Abstract
Human thioredoxin and antibacterial peptide, PR39, have been shown to have potent antioxidant effects that may prolong survival of cells during hypoxia. The pSSCMV/human thioredoxin-PR39 vector was successfully constructed in this study and used to infect ECV304 cells. Transfected ECV304 cells were incubated at 1%, 5% hypoxic, and normal oxygen conditions. We found that the number of apoptotic cells after transfection with recombinant adeno-associated virus-human thioredoxin -PR39 was significantly lower than controls, suggesting a protective effect of the recombinant human thioredoxin-PR39 protein on hypoxic cells.
Keywords: human thioredoxin, antimicrobial peptide PR39, fusion gene, recombinant adeno-associated virus, gene therapy, apoptosis, hypoxia
Abbreviations:
hTRX, human thioredoxin; PR39, antibacterial peptide 9; rAAV, recombinant adeno-associated virus
INTRODUCTION
Gene therapy for central nervous system diseases often involves functional proteins; however, large molecules cannot cross the blood-brain barrier and thus are ineffective for the treatment of the brain. Therefore, there is an urgent need for improved delivery into the central nervous system. Human thioredoxin (hTRX) is a micromolecular protein that can act as both an oxidant and a reductant. The hTRX gene is 13 kb long and encodes 104 amino acids[1,2]. The hTRX protein homology consists of a protein cross-frame feature that provides available sites for binding active aptamer. The aptamer that has been inserted into the cross-frame, becomes more stable than free peptide and is more prone to transfer into cells[3]. As hTRX is a natural human protein with low immunogenicity, it serves as a cross-frame protein to construct a gene fusion expression system and can significantly increase the activity of expression products and activated soluble proteins[4].
Antibacterial peptide 9 (PR39) is a short peptide that is extremely unstable and is prone to inactivating conformational changes. Thus, therapeutic use of PR39 requires it to be expressed as a recombinant protein. Currently, studies of PR39 have mainly focused on two issues. (1) Direct synthesis of PR39 shows that it is significantly effective for a short time, but the half-life is relatively short; PR39 is rapidly degraded in vivo and expressed for only a short time, and since the cost of production is high, it has limited clinical application. (2) Adenoviruses can mediate PR39 expression in vascular endothelial cells and promote effects on blood vessels in the ischemic myocardium. Adenoviruses can act as gene expression vectors because of their ability to reach high titers, can infect a variety of cells, including non-dividing cells, and exogenous genes can be instantaneously and highly expressed. However, adenoviruses often induce inflammatory or toxic reactions that result in the recombinant gene not being expressed long term[5].
The hTRX protein can provide a framework for the expression of PR39 as a therapeutic aptamer. It has been previously shown that insertion of PR39 into the hTRX framework results in increased stability of PR39 compared with the free peptide with hTRX providing blood-brain barrier penetration[6,7]. Thus, the hTRX-PR39 chimeric protein provides structural compatibility to ensure both the directed bioavailability of PR39 at the target site and added stability of the protein. This study aimed to construct a recombinant adeno-associated virus (rAAV) vector containing a hTRX-PR39 chimeric gene to produce sustained, stable, and efficient expression of gene products in hypoxic ECV304 cells. These studies provide the basis for the investigation of in vivo expression of the chimeric protein to limit the effects of hypoxia.
RESULTS
Successful construction of recombinant plasmid pSSCMV/hTRX-PR39
Target gene segments of 5 020 and 438 bp were obtained after EcoRI and BamHI digestion of recombinant plasmids indicating that the hTRX-PR39 fragment had been inserted into the pGEM-T easy vector (Figure 1; supplementary Figures 1, 2 online).
Figure 1.
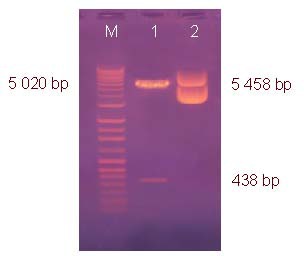
Identification of recombinant plasmid pSSCMV/hTRX-PR39 by restriction enzyme digestion.
Lane M: DNA molecular weight marker; 1: pSSCMV/hTRX-PR39 digested with EcoRI, BamHI; 2: pSSCMV/hTRX-PR39 (5 458 bp). hTRX: Human thioredoxin; PR39: antibacterial peptide 9.
Titers of hTRX-PR39 recombinant AAV
Viral titers were determined using dot blot assay (Figure 2). For comparison, the concentration of viral DNA was determined to be 10-100 ng/μL, which correlated to 3.46 × (109 - 1010) virus particles/μL.
Figure 2.

Titer of recombinant adeno-associated virus determined by quantitative dot-blot hybridization analysis.
(A) Standard pSSCMV/human thioredoxin-antibacterial peptide 9 plasmid DNA.
(B) Viral DNA samples.
Recombinant hTRX-PR39 expressed in ECV304 cells
Microscopy of stained cells revealed that a large number of hTRX brown granules (98.5 ± 1.5%) were visible within the cytoplasm of ECV304 cells in the rAAV-hTRX-PR39 treated group, while no hTRX expression was observed in the control group (1.5 ± 0.6%; P < 0.01). This is evidence that recombinant fusion protein, hTRX-PR39, was expressed in ECV304 cells (Figure 3).
Figure 3.
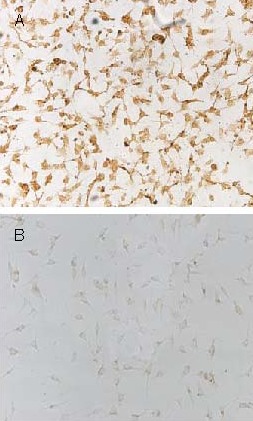
Expression of human thioredoxin (hTRX)-antibacterial peptide 9 (PR39) in ECV304 cells (× 100).
(A) Stained brown granules indicate expression of hTRX protein in the recombinant adeno-associated virus-hTRX-PR39 treated group.
(B) A small number of hTRX proteins were expressed in the untreated control group.
Recombinant hTRX-PR39 improved hypoxia-induced injury in ECV304 cells
Upon incubation under hypoxic conditions, the number of cells in the control group was significantly reduced and cells became thinner with several processes. However, cells in the rAAV-hTRX-PR39 group were only slightly reduced in number and exhibited a complete form, but severe hypoxia led to great changes in cell morphology (Figure 4).
Figure 4.
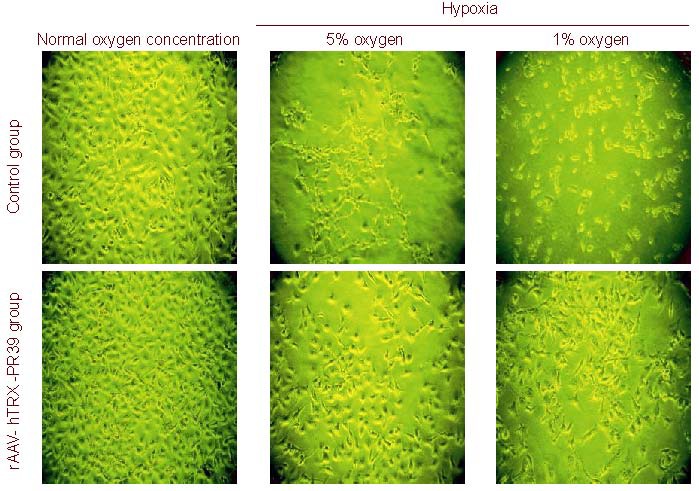
Changes of ECV304 cell morphology at different oxygen concentrations.
In control group, the number of cells was significantly reduced, cell morphology became sharpened, and several processes formed. Severe hypoxia could lead to great cell damage. The cell morphology was improved after recombinant adeno-associated virus (rAAV)-human thioredoxin (hTRX)-antibacterial peptide 9 (PR39) treatment.
Recombinant hTRX-PR39 inhibited hypoxia-induced apoptosis of ECV304 cells
After ECV304 cells were cultured at different oxygen concentrations for 24, 48, 72 hours, the number of viable cells in each group was the same at the beginning of the experiment as determined by inverted fluorescence microscopy (P > 0.05). Under hypoxic conditions, the number of viable cells was significantly increased after treatment with rAAV-hTRX-PR39 (P < 0.01; Table 1). Flow cytometric analysis of cells cultured under severe hypoxia (1% oxygen) revealed that the apoptotic rate in the rAAV-hTRX-PR39 group was significantly lower than that in the control group (7.35 ± 0.43% vs. 32.58 ± 0.39%; P < 0.05), suggesting that the recombinant hTRX-PR39 proteins can prevent hypoxia-induced apoptosis at a higher rate than cells in the control group (Figure 5).
Table 1.
The number of viable cells (/200-fold field) in each group after treatment with rAAV-hTRX-PR39 and different concentrations of oxygen
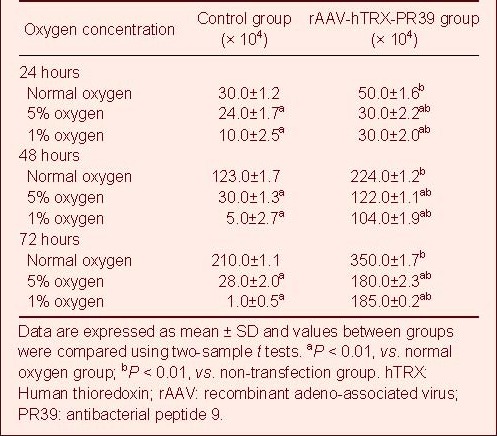
Figure 5.
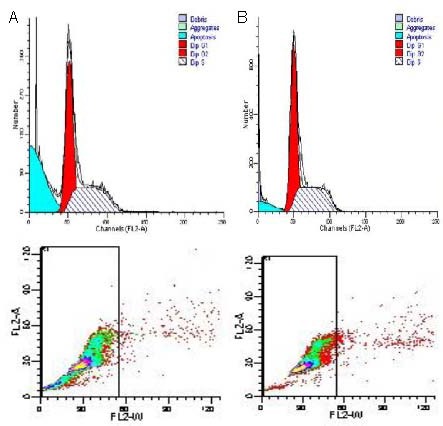
Cell cycle analysis of ECV304 cells cultured with 1% oxygen determined by flow cytometry. Red dots represent propidium iodide stained cells, green dots represent FITC stained cells, and the green area represents apoptotic cells.
(A) Control group apoptosis rate was 32.58 ± 0.39%.
(B) rAAV-hTRX-PR39 group apoptosis rate was 7.35 ± 0.43%.
rAAV: Recombinant adeno-ssociated virus; hTRX: human thioredoxin; PR39: antibacterial peptide 9; FITC: fluoresceine isothiocyanate.
DISCUSSION
This study showed that rAAV carrying hTRX-PR39 was successfully constructed and expressed in ECV304 cells. Therefore, the hTRX-PR39 fusion gene can be expressed in cells, and rAAV can deliver the gene into cells to produce the bioactive short-peptide in eukaryotic cells.
Under normoxic conditions, ECV304 cell morphology and viability was not significantly different between the control group and the rAAV-hTRX-PR39 treated group, and the number of viable cells was much higher than the control group regardless of hTRX-PR39 transfection.
Under hypoxic (1% and 5% oxygen) conditions, the number of cells was significantly reduced in the control group, with cell morphology changing to thin with several processes. However, for the rAAV-hTRX-PR39 treated group, the number of cells only decreased slightly and maintained a typical round shape. After hTRX-PR39 transfection, the number of surviving cells was significantly higher than that of non-transfected cells, and the cell survival rate decreased with increasing oxygen concentration. This is evidence that hTRX-PR39 can inhibit cellular apoptosis and protect cells against hypoxia. Flow cytometric analysis of apoptosis also agreed with these results.
The mechanism of protection that PR39 affords may be protection of IAP-2 (an inhibitor of apoptosis) and decreased activity of caspase-3, leading to suppression of apoptosis[8,9,10]. Increasing the concentration of PR39 in hypoxic tissue has been shown to prevent apoptosis and promote vascular growth[11,12,13,14]. Growing evidence indicates that PR39 can reduce infarction size during the early ischemic stage, promote angiogenesis in ischemic regions, establish effective collateral circulation, and prevent microcirculation dysfunction[15,16,17]. Likewise, the protective effects of hTRX on hypoxic cells may be related to antioxidant properties[18,19,20], anti-apoptosis, scavenging free-radicals, growth factors, cytokine-like effects[21,22,23,24], and a synergistic effect with PR39, as observed in the current study. Therefore, rAAV-hTRX-PR39 conferred a protective effect on hypoxic cells at the cellular level.
MATERIALS AND METHODS
Design
A cell biological experiment of vector construction and transfection.
Time and setting
Experiments were performed from April 2008 to December 2009 in the Laboratory of Xi’an Huaguang Biological Engineering, China.
Materials
The pGEM-T-hTRX-PR39 cloning vector containing hTRX-PR39 full-length gene sequence was constructed as previously described[25]. The pSSCMV viral vector, adenovirus plasmid PFG140, assistant plasmid pAAV/Ad, E. coli TOP10, ECV304 cell lines, and 293 cell lines were provided by Xi’an Huaguang Biological Engineering Co., Ltd., China[26].
Methods
Construction and identification of pSSCMV/hTRX-PR39 plasmid vector
The pGEM T-hTRX-PR39 plasmid was isolated from transformed E. coli using the standard alkaline lysis method[27]. The hTRX-PR39 cDNA fragment was obtained from this plasmid after restriction enzyme digestion with EcoRI and BamHI (Xi’an Huaguang Biological Engineering Co., Ltd., China). The resultant digested fragment was ligated to pSSCMV viral vectors using T4 DNA ligase (Xi’an Huaguang Biological Engineering, China), to obtain pSSCMV/hTRX-PR39. The plasmid was used to transform competent E. coli TOP10 using the CaCl2 transformation method[28]. Transformed bacteria were selected on LB agar plates containing ampicillin, cultured at 37°C for 16 hours. White colonies were used to inoculate liquid LB medium and incubated overnight, and then stored at 4°C. A small amount of plasmid DNA was prepared using the alkaline lysis method and electrophoresed through 0.1% agarose gel after EcoRI digestion. DNA bands were observed using a short-wave ultraviolet analyzer (Shanghai Huayan Instrument Factory, China). The pSSCMV/hTRX-PR39 plasmid was isolated using the alkali rupture method. The pSSCMV/hTRX-PR39 plasmid vector, adenovirus vector PFG140, and pAAV/Ad assistant plasmid were transfected into 293 cells (80% confluence) by the DNA-calcium phosphate coprecipitation method[29]. The formation of fine precipitates was visible on the cell surface by optical microscopy (Olympus, Tokyo, Japan). Cells were cultured in Dulbecco's modified Eagles medium containing 10% fetal bovine serum at 37°C in a CO2 incubator for 72 hours (Figure 6).
Figure 6.
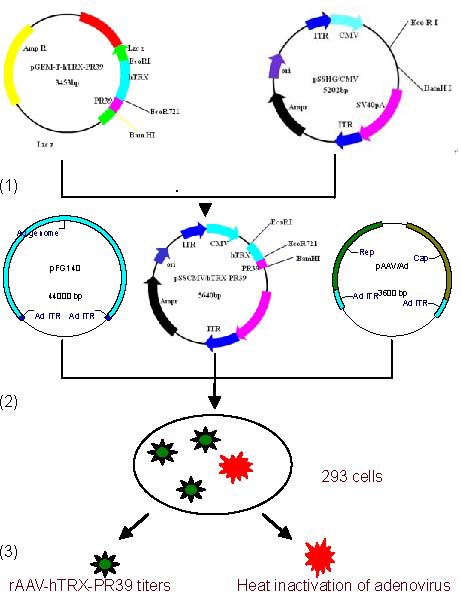
Strategy used to construct hTRX-PR39 recombinant adeno-associated virus (rAAV) vectors.
(1) pSSCMV/hTRX-PR39 vector plasmid was prepared by alkaline lysis.
(2) Co-transfection of three plasmids (pSSCMV/hTRX-PR39, pFG140, and adenovirus assistant plasmid, pAAV/Ad).
(3) Titration of heat-inactivated adenovirus. hTRX: Human thioredoxin; PR39: antibacterial peptide 9.
Recovery of recombinant viral particles and determination of viral titer
Cells were collected 72 hours after transfection and resuspended in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid medium, followed by three repeated freezing/thawing cycles of -20°C freeze and 37°C water bath. Cells were lysed using an ultrasonic cell crusher (Ningbo Xinyi Ultrasound Equipment, China) and adenovirus was inactivated at 56°C. Viral titers were determined by dot blot analysis of the DNA content[30].
Transfected ECV304 cells with pSSCMV/hTRX-PR39 recombinant adenovirus vector
ECV304 cell lines were transferred to coverslips placed in culture flasks along with Dulbecco's modified Eagle medium containing 10% fetal bovine serum and cultured at 37°C in a 5% CO2 incubator (Thermo Electron Corporation, USA). Recombinant virus was seeded into the culture medium and incubated at 37°C in a 5% CO2 incubator for 12 hours. Cells were divided into a control (untreated) group and a rAAV-hTRX-PR39 treated group treated with 3.4 × 108 pfu rAAV. Coverslips were transfected for 60 hours and removed for processing.
Expression of hTRX-PR39 in ECV304
Cells grown and infected on coverslips were fixed in acetone at room temperature for 15 minutes and rinsed three times with 0.1 M phosphate-buffered saline (pH 7.4) for 5 minutes. Cells were then treated with 50 μL 0.75% H2O2 at 37°C for 30 minutes to block endogenous peroxidase activity. Cells were rinsed again three times with phosphate buffered saline for 5 minutes, and incubated with 50 μL 0.5% Triton X-100 for 30 minutes. Coverslips were then washed three times of phosphate buffered saline for 30 minutes each. Cells were blocked with 50 μL 20% bovine serum albumin at 37°C in a humidified chamber for 30 minutes to eliminate non-specific staining. Cells were then incubated with mouse anti-hTRX monoclonal antibody (1:500; Xi’an Huaguang Biological Engineering, China) for 4 hours in a humidified chamber, followed by five washes with phosphate buffered saline for 5 minutes. Cells were incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (1:500; Beijing Zhongshan Company, China) at 37°C for 2 hours in a humidified chamber, followed by three washes with phosphate buffered saline for 5 minutes. Cells were finally stained using 0.5 mg/mL diaminobenzidine and 0.03% H2O2 for 10-20 minutes. Gene expression was detected using an inverted microscope (model 1X71; Olympus, Japan) where the number of positive cells among 100 randomly selected cells from 10 fields-of-view at 200 × magnification were counted. Thus, the percentage of positive cells represented the level of protein expression.
Hypoxic intervention
Both control group and rAAV-hTRX-PR39 group cells were divided into three sub-groups and incubated at 1% oxygen, 5% oxygen, and normal concentration of oxygen. All cells were grown at 37°C in 5% CO2 incubator for 24, 48, 72 hours. Cellular growth was observed using an inverted fluorescence microscope.
Cell viability
At 24, 48, 72 hours culture, cells were resuspended and mixed with 60% trypan blue saline solution for 1 minute. Viable cells were quantified by microscopy (model 1X71; Olympus, Japan).
Cell apoptosis by flow cytometry
At 24, 48, 72 hours of culture, the ECV304 cells in the control group (1% oxygen condition) and rAAV-PR39 group were washed twice with cold phosphate buffered saline and resuspended to 1 × 106/mL in binding buffer. Three 100 μL aliquots of cells were incubated with 5 μL Annexin V-fluoresceine isothiocyanate and 5 μL propidium iodide using gentle oscillation at room temperature for 15 minutes while being protected from light. Cell apoptosis was detected by using 400 μL staining buffer and flow cytometer (Shanghai Jieweifu Industrial Co., Ltd., China).
Statistical analysis
Data were expressed as mean ± SD using SPSS analysis of variance and homogeneity of variance test according to completely randomized design.
Comparisons between groups were performed using the two-sample t-test. A P-value of < 0.05 was considered statistically significant.
Acknowledgments
We would like to thank Professor Qingyong Liu, from Jinan Central Hospital Affiliated to Shandong University, for great support and dedication in the study design and provision of reagents; Shibao Zhang, Bo Liu and Shuangqing Liu, from Jinan Central Hospital Affiliated to Shandong University, for data collection, statistical analyses and image acquisition; Professor Jianzhong Bi, from the Second Affiliated Hospital of Shandong University, for research guidance; and Professor Aiqin Song, from the Department of Statistics at Jining Medical College for strong statistical support.
Footnotes
Funding: This study was sponsored by the National Natural Science Foundation of China, No. 30970992.
Conflicts of interest: None declared.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org, and entering Vol. 7, No. 9, 2012 item after selecting the “NRR Current Issue” button on the page.
(Edited by Li XG, Zhang GH/Yang Y/Wang L)
REFERENCES
- [1].Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- [2].Spector A, Yan GZ, Huang RR, et al. The effect of H2O2 upon thioredoxin-enriched lens epithelial cells. J Biol Chem. 1988;263(10):4984–4990. [PubMed] [Google Scholar]
- [3].Anbanandam A, Albarado DC, Tirziu DC, et al. Molecular basis for proline- and arginine-rich peptide inhibition of proteasome. J Mol Biol. 2008;384(1):219–227. doi: 10.1016/j.jmb.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Borghouts C, Kunz C, Delis N, et al. Monomeric recombinant peptide aptamers are required for efficient intracellular uptake and target inhibition. Mol Cancer Res. 2008;6(2):267–281. doi: 10.1158/1541-7786.MCR-07-0245. [DOI] [PubMed] [Google Scholar]
- [5].Post MJ, Sato K, Murakami M, et al. Adenoviral PR39 improves blood flow and myocardial function in a pig model of chronic myocardial ischemia by enhancing collateral formation. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R494–500. doi: 10.1152/ajpregu.00460.2005. [DOI] [PubMed] [Google Scholar]
- [6].Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57(4):529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- [7].Deshayes S, Morris MC, Divita G, et al. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci. 2005;62(16):1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hermann DM, Kilic E, Kügler S, et al. Adenovirus-mediated GDNF and CNTF pretreatment protects against striatal injury following transient middle cerebral artery occlusion in mice. Neurobiol Dis. 2001;8(4):655–666. doi: 10.1006/nbdi.2001.0399. [DOI] [PubMed] [Google Scholar]
- [9].Muinck ED, Nagy N, Tirziu D, et al. Protection against myocardial ischemia-reperfusion injury by the angiogenic Masterswitch protein PR 39 gene therapy: the roles of HIF1alpha stabilization and FGFR1 signaling. Antioxid Redox Signal. 2007;9(4):437–445. doi: 10.1089/ars.2006.1501. [DOI] [PubMed] [Google Scholar]
- [10].Wu J, Parungo C, Wu G, et al. PR39 inhibits apoptosis in hypoxic endothelial cells: role of inhibitor apoptosis protein-2. Circulation. 2004;109(13):1660–1667. doi: 10.1161/01.CIR.0000124067.35915.E0. [DOI] [PubMed] [Google Scholar]
- [11].Nie XW, Sun LJ, Hao YW, et al. Construction of a general AAV vector regulated by minimal and artificial hypoxic-responsive element. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011;27(3):278–280. 283. [PubMed] [Google Scholar]
- [12].Hao YW, Sun LJ, Liu Y, et al. Secretory expression of PR39 following adeno-associated viral-encoding fusion gene transfer induces angiogenesis in hypoxia chick embryo. Zhonghua Xin Xue Guan Bing Za Zhi. 2009;37(8):746–749. [PubMed] [Google Scholar]
- [13].Wen YA, Yu XL, Xia QF, et al. Macrophage-specific overexpression of antimicrobial peptide PR-39 inhibits intracellular growth of Salmonella enterica serovar Typhimurium in macrophage cells. Int J Antimicrob Agents. 2009;34(4):315–321. doi: 10.1016/j.ijantimicag.2009.04.014. [DOI] [PubMed] [Google Scholar]
- [14].Rodríguez-Martínez S, Cancino-Diaz JC, Vargas-Zuñiga LM, et al. LL-37 regulates the overexpression of vascular endothelial growth factor (VEGF) and c-IAP-2 in human keratinocytes. Int J Dermatol. 2008;47(5):457–462. doi: 10.1111/j.1365-4632.2008.03340.x. [DOI] [PubMed] [Google Scholar]
- [15].Korthuis RJ, Gute DC, Blecha F, et al. PR-39, a proline/arginine- rich antimicrobial peptide, prevents postischemic microvascular dysfunction. Am J Physiol. 1999;277(3 Pt 2):H1007–1013. doi: 10.1152/ajpheart.1999.277.3.H1007. [DOI] [PubMed] [Google Scholar]
- [16].Hoffmeyer MR, Scalia R, Ross CR, et al. PR-39, a potent neutrophil inhibitor, attenuates myocardial ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2000;279(6):H2824–2828. doi: 10.1152/ajpheart.2000.279.6.H2824. [DOI] [PubMed] [Google Scholar]
- [17].Gennaro R, Zanetti M, Benincasa M, et al. Pro-rich antimicrobial peptides from animals: structure, biological functions and mechanism of action. Curr Pharm Des. 2002;8(9):763–778. doi: 10.2174/1381612023395394. [DOI] [PubMed] [Google Scholar]
- [18].Yegorova S, Yegorov O, Lou MF. Thioredoxin induced antioxidant gene expressions in human lens epithelial cells. Exp Eye Res. 2006;83(4):783–792. doi: 10.1016/j.exer.2006.03.018. [DOI] [PubMed] [Google Scholar]
- [19].Kaimul Ahsan M, Nakamura H, Tanito M, et al. Thioredoxin-1 suppresses lung injury and apoptosis induced by diesel exhaust particles (DEP) by scavenging reactive oxygen species and by inhibiting DEP-induced downregulation of Akt. Free Radic Biol Med. 2005;39(12):1549–1559. doi: 10.1016/j.freeradbiomed.2005.07.016. [DOI] [PubMed] [Google Scholar]
- [20].Yegorova S, Yegorov O, Lou MF. Thioredoxin induced antioxidant gene expressions in human lens epithelial cells. Exp Eye Res. 2006;83(4):783–792. doi: 10.1016/j.exer.2006.03.018. [DOI] [PubMed] [Google Scholar]
- [21].Kobayashi-Miura M, Nakamura H, Yodoi J, et al. Thioredoxin, an anti-oxidant protein, protects mouse embryos from oxidative stress-induced developmental anomalies. Free Radic Res. 2002;36(9):949–956. doi: 10.1080/1071576021000006626. [DOI] [PubMed] [Google Scholar]
- [22].Isowa N, Yoshimura T, Kosaka S, et al. Human thioredoxin attenuates hypoxia-reoxygenation injury of murine endothelial cells in a thiol-free condition. J Cell Physiol. 2000;182(1):33–40. doi: 10.1002/(SICI)1097-4652(200001)182:1<33::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [23].Sasada T, Iwata S, Sato N, et al. Redox control of resistance to cis-diamminedichloroplatinum (II) (CDDP): protective effect of human thioredoxin against CDDP-induced cytotoxicity. J Clin Invest. 1996;97(10):2268–2276. doi: 10.1172/JCI118668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maruyama T, Sachi Y, Furuke K, et al. Induction of thioredoxin, a redox-active protein, by ovarian steroid hormones during growth and differentiation of endometrial stromal cells in vitro. Endocrinology. 1999;140(1):365–372. doi: 10.1210/endo.140.1.6455. [DOI] [PubMed] [Google Scholar]
- [25].Ruan XY, Bi JZ, Liu QY, et al. Construction and identification of recombinant plasmids expressing hTRX-PR39. Shandong Daxue Xuebao: Yixue Ban. 2009;47(3):30–34. [Google Scholar]
- [26].Liu QY, Ruan XY, Liu XG, et al. Cloning and expression of a cDNA sequence for human thioredoxin. Xi’an Jiaotong Daxue Xuebao. 2003;15(2):183–188. [Google Scholar]
- [27].Feliciello I, Chinali G. A modified alkaline lysis method for the preparation of highly purified plasmid DNA from Escherichia coli. Anal Biochem. 1993;212(2):394–401. doi: 10.1006/abio.1993.1346. [DOI] [PubMed] [Google Scholar]
- [28].Lu SD. 2nd ed. Beijing: Peking Union Medical College Press; 1999. Current Protocols for Molecular Biology. [Google Scholar]
- [29].Gray-Munro JE, Strong M. The mechanism of deposition of calcium phosphate coatings from solution onto magnesium alloy AZ31. J Biomed Mater Res A. 2009;90(2):339–350. doi: 10.1002/jbm.a.32107. [DOI] [PubMed] [Google Scholar]
- [30].Nishizawa T, Okamoto H, Konishi K, et al. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241(1):92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]


