Abstract
Lycium barbarum, a traditional Chinese anti-aging herb, has been shown to protect retinal ganglion cells (RGCs) in a rat chronic ocular hypertension (COH) model. Here, we investigated the expression of endothelin-1 (ET-1), a strong vasoconstrictor, and its receptors, ETA and ETB, in the COH model and assessed the effects of Lycium barbarum on the ET-1 axis. Elevated intraocular pressure (IOP) was induced in the right eye of SD rats using argon laser photocoagulation. (1) The expression of ET-1, ETA and ETB in normal and COH retinas was studied. (2) Some COH rats were fed daily with Lycium barbarum Polysaccharides (LBP) using 1 mg/kg or phosphate-buffered saline (PBS) for 3 weeks (started 1 week before photocoagulation). The effects of LBP on the expression of ET-1 and its receptors, ETA and ETB, in COH retina were evaluated. A semi-quantitative analysis of staining intensity was used to evaluate the expression levels of ET-1, ETA and ETB in retinal vasculature. We found that (1) Under COH condition, the immunoreactivity of ET-1 was increased in retina associated with an increase of ETB receptor immunoreactivity and a decrease of ETA receptor immunoreactivity. (2) After feeding COH rats with LBP, the expression of ET-1 was decreased with an increase of ETA expression and a decrease of ETB expression in the retina, especially in RGCs. (3) By comparing the staining intensity in the vasculature of COH retina in LBP-fed group with PBS-fed group, there was a decrease in the expression of ET-1 and ETA and an increase in ETB. In summary, ET-1 expression was up-regulated in the retina in COH model. LBP could decrease the expression of ET-1 and modulate the expression of its receptors, ETA and ETB, under the condition of COH. The neuroprotective effect of LBP on RGCs might be related to its ability to regulate the ET-1-mediated biological effects on RGCs and retinal vasculature.
Keywords: retinal ganglion cell, Lycium barbarum Polysaccharides, glaucoma, endothelin-1
Abbreviations:
COH, chronic ocular hypertension; LBP, Lycium barbarum Polysaccharides; ET-1, endothelin-1
INTRODUCTION
Glaucoma is a neurodegenerative disease of the retinal ganglion cells (RGCs) and optic nerve, the second leading cause of blindness worldwide[1]. Pathologically, glaucoma is characterized by death of RGCs and elevated intraocular pressure (IOP)[2]. Elevated IOP is an important factor contributing to primary open angle glaucoma (POAG)[3]. The elevation of IOP could induce many changes involved in the pathogenesis of glaucoma, e.g. oxidative stress, glutamate toxicity, as well as ischemia[4]. Many investigations hypothesize that glaucoma could be linked to an insufficient ocular blood supply to the eye, which is regulated to some extent by endothelin-1 (ET-1)[4,5,6,7,8]. Thus, it is believed that ET-1 plays an important role in the pathogenesis of glaucoma.
As a potent vasoconstrictor, ET-1 is released by blood vessel endothelial cells[9]. Clinical studies have found that there are increased levels of ET-1 in the plasma of both POAG and normal tension glaucoma (NTG) patients[10,11]. Moreover, ET-1 was considered to be the main factor to induce vascular dysfunction in glaucoma[12]. In ocular tissues, ET-1 could regulate blood flow by binding to its two receptors, ETA and ETB[3]. ETA contracts blood vessels; in contrast, ETB dilates blood vessels[3].
Astrocytes that express ETB undergo astrogliosis, which contributes to RGC death through retrograde axonal transport[13]. ET-1 could mediate the apoptosis of RGC-5 through ETB receptor by activating c-Jun N-terminal kinases (JNK1/2) signaling cascades in vitro[3,14]. Both the antagonists for ETA and ETB have been shown to be beneficial to glaucoma[14,15,16]. Delivering ET-1 could kill RGCs directly through inducing ischemia in the optic nerve head[17]. More recently, it was reported that molecular clustering study identifies induction of endothelin as an early event in a mouse model of glaucoma[18]. Thus, investigation on the endothelin system is important for understanding the mechanism and treatment of glaucoma.
Lycium barbarum (Wolfberry) has been used as a traditional anti-aging herb in Chinese pharmacopoeia for a long history[19,20]. Lycium barbarum Polysaccharides (LBP), the main effective ingredient of Lycium barbarum, has been reported to exhibit cytoprotective effects on neurons in the eye and brain as well as on hepatocytes in our previous studies[21,22,23,24,25,26,27,28,29,30,31] and could decrease the leakage of blood vessels in a retinal ischemic model[24]. LBP significantly decreases RGC death, but does not lower the elevated IOP in laser-induced chronic ocular hypertension (COH) model of rats[21]. The possible mechanisms include modulating retinal microglia[22] and up-regulating the expression of crystallins in RGCs[23]. A previous study has shown that LBP dilates blood vessels, enhancing blood flow in renal hypertensive rats. Thus, we hypothesize that neuroprotective effects of LBP may be related to regulating the endothelin system. This study aimed to investigate the role ET-1 and its receptors in the degeneration of RGCs in COH rats, and whether LBP could affect the expression of ET-1, ETA and ETB on RGCs and retinal blood vessels. This study is the first to investigate the relationship between LBP and ET-1 system in the pathogenesis of glaucoma and also provides further information on how LBP exerts its neuroprotective effects.
RESULTS
The expression of ET-1 and its receptors in the normal rat retina
To understand the role of ET-1 and its receptors, ETA and ETB, the location of their expression pattern were firstly detected in normal rat retinas. ET-1 immunoreactivity was diffusely presented in the whole retina, such as ganglion cell layer (GCL), retinal pigment cells (RPEs), neurons in the inner nuclear layer (INL), inner plexiform layer (IPL), outer plexiform layer (OPL), and outer nuclear layer (ONL) (Figure 1A). The immunoreactivity of ETA and ETB were found in GCL, IPL, INL, OPL and weakly in ONL (Figures 1D, G).
Figure 1.
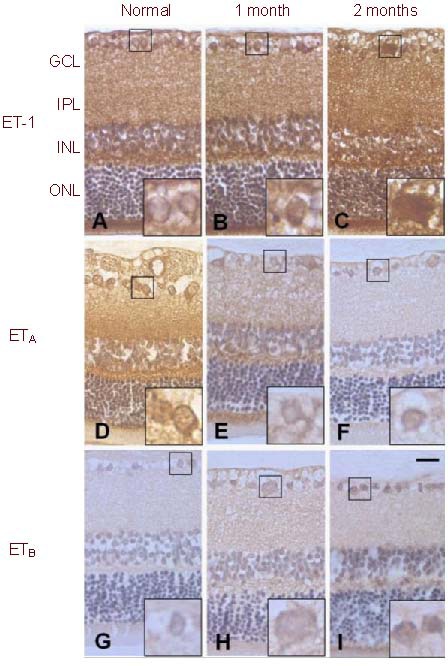
Immunoreactivity of ET-1, ETA and ETB in COH retina compared to the normal.
The immunoreactivity of ET-1 was increased at 1-month and 2-month in COH (B, C) when compared with the normal (A); the immunoreactivity of ETA receptor was decreased at 1-month and 2-month in COH (E, F) when compared with the normal (D) and the immunoreactivity of ETB receptor was increased at 1-month and 2-month in COH (H, I) when compared with the normal (G). Scale bar: 20 μm, n = 3.
ET-1: Endothelin-1; ETA: endothelin A receptor; ETB: endothelin B receptor; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; ONL: outer nuclear layer; COH: chronic ocular hypertension.
Alteration of ET-1 system correlated with the RGC numbers after ocular hypertension
The density of RGCs in the normal control eyes was 2241 ± 27 cells/mm2. Laser photocoagulation induced about 18% RGC loss at 1 month and continuous loss to about 21% at 2 months[21].
After induction of the COH, at month 1 and month 2, the immunoreactivity of ET-1 was increased in the RGCs, as well as in the whole retina (Figures 1B, C) when compared with the normal control (Figure 1A). However, ETA immunoreactivity was decreased and ETB immunoreactivity was increased in RGCs (Figures 1E, F, H and I) when compared with the normal control (Figures 1D, G).
Administration of LBP changed the expression of ET-1 and its receptors after ocular hypertension which was correlated with the survival number of RGCs
At 2 weeks after COH, loss of RGC was significantly decreased from 17% in COH + phosphate-buffered saline (PBS) group to about 1% in COH + LBP group[23]. To detect the effect of LBP treatment on the eye, the immunostaining of ET-1, ETA and ETB was examined at day 14 under COH condition. Compared with PBS group (COH + PBS) (Figures 2A, C and E enlarged by Figures 2G, I, K), 1 mg/kg LBP daily feeding markedly decreased the ET-1 expression level in the surviving RGCs (COH + LBP group) (Figure 2B), with increased ETA (Figure 2D) and decreased ETB immunoreactivity (Figure 2F, enlarged by Figures 2H, J, L).
Figure 2.
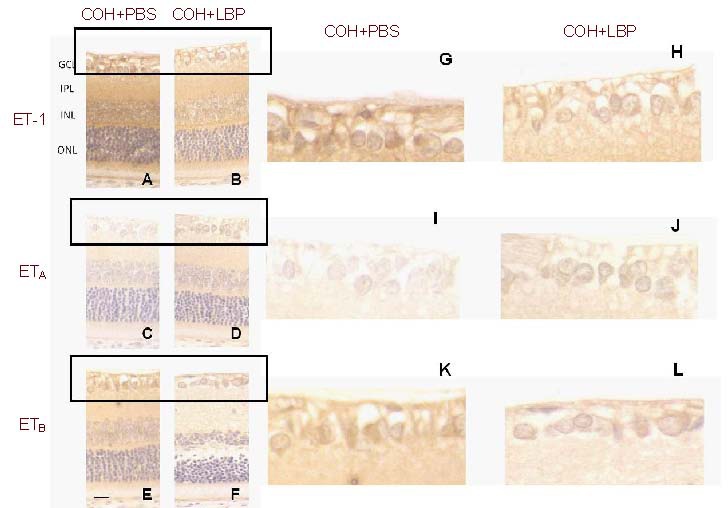
Immunoreactivity of ET-1, ETA and ETB in retinas with different treatment of LBP and PBS at day 14 post COH. The immunoreactivity of ET-1 and ETB was decreased whereas ETA immunoreactivity increased in COH+LBP group (B, D, F), when compared with COH+PBS group (A, C, E). G-L were enlarged pictures of GCL from A-F. Scale bar: 20 μm, n = 3.
COH: Chronic ocular hypertension; PBS: phosphate-buffered saline; LBP: Lycium barbarum Polysaccharides; COH+PBS: COH with feeding PBS group; COH+LBP: COH with feeding LBP group; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; ONL: outer nuclear layer.
To further analyze the vasculature in different areas of the eye, the blood vessels in GCL and choroid as well as ciliary blood vessels around the optic nerve in COH + LBP and COH + PBS groups were examined.
Interestingly, stronger expression levels of ET-1 (4.05 ± 0.17) and ETA (3.98 ± 0.16) with a lower expression level of ETB (2.04 ± 0.32) were found in COH + PBS group, while decreased expression levels of ET-1 (3.04 ± 0.3) and ETA (2.13 ± 0.35) with a higher expression level of ETB (3.62 ± 0.19) in COH + LBP group were observed in all of the above areas. After semi-quantitative analysis of the vasculature staining, these changes were confirmed with statistic difference (Figures 3A–F, Figures 4A–F and Figures 5A–F).
Figure 3.
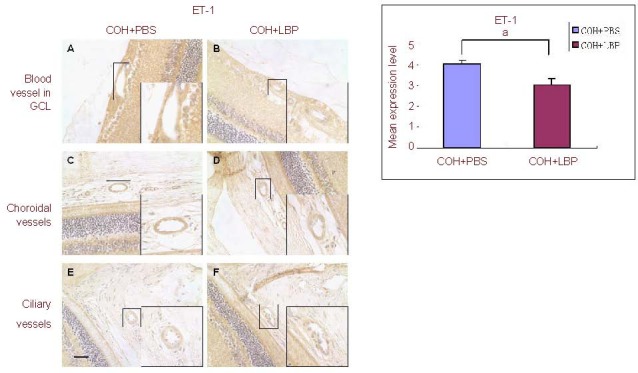
The immunoreactivity of ET-1 in vasculature of different areas in the eye at day 14 post COH. The immunoreactivity of ET-1 was decreased in COH+LBP group (B, D, F) when compared with COH+PBS group (A, C, E). Scale bar: 30 μm, n = 3, aP = 0.043.
ET-1: Endothelin-1; GCL: ganglion cell layer; COH: chronic ocular hypertension; PBS: phosphate-buffered saline; LBP: Lycium barbarum Polysaccharides; COH+PBS: COH with feeding PBS group; COH+LBP: COH with feeding LBP group.
Figure 4.
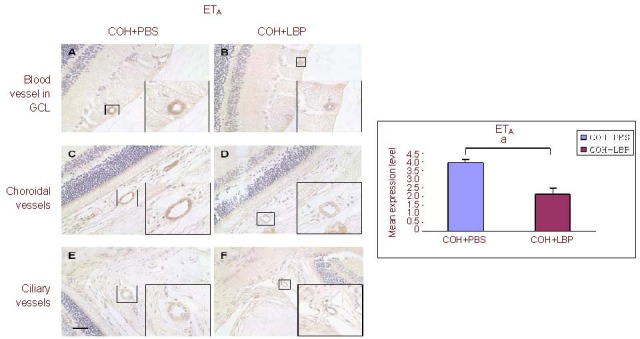
The immunoreactivity of ETA receptor in vasculature of different areas in the eye at day 14 post COH. The immunoreactivity of ETA receptor was decreased in COH+LBP group (B, D, F) when compared with COH+PBS group (A, C, E). Scale bar: 30 μm, n =3, aP=0.009.
ETA: Endothelin A receptor; GCL: ganglion cell layer; COH: chronic ocular hypertension; PBS: phosphate-buffered saline; LBP: Lycium barbarum Polysaccharides; COH+PBS: COH with feeding PBS group; COH+LBP: COH with feeding LBP group.
Figure 5.
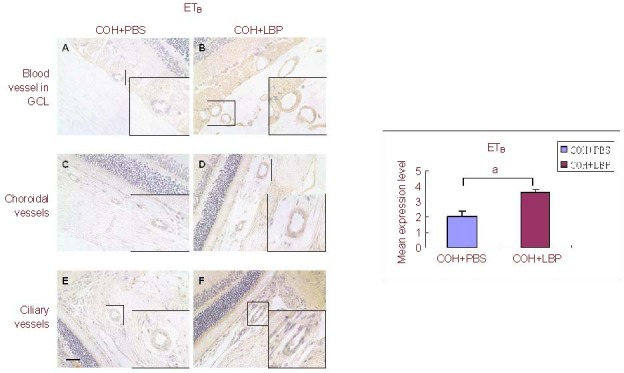
The immunoreactivity of ETB receptor in vasculature of different areas in the eye at day 14 post COH. The immunoreactivity of ETB receptor was increased in COH+LBP group (B, D, F) when compared with COH+PBS group (A, C, E). Scale bar: 30 μm, n = 3, aP = 0.012.
ETB: Endothelin B receptor; GCL: ganglion cell layer; COH: chronic ocular hypertension; PBS: phosphate-buffered saline; LBP: Lycium barbarum Polysaccharides; COH+PBS: COH with feeding PBS group; COH+LBP: COH with feeding LBP group.
DISCUSSION
Argon laser coagulation-induced COH in rats has shown a significant loss of RGCs and the sustaining IOP elevation lasting more than 2 months in our previous studies[21,22,23]. Here we show for the first time, there was an increased expression of ET-1 associated with the alteration of ETA and ETB in the retina, which is correlated to the loss of RGCs in this model.
The elevation of IOP has been considered as the most important factor to induce the RGC death in glaucoma[33]. A previous study using the Morrison rat model of glaucoma found an increased level of ET-1 in aqueous humor and optic nerve head[5]. Other studies showed that ET-1 could target the activation of astrocytes in optic nerve head leading to contribute to RGC death[34,35]. Blocking the biological effect of ET-1 in the anterior chamber could lower the elevated IOP in glaucoma[18]. All the evidence have demonstrated that ET-1 plays a role in the pathogenesis of glaucoma by an indirect mode through regulating the IOP and activating astrocytic gliosis of optic nerve head. In this experiment, we showed the elevated IOP resulted in an increased expression of ET-1 on RGCs. It could serve as an evidence for the role of ET-1 to be involved in the death of RGCs of glaucoma directly. Furthermore, in the COH retina, we also found the up-regulation of ETB receptor on RGCs. Taken together, the results suggest that the binding of ET-1 with ETB receptor on RGCs could be activated by the elevated IOP in glaucoma, which would contribute to the apoptosis of RGCs[3]. It also provides an in vivo evidence to reflect the in vitro finding showing that ET-1 could mediate apoptosis of RGC-5 cells directly via the increased expressions of ETB-receptor[14]. In this COH model, we also detected the increase of ET-1 with up-regulation of ETA receptor in retinal blood vessels, which is also due to the elevation of IOP. The binding of ET-1 with ETA receptor in blood vessels could induce vasoconstriction[8]. From the previous studies, it is known that ET-1-induced vasoconstriction is also an important mechanism involved in the pathogenesis of glaucoma[6,36].
Finding the effective bio-markers for early diagnosis of glaucoma has always been an important goal in the clinical research. However, since there are many mechanisms and risk factors involved in the pathogenesis of glaucoma, it is difficult to find a unique indicator to reflect glaucoma in different population and the different stages of the disease. ET-1 could be a promising candidate because ET-1 has been reported as the most sensitive indicator to be detected in glaucoma patients from other research groups[7,10,11]. Previous study has shown that sustained high level of ET-1 was found in the aqueous humor in the Morrison model of glaucoma rats[5]. Indeed, our COH model also shows sustained high levels of ET-1 in the retina tissue. Both of these studies may provide an experimental foundation to support the notion that ET-1 could be an effective bio-marker to detect the early defection of glaucoma. Survival of RGCs has been one main therapeutic goal for neuroprotection in glaucoma. It was showed that the activation of microglial cells was involved in the process of RGC degeneration in our laser-induced COH model[22]. Modulating microglia was one possible mechanism for neuroprotective effects of LBP[22]. The present study demonstrated another possible mechanism, of neuroprotective effect of LBP, since LBP could decrease the expression of ET-1. In blood vessels of COH retina, LBP could reduce the ET-1/ETA signal pathway while activate ET-1/ETB pathway by down-regulating the expression of ETA and up-regulating ETB. This kind of regulation by LBP to ETA and ETB receptors may induce an effect of increasing the blood supply to the retina and optic nerve head, which would be beneficial to the survival of RGCs under the COH conditions. The similar effects of LBP in the blood vessels were also reported in a renal hypertension model of rats[32]. Thus, it is reasonable to believe that to regulate the expression of ETA and ETB receptors on blood vessels could be one of the mechanisms of LBP in vascular regulation in COH rats. Whether the effect of LBP on ET-1 system suggested in this study plays a role in mediating the activation of retinal microglia should be further investigated.
MATERIALS AND METHODS
Materials
Female adult Sprague-Dawley rats (250–280 g) were used in this study. The rats were maintained on a 12 hour: 12-hour light-dark cycle and received food and water ad libitum. All animal experimental protocols were approved by the Committee for the Use of Live Animals in Teaching and Research (CULATR: 1664-08) at the University of Hong Kong.
Methods
COH model generation and animal treatment
In the present study, we used a chronic ocular hypertension (COH) model to study the effect of LBP on ET-1 expression. COH was induced in the right eye of rats with Argon laser coagulation of the limbal and episcleral veins[21,37]. The increased IOP could last for at least 2 months. We confirmed the significant loss of RGCs using Fluoro-Gold (FG) retrograde labeling from bilateral superior colliculi[22,23]. This method was routinely used in our laboratory for RGC survival and degeneration studies[38,39,40].
Rats were grouped and fed daily with LBP or phosphate-buffered saline (PBS, 0.01 M, pH 7.4) vehicle for 3 weeks (started 1 week before photocoagulation)[22]. LBP extracts was from Lycium barbarum, the preparation method has been reported by our previous study[22]. LBP solution for feeding was prepared using freeze-dried powder dissolving into PBS (0.01 M, pH 7.4). The feeding dosage was 1 mg/kg.
Sample collection and tissue processing
To study the time course profile of ET-1 and its receptors in COH retina, the eye samples were collected at 1 and 2 months after COH procedure compared with the normal control (Figure 6A). To evaluate the effect of LBP on ET-1 and its receptors, the eye samples were collected at the time point of 14-day post COH. Immunostaining was compared between the LBP-fed COH group (COH + LBP) and PBS-fed COH group (COH + PBS) (Figure 6B).
Figure 6.
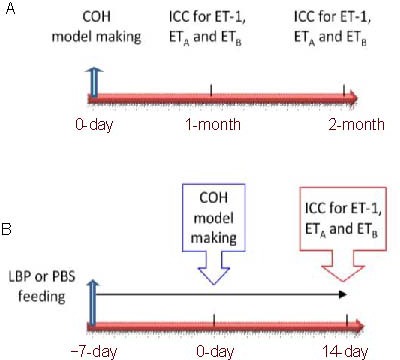
The schedule of procedure for COH model making, animal intragastric administration and time points of sample collection. (A) Time course of the expression of ET-1 and its receptors, ETA and ETB. (B) LBP effect on the expression of ET-1 and its receptors, ETA and ETB. n = 3.
ET-1: Endothelin-1; COH: chronic ocular hypertension; LBP: Lycium barbarum Polysaccharides; ICC: immunocytochemistry; ETA: endothelin A receptor; ETB: endothelin B receptor.
After an overdose of pentobarbital sodium (150 mg/kg), the animals were perfused with 0.9% saline solution. The eye-cups were immediately fixed in 4% paraformaldehyde overnight at 4°C, and then dehydrated with a graded series of ethanol and xylene and embedded in paraffin wax. Serial sections (4 μm thickness) were prepared for immunohistochemistry.
Immunohistochemistry for ET-1, ETA and ETB
Paraffin sections of retinas were deparaffinized in xylene and rehydrated with a graded series of ethanol. After washing in 0.01 M PBS, sections were blocked with 3% hydrogen peroxide for 15 minutes, and followed with 2% normal goat serum blocking for 1 hour, and then incubated with the following antibodies: anti-ET-1 (1:800, polyclonal, Peninsula Laboratories, San Carlos, CA, USA), anti-ETA (1:100, polyclonal, Calbiochem Merck, Darmstadt, Germany) and anti-ETB (1:200, polyclonal, Calbiochem Merck, Darmstadt, Germany) respectively, at 4°C overnight. Sections were then incubated with secondary antibodies accordingly (1:200) for 2 hours at room temperature. Avidin-biotin-peroxidase complex (ABC-Elite Kit, Vector Laboratories, Burlingame, CA, USA) was added onto each section and incubated for 30 minutes, then subsequently treated with stable 3,3’-diaminobenzidine tetrahydrochloride as a peroxidase substrate for color development. Finally, sections were counterstained with Hematoxylin for nuclear staining. All the pictures taken for analysis were from sections stained in the batch concurrently.
Statistic analysis
To semi-quantitatively analyze the staining of the blood vessels, a classification of DAB staining color was defined as grade 1-5 with scoring 1–5, e.g. grade 1 with score 1 for white color, grade 2 with score 2 for light yellow, grade 3 for golden, grade 4 for brown and grade 5 for dark brown. The average staining level was given to each retinal section by scoring each blood vessel together versus the total number of blood vessels on the section. Twelve sections per group were analyzed by observers blinded to the identification of the animals. Data were represented as mean ± SEM for average staining level. Kruskal-Wallis test followed by the Mann-Whitney U test was performed to compare the staining scores of these LBP and PBS groups using SPSS 12.0 software, and P < 0.05 or P < 0.01 were considered statistically significant.
Footnotes
Funding: This work is supported by the Azalea (1972) Education fund to KFSo and RCCC; Fundamental Research Fund for The Centre Universities, No. 21609101.
Ethical approval: This study received permission from the Committee for the Use of Live Animals in Teaching and Research (CULATR: 1664-08) at the University of Hong Kong, China.
Conflicts of interest: None declared.
(Edited by You SW, Lou XG/Zhao M/Wang L)
REFERENCES
- [1].Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Clark AF, Yorio T. Ophthalmic drug discovery. Nat Rev Drug Discov. 2003;2:448–459. doi: 10.1038/nrd1106. [DOI] [PubMed] [Google Scholar]
- [3].Prasanna G, Narayan S, Krishnamoorthy RR, et al. Eyeing endothelins: a cellular perspective. Mol Cell Biochem. 2003;253:71–88. doi: 10.1023/a:1026005418874. [DOI] [PubMed] [Google Scholar]
- [4].Flammer J, Mozaffarieh M. What is the present pathogenetic concept of glaucomatous optic neuropathy? Surv Ophthalmol. 2007;52(Suppl 2):S162–173. doi: 10.1016/j.survophthal.2007.08.012. [DOI] [PubMed] [Google Scholar]
- [5].Prasanna G, Hulet C, Desai D, et al. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol Res. 2005;51:41–50. doi: 10.1016/j.phrs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- [6].Delaney Y, Walshe TE, O’Brien C. Vasospasm in glaucoma: clinical and laboratory aspects. Optom Vis Sci. 2006;83:406–414. doi: 10.1097/01.opx.0000225877.13217.01. [DOI] [PubMed] [Google Scholar]
- [7].Ghanem AA, Elewa AM, Arafa L. Endothelin-1 and nitric oxide levels in patients with glaucoma. Ophthalmic Res. 2011;46:98–102. doi: 10.1159/000323584. [DOI] [PubMed] [Google Scholar]
- [8].Yorio T, Krishnamoorthy R, Prasanna G. Endothelin: is it a contributor to glaucoma pathophysiology? J Glaucoma. 2002;11:259–270. doi: 10.1097/00061198-200206000-00016. [DOI] [PubMed] [Google Scholar]
- [9].Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38(Suppl):S3–6. doi: 10.1016/0039-6257(94)90041-8. [DOI] [PubMed] [Google Scholar]
- [10].Emre M, Orgul S, Haufschild T, et al. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol. 2005;89:60–63. doi: 10.1136/bjo.2004.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cellini M, Possati G, Profazio V, et al. Color doppler imaging and plasma levels of endothelin-1 in low-tension glaucoma. Acta Ophthalmol Scand Suppl. 1997;224:11–13. doi: 10.1111/j.1600-0420.1997.tb00448.x. [DOI] [PubMed] [Google Scholar]
- [12].Grieshaber MC, Mozaffarieh M, Flammer J. What is the link between vascular dysregulation and glaucoma? Surv Ophthalmol. 2007;52:S144–S154. doi: 10.1016/j.survophthal.2007.08.010. [DOI] [PubMed] [Google Scholar]
- [13].Wang X, LeVatte TL, Archibald ML, et al. Increase in endothelin B receptor expression in optic nerve astrocytes in endothelin-1 induced chronic experimental optic neuropathy. Exp Eye Res. 2009;88:378–385. doi: 10.1016/j.exer.2008.09.009. [DOI] [PubMed] [Google Scholar]
- [14].Krishnamoorthy RR, Rao VR, Dauphin R, et al. Role of the ETB receptor in retinal ganglion cell death in glaucoma. Can J Physiol Pharmacol. 2008;86:380–393. doi: 10.1139/Y08-040. [DOI] [PubMed] [Google Scholar]
- [15].Resch H, Karl K, Weigert G, et al. Effect of dual endothelin receptor blockade on ocular blood flow in patients with glaucoma and healthy subjects. Invest Ophthalmol Vis Sci. 2009;50:358–363. doi: 10.1167/iovs.08-2460. [DOI] [PubMed] [Google Scholar]
- [16].Zhao Z, Chen Y, Wang J, et al. Age-related retinopathy in NRF2-deficient mice. PLoS One. 2011;6:e19456. doi: 10.1371/journal.pone.0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chauhan BC, LeVatte TL, Jollimore CA, et al. Model of endothelin-1-induced chronic optic neuropathy in rat. Invest Ophthalmol Vis Sci. 2004;45:144–152. doi: 10.1167/iovs.03-0687. [DOI] [PubMed] [Google Scholar]
- [18].Howell GR, Macalinao DG, Sousa GL, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121:429–1444. doi: 10.1172/JCI44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang RCC, So KF. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28:643–652. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ho YS, So KF, Chang RC. Drug discovery from Chinese medicine against neurodegeneration in Alzheimer's and vascular dementia. Chin Med. 2011;6:15. doi: 10.1186/1749-8546-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chan HC, Chang RC, Koon-Ching Ip A, et al. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203:269–273. doi: 10.1016/j.expneurol.2006.05.031. [DOI] [PubMed] [Google Scholar]
- [22].Chiu K, Chan HC, Yeung SC, et al. Modulation of microglia by Wolfberry on the survival of retinal ganglion cells in a rat ocular hypertension model. J Ocul Biol Dis Infor. 2009;2:27–136. doi: 10.1007/s12177-009-9023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chiu K, Zhou Y, Yeung SC, et al. Up-regulation of crystallins is involved in the neuroprotective effect of wolfberry on survival of retinal ganglion cells in rat ocular hypertension model. J Cell Biochem. 2010;110:311–320. doi: 10.1002/jcb.22539. [DOI] [PubMed] [Google Scholar]
- [24].Li SY, Yeung CM, Yu WY, et al. Lycium barbarum Polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS One. 2011;6:e16380. doi: 10.1371/journal.pone.0016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ho YS, Yu MS, Lai CS, et al. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on [beta]-amyloid peptide neurotoxicity. Brain Res. 2007;1158:123–134. doi: 10.1016/j.brainres.2007.04.075. [DOI] [PubMed] [Google Scholar]
- [26].Ho YS, Yu MS, Yik SY, et al. Polysaccharides from wolfberry antagonizes glutamate excitotoxicity in rat cortical neurons. Cell Mol Neurobiol. 2009;29:1233–1244. doi: 10.1007/s10571-009-9419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xiao J, Liong EC, Ching YP, et al. Lycium barbarum polysaccharides protect mice liver from carbon tetrachloride-induced oxidative stress and necroinflammation. J Ethnopharmacol. 2012;139:462–470. doi: 10.1016/j.jep.2011.11.033. [DOI] [PubMed] [Google Scholar]
- [28].Ho YS, Yu MS, Yang XF, et al. Neuroprotective effects of polysaccharides from wolfberry, the fruits of Lycium barbarum, against homocysteine-induced toxicity in rat cortical neurons. J Alzheimers Dis. 2010;19:813–827. doi: 10.3233/JAD-2010-1280. [DOI] [PubMed] [Google Scholar]
- [29].Ho YS, So KF, Chang RC. Anti-aging herbal medicine--how and why can they be used in aging-associated neurodegenerative diseases? Ageing Res Rev. 2010;9:354–362. doi: 10.1016/j.arr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- [30].Yu M, Lai C, Ho Y, et al. Characterization of the effects of anti-aging medicine Fructus lycii on β-amyloid peptide neurotoxicity. Int J Mol Med. 2007;20:261–268. [PubMed] [Google Scholar]
- [31].Yu MS, Leung SK, Lai SW, et al. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against β-amyloid peptide neurotoxicity. Exp Gerontol. 2005;40:716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]
- [32].Jia Y, Dong J, Wu X, et al. The effect of lycium barbarum polysaccharide on vascular tension in two-kidney, one clip model of hypertension. Sheng Li Xue Bao. 1998;50:309–314. [PubMed] [Google Scholar]
- [33].Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- [34].He S, Prasanna G, Yorio T. Endothelin-1-mediated signaling in the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in astrocytes. Invest Ophthalmol Vis Sci. 2007;48:3737–3745. doi: 10.1167/iovs.06-1138. [DOI] [PubMed] [Google Scholar]
- [35].Prasanna G, Krishnamoorthy R, Yorio T. Endothelin, astrocytes and glaucoma. Exp Eye Res. 2011;93:170–177. doi: 10.1016/j.exer.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yu MS, Leung SK, Lai SW, et al. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against β-amyloid peptide neurotoxicity. Exp Gerontol. 2005;40:716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]
- [37].Chiu K, Chang RC, So KF. Laser-induced chronic ocular hypertension model on SD rats. J Vis Exp. 2007;10:594. doi: 10.3791/549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li RS, Tay DK, Chan HH, et al. Changes of retinal functions following the induction of ocular hypertension in rats using argon laser photocoagulation. Clin Experiment Ophthalmol. 2006;34:575–583. doi: 10.1111/j.1442-9071.2006.01279.x. [DOI] [PubMed] [Google Scholar]
- [39].Ji JZ, Elyaman W, Yip HK, et al. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19:265–272. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- [40].Fu QL, Hu B, Wu W, et al. Blocking LINGO-1 function promotes retinal ganglion cell survival following ocular hypertension and optic nerve transection. Invest Ophthalmol Vis Sci. 2008;49:975–985. doi: 10.1167/iovs.07-1199. [DOI] [PubMed] [Google Scholar]


