Abstract
PC12 cell injury was induced using 20 μM amyloid β-protein 25–35 to establish a model of Alzheimer's disease. The cells were then treated with 5, 10, and 25 μM Schisandrin B. Methylthiazolyldiphenyl-tetrazolium bromide assays and Hoechst 33342 staining results showed that with increasing Schisandrin B concentration, the survival rate of PC12 cells injured by amyloid β-protein 25–35 gradually increased and the rate of apoptosis gradually decreased. Reverse transcription-PCR, immunocytochemical staining and western blot results showed that with increasing Schisandrin B concentration, the mRNA and protein expression of vacuolar protein sorting 35 and amyloid precursor protein were gradually decreased. Vacuolar protein sorting 35 and amyloid precursor protein showed a consistent trend for change. These findings suggest that 5, 10, and 25 μM Schisandrin B antagonizes the cellular injury induced by amyloid β-protein 25–35 in a dose-dependent manner. This may be caused by decreasing the expression of vacuolar protein sorting 35 and amyloid precursor protein.
Keywords: Schisandrin B, PC12 cells, amyloid β-protein 25–35, amyloid precursor protein, vacuolar protein sorting 35, neural protection
Abbreviations:
APP, amyloid precursor protein; Aβ, amyloid β-protein; AD, Alzheimer's disease; VPS35, vacuolar protein sorting 35
INTRODUCTION
Amyloid precursor protein (APP), a transmembrane glycoprotein that ubiquitously exists on cell membranes, can be hydrolyzed into the neurotoxic amyloid β-protein (Aβ) by β-secretases and γ-secretases. The generation and accumulation of Aβ leads to toxic injury of nerve cells and finally brings about dementia[1,2], which is the most important pathological manifestation of Alzheimer's disease (AD)[3]. However, the latest research shows that retromer complex[4], a sorting nexin protein complex, plays an important role in AD. Vacuolar protein sorting 35 (VPS35), the core component of the retromer complex, can selectively identify and transfer cargo proteins. Thus, it is frequently referred to in the literature as the “cargo-selective subcomplex”.
Deficiencies in VPS35 cause receptor protein transport defects, abnormal Wnt signaling, toxicity transport disorder and neurodegenerative diseases.
Schisandrin B, a type of lignan, is an active compound extracted from Schisandra. Many studies have shown that Schisandrin B has a strong effect in antioxidation and eliminating radicals[5,6]. However, current research on Schisandrin B is mainly concerned with drug content measurement, liver and kidney protection, treating drug-resistant tumors, and improving pulmonary function[7]. We hypothesized that Schisandrin B is protective against oxidative damage to neurons.
Previous research from our group has shown that, 5, 10, and 25 μM Schisandrin B has low toxicity (toxicity < 10%) to PC12 cells[8]. First, we will establish an in vitro neuronal damage model with Aβ25-35, then observe whether Schisandrin B at low-toxicity concentrations (5, 10, and 25 μM) has protective effects on the damaged PC12 cells, and explore the possible protective mechanisms involving APP and VPS35.
RESULTS
Viability of PC12 cells treated with Schisandrin B
The methylthiazolyldiphenyl-tetrazolium bromide assay showed that in the Aβ25-35 group (PC12 cells treated with 20 μM Aβ25-35), the viability of PC12 cells significantly decreased compared with the control group (untreated PC12 cells; P < 0.05). After treatment with 5, 10, and 25 μM Schisandrin B, PC12 cell viability significantly increased (P < 0.05) in a dose-dependent manner (Figure 1).
Figure 1.
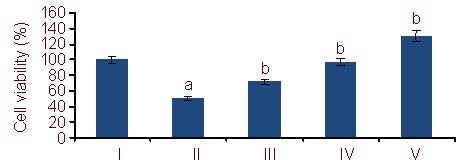
Viability of PC12 cells (MTT assay). The data are expressed as mean ± SD. The experiments were repeated at least three times (n = 6).
aP < 0.05, vs. control group; bP < 0.05, vs. Aβ25-35 group (one-way analysis of variance and least significant difference method). MTT: methylthiazolyldiphenyl- tetrazolium bromide; Aβ: amyloid β protein; I–V: control, Aβ25-35, 5, 10, 25 μM Schisandrin B groups, respectively.
Apoptosis of PC12 cells treated with Schisandrin B
Hoechst 33342 staining was used to examine cell apoptosis. Under a fluorescence microscope, most cells in the control group were equal in size, cell membranes were intact, and the nuclei had non-erratic borders with even, low intensity blue fluorescence. With Aβ25-35 treatment, PC12 apoptosis was significantly increased (P < 0.05). Many cells seemed decreased in size, showing chromatin agglutination and marginalization, which presented as brilliant blue fluorescence; some of the nuclei exhibited pyknosis and fragmentation visualized as a ring of fluorescence. These are changes associated with cell apoptosis. After being treated with 5, 10, and 25 μM Schisandrin B, the rate of PC12 cell apoptosis decreased significantly in a dose-dependent manner compared with the Aβ25-35 group (P < 0.05) (Figures 2, 3).
Figure 2.
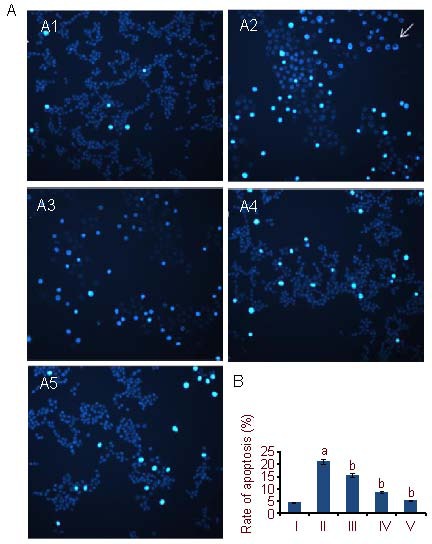
PC12 cell apoptosis (Hoechst 33342 staining).
(A) PC12 cell apoptosis under fluorescence microscope (nuclear staining, × 400).
(A1) Control group: nuclei have non-erratic borders with even, low intensity blue fluorescence.
(A2) Aβ25-35 group: chromatin exhibited agglutination and marginalization with pyknotic brillant blue fluorescence of the nucleus; some nuclei exhibited a fluorescence ring (arrow).
(A3–A5) Aβ25-35 + Schisandrin B groups (5 μM, 10 μM, 25 μM): The number of cells with brillant blue fluorescent nuclei is decreased in a dose-dependent manner, cells are even and intact compared with the Aβ25-35 group.
(B) Quantification of cell apoptosis. Rate of apoptosis (%) = the number of apoptotic cells/the number of all counted cells × 100%. Aβ: Amyloid β protein; I-V: control, Aβ25-35, 5, 10, 25 μM Schisandrin B groups, respectively.
The data are expressed as mean ± SD. The experiments were repeated at least three times (n = 3). aP < 0.05, vs. control group. bP < 0.05, vs. Aβ25-35 group (one-way analysis of variance and least significant difference method).
Figure 3.
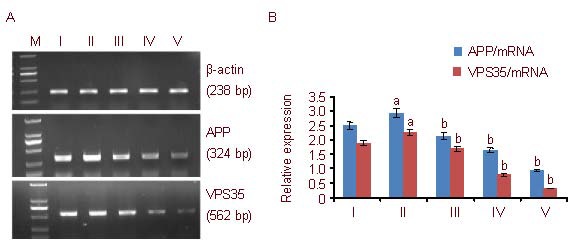
Amyloid precursor protein (APP) and vacuolar protein sorting 35 (VPS35) mRNA expression in PC12 cells (reverse transcription-PCR).
(A) APP and VPS35 mRNA expression in PC12 cells. In each group, APP expression is higher than that of VPS35. Aβ: Amyloid β protein; M: Marker; I-V: control, Aβ25-35, 5, 10, 25 μM Schisandrin B groups, respectively.
(B) Quantification of APP and VPS35 mRNA expression. The data are expressed as mean ± SD of band absorbance ratio of target gene to β-actin, and analyzed by one-way analysis of variance and least significant difference method.
The experiments were repeated at least three times (n = 3). aP < 0.05, vs. control group; bP < 0.05, vs. Aβ25-35 group. Furthermore, there were significant differences (P < 0.05) between the three Schisandrin B groups with different concentrations.
APP and VPS35 mRNA expression in PC12 cells treated with Schisandrin B
The reverse transcription (RT)-PCR results show that, after being treated with Aβ25-35, the expression of APP and VPS35 mRNA significantly increased (P < 0.05) compared with the control group. In the 5, 10, and 25 μM Schisandrin B groups, the expression of APP and VPS35 mRNA significantly decreased in a dose-dependent manner (P < 0.05); APP and VPS35 mRNA expression changed in the same direction. There were significant differences in APP and VPS35 mRNA expression between the three doses of Schisandrin B (P < 0.05; Figure 3).
Expression of APP and VPS35 protein in PC12 cells treated with Schisandrin B
After streptavidin-peroxidase (S-P) staining, PC12 cell nuclei were blue; APP and VPS35 proteins, which were expressed in the endochylema, appeared brown. Compared with the control group, endochylema staining of the Aβ25-35 group was darker, and the average absorbance of APP and VPS35 proteins also increased (P < 0.05). However, in the 5, 10, and 25 μM Schisandrin B groups, endochylema staining became progressively lighter and the absorbance values of APP and VPS35 were accordingly reduced compared with the Aβ25-35 group, which indicates that the expression of APP and VPS35 protein gradually decreased (P < 0.05) (Figure 4). Western blot results showed that the expression of APP and VPS35 proteins had the same trend for change. In the Aβ25-35 group, the expression of both APP and VPS35 proteins increased compared with the control group (P < 0.05), while APP and VPS35 protein expression in the Schisandrin B groups gradually decreased compared with the Aβ25-35 group (P < 0.05). The expression of APP and VPS35 proteins was significantly different between the three Schisandrin B groups (P < 0.05) (Figure 5).
Figure 4.
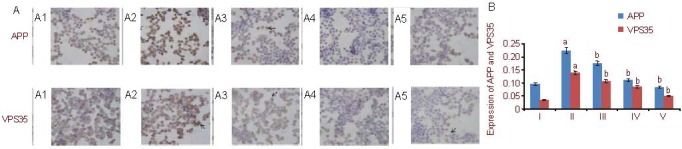
Amyloid precursor protein (APP) and vacuolar protein sorting 35 (VPS35) expression in PC12 cells (streptavidin-peroxidase method).
(A) Immunocytochemical staining of APP and VPS35 expression (endochylema staining, optical microscope, ×400).
(A1) Control group: cell nuclei are dyed blue, APP and VPS35 endochylema staining is brown (arrows).
(A2) Aβ25-35 group: endochylema staining (arrows) is much darker compared with the control group.
(A3–A5) Schisandrin B groups (5 μM, 10 μM, 25 μM): endochylema staining (arrows) gradually becomes lighter compared with the Aβ25-35 group.
In each group, endochylema staining of APP is more intense than that of VPS35.
(B) Quantification of APP and VPS35 protein expression. The data are expressed as mean ± SD and analyzed by one-way analysis of variance and least significant difference method. Image Pro Plus software was used to measure the expression of target proteins. The experiments were repeated at least three times (n = 3). aP < 0.05, vs. control group; bP < 0.05, vs. Aβ25-35 group. Furthermore, there were significant differences (P < 0.05) between the three Schisandrin B groups with different concentrations. Aβ: Amyloid β protein; I–V: control, Aβ25-35, 5, 10, 25 μM Schisandrin B groups, respectively.
Figure 5.
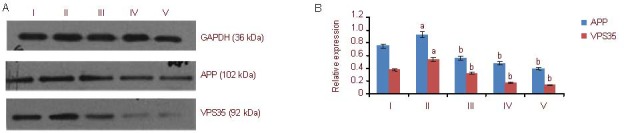
Amyloid precursor protein (APP) and vacuolar protein sorting 35 (VPS35) expression in PC12 cells (western blot method). I–V: control, Aβ25-35, 5, 10, 25 μM Schisandrin B groups, respectively.
(A) Western blot detection results of APP and VPS35 protein expression in PC12 cells. In each group, APP expression is higher than that of VPS35.
(B) Quantification of APP and VPS35 protein expression. The data are expressed as mean ± SD of the band absorbance value ratio of target protein to GAPDH, and analyzed by one-way analysis of variance and least significant difference method. The experiments were repeated at least three times (n = 3). aP < 0.05, vs. control group; bP < 0.05, vs. Aβ25-35 group. Furthermore, there were significant differences (P < 0.05) between the three Schisandrin B groups with different concentrations. Aβ: Amyloid β protein.
DISCUSSION
In our study, we established an in vitro model of Alzheimer's disease, using Aβ25-35 to induce damage in PC12 cells. The viability of PC12 cells decreases in the Aβ25-35 group and the rate of cell apoptosis increases, which shows that Aβ25-35 is toxic to PC12 cells, and that the in vitro model is successful.
Previous research has demonstrated that Schisandrin B improves the antioxidant function of chondriosomes and glutathione in mouse brain[9,10], and protects primary rat cortical cultures from Aβ1-42- and glutamate-induced toxicity[5,11]. In vitro, Schisandrin B can relieve hydrogen peroxide-induced oxidation on PC12 cells[8], prevent M146 cells from secreting Aβ[12], and can also help inhibit ataxia telangiectasia and Rad3-related (ATR) protein kinase activity to lessen the DNA damage response[13]. In our study, the results from the MTT assay and the Hoechst 33342 staining demonstrate that Aβ25-35 is harmful to PC12 cells, but Schisandrin B can inhibit the damage induced by Aβ25-35 and improve cell viability. Moreover, after treatment with different concentrations of Schisandrin B, cell viability increases and the rate of apoptosis decreases gradually, meaning that Schisandrin B protects PC12 cells from Aβ25-35-induced damage in a dose-dependent manner.
In studies of the mechanism of AD pathology, the amyloid theory, the tau theory, the cholinergic theory and the radical damage theory have occupied important positions[14]. However, the retromer complex[1], a complex of sorting proteins, also plays an important role in the occurrence and development of AD[15]. VPS35 is the core part of the retromer complex[1]. RT-PCR, immunocytochemical staining and western blot results showed that the expression of APP and VPS35 in the Aβ25-35 group is stronger than in the control group, and in the different Schisandrin B groups, the expression of APP and VPS35 is gradually inhibited, with a consistent trend. APP expression in each group is much higher than that of VPS35. APP is necessary for AD pathological changes, so we assumed that VPS35 is an important upstream source of APP, and may be one of the numerous ways to regulate and control APP. This assumption is consistent with previous studies, and helps to provide evidence for a greater role for VPS35 in AD.
RT-PCR, immunocytochemical staining and western blot analysis show that the mRNA and protein expression of APP and VPS35 in the Aβ25-35 groups all increased compared with the control group. However, after the cells were treated with Schisandrin B, the expression of APP and VPS35 decreased. These findings suggest that (1) the Aβ25-35 toxicity to PC12 cell is not only induced by increased APP expression, but also by increased VPS35 expression, possibly due to the aggregation of Aβ25-35, which leads to damaging changes in cell function; under these conditions of stress, the expression of VPS35 increases to strengthen its transfer of APP and decrease APP degradation; (2) different concentrations of Schisandrin B can protect PC12 cells from Aβ25-35-induced injury, and the expression of both VPS35 and APP is decreased. A possible mechanism is that, after addition of Schisandrin B, VPS35 expression decreases, which leads to secondary reductions in sorting protein-related receptor containing LDLR class A repeats (SorLA), its cargo receptor[16]. SorLA is closely linked to the endocytic process of APP hydrolysis to Aβ[17,18]. Therefore, the degradation of APP is attenuated, inhibiting the production of toxic Aβ. Moreover, as VPS35 decreases, the transport time of APP from the endosome to the trans-Golgi network (TGN) increases, correspondingly reducing the time during which APP protein is associated with its catenase on the cell membrane, and finally inhibiting Aβ formation (Figure 6).
Figure 6.
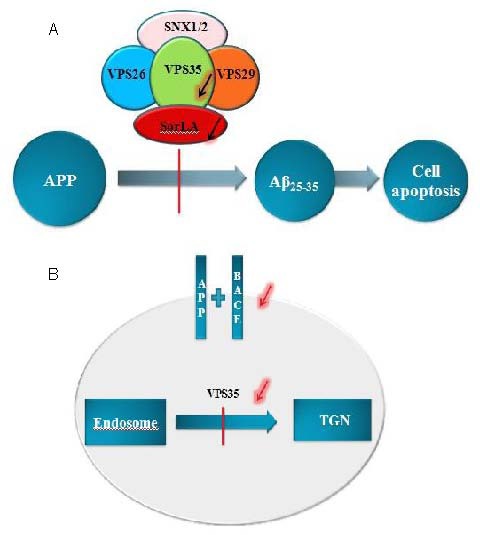
Schisandrin B protects PC12 cells from Aβ25-35 induced injury via regulation of amyloid precursor protein (APP) and vacuolar protein sorting 35 (VPS35). VPS35 is an important upstream source of APP, and VPS35 may regulate APP. Its mechanism may be as follows,
(A) VPS35 could hinder the process of APP hydrolysis into Aβ by reducing its cargo protein SorLA; (B) VPS35 could extend the transport time of its cargo protein APP from the endosome to the TGN, to reduce its degradation in the cell membrane.
Aβ: Amyloid β protein; SNX: sorting nexin; SorLA: sorting protein related receptor; BACE: β-site APP cleaving enzyme; TGN: trans Golgi network.
In summary, 5, 10, and 25 μM Schisandrin B antagonizes Aβ25-35 injury to PC12 cells in a dose-dependent manner. This is achieved through decreasing the expression of APP and sorting protein VPS35.
MATERIALS AND METHODS
Design
A cytological, comparative, and observational study.
Time and setting
Experiments were performed at the Department of Neurology, Renmin Hospital, Wuhan University in China from April 2010 to December 2010.
Materials
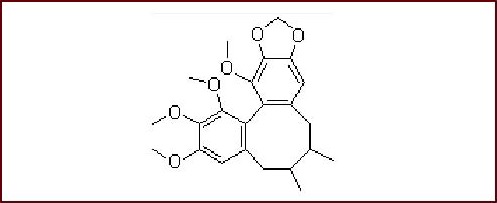
PC12 cells (Rattus norvegicus adrenal pheochromocytoma cells) were obtained from the Biology Store Center of Wuhan University, China. Schisandrin B at 98% purity was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (batch No.110765-200710). The molecular formula of Schisandrin B is C23H28O6 and the molecular weight is 400.46. Its chemical structure is as follows:
Methods
PC12 cell culture
PC12 cells were grown in RPMI-1640 culture solution (10% horse serum, 5% fetal calf serum, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM glutamine; Gibco, Grand Island, NY, USA) in a humidified atmosphere containing 5% CO2 at 37°C. The culture solution was changed once every 2 days and the growth of PC12 cells was observed using an invert microscope (Olympus, Japan). Cells at the logarithmic growth phase were used for experiments.
Drug dispensation
Aβ25-35 (Beijing Biosynthesis Biotechnology Co., Ltd., China) was dissolved at 1mM in autoclaved tri-distilled water, aliquoted and frozen at -20°C. The final concentration of Aβ25-35 used on the PC12 cells was 20 μM. 10 mM Schisandrin B was first dissolved in dimethyl sulfoxide, and then diluted with RPMI-1640 culture solution (Gibco) to get different working solutions (5, 10, and 25 μM). The MTT solution, at a working concentration of 5 mg/mL, was dissolved in phosphate-buffered solution (NaCI: 137 mM, KCI: 2.7 mM, Na2HPO4: 4.3 mM, KH2PO4: 1.4 mM, pH 7.2–7.4).
MTT for testing cell viability
An MTT kit (Beijing CellChip Biotechnology Co., Ltd., China) was used to examine the protective effects of Schisandrin B at different concentrations (5, 10, 25 μM) on the damaged PC12 cells treated with Aβ25-35. According to the instructions, PC12 cells at the logarithmic growth phase were plated at a density of 1 × 104/well on a 96-well tissue culture plate (Beyotime Institute of Biotechnology, China) in an environment with 5% CO2 at 37°C for 24 hours. The experiment contained six groups: blank group (RPMI-1640 culture solution), control group (PC12 cells, RPMI-1640 culture solution), Aβ25-35 treatment group (PC12 cells, RPMI-1640 culture solution, Aβ25-35), Aβ25-35 + 5, 10, and 25 μM Schisandrin B groups (PC12 cells, RPMI-1640 culture solution, Aβ25-35, Schisandrin B). After 48 hours of incubation, 20 μL per well of 5 mg/mL MTT was added to the medium. Four hours later, the culture solution was discarded, 150 μL DMSO was added, and the culture plate was shaken for 5-10 minutes until the dark purple crystalline particles were completely dissolved. Finally, the absorbance value at a wavelength of 570 nm in each group was obtained using a microplate reader (BioTek, USA). Cell viability (%) = (absorbance of experimental group – absorbance of blank group) / (absorbance of control group – absorbance of blank group) × 100%. The experiment was repeated at least three times.
Hoechst 33342 staining for detection of cell apoptosis
Sterilized slides were placed in a 6-well culture plate (Beyotime Institute of Biotechnology, China). After an equal amount of cell suspension was dropped onto the slides, the cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C for 3 hours. When the cells firmly adhered to the slides, the slides were taken out carefully and fixed with 4% paraformaldehyde. Hoechst 33342 staining solution (Beyotime Institute of Biotechnology, China) was added to cover the samples. Cells were washed twice or three times with phosphate-buffered solution after incubation for 3-5 minutes at room temperature, and the apoptotic cells among 500 cells were counted using a fluorescence microscope (Olympus, Tokyo, Japan). Apoptosis rate (%) = the number of apoptotic cells / the number of all counted cells × 100%.
RT-PCR for detection of APP and VPS35 mRNA expression
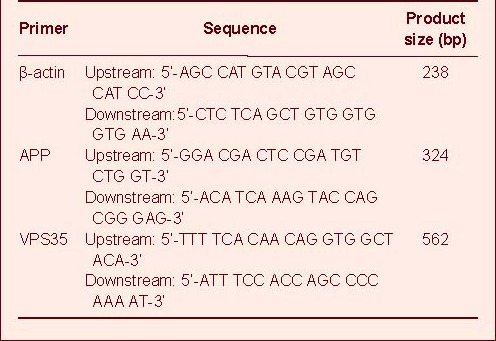
PC12 cells in 6-well tissue culture plates (Beyotime Institute of Biotechnology, China) were cultivated and then incubated for 48 hours after adding the corresponding drugs. The total cell RNA was extracted in Trizol (Invitrogen, Carlsbad, CA, USA) and cDNA was obtained using a reverse transcription kit (Promega Company, Mexico) according to the manufacturer's instructions. Primers were synthesized by Shanghai Biological Technology Company, China. RT-PCR was performed in accordance with the Promega instructions. The primer sequences are shown in Table 1.
Table 1.
The primer sequences of APP and VPS35 mRNA
PCR was performed according to the following protocol: pre-denaturation at 95°C for 5 minutes, then 30 cycles of denaturation at 95°C for 45 seconds, annealing at 54.2°C/56.6°C for 30 seconds, extension at 72°C for 40 seconds; and a single re-extension at 72°C for 10 minutes. Then, 1% agarose gel electrophoresis was used for DNA separation and the absorbance of the spectral band in the electrophoresis results was analyzed using BioCaptMW software (Vilber Lourmat, Mame-La-Vallee, France). The ratio of the target gene spectral band parameter to the corresponding β-actin spectral band parameter was used as the value of mRNA expression in each sample.
Immunocytochemistry for detecting the expression of APP and VPS35 protein
Cell slides were made as above. According to the instructions in the S-P staining kit (Beijing Zhongshan Biological Reagent Company, Beijing, China), the cells were fixed with 2% paraformaldehyde and microwave antigen retrieval was performed. Following addition of 3% H2O2 solution and non-immune goat serum to block peroxidase activity and nonspecific antigens, cells were incubated with primary antibodies [polyclonal rabbit anti-APP (1:500; Protein Tech Group, Inc., Chicago, IL, USA), polyclonal rabbit anti-VPS35 (1:200; Protein Tech Group, Inc.)] at 4°C overnight, followed by secondary antibody [biotinylated goat anti-rabbit IgG (1:1 000; Beijing Zhongshan Biological Reagent Company, Beijing, China)] at 37°C for 15 minutes. Diaminobenzidine was then applied as the chromogen. Strength of staining was controlled under a microscope (Olympus). Finally, the cells were counterstained with hematoxylin and the expression of APP and VPS35 proteins was detected through using an optical microscope (Olympus). At least four slides were selected from each group, and five non-overlapping fields of vision were randomly selected from each slide. Image Pro Plus software (Olympus Sales and Service Co., Ltd., Beijing, China) was used to measure the expression of target proteins.
Western blot analysis for detecting the expression of APP and VPS35 proteins
According to the BCA kit instructions (Promega Company, Mexico), cells were washed twice for 5 minutes at 100°C. The same amount of protein was placed in each sample. Cell proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, then transferred to a polyvinylidene difluoride membrane (Beyotime Institute of Biotechnology, China), blocked with 5% non-fat milk (Inner Mongolia Yili Industrial Group Co., Ltd., China) which was dissolved in phosphate-buffer for 1 hour at room temperature, incubated with primary antibodies [polyclonal rabbit anti-APP (1:200; Protein Tech Group, Inc.), polyclonal rabbit anti-VPS35(1:200; Protein Tech Group, Inc.)] at 4°C overnight, then incubated with diluted secondary antibody [goat anti-rabbit (1:10 000; Gibco)] for another hour at room temperature, and developed in the dark with enhanced chemiluminescent reagent (Gibco). All antibodies were diluted in antibody diluent (Beyotime Institute of Biotechnology). GAPDH was used as the internal reference for the experiment. Bandscan 5.0 software (Glyko, USA) was used to analyze APP and VPS35 protein expression. The results were expressed as the band absorbance ratio of target protein to GAPDH.
Statistical analysis
The data were analyzed using SPSS 16.0 (SPSS, Chicago, IL, USA) software and were expressed as mean ± SD. One-way analysis of variance and the least significant difference method were used for statistical analysis. A level of P < 0.05 was considered statistically significant.
Acknowledgments
We thank the staff of the Central Laboratory in Renmin Hospital of Wuhan University in China, for technical guidance.
Footnotes
Funding: This study was supported by the National 985 Project “linguistic science technology and the construction of interdisciplinary innovation platform in current society”, No. 985yk002 and the National 985 Project “cognitive and neural information science platform”, No. 904273258.
Conflicts of interest: None declared.
(Edited by Jiang H, Li ZH/Song LP)
REFERENCES
- [1].Zheng CY, Zhang HY, Tang XC. Toxicity of intracellular amyloid-β peptide and mitochondrial permeability transition pore. Shengming Kexue. 2008;20(4):593–598. [Google Scholar]
- [2].Xiao XQ, Wang R, Han YF, et al. Protective effects of huperzine A on beta-amyloid(25-35) induced oxidative injury in rat pheochromocytoma cells. Neurosci Lett. 2000;286(3):155–158. doi: 10.1016/s0304-3940(00)01088-0. [DOI] [PubMed] [Google Scholar]
- [3].Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- [4].Attar N, Cullen PJ. The retromer complex. Adv Enzyme Regul. 2010;50(1):216–236. doi: 10.1016/j.advenzreg.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [5].Kim SR, Lee MK, Koo KA, et al. Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. J Neurosci Res. 2004;76(3):397–405. doi: 10.1002/jnr.20089. [DOI] [PubMed] [Google Scholar]
- [6].Wang L, Tang Y, Huang S. Protective effects of schisandrin B and schisandrin on oxidative damage in PC12 Cells. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2006;10(47):64–66. [Google Scholar]
- [7].Lu H, Liu GT. Effect of dibenzo[a,c]cyclooctene lignans isolated from Fructus schizandrae on lipid peroxidation and anti-oxidative enzyme activity. Chem Biol Interact. 1991;78(1):77–84. doi: 10.1016/0009-2797(91)90104-f. [DOI] [PubMed] [Google Scholar]
- [8].Yan MM, Mao SP, Liu BH, et al. The protective effect of Schisandrin B on PC12 Cells injuries induced by A β25-35. Zuzhong yu Shenjing Jibing. 2010;17(4):212–216. [Google Scholar]
- [9].Chiu PY, Leung HY, Poon MK, et al. Chronic schisandrin B treatment improves mitochondrial antioxidant status and tissue heat shockprotein production in various tissues of young adult and middle-aged rats. Biogerontology. 2006;7(4):199–210. doi: 10.1007/s10522-006-9017-y. [DOI] [PubMed] [Google Scholar]
- [10].Ko KM, Lam BY. Schisandrin B protects against tert-butylhydroperoxide induced cerebral toxicity by enhancing glutathione antioxidant status in mouse brain. Mol Cell Biochem. 2002;238(1-2):181–186. doi: 10.1023/a:1019907316129. [DOI] [PubMed] [Google Scholar]
- [11].Wang B, Wang XM. Schisandrin B protects rat cortical neurons against Abeta1-42-induced neurotoxicity. Pharmazie. 2009;64(7):450–454. [PubMed] [Google Scholar]
- [12].Xiao F, Luo HM, Li XG, et al. Inhibition of γ-schisandrin on the production of amyloid β-protein from M146L cells. Zhongguo Xinyao Zazhi. 2005;14(3):290–292. [Google Scholar]
- [13].Nishida H, Tatewaki N, Nakajima Y, et al. Inhibition of ATR protein kinase activity by schisandrin B in DNA damage response. Nucleic Acids Res. 2009;37(17):5678–5689. doi: 10.1093/nar/gkp593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chang Y, Xue YL. Progress of Alzheimer disease's pathogenesis. Zhongguo Linchuang Kangfu. 2004;8(4):693–695. [Google Scholar]
- [15].Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008;60(4):534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nielsen MS, Gustafsen C, Madsen P, et al. Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA. Mol Cell Biol. 2007;27(19):6842–6851. doi: 10.1128/MCB.00815-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andersen OM, Reiche J, Schmidt V, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102(38):13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Spoelgen R, von Arnim CA, Thomas AV, et al. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J Neurosci. 2006;26(2):418–428. doi: 10.1523/JNEUROSCI.3882-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


