negative correlation between neurological scores and cortical width indices in rats following ischemic stroke. These experimental findings suggested that recombinant human erythropoietin promoted cerebral cortical hyperplasia, increased cortical neurogenesis, and enhanced functional recovery following ischemic stroke.
Keywords: cerebral infarction, erythropoietin, neural regeneration, neurogenesis
Abbreviations:
rhEPO, recombinant human erythropoietin; MCAO, middle cerebral artery occlusion; BrdU, 5'-bromo-2'-deoxyuridine-5'-monophosphate; NeuN, neuronal nuclei
INTRODUCTION
Stroke is increasingly recognized as the leading cause of mortality and long-term disability. Thrombolysis in combination with recombinant tissue plasminogen activator, the only licensed drug for acute stroke, is applied to a restricted number of stroke patients due to multiple contraindications[1,2], and its efficacy has been limited by toxicity and reperfusion injury[3]. Therefore, there is an urgent need for novel therapies for acute ischemic stroke patients who are not qualified for recombinant tissue plasminogen activator treatment. Recent studies have focused on the role of a local protective mechanism that underlies an anti-inflammatory response and anti-apoptotic actions during the acute phase of ischemic stroke[4,5]. Angiogenesis and neurogenesis have been shown to contribute to neuroprotection and functional recovery after stroke[6,7,8], and a promising neuroprotective therapy can improve histological functions, as well as neurological functions. To date, erythropoietin and its analogues have been widely tested in animal models and clinical trials, showing the protective effects against cerebral ischemic impairment[9,10,11,12,13].
Erythropoietin prevents neuronal apoptosis induced by a variety of stimuli[14]. Erythropoietin affords significant neuroprotection against ketamine-induced injury in neurons via the PI3K/Akt-mediated signaling pathway[15] and protects cortical neurons from glutamic acid toxicity, which could be related to signal transduction of nuclear factor-kappa B[16]. Erythropoietin also promotes proliferation and angiogenesis in vitro and in the subventricular zone[17,18,19]. Erythropoietin significantly decreases expansion of the subventricular zone unilaterally and improves functional outcomes following experimental neonatal stroke[20]. However, little is known about the effects of erythropoietin on morphological changes due to ischemic cerebral cortex, as well as the relationship between these changes and neurological functional improvement. The present study evaluated the effects of recombinant human erythropoietin (rhEPO) on neurological functional improvement, neurogenesis, and cerebral cortical expansion following intraperitoneal injection into a permanent middle cerebral artery occlusion (MCAO) CB-17 mouse model. The relationship between neurological functional improvement and cerebral cortical expansion was also evaluated.
RESULTS
Quantitative analysis of experimental animals
A total of 20 CB-17 male mice were selected to establish MCAO models. No mice died during the study, and model establishment was considered successful. Twenty surviving mice were randomly assigned to two groups: rhEPO (treated with rhEPO; n = 10) and model (treated with phosphate-buffered saline; n = 10). All 20 mice were included in the final analysis, with no loss.
rhEPO-induced cerebral cortex expansion following ischemic stroke
To determine cerebral cortical expansion, the cortical width index was utilized as a quantitative hallmark. Quantitative analysis revealed that the cortical width index significantly increased in the rhEPO treatment group compared with the model group at 35 days post-stroke (P < 0.05; Figure 1).
Figure 1.
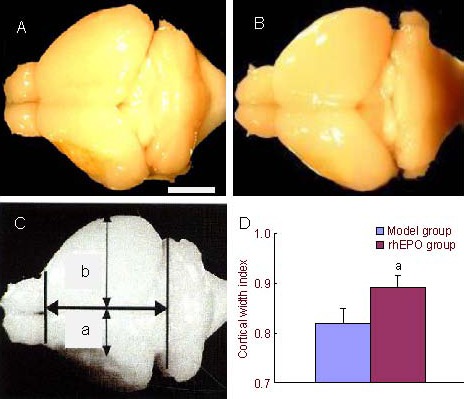
Recombinant human erythropoietin (rhEPO) induced cerebral cortical expansion in mice after ischemic stroke. Using a digital camera system, whole brain images were captured. Scale bar: 2 mm.
(A) Model group.
(B) rhEPO treatment at 35 days post-stroke.
(C) Cortical width index measured using NIH image software; a: maximum width from midpoint to edge of infarcted hemisphere; b: maximum width from midpoint to edge of non-infarcted hemisphere;
(D) Cortical width index after treating with phosphate-buffered saline and rhEPO (mean ± SD, n = 10, two-sample t-test). aP < 0.05, vs. model group.
rhEPO-increased neurogenesis at the infarction edge
Immunofluorescence stainings were performed with 5'-bromo-2'-deoxyuridine-5'-monophosphate (BrdU; synthetic nucleoside thymidine analogue used for detection of proliferating cells) and neuronal nuclei (NeuN; neuronal-specific nuclear protein) monoclonal antibody to determine neurogenesis at the infarction edge at 35 days post-stroke. Quantitative analysis revealed that the number of NeuN/BrdU-positive cells significantly increased in the rhEPO group compared with the model group (6.8 ± 2.3 vs. 2.5 ± 1.5, n = 10, P < 0.05; Figure 2).
Figure 2.
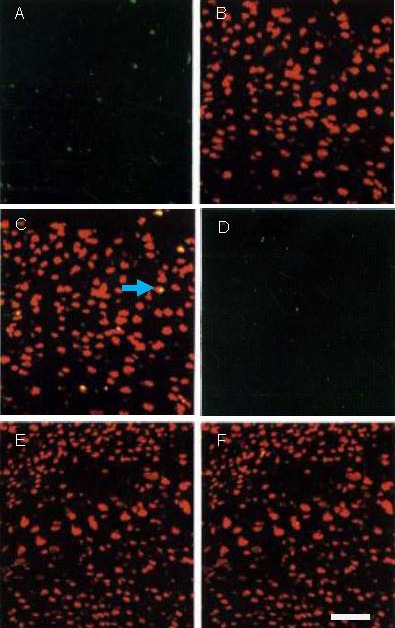
Neurogenesis at infarct edge following phosphate-buffered saline and recombinant human erythropoietin (rhEPO) treatment (immunofluorescence, laser scanning confocal microscopy).
(A–C) rhEPO group; (D–F) model group.
Green fluorescence indicates 5’-bromo-2’-deoxyuridine- 5’-monophosphate (BrdU)-positive cells (FITC), red fluorescence (Texas Red) indicates neuronal nuclei (NeuN)-positive cells; yellow fluorescence is an indicator of NeuN/BrdU double-labeling.
A greater number of NeuN/BrdU-positive cells is visible in the rhEPO group compared with model group at 35 days post-stroke. Arrow represents neurogenesis (NeuN/BrdU-positive cells). Scale bar: 50 μm.
rhEPO-accelerated neurological function recovery following ischemic stroke
Neurological function recovery was assessed using a modified neurological functional rating scale. Compared with the model group, mice treated with rhEPO exhibited accelerated functional recovery at 35 days post-stroke (neurological function score: 1.3 ± 0.3 vs. 2.4 ± 0.2, n = 10, P < 0.05). The neurological function score of ischemic mice negatively correlated with cortical width index (n = 10 per group, r = −0.23, P < 0.05).
Figure 3.
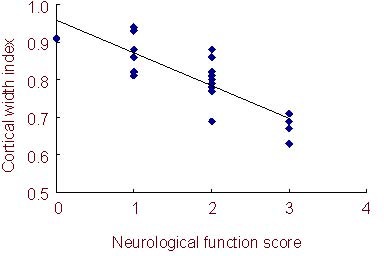
Correlation between cortical width index and neurological function in mice.
The neurological function score negatively correlates with the cortical width index (1.3 ± 0.3 vs. 2.4 ± 0.2, mean±SD, n = 10; regression analysis, P < 0.05, r = -0.23).
DISCUSSION
The MCAO animal model correlates with many human clinical characteristics and is recognized as a standard animal model for focal cerebral infarction studies. The present study introduced a highly reproducible model of permanent cerebral ischemia in CB-17 mice. Results showed that rhEPO significantly accelerated neurological function recovery and promoted neurogenesis at the infarct edge, as well as induced cortical expansion, at 35 days post-ischemic stroke. In addition, neurological function improvement directly correlated with cortical expansion.
Compared with the model group, rhEPO treatment resulted in significant neurological functional improvement. These experimental results were consistent with previous studies[20,21]. EPO treatment has been shown to significantly decrease infarct size, thereby maintaining cognitive function after ischemic stroke[22]. The subventricular zone in the rodent forebrain contains a population of neuronal progenitor cells throughout life[23]. Various types of injury have been shown to stimulate progenitor proliferation in the subventricular zone. Following MCAO, increased size and proliferation of progenitor cells in the subventricular zone have been shown in the adult and in a neonatal rodent stroke model[24]. Erythropoietin and its receptor play important roles in subventricular zone proliferation[25]. In contrast to results from other previous studies, the present study did not quantify subventricular zone progenitors. However, results demonstrated that rhEPO significantly increased the number of BrdU/NeuN-positive cells at the ischemic boundary. Expression of the EPO receptor has been described in a permanent MCAO animal model[26].
Erythropoietin is an important physiological determinant for mobilizing immature hematopoietic stem cells, including endothelial progenitor cells, which results in a significantly increased numbers of circulating endothelial progenitor cells[27,28,29], which subsequently have the capacity to participate in neovascularization of ischemic tissues and accelerate angiogenesis by incorporating into the neovasculature at the ischemic site, thereby limiting tissue damage[30,31]. Disruption of the blood-brain barrier after ischemic stroke might facilitate hematopoietic stem cell entry into ischemic tissue. Therefore, erythropoietin administration is thought to increase mobilization of circulating hematopoietic stem cells to the damaged area, subsequently stimulating cell division. Erythropoietin is also a survival factor for endothelial cells and indirectly acts on endothelial cells via activation of vascular endothelial growth factor receptor system[32]. Vascular endothelial growth factor is the most important specific regulator of endothelial cell growth and differentiation and a major angiogenesis factor. Following administration of rhEPO, there are a greater number of hematopoietic stem cells and newly formed vessels that promote neurogenesis.
Neovascularization is essential for neuronal regeneration after stroke, and therapeutic neovascularization is a potential effective means for enhancing functional recovery. Results from the present study indicated that rhEPO promoted neurogenesis at the infarction border, as well as significantly increased the number of cells labeled with BrdU and NeuN antibodies in the ischemic border. The increased neurogenesis might be due to proliferation of immature hematopoietic stem cells mobilized by rhEPO or migration from the striatum to the cortex via the hippocampus.
To the best of our knowledge, the present study identified, for the first time, that neurological function improvement directly correlated with cortical expansion after cerebral infarction. Cortical expansion in MCAO mice treated with rhEPO suggested ongoing neurogenesis. Erythropoietin is a potent inhibitor of ischemia-induced apoptosis[33] and provides neuroprotection by maintaining Bcl-2 and Bcl-x expression or inactivating caspases[34]. Therefore, the beneficial effects of rhEPO on neurological functional improvement comprise multiple mechanisms.
Some limitations exist in the present study. First, the effect of rhEPO on angiogenesis was not analyzed, nor the growth of new blood vessels from the existing vasculature, to explore probable mechanisms underlying neurogenesis. Second, the numbers of circulating hematopoietic stem cells were not quantified. Third, the effect of rhEPO on erythropoiesis was not determined and could be detrimental following stroke.
In conclusion, these results showed that rhEPO provided protection against cerebral infarction in a mouse MCAO model. Increased neural regeneration has been shown to contribute to long-term repair of the ischemic brain[35]. Early use of rhEPO promotes neurobehavioral development in preterm infants[36]. Results from the present study suggested that rhEPO provided neuroprotection and therapeutic effects, and might be helpful for treatment of patients following ischemic stroke.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
This experiment was performed at the Laboratory of Cerebral Circulation and Metabolism, the Second Affiliated Hospital of Soochow University, China from January to October 2009.
Materials
A total of 20 male, CB-17 mice, aged 8 weeks and weighing 10–12 g, were purchased from Animal Department of Soochow University, China (license No. SYKK (Su) 2009-0048). All mice were allowed access to food and tap water ad libitum. Powdered chow and sterilized water were also provided during the first 7 days after stroke induction. All experiments were conducted according to the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[37].
Methods
Establishment of cerebral ischemic infarction model
Permanent focal cerebral infarction was established by ligation and disconnection of the distal portion of the left middle cerebral artery, as previously described[38,39].
Briefly, general anesthesia was induced and maintained by inhalation of 3% and 1.5% halothane (7025 Rodent Ventilator, Ugo Basile, Italy) through a face mask, and a skin incision was made between the left eyeball and left ear hole. The left salivary gland and veins were carefully removed to visualize the zygoma. The left zygoma was dissected under a surgical microscope, and the jaw joint was detached to allow visualization of the middle cerebral artery through the cranial bone. A hole (2 mm diameter) was made in the bone using a dental drill. Subsequently, the dura matter was carefully removed to avoid damage to the brain surface. The middle cerebral artery was then isolated, electrocauterized, and disconnected distally to the olfactory tract (distal M1 portion of middle cerebral artery). The middle cerebral artery was coagulated from the distal to the proximal portion. After confirming no re-canalization, the skin incision was closed with sutures. Body temperature was maintained at 36.5-37.0°C through use of a heat lamp during surgery and for 2 hours after MCAO. Mice exhibiting decreased neurological function immediately after the procedure were used for subsequent experiments.
Drug administration
rhEPO (1 000 μg/kg, 0.1 mL; Kinrin, Japan) or phosphate-buffered saline (0.1 mL) was administered via subcutaneous injection at 24 hours after ischemic stroke, once daily for 3 days, after surgery[34]. Animals were administered BrdU (50 mg/kg intraperitoneally; Sigma, St. Louis, MO, USA) daily after ischemic stroke until day of sacrifice.
Neurological functional detection
To assess neurological functions, the mice were subjected to behavioral testing at 35 days post-stroke. Neurological deficits were scored using a modified neurological function rating scale, as previously described[34]: 0, no neurological deficit; 1, failure to fully extend left forepaw; 2, failure to circle to left; 3, loss of walking or righting reflex.
Assessment of cerebral cortical expansion after stroke
To assess cortical expansion, mice were perfusion-fixed with 100 mL periodate-lysine-paraformaldehyde fixative under deep anesthesia at 35 days post-stroke, and the brains were removed. Whole brain images were captured using a microscopic digital camera system (Olympus, Tokyo, Japan), and maximum width from the midpoint to edge of brain was measured using NIH image software (Olympus). The ratio of left/right cortical width was defined as the cortical width index (Figure 1C).
Evaluation of neurogenesis
At 35 days post-stroke, the mice were perfusion-fixed and entire brains were removed. Free-floating coronal sections (20 μm thick) were cut on a vibratome and were subjected to immunofluorescent analysis. Immunofluorescent staining of brain tissues was performed using mouse anti-rat BrdU monoclonal antibody (1:600; Boehringer Mannheim, Mannheim, Germany) and mouse anti-rat NeuN monoclonal antibody (1:100, 4°C, 48 hours; Roche, Welwyn Garden City, UK), followed by incubation in goat anti-mouse IgG secondary antibody(1:10, 35°C, 2 hours). Localization of BrdU and NeuN was conducted using laser scanning confocal microscopy (Olympus). For immunofluorescence, green (FITC for BrdU), red (Texas Red for NeuN), and yellow (double-stained with BrdU and NeuN) was visualized by laser beam excitation. Areas of interest were scanned with a 40 × oil-immersion objective lens. For quantitative analysis, the number of cells double-stained with BrdU and NeuN was quantified around the ischemic cortex edge using a high-power lens field under microscopy (Olympus).
Statistical analysis
Data were analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA), and the results were statistically evaluated in each group. Measurement data were expressed as mean ± SD, and the difference between two groups was evaluated using the two-sample t-test. Regression analysis was performed between cortical width index and neurological functional score. Statistical significance was set at P < 0.05.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethics Committee of Soochow University in China.
(Edited by Chen JF, Chen F/Yang Y/Song LP)
REFERENCES
- [1].Galldiks N, Zaro-Weber O, Dohmen C, et al. Systemic thrombolysis with rt-PA in patients under 40 years of age: a subgroup analysis of the Cologne Stroke Experience. Cerebrovasc Dis. 2010;30(5):514–518. doi: 10.1159/000319776. [DOI] [PubMed] [Google Scholar]
- [2].Machumpurath B, Davis SM, Yan B. Rapid neurological recovery after intravenous tissue plasminogen activator in stroke: prognostic factors and outcome. Cerebrovasc Dis. 2011;31(3):278–283. doi: 10.1159/000322564. [DOI] [PubMed] [Google Scholar]
- [3].Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- [4].Martinez-Vila E, Sieira PI. Current status and perspectives of neuroprotection in ischemic stroke treatment. Cerebrovasc Dis. 2001;11(Suppl 1):60–70. doi: 10.1159/000049127. [DOI] [PubMed] [Google Scholar]
- [5].Danton GH, Dietrich WD. The search for neuroprotective strategies in stroke. AJNR Am J Neuroradiol. 2004;25(2):181–194. [PMC free article] [PubMed] [Google Scholar]
- [6].Du Y, Shi L, Li J, et al. Angiogenesis and improved cerebral blood flow in the ischemic boundary area were detected after electroacupuncture treatment to rats with ischemic stroke. Neurol Res. 2011;33(1):101–107. doi: 10.1179/016164110X12714125204317. [DOI] [PubMed] [Google Scholar]
- [7].Miriam N, Anna R, Mar H, et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216(1):205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- [8].Maria K, Evgeny K, Oleg T, et al. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp Neurol. 2010;226(1):90–99. doi: 10.1016/j.expneurol.2010.08.006. [DOI] [PubMed] [Google Scholar]
- [9].Ruifrok WP, de Boer RA, Iwakura A, et al. Estradiol-induced, endothelial progenitor cell-mediated neovascularization in male mice with hind-limb ischemia. Vasc Med. 2009;14(1):129–136. doi: 10.1177/1358863X08096666. [DOI] [PubMed] [Google Scholar]
- [10].Liu X, Li Y, Liu Y, et al. Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy. Am J Pathol. 2010;176(1):504–515. doi: 10.2353/ajpath.2010.081152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosemeire M, Lauro M, Konstantinos H, et al. Effects of combination of proliferative agents and erythropoietin on left ventricular remodeling post-myocardial infarction. Clin Transl Sci. 2011;4(3):168–174. doi: 10.1111/j.1752-8062.2011.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40(12):e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- [13].Minnerup H, Herdrich J, Rogalewski A, et al. The efficacy of erythropoietin and its analogues in animal model: a meta-analysis. Stroke. 2009;40(9):3113–3120. doi: 10.1161/STROKEAHA.109.555789. [DOI] [PubMed] [Google Scholar]
- [14].Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11(Suppl 1):S37–44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- [15].Shang Y, Yao SL, Wu Y. Erythropoietin protects neuron against ketamine induced injuries. Zhonghua Yi Xue Za Zhi. 2008;88(13):876–879. [PubMed] [Google Scholar]
- [16].Dong WB, Hou HM, Wang Q, et al. NF-kappaB-mediated protective effect of erythropoitin on neuron against glutamate-induced damage. Xibao yu Fenzi Mianyixue Zazhi. 2008;24(6):584–585. [PubMed] [Google Scholar]
- [17].Osredkar D, Sall JW, Bickler PE, et al. Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol Dis. 2010;38(2):259–265. doi: 10.1016/j.nbd.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xiong Y, Mahmood A, Meng Y, et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2010;113(3):598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang L, Chopp M, Gregg SR, et al. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28(7):1361–1368. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chang YS, Mu D, Wendland M, et al. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr Res. 2005;58(1):106–111. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- [21].Lu D, Mahmood A, Qu C, et al. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22(9):1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- [22].Gonzalez FF, Abel R, Almli CR, et al. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31(5):403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ma DK, Bonaguidi MA, Ming GL, et al. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19(6):672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bacigaluppi M, Pluchino S, Martino G, et al. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265(1-2):73–77. doi: 10.1016/j.jns.2007.06.012. [DOI] [PubMed] [Google Scholar]
- [25].Hagg T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist. 2009;15(1):20–27. doi: 10.1177/1073858408324789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fletcher L, Kohli S, Sprague SM, et al. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111(1):164–170. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- [27].Heeschen C, Aicher A, Lehmann R, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102(4):1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- [28].Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- [29].Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105(6):732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- [30].Liu X, Li Y, Liu Y, et al. Endothelial progenitor cells (EPCs) mobilized and activated by neurotrophic factors may contribute to pathologic neovascularization in diabetic retinopathy. Am J Pathol. 2010;176(1):504–515. doi: 10.2353/ajpath.2010.081152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krenning G, van Luyn MJ, Harmsen MC. Endothelial progenitor cell-based neovascularization: implications for therapy. Trends Mol Med. 2009;15(4):180–189. doi: 10.1016/j.molmed.2009.02.001. [DOI] [PubMed] [Google Scholar]
- [32].Westenbrink BD, Lipsic E, van der Meer P, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28(16):2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- [33].Villa P, Bigini P, Mennini T, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198(6):971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Warren JS, Zhao Y, Yung R, et al. Recombinant human erythropoietin suppresses endothelial cell apoptosis and reduces the ratio of Bax to Bcl-2 proteins in the aortas of apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2011;57(4):424–433. doi: 10.1097/FJC.0b013e31820d92fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shi X, Kang Y, Hu Q, et al. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain Res. 2010;1317:257–267. doi: 10.1016/j.brainres.2009.12.061. [DOI] [PubMed] [Google Scholar]
- [36].He JS, Huang ZL, Yang H, et al. Early use of recombinant human erythropoietin promotes neurobehavioral development in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi. 2008;10(5):586–588. [PubMed] [Google Scholar]
- [37].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [38].Taguchi A, Wen Z, Myojin K, et al. Granulocyte colony-stimulating factor has negative effect on stroke outcome in a murine model. Eur J Neurosci. 2007;26(1):126–133. doi: 10.1111/j.1460-9568.2007.05640.x. [DOI] [PubMed] [Google Scholar]
- [39].Taguchi A, Kasahara Y, Nakagomi T, et al. A reproducible and simple model of permanent cerebral ischemia in CB-17 and SCID Mice. J Exp Stroke Transl Med. 2010;3(1):28–33. doi: 10.6030/1939-067x-3.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]


