Abstract
Three cycles of remote ischemic pre-conditioning induced by temporarily occluding the bilateral femoral arteries (10 minutes) prior to 10 minutes of reperfusion were given once a day for 3 days before the animal received middle artery occlusion and reperfusion surgery. The results showed that brain infarct volume was significantly reduced after remote ischemic pre-conditioning. Scores in the forelimb placing test and the postural reflex test were significantly lower in rats having undergone remote ischemic pre-conditioning compared with those who did not receive remote ischemic pre-conditioning. Thus, neurological function was better in rats having undergone remote ischemic pre-conditioning compared with those who did not receive remote ischemic pre-conditioning. These results indicate that remote ischemic pre-conditioning in rat hindlimb exerts protective effects in ischemia-reperfusion injury.
Keywords: cerebral ischemia-reperfusion, remote ischemic preconditioning, stroke, neural regeneration
Abbreviations:
RIPC, remote ischemic pre-conditioning; MCAO, middle cerebral artery occlusion; CBF, cerebral blood flow
INTRODUCTION
Many studies have been devoted to exploring an effective treatment against cerebral ischemia-reperfusion injury.
Though validated in animal models of ischemia, few of them have been effective in clinical applications. Ischemic preconditioning has been firmly established as an endogenous protective mechanism for the reduction of cerebral ischemia injury. To induce tolerance to a subsequent prolonged ischemia episode, ischemic preconditioning is initiated by short periods of ischemia-reperfusion applied 1 hour or a few days before a prolonged cerebral ischemic insult[1]. Ischemic preconditioning, first described by Murry et al[2] in 1986 in the canine heart model, has been extended to other organ systems, such as the brain, liver and gastrointestinal tract[3,4].
However, it is implausible for vital organs more sensitive to ischemia, such as heart and brain, to be subjected to intermittent ischemia and reperfusion. A less invasive method of ischemic preconditioning is required for clinical application. Przyklenk et al[5] first reported that brief episodes of ischemia in one vascular bed protect remote, virgin myocardium from subsequent sustained coronary artery occlusion in the canine model. Recently Zhou et al[6] showed that ischemic pre-conditioning of the left upper arm, could protect heart and lung against injury in infants. Remote ischemic pre-conditioning (RIPC), performed in a distant organ that is more tolerant to ischemia, can protect vital organs that are more sensitive to ischemia. Studies in ischemic animal models also showed that rats undergoing brief limb ischemia had smaller cerebral infarctions than those not exposed to this intervention before ischemia[7], though the mechanism of remote ischemic pre-conditioning remains unclear. This study investigated the neuroprotective effects of limb remote ischemic conditioning against ischemia using the intraluminal thread middle cerebral artery occlusion (MCAO) and reperfusion model in rats.
RESULTS
Quantitative analysis of experimental animals
A total of 24 rats were equally and randomly divided into RIPC, MCAO alone (control), and sham surgery groups. MCAO and reperfusion were performed in the RIPC and model groups by the suture method. The skin was incised in rats from the sham surgery group. The rats in the RIPC group received RIPC treatment induced by temporarily occluding the bilateral femoral arteries 3 days before cerebral ischemia-reperfusion injury. All rats were included in the final analysis without loss.
RIPC effects on cerebral blood flow (CBF) and blood pressure
We measured CBF 10 minutes before ischemia (baseline), immediately after ischemia, after 30 minutes of ischemia, immediately after reperfusion, and after 30 minutes of reperfusion. In addition, we measured CBF at the time of occluding-opening the femoral artery. There was no statistical significance (P > 0.05), which suggested that CBF was not affected by occluding-opening of the femoral artery (Table 1).
Table 1.
Effects of remote ischemic pre-conditioning on cerebral blood flow (CBF) and mean artery blood pressure (MABP) in rats

To investigate the effects of RIPC on the physiological parameters of rats following cerebral ischemia-reperfusion injury, we further measured mean artery blood pressure (MABP) in RIPC-treated animals. The results showed that RIPC did not affect MABP (Table 1).
RIPC effects on neurologic impairment
We assessed neurological function after 24 hours of cerebral ischemia-reperfusion injury. Rats in each group had neurological dysfunction including forelimb flexion. The score in the forelimb placing test in the RIPC group (3.00 ± 0.76) was significantly lower than in the control group (7.50 ± 1.60, P < 0.01). The neurological deficit score (normal score, 0; maximal score, 12) in the RIPC group (5.50 ± 0.76) was significantly lower than in the control group (9.50 ± 0.93, P < 0.01). No neurological impairment was detectable in the sham surgery group. RIPC improved neurological function scores after cerebral ischemia and reperfusion.
RIPC effects on infarct volume in rats
All rats were sacrificed 24 hours after ischemia and reperfusion to calculate brain infarct volume. The infarct tissue volume was calculated as a percentage of the contralateral hemisphere in rats. No cerebral infarction was visible in the sham surgery group. There were significant differences between the control group and the RIPC group (42.74 ± 8.13% vs. 25.28 ± 5.84%). The 40% reduction in infarct volume in the RIPC group versus the control group was significant (P < 0.01; Figure 1).
Figure 1.
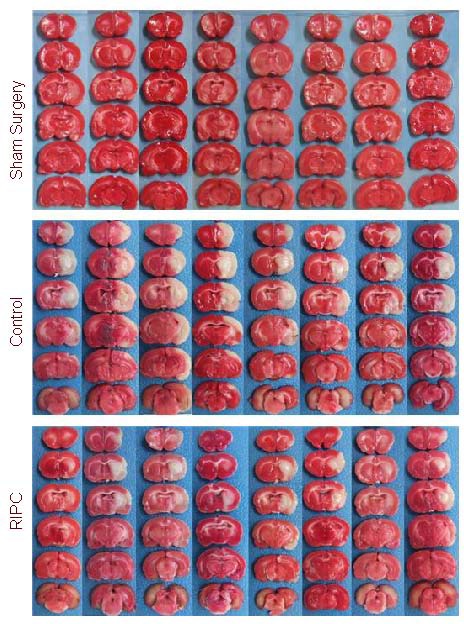
Brain coronal sections stained by 5-triphenyl-2H-tetrazolium chloride in the remote ischemic pre-conditioning (RIPC), control, and sham surgery groups. The white segment represents the infarction.
DISCUSSION
Birnbaum et al[8] published the first study suggesting that transient limb ischemia could remotely precondition the ischemic heart in 1997. Studies in patients undergoing coronary artery bypass surgery showed myocardial injury was reduced in patients who underwent three cycles of RIPC in the arm, induced by a blood pressure cuff[9]. In our study, the infarction volume was reduced by 40% in the RIPC group compared to the control group, and neurological function improved, which demonstrates that RIPC protects the brain against cerebral ischemia. Determining the time course for RIPC is a prerequisite for clinical outcome studies. Some studies showed that the immediate protection from pre-conditioning lasted just a few hours, but the “second window” of protection, which followed 24 hours later, lasted for 48 to 72 hours[10] and depended on the induction of protective proteins[11]. Loukogeorgakis et al[12] established that RIPC had early and late phases, which existed immediately, 24 hours, and 48 hours before endothelial ischemia-reperfusion injury, but not 4 hours before injury. Given the above studies, we suspect the “second window” or “late phase” of protection was involved in our study, in which RIPC was performed once a day for 3 days before cerebral ischemia. The RIPC time points were limited in this study, and we had no negative results in this study. Thus, the therapeutic time window needs to be further studied.
In Loukogeorgakis's study[13], three cycles of RIPC in the arms showed protection, but shortening the stimulus to two cycles was not protective. Two cycles of RIPC applied to the leg were effective, which demonstrated that the volume of tissue exposed to the preconditioning stimulus also determines the degree of protection. In our study, three cycles of RIPC, applied by occluding the bilateral femoral arteries once a day for three days before the middle artery occlusion and reperfusion surgery could protect the brain against injury. Nevertheless, the stimulus threshold of RIPC is unclear.
The mechanism of remote ischemic pre-conditioning remains unknown, but humoral and neurogenic pathways have been implicated. Humoral substances such as adenosine or bradykinin, produced by preconditioning, were released into systemic circulation and protected the remote region or organ[14,15]. Other mechanistic factors proposed include erythropoietin, activation of the KATP channel[16], nitric oxide[17], delta 1-opioid[18,19] and free radicals[19]. The ganglion blocker hexamethonium abolished the heart benefits of remote ischemic preconditioning-induced mesenteric artery occlusion, supporting the involvement of a neurogenic pathway[20,21,22,23,24]. Our results provide evidence that remote ischemic preconditioning given once a day for 3 days before ischemia can protect the brain against injury in experimental ischemic stroke, which may provide a basis for the clinical use of RIPC in cerebral protection.
MATERIALS AND METHODS
Design
A randomized controlled animal study.
Time and setting
This experiment was performed at the Cerebrovascular Diseases Research Institute of Capital Medical University, Beijing, China, from March to October 2010.
Materials
A total of 24 adult male specific pathogen-free Sprague-Dawley rats, aged 8-10 months and weighing 280-300 g, were used for the study. The rats were maintained at the Animal Center of the Xuanwu Hospital of Capital Medical University in China (Permission No. SCXK 2006-0009). Rats were housed in individual cages with free access to water and chow for 3-5 days before experiments, and fasted with free access to water for 12 hours prior to the surgeries. All animal experiments were performed in accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Methods
Establishment of cerebral ischemia-reperfusion models
Each rat was weighed and anesthetized with 4% enflurane, initially by facemask, then with 2% enflurane after endotracheal intubation, and mechanically ventilated with 70% nitrous oxide and 30% O2. Rectal temperature was maintained within normal limits using a feedback-controlled heating pad. A transcranial laser Doppler probe (PeriFlux System 5000; Perimed, Stockholm, Sweden) was used to measure brain blood flow velocity. An arterial blood sample was used for blood gas analysis[25]. Transient focal ischemia was induced by the intraluminal suture method[26]. Briefly, the right common carotid artery was exposed and the external carotid was ligated and transected. A length of 3/0 nylon suture with a 0.3-mm diameter silicone tip was introduced through the stump of the external carotid and advanced through the internal carotid until a sharp decrease in cerebral perfusion was observed using the transcranial laser Doppler probe. After 2 hours of ischemia, the thread was withdrawn and reperfusion confirmed by a return of the laser Doppler probe readings to baseline. Rectal temperature was maintained at 37.0 ± 0.5°C. After surgery, animals were returned to a quiet room, temperature-controlled at 25 ± 1°C. Rats in which subarachnoid hemorrhage occurred were excluded. Rats in the sham surgery group received the same procedure without middle cerebral artery and bilateral femoral artery occlusion.
RIPC
RIPC was applied by occluding the bilateral femoral arteries with aneurysm clips (FE 681K, Aesculap Inc, USA) for 10 minutes, followed by a 10-minute reperfusion. This was considered one cycle. For preconditioning, this cycle was repeated three times. Three cycles of RIPC were given once a day for 3 days before the animal underwent middle artery occlusion and reperfusion.
Regional CBF measurement
Regional CBF was monitored by a laser Doppler flowmetry probe. Measurements were taken at 3.0 mm posterior and 5.0 mm lateral to the bregma, at five different time points: 10 minutes before ischemia (baseline), immediately after ischemia, after 30 minutes of ischemia, immediately after reperfusion, and after 30 minutes of reperfusion.
Blood pressure measurement
Blood pressure was tested at the tail artery, with a monitor (MP100A-CE, BIOPAC Systems, Inc., CA, USA). Data were collected at the following time points: 10 minutes before ischemia (baseline), immediately after ischemia, after 30 minutes of ischemia, immediately after reperfusion, and after 30 minutes of reperfusion.
Behavioral tests
Behavioral tests were performed 24 hours after cerebral ischemia and reperfusion. The forelimb placing test was performed in accordance with the method developed by De Ryck et al[27]. The Neurological deficit scores examined sensorimotor integration in forelimb placing responses to visual, tactile, and proprioceptive stimuli and were assessed based on previously published criteria[28] (normal score, 0; maximal score, 12).
Cerebral infarct volume measurements
Acute infarct volume was measured as previously described[29,30]. Rats anesthetized with 1% chloral hydrate were sacrificed 24 hours after MCAO. The brains were quickly removed and sectioned into eight consecutive 2-mm thick coronal slices, from the frontal pole to the occipital pole of the brain. The slices were stained by immersion in 2% 5-triphenyl-2H-tetrazolium chloride for 30 minutes at 37°C and then fixed with 8% formalin. Pictures of brain slices were taken 1 day after fixation. Brain infarct volumes were analyzed with Image-Pro Plus software (Alpha Innotech, San Leandro, CA, USA). The border between infarct and non-infarct tissue was outlined, and the area of infarction was measured by subtracting the area of the non-lesioned ipsilateral hemisphere from that of the contralateral side. The infarction volume was calculated by integration of lesion areas. The infarction area was determined by subtracting the total volume of the non-ischemic hemisphere from that of the ischemic hemisphere[31].
Statistical analysis
All data were shown as mean ± SD. The significance of difference between means was assessed by Student's t-test (single comparisons) or by analysis of variance and post hoc Scheffe's tests. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We would like to thank the Animal Center of Xuanwu Hospital of Capital Medical University, China for raising the rats.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China (The mechanism of the remote ischemia postconditioning and its time therapeutic window), No. 30870854; (The cerebral protection of remote ischemia postconditioning and its mechanism), No. 30770743; (The effect and its mechanism of EPO intravascular injection on the thrombolysis time window of tPA on cerebral infarction in rats), No. 81071058.
Conflicts of interest: None declared.
Ethical approval: Experiments were carried out in accordance with guidelines formulated by Institutional Animal Care and Use Committee of Capital Medical University, China.
(Edited by Li TF, Yin SM/Qiu Y/Song LP)
REFERENCES
- [1].Schaller B, Graf R. Cerebral ischemic preconditioning. An experimental phenomenon or a clinical important entity of stroke prevention? J Neurol. 2002;249(11):1503–1511. doi: 10.1007/s00415-002-0933-8. [DOI] [PubMed] [Google Scholar]
- [2].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [3].Steiger HJ, Hänggi D. Ischaemic preconditioning of the brain, mechanisms and applications. Acta Neurochir (Wien) 2007;149(1):1–10. doi: 10.1007/s00701-006-1057-1. [DOI] [PubMed] [Google Scholar]
- [4].Liu XQ, Sheng R, Qin ZH. The neuroprotective mechanism of brain ischemic preconditioning. Acta Pharmacol Sin. 2009;30(8):1071–1080. doi: 10.1038/aps.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- [6].Zhou W, Zeng D, Chen R, et al. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010;31(1):22–29. doi: 10.1007/s00246-009-9536-9. [DOI] [PubMed] [Google Scholar]
- [7].Ren C, Gao X, Steinberg GK, et al. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151(4):1099–1103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96(5):1641–1646. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- [9].Hoole SP, Heck PM, Sharples L, et al. Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119(6):820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- [10].Baxter GF, Goma FM, Yellon DM. Characterisation of the infarct-limiting effect of delayed preconditioning: timecourse and dose-dependency studies in rabbit myocardium. Basic Res Cardiol. 1997;92(3):159–167. doi: 10.1007/BF00788633. [DOI] [PubMed] [Google Scholar]
- [11].Bolli R, Li QH, Tang XL, et al. The late phase of preconditioning and its natural clinical application&gene therapy. Heart Fail Rev. 2007;12(3-4):189–199. doi: 10.1007/s10741-007-9031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, et al. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46(3):450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- [13].Loukogeorgakis SP, Williams R, Panagiotidou AT, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation. 2007;116(12):1386–1395. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- [14].Liem DA, Verdouw PD, Ploeg H, et al. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283(1):H29–37. doi: 10.1152/ajpheart.01031.2001. [DOI] [PubMed] [Google Scholar]
- [15].Diwan V, Kant R, Jaggi AS, et al. Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem. 2008;315(1-2):195–201. doi: 10.1007/s11010-008-9808-3. [DOI] [PubMed] [Google Scholar]
- [16].Kristiansen SB, Henning O, Kharbanda RK, et al. Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol. 2005;288(3):H1252–1256. doi: 10.1152/ajpheart.00207.2004. [DOI] [PubMed] [Google Scholar]
- [17].Chen XG, Wu BY, Wang JK, et al. Mechanism of the protective effects of noninvasive limbs preconditioning on myocardial ischemia-reperfusion injury. Chin Med J (Engl) 2005;118(20):1723–1727. [PubMed] [Google Scholar]
- [18].Weinbrenner C, Schulze F, Sárváry L, et al. Remote preconditioning by infrarenal aortic occlusion is operative via deltal-opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res. 2004;61(3):591–599. doi: 10.1016/j.cardiores.2003.10.008. [DOI] [PubMed] [Google Scholar]
- [19].Patel HH, Moore J, Hsu AK, et al. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol. 2002;34(10):1317–1323. doi: 10.1006/jmcc.2002.2072. [DOI] [PubMed] [Google Scholar]
- [20].Lang SC, Elsässer A, Scheler C, et al. Myocardial preconditioning and remote renal preconditioning&identifying a protective factor using proteomic methods? Basic Res Cardiol. 2006;101(2):149–158. doi: 10.1007/s00395-005-0565-0. [DOI] [PubMed] [Google Scholar]
- [21].Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- [22].Shimizu M, Tropak M, Diaz RJ, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117(5):191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- [23].Konstantinov IE, Arab S, Kharbanda RK, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19(1):143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- [24].Shimizu M, Saxena P, Konstantinov IE, et al. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res. 2010;158(1):155–161. doi: 10.1016/j.jss.2008.08.010. [DOI] [PubMed] [Google Scholar]
- [25].Konorova IL, Veiko NN, Novikov VE. Influence of plasma DNA on acid-base balance, blood gas measurement, and oxygen transport in health and stroke. Ann N Y Acad Sci. 2008;1137:278–282. doi: 10.1196/annals.1448.040. [DOI] [PubMed] [Google Scholar]
- [26].Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- [27].De Ryck M, Van Reempts J, Borgers M, et al. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20(10):1383–1390. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- [28].Belayev L, Alonso OF, Busto R, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616–1623. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- [29].Ren C, Gao X, Niu G, et al. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS One. 2008;3(12):e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008;86(11):2505–2511. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- [31].Ren C, Gao M, Dornbos D, 3rd, et al. Remote ischemic post-conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol Res. 2011;33(5):514–519. doi: 10.1179/016164111X13007856084241. [DOI] [PubMed] [Google Scholar]


