Abstract
A mixture of ibotenic acid and β-amyloid 1–42 was injected into the hippocampus of a rat model of Alzheimer's disease, followed by intragastric administration of a traditional Chinese medicine Buyuan Congnao decoction (main components included radix astragali, radix polygoni multiflori preparata, rhizoma acori talarinowii, radix polygalae, fructus alpiniae oxyphyllae, and radix glycyrrhizae preparata) and a piracetam suspension. Following treatment with traditional Chinese medicine or western medicine, β-amyloid expression decreased and neuronal morphology was normal in the rat hippocampal CA1 region, in addition to significantly shortened average latency in the Morris water navigation task. These findings suggested that compound prescription of Buyuan Congnao decoction, similar to the curative effects of piracetam, decreased hippocampal β-amyloid expression in a rat model of Alzheimer's disease, as well as improved learning and memory.
Keywords: Alzheimer's disease, Buyuan Congnao decoction, β-amyloid, immunofluorescence, Morris water maze, neural regeneration
Abbreviations:
AD, Alzheimer's disease; IBO, ibotenic acid; Aβ, β-amyloid
INTRODUCTION
Previous studies have shown that the imbalance between generation and clearance of β-amyloid (Aβ) leads to Aβ deposition in the brain parenchyma, which is an important pathogenic factor of Alzheimer's disease (AD)[1,2]. Experimental results and clinical data have demonstrated that Aβ neurotoxicity, which persistently damages cerebral neurons, is a common pathway for many factors that lead to AD. This neurotoxicity subsequently leads to progressive cognitive deficits and the formation of senile plaques, which are involved in formation and development of AD neuropathological processes[3,4,5,6,7]. Previous studies have also shown that Aβ40 and Aβ42 are the two main forms of Aβ and co-exist in senile plaques; Aβ42 is more neurotoxic and more easily accumulates than Aβ40[8,9]. Ibotenic acid (IBO) exhibits strong neurotoxicity and damages the cholinergic system, which is associated with learning and memory. The hippocampus is the structure that most closely correlates with learning and memory, and hippocampal atrophy is more prominent in AD[10]. Therefore, the present study injected a mixture of IBO and Aβ1-42 into hippocampal tissue to establish an experimental AD rat model. The animals exhibited behavioral changes (learning and memory deficits) and typical pathological features (Aβ plaque deposition and neuronal apoptosis)[11]. The main component of traditional Chinese medicine Buyuan Congnao decoction comprises radix astragali, radix polygoni multiflori preparata, rhizoma acori talarinowii, radix polygalae, fructus alpiniae oxyphyllae, and radix glycyrrhizae preparata. Radix astragali contains flavones, saponins, and polysaccharides, which protect cells against oxidation[12,13]. Radix polygoni multiflori preparata contains phospholipids, which improve learning and memory, protect the cholinergic system, and inhibit apoptosis[14,15]. The extract of radix polygalae significantly increases choline acetyl transferase and promotes the secretion of nerve growth factor[16,17]; the active ingredient inhibits β-secretase, thereby antagonizing Aβ toxicity[18]. Fructus alpiniae oxyphyllae contains volatile oils, terpenoids, and flavonoids, which exhibit anti-aging and cognitive-increasing effects[19]. The volatile oil of rhizoma acori talarinowii significantly improves dementia symptoms[20], and radix glycyrrhizae contains liquiritin, which exhibits satisfactory neuroprotective effects[21,22,23].
For this reason, the present study utilized immunofluorescence techniques combined with Morris water maze to detect the intervention effects of Buyuan Congnao decoction in an AD rat model.
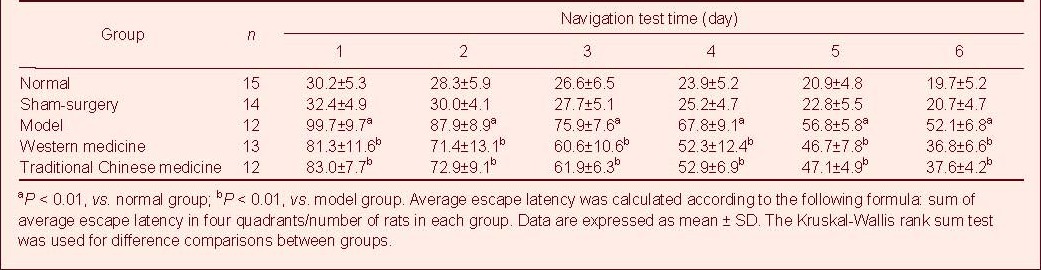
Table 1.
Comparison of average escape latency after treatment
RESULTS
Quantitative analysis of experimental animals
A total of 75 male, Sprague-Dawley rats were randomly assigned to five groups with 15 rats in each group: normal, sham surgery, model (AD model), western medicine (AD model + piracetam intervention), traditional Chinese medicine (AD model + Buyuan Congnao decoction intervention). During experimentation, nine rats died due to anesthesia, drowning in the water maze, or improper intragastric administration. In total, 15 rats from the normal group, 14 from the sham-surgery group, 12 from the model group, 13 from the western medicine group, and 12 from the traditional Chinese medicine group were included in the final analysis.
Neuronal morphology and Aβ expression in the AD rat hippocampus
In the normal group, immunofluorescence revealed neurons with clear morphology, clear nuclear structures, normal axons and dendrites, uneven cytoplasm, and some scattered apoptotic cells and Aβ deposition. In the sham-surgery group, neuronal morphological structure was slightly altered, the number of axons and dendrites decreased, the cytoplasm was uneven, and apoptotic cells and Aβ deposition were increased compared with the normal group. In the model group, neuronal morphology was irregular. Axons, dendrites, and cytoplasm were missing, and there was a large number of apoptotic cells and Aβ deposition. In the western medicine group, neuronal morphology was relatively normal, and compared with the model group, there was an increase in the number of axons and dendrites, although the number of apoptotic cells and Aβ deposition decreased. In the traditional Chinese medicine group, there was a large number of neurons with relatively regular cell morphology, as well as significantly increased cytoplasm, decreased apoptotic cells and Aβ deposition (Figure 1).
Figure 1.
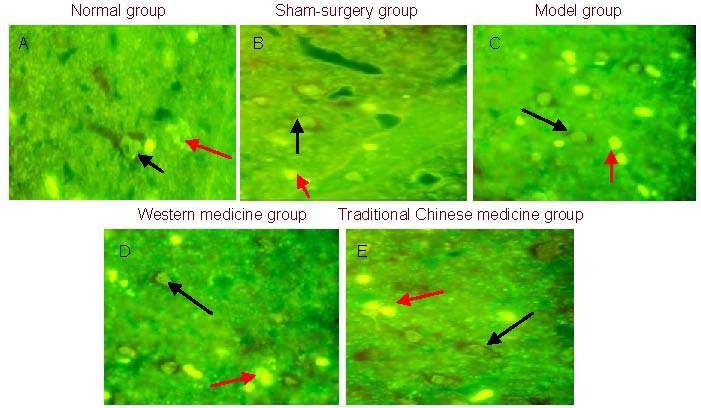
Neuronal expression in the rat hippocampus at 4 weeks post-treatment (immunofluorescence, × 400). Black arrows indicate neurons, red arrows indicate β-amyloid deposition.
In the normal (A) and sham-surgery (B) groups, apoptotic cells and β-amyloid deposition are reduced. In the model group (C), there is a large number of apoptotic cells and β-amyloid deposition. In the western medicine group (D) and traditional Chinese medicine group (E), apoptotic cells and β-amyloid deposition are reduced compared with the model group.
There were significantly more Aβ-positive cells in the rat hippocampus of the model group (25.0 ± 7.38/400-fold visual field) than in the normal group (10.2 ± 2.78/400-fold visual field) or sham-surgery group (10.5 ± 3.69/400-fold visual field, P < 0.01). Compared with the model group, the number of hippocampal Aβ-positive cells significantly decreased in the traditional Chinese medicine group (14.9 ± 2.08/400-fold visual field) and in the western medicine group (15.5 ± 2.76/400-fold visual field, P < 0.01), but there were significantly more hippocampal Aβ-positive cells than in the normal group (P < 0.01). There was no significant difference in the number of Aβ-positive cells between traditional Chinese medicine group and western medicine group (P > 0.05).
Learning and memory in AD rats
Rats from each group were subjected once daily to the Morris water maze for a total of 6 days. Average escape latency was significantly longer in the model group than in the normal group or sham-surgery group (P < 0.01). These results demonstrated that learning and memory significantly decreased. Average escape latency in the western medicine group and traditional Chinese medicine group was significantly less than in the model group (P < 0.01).
DISCUSSION
Clinical manifestations of AD patients include mental deterioration and short-term memory loss. Therefore, learning and memory analysis remains a primary indicator for observing the effects of drug treatments in an AD model[24,25].
The Morris water maze allows rats and mice to learn to find a hidden platform in opaque water. The test is widely used to test spatial learning and memory, and has been widely applied to the study of the hippocampus and the effect of damage outside the hippocampus and drug applications[26]. The present study utilized the Morris water maze for animal selection and to study behavior in AD rats.
Rat learning and memory deficits were successfully induced and both piracetam and the traditional Chinese medicine Buyuan Congnao decoction significantly improved learning and memory in the AD rats.
Inflammatory changes, synaptic loss, and the proliferation of astrocyte and microglia ultimately lead to neuronal apoptosis, especially in regions with obvious Aβ deposition[17,18,19,20,21]. Detecting Aβ deposition in the hippocampal tissue is necessary for analyzing mechanisms of drug treatment and validating the success of AD induction. Aβ expression detection results showed that Aβ injection into the hippocampus increased Aβ expression around hippocampal neurons and that western and traditional Chinese medicines decreased Aβ expression in the hippocampus of AD model rats.
The present study used immunofluorescence to detect Aβ deposition and neuronal expression in the hippocampus to analyze the neuroprotective effects of Buyuan Congnao decoction in AD rats.
Experimental results demonstrated that learning and memory were significantly improved after treatment. In addition, excessive Aβ deposition in the brain was reduced, leading to neuronal repair and regeneration, which suggested reduced Aβ neurotoxicity due to Buyuan Congnao treatment.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
Experiments were performed at the Experiment Center of Liaoning University of Traditional Chinese Medicine from March to September in 2009.
Materials
Animals
A total of 75 healthy, male, Sprague-Dawley rats, aged 18–22 weeks and weighing 280 ± 20 g, were provided by the Laboratory Animal Center, Liaoning University of Traditional Chinese Medicine. Excessively clumsy or sensitive rats were rejected in the water maze test; the remaining rats were randomly assigned to separate cages. Rats were allowed free access to food and water at 20-22°C with 50-70% relative humidity. Experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[27].
Traditional Chinese medicine
Buyuan Congnao decoction comprised radix astragali, radix polygoni multiflori preparata, rhizoma acori talarinowii, radix polygalae, fructus alpiniae oxyphyllae, and radix glycyrrhizae preparata, and was provided by the Department of Pharmacy, Affiliated Hospital of Liaoning University of Traditional Chinese Medicine. A single drug dose contained 185 g crude drug and was prepared into 1 g/mL suspension for use.
Methods
Preparation of condensed matter Aβ1-42
A total of 1 mg Aβ1-42 peptide (Beijing Boao Sen Biotechnology, Beijing, China) was dissolved in 100 μL sterile saline (10 μg/μL solution). The solution was sealed and incubated for 1 week at 37°C to condense. IBO (1 mg) (Sigma, St. Louis, MO, USA) was dissolved in the condensed matter to prepare an IBO and Aβ1-42 mixture. The precise method was in accordance with previously described methods[28].
Establishment of an AD rat model and drug intervention
Model group: following anesthesia by intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g), the rats were fixed into a brain stereotaxic apparatus. A 2.0-cm longitudinal incision was made along the parietal midline. Following hemostasis, the periosteum was bluntly separated and the skull was exposed. According to the stereotactic brain atlas[29], the hippocampus was located (3.5 mm posterior to bregma, 3 mm left lateral to midline, and 5.5 mm below the bregma), and a small 1-mm diameter hole was drilled through the skull with a dental drill. A microinjector was vertically inserted into the hippocampus at a depth of 5.5 mm from the skull surface. The Aβ1-42 and IBO (1 μL) mixture was slowly injected into the hippocampus over a period of 5 minutes, and the needle was then maintained in place for an additional 10 minutes. The needle was then slowly withdrawn. After sprinkling sulfanilamide powder on the incision, the skin was sutured[20].
Sham-surgery group: 1 μL sterile normal saline was injected into the same hippocampal region as the model group.
Normal group: no intervention.
At 2 weeks after AD induction, Morris water maze test[30] results showed significantly longer average escape latency in the model group, which suggested successful AD model establishment. The AD rats were randomly assigned to three groups, with 15 rats in each group: model, western, and traditional Chinese medicine. At 2 weeks after AD induction, the traditional Chinese medicine rats were intragastrically administered Buyuan Congnao decoction. Rats from the western medicine group were intragastrically administered a 30 mg/mL piracetam suspension (Northeast General Pharmaceutical Factory, Shenyang, Liaoning Province, China). According to body mass per unit, the rat drug dose was 6.3 greater than that used in adult humans.
Therefore, the drug dose used for intragrastric administration was 11.1 g/kg per day for traditional Chinese medicine and 0.324 g/kg per day for western medicine. Rats from the normal, sham-surgery, and model groups were intragrastrically administered 3 mL normal saline each morning for a total of 28 days.
Rat behavioral changes, as detected by Morris water maze test
At 2 weeks after AD induction, each rat was subjected to the Morris water maze test once daily, for 6 successive days[31]. Learning and memory were analyzed by determining average escape latency. The rats were placed into the water facing the pool wall in the order of southeast, southwest, northwest, and northeast quadrants. The longest swim time was designated 120 seconds. The time required for rats to locate the platform was recorded as escape latency. Data acquisition and processing was automatically performed using the Morris water maze image process system (Chengdu Tai Meng Technology, Chengdu, Sichuan Province, China).
Following treatment, the Morris water maze test was performed once again.
Histomorphological changes in AD model rats, as detected by Aβ immunofluorescence
Following Morris water maze testing, five rats from each group were randomly selected. Following successful anesthesia by intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g), the abdominal cavity was opened to expose the heart. The right auricle of the heart was incised and 4°C normal saline was rapidly perfused. When clear fluid flowed from the right auricle, 10% formalin was perfused at a speed of 10 mL/min until the body became stiff. The brain was the quickly removed, and hippocampal coronal sections were fixed in 4% paraformaldehyde and further sliced into 35 μm-thick sections through the use of a 1850 Leica microsystem (Leica, Nussloch, Germany).
The sections were incubated in freshly prepared 3% H2O2 solution and pure methanol solution (1:50) for 5-10 minutes at room temperature to inactivate endogenous peroxidase and RNAse. After three washes in distilled water, the sections were incubated with mouse anti-Aβ primary antibody (1:100; Wuhan Boster Bioengineering, Wuhan, Hunan Province, China) for 2 hours at 37°C, followed by wash steps in phosphate-buffered saline, incubation with biotinylated goat anti-mouse IgG (ready to use; Wuhan Boster Bioengineering) for 30 minutes at room temperature, two wash steps in phosphate-buffered saline, incubation with fluorescein isothiocyanate-labeled goat anti-rabbit IgG (ready to use; Wuhan Boster Bioengineering) for 1-2 hours at 37°C, and mounted with 50% glycerin. Finally, the sections were observed and photographed using a BX51 fluorescence microscope (Olympus, Tokyo, Japan) and Aβ-positive cells were quantified through the use of image analysis system[32].
Statistical analysis
The data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA), and the results were expressed as mean ± SD. The means of different groups were analyzed using the Kruskal-Wallis rank sum test. A value of P < 0.05 was considered statistically significant.
Acknowledgments
We would like to express our thanks to the teachers at the Experimental Center and Basic Medical College of Liaoning University of Traditional Chinese Medicine for their great help.
Footnotes
Funding: This work was supported by Liaoning Provincial Education Department, No. 20060551.
Conflicts of interest: None declared.
Ethical approval: The experiment was approved by the Ethics Committee of Liaoning University of Traditional Chinese Medicine in China.
(Edited by Chen GH, Liu SX/Song LP)
REFERENCES
- [1].Vassar R. Beta-Secretase, APP and Abeta in Alzheimer's disease. Subcell Biochem. 2005;38:79–103. [PubMed] [Google Scholar]
- [2].Yang WM, Yang JF, Chen B, et al. Effects of zhinao capsule on learning, memory and cerebral cholinergic neurotransmitter system in rat models of Alzheimer disease. Zhongguo Linchuang Kangfu. 2004;8(28):6262–6264. [Google Scholar]
- [3].Butterfield DA, Poon HF. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer's disease. Exp Gerontol. 2005;40(10):774–783. doi: 10.1016/j.exger.2005.05.007. [DOI] [PubMed] [Google Scholar]
- [4].Billings LM, Oddo S, Green KN, et al. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- [5].Nilsberth C, Westlind-Danielsson A, Eckman CB, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4(9):887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- [6].Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- [7].Holtzman DM, Fagan AM, Mackey B, et al. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer's disease model. Ann Neurol. 2000;47(6):739–747. [PubMed] [Google Scholar]
- [8].Wu Y, JI SR, Jiang WL, et al. Comparison of the aggregation of beta-amyloid peptide 1-42 and beta-amyloid peptide 1-40. Dianzi Xianwei Xuebao. 2003;22(1):21–25. [Google Scholar]
- [9].Cole GM, Frautschy SA. Alzheimer's amyloid story finds its star. Trends Mol Med. 2006;12(9):395–396. doi: 10.1016/j.molmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- [10].Chen X, Zhang ZX, Huang JB, et al. Comparative study on clinical features of Alzheimer's disease and vascular dementia diagnosed on the basis of neuroimaging results. Zhonghua Shenjingke Zazhi. 2004;37(2):109–113. [Google Scholar]
- [11].Kong MW, Wang P, Tian DZ, et al. Establishment and evaluation of Aβ_(25-35) and IBO induced the aged rat model of Alzheimer's disease. Zhongguo Laonianxue Zazhi. 2008;28(10):945–947. [Google Scholar]
- [12].Ouyang H. Should be strengthened in TCM characteristic of positive cognition. Hunan Zhongyi Xueyuan Xuebao. 2006;26(4):6–7. [Google Scholar]
- [13].Zhang J. Talk about “astragalus” pharmacological activities. Zhongwai Yiliao. 2009;28(8):83. [Google Scholar]
- [14].Zhou L, Yuan MS, Xia J, et al. Effect of polygonum multiflorum thumb on apoptosis of hippocampal neurons induced by Aβ. Zhongfeng yu Shenjing Jibing Zazhi. 2006;23(2):143–145. [Google Scholar]
- [15].Liang YS, Ma L. Effect of radix polygoni multiflori preparata polycose on senile dementia in rabbit model. Neimenggu Yixue Zazhi. 2007;39(1):15–17. [Google Scholar]
- [16].Mu JX, Li XY. Effect of yuanzhi on learning and memory and AchE activity of model rats with Alzheimer's disease. Shijie Zhongxiyi Jiehe Zazhi. 2007;2(1):18–20. [Google Scholar]
- [17].Zeng H, Deng BX, Yan J, et al. Effect of saponins of polygala on learning, memory and activities of AchE and ChAT in hippocampus in AD rats. Hunan Zhongyi Xueyuan Xuebao. 2009;29(3):30–32. [Google Scholar]
- [18].Peng Y, Lee DY, Jiang L. Huperzine A regulates amyloid precursor protein processing via protein kinase C and mitogen-activated protein kinase pathways in neuroblastoma SK-N-SH cells over-expressing wild type human amyloid precursor protein 695. Neuroscience. 2007;150(2):386–395. doi: 10.1016/j.neuroscience.2007.09.022. [DOI] [PubMed] [Google Scholar]
- [19].You WH, He S. Effect of alp inia oxyphyllaMiq. feut on metabolism of free radicals and ultrastructure of liver in exercised mice. Disi Junyi Daxue Xuebao. 2007;28(23):2160–2162. [Google Scholar]
- [20].Zhang LT. Research Acorustatarinowii Schott central nervous system. Zhongguo Xiandai Yixue Zazhi. 2007;3(9):141–142. [Google Scholar]
- [21].Liu RT, Bian GX, Zou LB, et al. Neuroprotective effects of liquiritin and its inhibitory actions on cholinesterase activity. Zhongguo Xinyao Zazhi. 2008;17(7):574–581. [Google Scholar]
- [22].Zhang MT, Qian YH, Yang J, et al. Protection of tanshinone ⅡA on cellular damage induced by glutamate and β-amyloid protein. Zhongguo Laonianxue Zazhi. 2009;29(19):2465–2468. [Google Scholar]
- [23].Ji ZH, Yu XY, Chen X. The AOF's improving function of memory disorders of rats in cerebral ischemia. Zhonghua Xingwei Yixue he Naokexue Zazhi. 2009;18(7):580–582. [Google Scholar]
- [24].Blusztajn JK, Berse B. The cholinergic neuronal phenotype in Alzheimer's disease. Metab Brain Dis. 2000;15(1):45–64. doi: 10.1007/BF02680013. [DOI] [PubMed] [Google Scholar]
- [25].Jankowsky JL, Melnikova T, Fadale DJ, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2005;25(21):5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fassbender K, Masters C, Beyreuther K. Alzheimer's disease: molecular concepts and therapeutic targets. Naturwissenschaften. 2001;88(6):261–267. doi: 10.1007/s001140100237. [DOI] [PubMed] [Google Scholar]
- [27].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [28].Wang J, Chen HX, Chen M. Buyuan Congnao decoction on the effects of ad's cholinergic system. 2010;4:88–89. [Google Scholar]
- [29].Paxions G, Watson C. 2nd ed. San Diego: Academic Press; 1986. The Rat Brain in Stereotaxic Coodinates. [Google Scholar]
- [30].Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- [31].Zhang LH, Wang X, Zheng W, et al. The localization of zinc transporter1 (ZNT1) in Alzheimer's disease brain. Jiepouxue Jinzhan. 2007;13(4):310–313. [Google Scholar]
- [32].Jiang SJ, Yang XF, Li JM. The expression of β-amyloid precursor protein in brain tissue of pharmacoresistant epilepsy patients by immunofluorescence examination. Huaxi Yixue. 2009;24(1):32–34. [Google Scholar]


