Abstract
Neurofilament protein is a component of the mature neuronal cytoskeleton, and it interacts with the zygosome, which is mediated by neurofilament-related proteins. Neurofilament protein regulates enzyme function and the structure of linker proteins. In addition, neurofilament gene expression plays an important role in nervous system development. Previous studies have shown that neurofilament gene transcriptional regulation is crucial for neurofilament protein expression, especially in axonal regeneration and degenerative diseases. Post-transcriptional regulation increased neurofilament protein gene transcription during axonal regeneration, ultimately resulting in a pattern of neurofilament protein expression. An expression imbalance of post-transcriptional regulatory proteins and other disorders could lead to amyotrophic lateral sclerosis or other neurodegenerative diseases. These findings indicated that after transcription, neurofilament protein regulated expression of related proteins and promoted regeneration of damaged axons, suggesting that regulation disorders could lead to neurodegenerative diseases.
Keywords: axonal regeneration, nerve injury, neurodegenerative diseases, neurofilament protein, post-transcriptional regulation, reviews
Abbreviations:
NF, neurofilament; NF-L, light neurofilament; NF-M, midsized neurofilament; NF-H, heavy neurofilament; hnRNA, heterogeneous nuclear RNA
INTRODUCTION
In the 1830s, the structure of neurofilament (NF) protein was first reported[1], but the shape and accurate positioning in neurons was further confirmed with the development of electron microscopy. In recent years, NF structure, expression, modification, protein interactions, and other biological characteristics, as well as post-transcriptional gene regulation, have become increasingly important in the fields of neurodegenerative diseases and neural regeneration. NF expression is closely associated with axonal growth and maintenance of neuronal homeostasis. Current evidence indicates that NF plays a key role in axonal regeneration and is dependent on post-transcriptional mRNA transport, translation, and stability[2]. Recent studies have demonstrated that post-transcriptional regulation increases NF gene transcription during axonal regeneration, ultimately resulting in NF protein expression patterns[3]. However, disordered expression of post-transcriptional regulatory proteins and other variant proteins can lead to amyotrophic lateral sclerosis[4]. Recent studies have shown that NF protein expression is closely associated with continued growth of normal axons and the regeneration of impaired axons. These NF functions contribute to the transcription process, as well as the regulation of mRNA transport, translation, and stability.
Post-transcriptional regulation plays an important role in maintaining stability of adult neurons, and regulation disorders may be the cause of some neurodegenerative diseases[5]. These previous studies proposed explanations regarding how neurons coordinate with function-related proteins to promote axonal regeneration, revealing that regulation disorders can lead to neurodegenerative diseases. This paper summarizes current knowledge on NF biology, including NF structure, expression, modification, protein interactions, and post-transcriptional gene regulation related to neural regeneration and neurodegenerative diseases.
NF STRUCTURE, EXPRESSION, MODIFICATION, AND INTERACTIONS WITH PROTEIN
NF structure
The neuronal cytoskeleton is composed of actin filament protein (16 nm diameter), tubulin (6 nm diameter), and intermediate filament protein (10 nm diameter)[6]. Intermediate filaments are unique, because they are made of different proteins in different tissues. NFs are abundant in neurons, especially in large-diameter axons[7]. In vitro studies have shown that axonal NFs are a parallel array arranged between NFs and microtubules, or membranous organelles, 10 nm long[8]. The light neurofilament (NF-L) subunit itself is a 10-nm core filament[9]. Although these three types of protein structures have been previously described, midsized neurofilament (NF-M) and heavy neurofilament (NF-H) have a long, highly variable carboxyl-terminal tail (NF-M 439 amino acids, NF-H 660 amino acids), which contains several specific protein phosphorylation targets. There are three kinds of cytoskeleton, namely intermediate filaments, microtubules, and microfilaments, and each have unique structure and assembly properties[6].
NFs are the main component of the cytoskeleton in mature neurons, as well as the intermediate filaments of neurons. NFs are abundant at myelinated axons within cytoskeleton polymers in the vertebrate[10]. The estimated molecular weights of human NF-L, NF-M, and NF-H subunits in DNA sequences are 61.5, 102.5 and 112.5 kDa, respectively. However, the presence of a high content of negatively charged amino acids (glutamic acid), as well as wide post-translational modification (phosphorylation and glycosylation), allows for higher molecular weight in sodium dodecyl sulfate polyacrylamide gel electrophoresis. NF-M or NF-H alone cannot synthesize fibroin if NF-L is not involved. NF-L transfection alone in human cells is able to synthesize the homopolymer in vitro, but such an efficacy is not available in mice and other rodents. In rodents, NFs synthesize heteropolymers according to a 4:2:1 ratio of NF-L: NF-M: NF-H subunits[11], and this ratio is continuously altered during neuronal formation. Currently, it is widely held that these proteins are integral components of NFs, along with NFL, NFM and NFH. In addition to NF-L, NF-M, and NF-H, Yuan et al[12] showed that α-tubulin in the central nervous system acts as a fourth type of NF subunit. Different NF subunits form intermediate filament proteins; although nucleotide binding or hydrolysis is not required, the formation depends on ionic strength, pH values, and temperature[13]. The first step for NF formation consists of NF-L and NF-H forming a parallel coiled dimer with a relatively stable bar area; this coiled dimer produces an anti-parallel tetramer in a half-staggered manner[14]. Accordingly, the tetramer is incorporated into the protofilament and subsequently assembles 10-nm filaments. In vitro studies have demonstrated that the interaction between NFs results in the deformation of a visco-elastic network, which highlights the significance of microfilaments on NF mechanical properties[13,15]. Kreplak et al[16] verified the NF mechanical properties using a mechanical microscope, showing that NFs are extended over 3 folds, with an average of 2.6 folds, indicating that NF functions to absorb mechanical shock in vivo.
NF expression
NF-L and NF-M genes are closely linked with chromosome 8 in humans, while the NF-H gene is located on chromosome 22[17]. In mice, NF-L and NF-M genes are located on chromosome 14, while the NF-H gene is located on chromosome 11[18]. Expression of the three NF subunits is regulated by nervous system development and ongoing neuronal differentiation. Expression levels modulate location, stability, and conversion efficiency at both the transcriptional level and mRNA post-transcriptional level[19]. To ensure axonal growth, NF-L, together with intein and peripherin, is the first expressed subunit, followed by NF-M expression[20]. NF protein mRNA sequential expression occurs in the order of NF-L, NF-M, and NF-H[21]. In the peripheral nervous system and central nervous system, NF expression is significantly down-regulated at the early period following axonal transection[22], leading to reduced NF transport at injured neurons. In contrast, NG subunit expression is significantly increased during the regeneration of damaged neurons[23]. However, this increase does not occur when regeneration is prevented or in mammalian central nervous system axons that normally do not regenerate[24].
NF translational modification
NF modification includes phosphorylation and glycosylation, which contributes to NF morphology and function.
Phosphorylation
NF phosphorylation regulates NF axonal transport in neurons, plays a significant role in NF translocation, morphology, and function, and is involved in the pathogenesis of some neurodegenerative diseases[25]. NF protein phosphorylation most widely occurs in the area from 51 site to NF-H C-terminal region[26].
Phosphorylation is dense in the axonal head-domain, but scarce in the cell body and dendrites. Phosphorylation mainly occurs in the N-terminal and carboxyl terminal of three subunits, which are second messenger-dependent kinase and non-dependent kinase targets[27].
Head-domain phosphorylation occurs soon after NF synthesis in cells, while tail-domain phosphorylation begins when it transfers to axons[28].
NF phosphorylation helps to maintain a dynamic balance between kinases and phosphatases in different nerve segments. Because head-domain phosphorylation inhibits NF assembly, dephosphorylation is necessary prior to NF transport into the axons[29]. A wide range of enzyme dephosphorylation events result in a gradual loss of NF functions, a loss of interconnecting in vitro into a reticulated network, measured by the formation of highly viscous gels in purified preparations of NFs[30].
Finally, dephosphorylation of the NF termination contributes to degradation and regulates their interactions with other cytoskeletal proteins[31]. NF subunit dephosphorylation is catalytically achieved via phosphatase 2A and is assisted by phosphatase 1[32]. Lobsiger et al[33] studied NF-H and NF-M phosphorylation in gene knockout mice, demonstrating that amyotrophic lateral sclerosis is delayed compared to normal animal models with identical total NF levels, suggesting that abnormal NF phosphorylation induces amyotrophic lateral sclerosis.
Glycosylation
NFs are modified by O-linked N-acetyl glucosamine or by post-translational serine and acetyl glucosamine residues, which regulate protein stability, subcellular localization, and protein-protein interactions[34]. Similar to phosphorylation, O-glycosylation is a dynamic process that alternates with phosphorylation. Glycosylated NF is present around peripheral nerves in diabetic patients, which may be related to peripheral neuropathy[35]. Post-translational modified NFs may be significant in family and single amyotrophic lateral sclerosis[36].
NF interactions with proteins and organelles
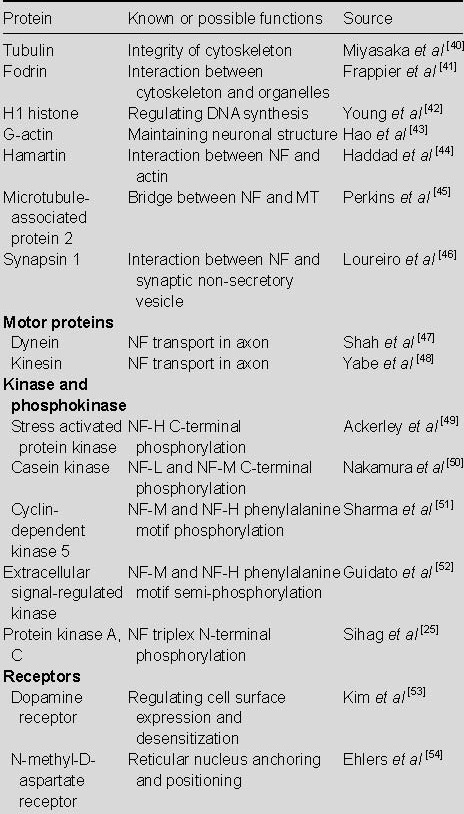
As a complex, dynamic network, the interactions between NF and its zygosome are mediated by NF-associated proteins, which regulate enzyme function and NF linker protein structure. Linker proteins are responsible for the interaction between actin and cell organelles, while kinase and phosphatase are involved in regulating NF structure, assembly, and spacing. According to Tashiro et al[37], studies of the rat sciatic nerve showed that phosphorylated NF significantly stabilizes microtubules within axons, thereby promoting axonal regeneration. Other results have shown that dynein and kinesin in molecular motors together with C-terminal NF phosphorylation, promote NF transport along axons and dendrites[38]. Using a yeast double-hybrid and affinity chromatography model, dynein intermediate chain binding NF-M was shown to bilaterally participate in NF transport in axons[39]. This study summarized and analyzed well-known or likely proteins, enzymes, and receptors that interact with NF (Table 1).
Table 1.
Protein interactions with neurofilament (NF)
NF GENE EXPRESSION, HIERARCHICAL CONTROL, AND POST-TRANSCRIPTIONAL REGULATION
NF gene expression plays an important role in nervous system development, as well as repair of damaged nervous system and neurodegenerative diseases[55]. Results have suggested that the regulation of NF gene transcription is crucial for the control of NF expression, especially in neural regeneration and neurodegenerative diseases[56].
Effect of altered NF expression on axon growth, regeneration, and neurodegenerative diseases
NF subunit expression is continuously changing in the developing vertebrate, indicating that expression correlates with nervous system regulation. As a protein expressed in the peripheral nerve system, NF-L maintains a high level in axons[57]. In the central nervous system, type III intermediate filament protein and vimentin expressions disappear in newly differentiated neurons, which is similar to the role of peripheral proteins[8]. Unexplained type III intermediate filament protein expression is conducive to axonal growth, and the majority of organisms survive due to wide axonal regeneration following severe nerve injury. The appropriate regulation of NF expression delays neural aging, and immunoblotting assays have shown that neuronal growth in aged rats is less than in young rats, because NF-L expression decreases in distal axons, while NF-M and NF-H mRNA expression is also selectively reduced[58]. Therefore, NF dysfunction leads to neurodegenerative diseases.
Hierarchical control of NF protein expression
Regulation of gene expression was originally found to occur at the transcriptional level. Clearly, the transcriptional controlling effect is effective for NF expression and specificity of neuronal growth[59]. Gene expression includes transcriptional and post-transcriptional control, and the latter is of equal significance in NF expression. Post-transcriptional regulation of gene expression makes protein synthesis more effective and can coordinate related proteins. With a better understanding of eukaryotic gene expression transcription and post-transcriptional regulatory mechanisms, current studies focus on regulation points of gene expression (Figure 1).
Figure 1.
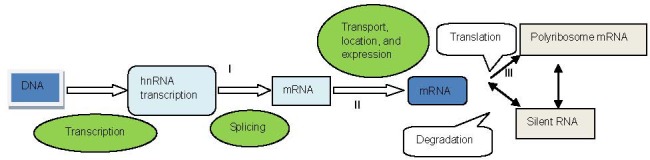
Coding gene chromosomal DNA was transcribed into heterogeneous nuclear RNA (hnRNA), thus producing messenger RNA (mRNA).
This nuclear mRNA forms complexes with ribonucleoproteins, some of which escort the mRNA into the cytosol. In the cytosol, mRNAs can join additional mRNAs and ribonucleoproteins to form transport granules, where they await signals that initiate translation. mRNA translation results in polymers containing several ribosomes. I, II, III: Modification points.
Translated mRNA in axons containing ribosomes is restricted to a specific segment of the axon. Willis et al[60] discovered limitations of NF mRNA within growth cone axons. Similarly, post-transcriptional regulation of NF subtype expression was observed to be significant, and NF-L protein expression was significantly increased compared to NF-H in gene dosage tolerance in transgenic mice, which directly relates to post-transcriptional inhibition of NF-L mRNA expression[61].
Transcription and post-transcriptional interaction controls NF axonal regeneration in the central nervous system
A number of studies on NF expression in Xenopus optic nerve regeneration have provided clear evidence for the role of transcriptional and post-transcriptional control on NF expression[62]. In these studies, heterogeneous nuclear RNA (hnRNA)-related mRNA changes were used as alternative measurements of gene transfer following optic nerve damage. hnRNA is an intermediate during transcription; it exists transiently and can be determined by introns that lack mature mRNA. Polysomal artherectomy serves as an analysis indicator of NF messenger RNA translation efficiency. Using introns and exons as probes, quantitative analysis of reverse transcription-polymerase chain reaction showed that NF-M transcription and linker protein subunit expression are similar to the normal side following optic nerve injury. In addition, NF mRNA expression is decreased in the surgical side of the optic nerve, although transcription was blocked during the initial regeneration phase. Even at the peak of axonal regeneration, hnRNA levels remain several times greater than mRNA levels[63]. During regeneration, NF information transcription efficacy fluctuates, with a greater amount of NF mRNA in polyribosomes at the operated side of the optic nerve[64]. Motor neurons from the normal side (no injury) mediate the injured side via the spinal cord, and similar signal transductions occur following retinal damage. Multi-path synaptic pathways are transferred from both eyes to the thalamus and visual nerve center. A small amount of NF-M mRNA is transformed to polyribosomes in the eyes and brain of the non-operated side, whereas about 85% of NF-M mRNA transform into polyribosomes in eyes from the surgical side when neural regeneration peaks, indicating that NF mRNA protein aggregation requires more NF-M structural proteins for neural regeneration[65].
Increased NF-M transcription on the normal and operated sides of the optic nerve reflects both optic nerve pathways in response to the initial fracture. For example, following peripheral nerve transection, instantaneous peripheral nervous system regeneration occurs in contralateral non-damaged neurons in rodent models. These neurons germinate towards the injury side, which is mediated by the spinal cord[66]. Similar signal transduction is also observed in the damaged retina, because signals are transferred from the thalamus and visual center to the eyes via a multi-synaptic pathway. Another possibility is that signals are mediated by body fluids, although the differences between these two mechanism require further studies[67].
Results have shown that NF expression regulates several genes that are involved in the repair of central nervous system injury[68], which suggests that post-transcriptional regulation meet requirements for NF subtypes in axonal regeneration, thereby promoting neural regeneration. Another study showed that NF post-transcriptional gene regulation mechanism is activated to promote repair of damaged nerves in the adult central nervous system, but also raises the question that inappropriate signal activation under the same mechanisms could be related to disease occurrence[59].
NF post-transcriptional regulation in neurodegenerative diseases
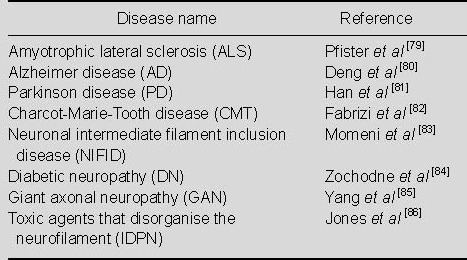
NF-containing polymers exhibit unique characteristics in various neurodegenerative diseases. Amyotrophic lateral sclerosis is closely related to out-of-control post-transcriptional regulation of NF mRNA. Using in situ hybridization and reverse transcription-polymerase chain reaction, NF-L mRNA has been shown to be selectively inhibited at the steady state in degenerated spinal cord motor neurons in amyotrophic lateral sclerosis, although NF-M and NF-H are not suppressed[69]. In contrast, although spinal cord motor neurons are a secondary factor for total RNA content in ventral spinal cord tissue, NF-L levels and peripheral protein in ventral spinal cord tissue are increased compared to mRNA from non-affected tissue[70]. However, the exact causes remain to be determined. Thermal denaturation or unaffected spinal cord homogenate treatment in patients with amyotrophic lateral sclerosis results in increased NF-L mRNA stability; therefore, it is also possible that unstable NF-L mRNA differential expression in amyotrophic lateral sclerosis exhibits the opposite effects[71]. The turnover of NF-L mRNAs regulated by a RNA-binding complex, a 43-kDa DNA-binding protein trans-acting region[72], 14-3-3 protein[73], as well as original or mutant soluble superoxide dismutase[74]. Various proteins in amyotrophic lateral sclerosis are transformed into polymers, and further implicating RNA binding proteins in human disease. In addition, NFL subunit content change in NF-L knockout mice is related to polymers within neurons, as well as proliferation of astrocytes and microglia activation, which are characteristic signs of amyotrophic lateral sclerosis[75]. Surprisingly, overexpression of the NF-L 3'-untranslated region leads to nerve degeneration[76], which suggests a complexity of NF-L mRNA regulation in the human and murine central nervous system. Ludemann et al[77], studying spinal cord tissues from a transgenic rat model of amyotrophic lateral sclerosis, found that dynamic alteration of NF-M over-phosphorylation and glycosylation in neurons leads to amyotrophic lateral sclerosis. Another study found significantly increased levels of NF-L and NF-H in Parkinson's disease with multiple system atrophy and various protein labeling[78]. Recent studies addressing NF-related neurodegenerative diseases are shown in Table 2.
Table 2.
Neurofilament-related neurodegenerative diseases
CONCLUSION
NF protein expression is closely linked with continuous growth of normal axons and impaired axonal regeneration. NF protein post-transcriptionally regulated changes in mRNA transport, translation and stability are now thought to work together with transcription to achieve the final expression pattern. Post-transcriptional regulation plays an important role in the maintenance of adult neuronal stability, and this regulation loss may result in neurodegenerative disease. NF post-transcriptional regulation is similar to axonal growth-related proteins, suggesting that post-transcriptional regulatory factors could act on critical axon proteins. In recent years, the use of transgenic mouse models allows researchers to determine the role of NF in axonal caliber during nerve growth. NF post-transcriptional gene regulation plays a role in axonal regeneration and neurodegenerative diseases.
Footnotes
Funding: The present study was supported by the National Natural Science Foundation of China, No. 30872609.
Conflicts of interest: None declared.
(Edited by Hu WX, Bai J/Yang Y/Song LP)
REFERENCES
- [1].Valentin G. Breslau: Gedruckt bei Grass, Barth und Comp; 1836. Über den Verlauf und die letzten Enden der Nerven. [Google Scholar]
- [2].Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- [3].Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xiao S, McLean J, Robertson J. Neuronal intermediate filaments and ALS: a new look at an old question. Biochim Biophys Acta. 2006;1762:1001–1012. doi: 10.1016/j.bbadis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [5].Liu Y, Gervasi C, Szaro BG. A crucial role for hnRNP K in axon development in Xenopus laevis. Development. 2008;135:3125–3135. doi: 10.1242/dev.022236. [DOI] [PubMed] [Google Scholar]
- [6].Coulombe PA, Bousquet O, Ma L, et al. The ‘ins’ and ‘outs’ of intermediate filament organization. Trends Cell Biol. 2000;10:420–428. doi: 10.1016/s0962-8924(00)01828-6. [DOI] [PubMed] [Google Scholar]
- [7].Goldman RD, Grin B, Mendez MG, et al. Intermediate filaments: versatile building blocks of cell structure. Curr Opin Cell Biol. 2008;20:28–34. doi: 10.1016/j.ceb.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Helfand BT, Mendez MG, Pugh J, et al. A role for intermediate filaments in determining and maintaining the shape of nerve cells. Mol Biol Cell. 2003;14(12):5069–5081. doi: 10.1091/mbc.E03-06-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hisanaga S, Hirokawa N. Structure of the peripheral domains of neurofilaments revealed by low angle rotary shadowing. Mol Biol. 1988;202(2):297–305. doi: 10.1016/0022-2836(88)90459-7. [DOI] [PubMed] [Google Scholar]
- [10].Perrot R, Berges R, Bocquet A, et al. Review of the multiple aspects of neurofilament functions and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38:27–65. doi: 10.1007/s12035-008-8033-0. [DOI] [PubMed] [Google Scholar]
- [11].Carter J, Gragerov A, Konvicka K, et al. Neurofilament (NF) assembly; divergent characteristics of human and rodent NF-L subunits. J Biol Chem. 1998;273:5101–5108. doi: 10.1074/jbc.273.9.5101. [DOI] [PubMed] [Google Scholar]
- [12].Yuan A, Rao MV, Sasaki T, et al. Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci. 2006;26(39):10006–10019. doi: 10.1523/JNEUROSCI.2580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rammensee S, Janmey PA, Bausch AR. Mechanical and structural properties of in vitro neurofilament hydrogels. Eur Biophys J. 2007;36:661–668. doi: 10.1007/s00249-007-0141-7. [DOI] [PubMed] [Google Scholar]
- [14].Heins S, Wong PC, Muller S, et al. The rod domain of NF-L determines neurofilament architecture, whereas the end domains specify filament assembly and network formation. J Cell Biol. 1993;123:1517–1533. doi: 10.1083/jcb.123.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Leterrier JF, Käs J, Hartwig J, et al. Mechanical effects of neurofilament cross-bridges. Modulation by phosphorylation, lipids, and interactions with F-actin. J Biol Chem. 1996;271:15687–15694. doi: 10.1074/jbc.271.26.15687. [DOI] [PubMed] [Google Scholar]
- [16].Kreplak L, Bar H, Leterrier JF, et al. Exploring the mechanical behavior of single intermediate filaments. J Mol Biol. 2005;354:569–577. doi: 10.1016/j.jmb.2005.09.092. [DOI] [PubMed] [Google Scholar]
- [17].Chang R, Kwak Y, Gebremichael Y. Structural properties of neurofilament sidearms: sequence-based modeling of neurofilament architecture. J Mol Biol. 2009;391:648–660. doi: 10.1016/j.jmb.2009.06.045. [DOI] [PubMed] [Google Scholar]
- [18].Lees JF, Shneidman PS, Skuntz SF, et al. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988;7:1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thyagarajan A, Strong MJ, Szaro BG. Post-transcriptional control of neurofilaments in development and disease. Exp Cell Res. 2007;313:2088–2097. doi: 10.1016/j.yexcr.2007.02.014. [DOI] [PubMed] [Google Scholar]
- [20].Zhu Y, Lee HC, Zhang L. An examination of heme action in gene expression: heme and heme deficiency affect the expression of diverse genes in erythroid k562 and neuronal PC12 cells. DNA Cell Biol. 2002;21:333–346. doi: 10.1089/104454902753759744. [DOI] [PubMed] [Google Scholar]
- [21].Huang F, Dong X, Zhang L, et al. GM1 and nerve growth factor modulate mitochondrial membrane potential and neurofilament light mRNA expression in cultured dorsal root ganglion and spinal cord neurons during excitotoxic glutamate exposure. J Clin Neurosci. 2010;17:495–500. doi: 10.1016/j.jocn.2009.07.112. [DOI] [PubMed] [Google Scholar]
- [22].Hoffman PN, Pollock SC, Striph GG. Altered gene expression after optic nerve transection: reduced neurofilament expression as a general response to axonal injury. Exp Neurol. 1993;119:32–36. doi: 10.1006/exnr.1993.1004. [DOI] [PubMed] [Google Scholar]
- [23].Cañete-Soler R, Wu J, Zhai J, et al. p190RhoGEF Binds to a destabilizing element in the 3’ untranslated region of light neurofilament subunit mRNA and alters the stability of the transcript. J Biol Chem. 2001;276:32046–32050. doi: 10.1074/jbc.M104104200. [DOI] [PubMed] [Google Scholar]
- [24].Shokouhi BN, Wong BZ, Siddiqui S, et al. Microglial responses around intrinsic CNS neurons are correlated with axonal regeneration. BMC Neurosci. 2010;11:13. doi: 10.1186/1471-2202-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sihag RK, Inagaki M, Yamaguchi T, et al. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res. 2007;313:2098–2109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lee S, Sunil N, Shea TB. C-terminal neurofilament phosphorylation fosters neurofilament-neurofilament associations that compete with axonal transport. Cytosleleton. 2011;68:8–17. doi: 10.1002/cm.20488. [DOI] [PubMed] [Google Scholar]
- [27].Stevenson W, Chang R, Gebremichael Y. Phosphorylation-mediated conformational changes in the mouse neurofilament architecture: insight from a neurofilament brush model. Mol Biol. 2011;405:1101–1118. doi: 10.1016/j.jmb.2010.11.022. [DOI] [PubMed] [Google Scholar]
- [28].Nixon RA, Lewis SE. Differential turnover of phosphate groups on neurofilament subunits in mammalian neurons in vivo. J Biol Chem. 1986;261:16298–16301. [PubMed] [Google Scholar]
- [29].Sihag RK, Jaffe H, Nixon RA, et al. Serine-23 is a major protein kinase A phosphorylation site on the aminoterminal head domain of the middle molecular mass subunit of neurofilament proteins. J Neurochem. 1999;72:491–499. doi: 10.1046/j.1471-4159.1999.0720491.x. [DOI] [PubMed] [Google Scholar]
- [30].Wagner OI, Rammensee S, Korde N, et al. Softness, strength and self-repair in intermediate filament networks. Exp Cell Res. 2007;313:2228–2235. doi: 10.1016/j.yexcr.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kushkuley J, Metkar S, Chan WK, et al. Aluminum induces neurofilament aggregation by stabilizing cross-bridging of phosphorylated c-terminal sidearms. Brain Res. 2010;1322:118–123. doi: 10.1016/j.brainres.2010.01.075. [DOI] [PubMed] [Google Scholar]
- [32].Strack S, Westphal RS, Colbran RJ, et al. Protein serine/threonine phosphatase 1 and 2A associate with and dephosphorylate neurofilaments. Brain Res Mol Brain Res. 1997;49:15–28. doi: 10.1016/s0169-328x(97)00117-4. [DOI] [PubMed] [Google Scholar]
- [33].Lobsiger CS, Garcia ML, Ward CM, et al. Altered axonal architecture by removal of the heavily phosphorylated neurofilament tail domains strongly slows superoxide dismutase 1 mutant-mediated ALS. Proc Natl Acad Sci U S A. 2005;102:10351–10356. doi: 10.1073/pnas.0503862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Slawson C, Hart GW. Dynamic interplay between OGIcNAc and O-phosphate: the sweet side of protein regulation. Curr Opin Struct Biol. 2003;13:631–636. doi: 10.1016/j.sbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- [35].Ryle C, Leow CK, Donaghy M. Nonenzymatic glycation of peripheral and central nervous system proteins in experimental diabetes mellitus. Muscle Nerve. 1997;20:577–584. doi: 10.1002/(sici)1097-4598(199705)20:5<577::aid-mus6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [36].Chou SM, Wang HS, Taniguchi A, et al. Advanced glycation end products in neurofilament conglomeration of motoneurons in familial and sporadic amyotrophic lateral sclerosis. Mol Med. 1998;4:324–332. [PMC free article] [PubMed] [Google Scholar]
- [37].Tashiro T, Imai R, Komiya Y. Early effects of beta, beta’-iminodipropionitrile on tubulin solubility and neurofilament phosphorylation in the axon. J Neurochem. 1994;63:291–300. doi: 10.1046/j.1471-4159.1994.63010291.x. [DOI] [PubMed] [Google Scholar]
- [38].Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- [39].Wagner OI, Ascano J, Tokito M, et al. The interaction of neurofilaments with the microtubule motor cytoplasmic dynein. Mol Biol Cell. 2004;15:5092–5100. doi: 10.1091/mbc.E04-05-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Miyasaka H, Okabe S, Ishiguro K, et al. Interaction of the tail domain of high molecular weight subunits of neurofilaments with the COOH-terminal region of tubulin and its regulation by tau protein kinase II. J Biol Chem. 1993;268:22695–22702. [PubMed] [Google Scholar]
- [41].Frappier T, Stetzkowski-Marden F, Pradel LA. Interaction domains of neurofilament light chain and brain spectrin. Biochem J. 1991;275:521–527. doi: 10.1042/bj2750521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Young KG, Kothary R. Dystonin/Bpag1--a link to what? Cell Motil Cytoskeleton. 2007;64:897–905. doi: 10.1002/cm.20235. [DOI] [PubMed] [Google Scholar]
- [43].Hao R, MacDonald RG, Ebadi M, et al. Stable interaction between G-actin and neurofilament light subunit in dopaminergic neurons. Neurochem Int. 1997;31:825–834. doi: 10.1016/s0197-0186(97)00027-2. [DOI] [PubMed] [Google Scholar]
- [44].Haddad LA, Smith N, Bowser M, et al. The TSC1 tumor suppressor hamartin interacts with neurofilament-L and possibly functions as a novel integrator of the neuronal cytoskeleton. J Biol Chem. 2002;277:44180–44186. doi: 10.1074/jbc.M207211200. [DOI] [PubMed] [Google Scholar]
- [45].Perkins GA, Sosinsky GE, Ghassemzadeh S, et al. Electron tomographic analysis of cytoskeletal cross-bridges in the paranodal region of the node of Ranvier in peripheral nerves. J Struct Biol. 2008;161:469–480. doi: 10.1016/j.jsb.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Loureiro SO, Heimfarth L, Pelaez Pde L, et al. Hyperhomocysteinemia selectively alters expression and stoichiometry of intermediate filament and induces glutamate- and calcium-mediated mechanisms in rat brain during development. Int J Dev Neurosci. 2010;28:21–30. doi: 10.1016/j.ijdevneu.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [47].Shah JV, Flanagan LA, Janmey PA, et al. Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol Biol Cell. 2000;11:3495–3508. doi: 10.1091/mbc.11.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yabe JT, Jung C, Chan WK, et al. Phospho-dependent association of neurofilament proteins with kinesin in situ. Cell Motil Cytoskeleton. 2000;45:249–262. doi: 10.1002/(SICI)1097-0169(200004)45:4<249::AID-CM1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [49].Ackerley S, Grierson AJ, Banner S, et al. p38alpha stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofilament pathology in amyotrophic lateral sclerosis. Mol Cell Neurosci. 2004;26:354–364. doi: 10.1016/j.mcn.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [50].Nakamura Y, Hashimoto R, Kashiwagi Y, et al. Casein kinase II is responsible for phosphorylation of NF-L at Ser-473. FEBS Lett. 1999;455:83–86. doi: 10.1016/s0014-5793(99)00832-7. [DOI] [PubMed] [Google Scholar]
- [51].Sharma M, Sharma P, Pant HC. CDK-5-mediated neurofilament phosphorylation in SHSY5Y human neuroblastoma cells. J Neurochem. 1999;73:79–86. doi: 10.1046/j.1471-4159.1999.0730079.x. [DOI] [PubMed] [Google Scholar]
- [52].Guidato S, Tsai LH, Woodgett J, et al. Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cyclin-dependent kinase-5. J Neurochem. 1996;66:1698–1706. doi: 10.1046/j.1471-4159.1996.66041698.x. [DOI] [PubMed] [Google Scholar]
- [53].Kim OJ, Ariano MA, Lazzarini RA, et al. Neurofilament-Minteracts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci. 2002;22:5920–5930. doi: 10.1523/JNEUROSCI.22-14-05920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ehlers MD, Fung ET, O’Brien RJ, et al. Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J Neurosci. 1998;18:720–730. doi: 10.1523/JNEUROSCI.18-02-00720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dubey M, Hoda S, Chan WK, et al. Reexpression of vimentin in differentiated neuroblastoma cells enhances elongation of axonal neurites. J Neurosci Res. 2004;78:245–249. doi: 10.1002/jnr.20146. [DOI] [PubMed] [Google Scholar]
- [56].Lariviere RC, Julien JP. Functions of intermediate filaments in neuronal development and disease. J Neurobiol. 2004;58:131–148. doi: 10.1002/neu.10270. [DOI] [PubMed] [Google Scholar]
- [57].Undamatla J, Szaro BG. Differential expression and localization of neuronal intermediate filament proteins within newly developing neurites in dissociated cultures of Xenopus laevis embryonic spinal cord. Cell Motil Cytoskeleton. 2001;49:16–32. doi: 10.1002/cm.1017. [DOI] [PubMed] [Google Scholar]
- [58].Uchida A, Tashiro T, Komiya Y, et al. Morphological and biochemical changes of neurofilaments in aged rat sciatic nerve axons. J Neurochem. 2004;88:735–745. doi: 10.1046/j.1471-4159.2003.02201.x. [DOI] [PubMed] [Google Scholar]
- [59].Perrot R, Eyer J. Neuronal intermediate filaments and neurodegenerative disorders. Brain Res Bull. 2009;80:282–295. doi: 10.1016/j.brainresbull.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [60].Willis DE, van Niekerk EA, Sasaki Y, et al. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Thyagarajan A, Szaro BG. Dynamic endogenous association of neurofilament mRNAs with K-homology domain ribonucleoproteins in developing cerebral cortex. Brain Res. 2008;1189:33–42. doi: 10.1016/j.brainres.2007.11.012. [DOI] [PubMed] [Google Scholar]
- [62].Ananthakrishnan L, Gervasi C, Szaro BG. Dynamic regulation of middle neurofilament (NF-M) RNA pools during optic nerve regeneration. Neuroscience. 2008;153:144–153. doi: 10.1016/j.neuroscience.2008.02.001. [DOI] [PubMed] [Google Scholar]
- [63].Ananthakrishnan L, Szaro BG. Transcriptional and translational dynamics of light neurofilament subunit RNAs during Xenopus laevis optic nerve regeneration. Brain Res. 2009;1250:27–40. doi: 10.1016/j.brainres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- [64].Vogelaar CF, Gervasi NM, Gumy LF, et al. Axonal mRNAs: characterisation and role in the growth and regeneration of dorsal root ganglion axons and growth cones. Mol Cell Neurosci. 2009;42:102–115. doi: 10.1016/j.mcn.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Volkening K, Leystra-Lantz C, Strong MJ. Human low molecular weight neurofilament (NFL) mRNA interacts with a predicted p190RhoGEF homologue (RGNEF) in humans. Amyotroph Lateral Scler. 2010;11:97–103. doi: 10.3109/17482960902995584. [DOI] [PubMed] [Google Scholar]
- [66].Rotshenker S, Tal M. The transneuronal induction of sprouting and synapse formation in intact mouse muscles. J Physiol. 1985;360:387–396. doi: 10.1113/jphysiol.1985.sp015623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Rotshenker S, Tal M. The transneuronal induction of sprouting and synapse formation in intact mouse muscles. J Physiol. 1985;360:387–396. doi: 10.1113/jphysiol.1985.sp015623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Moskowitz PF, Oblinger MM. Transcriptional and posttranscriptionalmechanisms regulating neurofilament and tubulingene expression during normal development of the rat brain. Mol. Brain Res. 1995;30:211–222. doi: 10.1016/0169-328x(95)00006-e. [DOI] [PubMed] [Google Scholar]
- [69].Menzies FM, Grierson AJ, Cookson MR, et al. Selective loss of neurofilament expression in Cu/Zn superoxide dismutase (SOD1) linked amyotrophic lateral sclerosis. J Neurochem. 2002;82:1118–1128. doi: 10.1046/j.1471-4159.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- [70].Strong MJ, Leystra-Lantz C, Ge WW. Intermediate filament steady-state mRNA levels in amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2004;316:317–322. doi: 10.1016/j.bbrc.2004.02.051. [DOI] [PubMed] [Google Scholar]
- [71].Ge WW, Leystra-Lantz C, Wen W, et al. Selective loss of trans-acting instability determinants of neurofilament mRNA in amyotrophic lateral sclerosis spinal cord. J Biol Chem. 2003;278:26558–26563. doi: 10.1074/jbc.M302886200. [DOI] [PubMed] [Google Scholar]
- [72].Strong MJ, Volkening K, Hammond R, et al. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol Cell Neurosci. 2007;35:320–327. doi: 10.1016/j.mcn.2007.03.007. [DOI] [PubMed] [Google Scholar]
- [73].Ge WW, Volkening K, Leystra-Lantz C, et al. 14-3-3 protein binds to the low molecular weight neurofilament (NFL) mRNA 30UTR. Mol Cell Neurosci. 2007;34:80–87. doi: 10.1016/j.mcn.2006.10.001. [DOI] [PubMed] [Google Scholar]
- [74].Ge WW, Wen W, Strong W, et al. Mutant copper/zinc superoxide dismutase binds to and destabilizes human low molecular weight neurofilament mRNA. J Biol Chem. 2005;280:118–124. doi: 10.1074/jbc.M405065200. [DOI] [PubMed] [Google Scholar]
- [75].McLean JR, Sanelli TR, Leystra-Lantz C, et al. Temporal profiles of neuronal degeneration, glial proliferation, and cell death in hNFL(+/+) and NFL(-/-) mice. Glia. 2005;52:59–69. doi: 10.1002/glia.20218. [DOI] [PubMed] [Google Scholar]
- [76].Nie Z, Wu J, Zhai J, et al. Untranslated element in neurofilament mRNA has neuropathic effect on motor neurons of transgenic mice. J Neurosci. 2002;22:7662–7670. doi: 10.1523/JNEUROSCI.22-17-07662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lüdemann N, Clement A, Hans VH, et al. O-glycosylation of the tail domain of neurofilament protein M in human neurons and in spinal cord tissue of a rat model of amyotrophic lateral sclerosis (ALS) J Biol Chem. 2005;280:31648–31658. doi: 10.1074/jbc.M504395200. [DOI] [PubMed] [Google Scholar]
- [78].Abdo WF, Bloem BR, Van Geel WJ, et al. CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson's disease. Neurobiol Aging. 2007;28:742–747. doi: 10.1016/j.neurobiolaging.2006.03.010. [DOI] [PubMed] [Google Scholar]
- [79].Pfister EL, Kennington L, Straubhaar J, et al. Five siRNAs targeting three SNPs may provide therapy for three-quarters of Huntington's disease patients. Curr Biol. 2009;19:774–778. doi: 10.1016/j.cub.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Deng Y, Li B, Liu F, et al. Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J. 2008;22:138–145. doi: 10.1096/fj.07-8309com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Han F, Bulman DE, Panisset M, et al. Neurofilament M gene in a French-Canadian population with Parkinson's disease. Can J Neurol Sci. 2005;32:68–70. doi: 10.1017/s0317167100016905. [DOI] [PubMed] [Google Scholar]
- [82].Fabrizi GM, Cavallaro T, Angiari C, et al. Charcot-Marie-Tooth disease type 2E, a disorder of the cytoskeleton. Brain. 2007;130:394–403. doi: 10.1093/brain/awl284. [DOI] [PubMed] [Google Scholar]
- [83].Momeni P, Cairns NJ, Perry RH, et al. Mutation analysis of patients with neuronal intermediate filament inclusion disease (NIFID) Neurobiol Aging. 2006;27:778.e1–778.e6. doi: 10.1016/j.neurobiolaging.2005.03.030. [DOI] [PubMed] [Google Scholar]
- [84].Zochodne DW, Sun HS, Cheng C, et al. Accelerated diabetic neuropathy in axons without neurofilaments. Brain. 2004;127:2193–2200. doi: 10.1093/brain/awh251. [DOI] [PubMed] [Google Scholar]
- [85].Yang Y, Allen E, Ding J, et al. Giant axonal neuropathy. Cell Mol Life Sci. 2007;64:601–609. doi: 10.1007/s00018-007-6396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jones LG, Prins J, Park S, et al. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. J Comp Neurol. 2008;506:1003–1017. doi: 10.1002/cne.21563. [DOI] [PubMed] [Google Scholar]


