Abstract
Objectives
The goal of this study was to determine the correlation of clinicopathological factors and the up-regulation of vascular endothelial growth factor (VEGF) expression in oral squamous cell carcinoma.
Materials and Methods
Immunohistochemical staining of VEGF and quantitative real-time polymerase chain reaction (RT-PCR) of VEGF mRNA were performed in 20 specimens from 20 patients with oral squamous cell carcinoma and another 20 specimens from 20 patients with carcinoma in situ as a controlled group.
Results
The results were as follows: 1) In immunohistochemical study of poorly differentiated and invasive oral squamous cell carcinoma, high-level staining of VEGF was observed. Significant correlation was observed between immunohistochemical VEGF expression and histologic differentiation, tumor size of specimens (Pearson correlation analysis, significance r>0.6, P<0.05). 2) In VEGF quantitative RT-PCR analysis, progressive cancer showed more VEGF expression than carcinoma in situ. Paired-samples analysis determined the difference of VEGF mRNA expression level between cancer tissue and carcinoma in situ tissue, between T1 and T2-4 (Student's t-test, P<0.05).
Conclusion
These findings suggest that up-regulation of VEGF may play a role in the angiogenesis and progression of oral squamous cell carcinoma.
Keywords: Squamous cell carcinoma, Vascular endothelial growth factor, Real-time polymerase chain reaction, Immunohistochemistry
I. Introduction
Angiogenesis is observed in inflammatory reactions, wound healing, and immune reactions. Within tumors, new blood vessel growth is essential for progression and metastasis1. The term angiogenesis was first used in 1971 by Folkman, and researchers have advised that tumors can grow by forming new blood vessels from the existing blood vascular system, and that angiogenesis is closely related to not only tumors, but also various other diseases such as proliferative retinopathies, age-related macular degeneration, and rheumatoid arthritis2,3. In the case of mammals, oxygen and nutrient supply are indispensable for survival; since diffusion-controlled oxygen supply to cells cannot function when cells are located farther than 100 to 200 µm from blood vessels, tumor cells cannot grow more than several cm or metastasize to other organs without angiogenesis4,5. This angiogenetic process is activated by factors produced by tumors to form new blood vessels through basal membrane decomposition by the protease secreted by tumor cells and to support the movement and proliferation of vascular endothelial cells6.
Approximately 20 different factors that induce the angiogenesis process are known, such as basic fibroblastic growth factor, placenta growth factor (PIGF-1), epidermal growth factor, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF)7,8. The most important angiogenic factor is VEGF, which was first identified by Ferrara and Henzel9 in a bovine pituitary gland follicle cell culture medium.
VEDF is a 46 kDa heparin-binding homodimeric glycoprotein. To date, in addition to VEGF-A, PIGF-1, VEGF-B, VEGF-C, and VEGF-D are known10,11,12. These VEGFs respectively bind to VEGF receptor-1 (VEGF-1 or Flt-1) and VEGF receptor-2 (VEGF-2 or KDR/Flk-1) to promote endothelial cell differentiation and proliferation. Many studies have demonstrated increases in the expression of VEGFs in the processes of carcinoma progression and proliferation13,14,15,16.
Oral cancers show lower than 50% long-term survival rates because of their high metastasis and recurrence rates, and their prognoses have not greatly improved, despite the development of various treatment methods, due to their high probability of local recurrence and metastasis17. In addition, although the treatment plans and prognoses of patients clinically diagnosed with oral squamous cell carcinoma mainly follow the TNM classification, treatment results do not coincide with these criteria in many cases. Accordingly, studies are needed to identify markers that will enable a more accurate patient prognosis based on the molecular biological characteristics of carcinomas. They are also elements that affect the occurrence, progression, and metastasis of carcinomas.
Therefore, the present study was conducted to examine the expression profiles of VEGFs involved in angiogenesis, which is important for carcinoma progression according to the histological characteristics of oral squamous cell carcinoma, and to find a correlation between patient clinical information and differences in VEGF expression according to TNM classification. In addition, for an indirect comparison of the relationship between carcinoma progression and VEGF expression, differences in the expression of oral squamous cell carcinoma and intraepithelial carcinoma were examined.
II. Materials and Methods
1. Study materials
For study materials, we used 20 tissue slices excised after surgery from 20 patients diagnosed with oral squamous cell carcinoma in the Department of Oral and Maxillofacial Surgery, College of Dentistry, Dankook University, and 20 tissue slices excised from 20 patients diagnosed with intraepithelial carcinoma. The specimens were divided into three groups of well, moderate, and poor histological differentiation. Tumor size classification based on TNM staging was performed, and the specimens were divided into four groups, T1 to T4.(Table 1)
Table 1. Correlation between immunohistochemical VEGF expression and clinical and pathological factors.
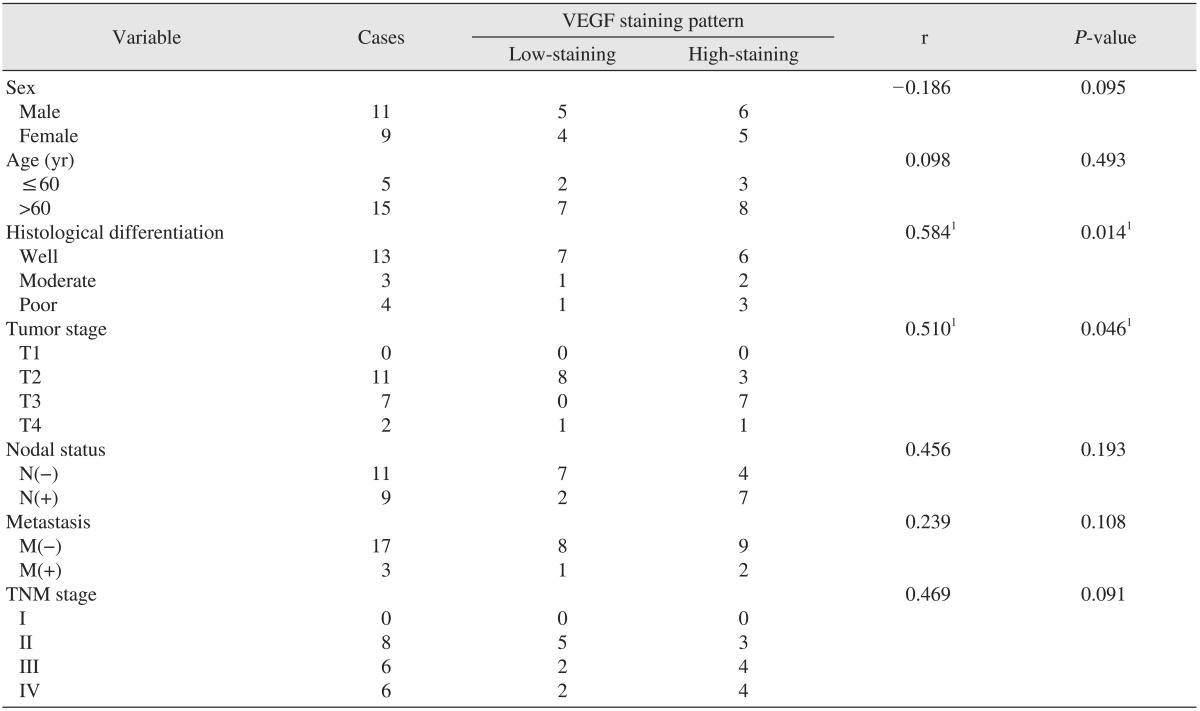
(VEGF: vascular endothelial growth factor)
1Pearson correlation analysis, significance r>0.6, P<0.05.
Values are presented as number.
The excised tissues were fixed for eight to 12 hours using 10% neutral-buffered formalin and then made into paraffin blocks in the usual method.
2. Study methods
1) Immunohistochemical staining of VEGF
The excised tissues were fixed and made into 4 µm paraffin slices on poly-L-lysine-treated slides. After removing the paraffin using the usual method, the tissues were treated with 0.01 M citrate buffer (pH 6.0) for 15 minutes in a pressure cooker to retrieve the antigens, treated with hydrogen peroxide/methanol for 15 minutes, and then treated with normal goat serum for 20 minutes to prevent nonspecific binding with endogenous peroxidase. A polyclonal antibody (Abcam, Cambridge, MA, USA) against VEGF was diluted 1 : 25 and applied to the tissues, which were then incubated for at least eight hours at 4℃. Thereafter, the tissues were washed three times with phosphate-buffered saline (PBS, pH 7.0), incubated for 20 minutes in a primary antibody enhancer in a Lab Vision kit (Thermo Scientific, Waltham, MA, USA), washed three times with PBS, and incubated with polymers for 40 minutes at room temperature. Then, the tissues were again washed three times using PBS, color-developed using diaminobenzidine, control-stained using hematoxylin, and observed using an optical microscope. When the staining was determined by a pathologist to be weak or negative, it was classified as low-level staining, and when the staining was even and strong, it was classified as high-level.
2) VEGF quantitative real-time polymerase chain reaction (qRT-PCR)
(1) Total RNA extraction
Three 15-µm slices from a paraffin block were placed into a xylene solution three times for five minutes each to remove paraffin and were then washed three times in 100% ethanol for five minutes each to remove xylene. Thereafter, the tissues were immersed in a graded ethanol solution prepared using diethyl pyrocarbonate (DEPC)-treated distilled water. Then, the tissues were briefly stained for 10 seconds using hematoxylin and washed with DEPC-treated distilled water. Thereafter, tumor tissues were placed into an Eppendorf tube for extraction of total RNA using a High Pure RNA Paraffin kit (Roche, Penzburg, Germany). The quantity and quality of the extracted RNA were measured using a NanoDrop spectrophotometer (Thermo Scientific).
(2) cDNA synthesis
Using 1 to 2 µg of total RNA as a template, cDNA was synthesized using a Maxime RT PreMix kit and random primers (iNtRON Biotechnology, Seongnam, Korea). That is, 20 µL of reactant was reacted for 60 minutes at 45°, and the reverse transcriptase was inactivated for five minutes at 95°.
(3) PCR
A PCR primer and probe were designed using Primer Express (PE Applied Biosystems, Foster City, CA, USA) software. The primer and probe sequences are shown below. An AccuPower DualStar PCR PreMix kit (Bioneer, Daejeon, Korea) was used for 20 µL reactions. The 20 µL of synthesized cDNA reactant was diluted in 80 µL of DEPC-treated distilled water, and 3 µL of the solution was used as a PCR template. The reaction medium composition was as follows: PCR forward primer, 10 pmol, 1 µL; PCR reward primer, 10 pmol, 1 µL; Taqman probe, 10 pmol, 1 µL; Template, 3 µL; DEPC-treated distilled water, 14 µL.
PCR was performed in an ExiCycler (Bioneer), and the individual reaction conditions were as follows: treatment for five minutes at 95°, followed by denaturation for 20 seconds at 95° and 50 cycles of annealing/extension for 30 seconds at 60°. Thereafter, each specimen was prepared in triplicate and analyzed.
The primers and probes of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and VEGF gene were as follows:
VEGF forward primer: 5'-GCACCCATGGCAGAAGG-3'
VEGF reverse primer: 5'-CTCGATTGGATGGCAGTAGCT-3'
VEGF probe: 5'-FAM-ACGAAGTGGTGAAGTTCATGGATGTCTATC
AC-TMARA-3'
GAPDH forward primer: 5'-GAAGGTGAAGGTCGGAGTC-3'
GAPDH reverse primer: 5'-GAAGATGGTGATGGGATTTC-3'
GAPDH probe: 5'-FAM-CAAGCTTCCCGTTCTCAGCCTAMRA-3'
The results of ΔCT=CT(VEGF)-CT(GAPDH), the relative calculation of the housekeeping gene GAPDH and VEGF expressions through analyses using a 96-channel optical unit, was converted into 2-ΔCT and is indicated as such.
3) Statistical analysis
Pearson's correlation analysis was used to examine the relationship between VEGF expression based on the results of immunohistochemical staining and the clinical and histological profiles of the carcinoma, and the significance level was set to a matching coefficient r>0.5 at P<0.05.
In addition, Student's t-test was used to examine the relationship between relative value of VEGF mRNA (VEGF/GAPDH) and difference in expression between carcinoma clinical profile and intraepithelial carcinoma tissues, and the significance level was set to P<0.05.
III. Results
1. Findings from immunohistochemical staining
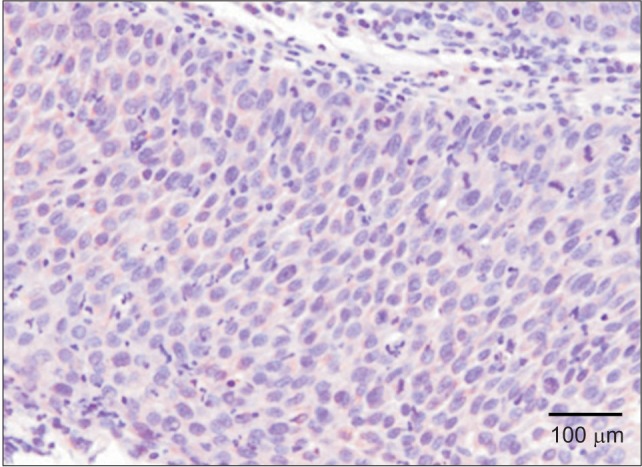
In normal oral squamous epithelial tissues or intraepithelial carcinoma, little VEGF expression was observed.(Fig. 1)
Fig. 1. Immunohistochemical staining of vascular endothelial growth factor of oral carcinoma in situ (×200).
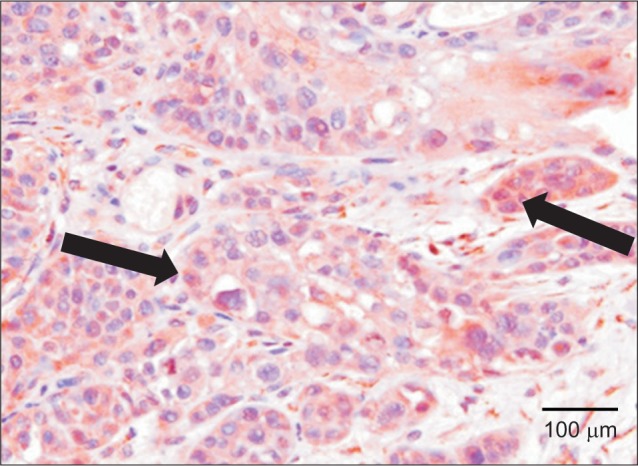
On the other hand, increased VEGF expression was observed in the cytoplasm of invasive tumor cells in moderately differentiated oral squamous cell carcinoma, remarkably increased VEGF expression was observed in marginally differentiated oral squamous cell carcinoma compared to normal tissues and intraepithelial carcinoma tumor cells.(Fig. 2, 3)
Fig. 2. Immunohistochemical staining for vascular endothelial growth factor (arrows) of moderate differentiated oral squamous cell carcinoma (×200).
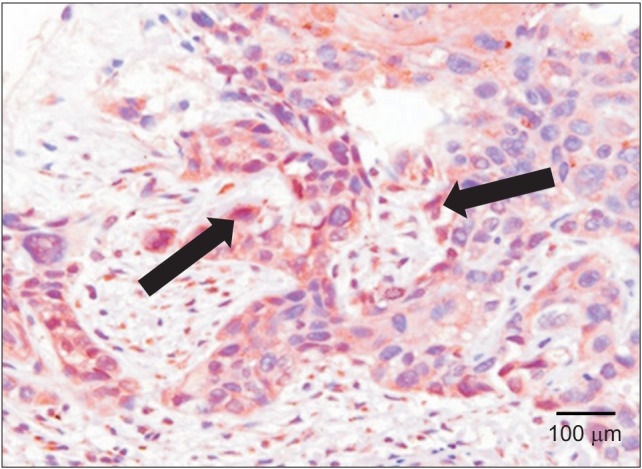
Fig. 3. Immunohistochemical staining for vascular endothelial growth factor (arrows) of poor differentiated and invasive oral squamous cell carcinoma (×200).
2. Clinical and histological relationships between VEGF expression and carcinoma according to immunohistochemical staining
Nine of 20 cases (45%) of VEGF staining were low-level, and the remaining 11 cases (55%) were high-level. The correlation between the profile of histological differentiation of carcinoma and VEGF expression was significant, as was the correlation between item classification according to tumor size of TNM classification and differences in VEGF expression. No correlation between any other factor and differences in VEGF expression was statistically significant.(Table 1)
3. qRT-PCR of VEGF
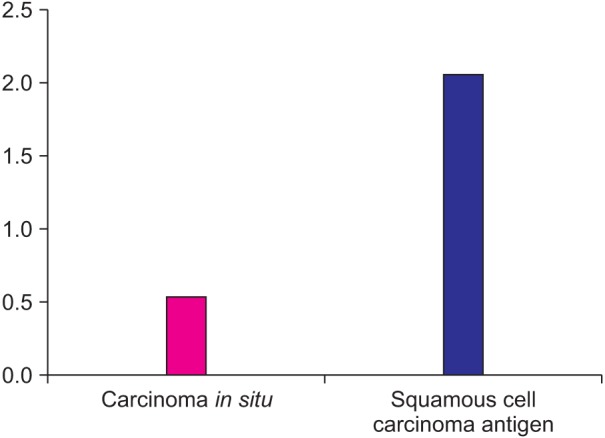
Although the relative VEGF mRNA expression (average 0.79) was weak in the intraepithelial carcinoma tissues used in the experiment, stronger relative VEGF mRNA expression (average 2.26) was observed in all 20 oral squamous cell carcinoma tissues.(Fig. 4)
Fig. 4. Quantitative real-time polymerase chain reaction of VEGF mRNA (VEGF/GAPDH×100). (VEGF: vascular endothelial growth factor, GAPDH: glyceraldehyde-3-phosphate dehydrogenase).
4. Relationship between relative VEGF mRNA level (VEGF/GAPDH) and clinical and pathological profiles of carcinomas
VEGF mRNA expression in the carcinoma was higher than that in intraepithelial carcinoma tissue, and the difference was statistically significant (Student's t-test, P<0.05). In addition, among tumor types classified according to size, T2 and larger tumors showed a significant increase in VEGF mRNA expression compared to that in T1. On the other hand, none of the correlations between clinical factors such as gender, age, nodal or remote metastasis, or TNM stage and VEGF expression were significant.(Table 2)
Table 2. Relationship between relative level of VEGF mRNA (VEGF/GAPDH) and clinical and pathological factors.

(VEGF: vascular endothelial growth factor, GAPDH: glyceraldehyde-3-phosphate dehydrogenase)
1VEGF mRNA expression derived from quantitative real-time polymerase chain reaction.
2Student's t-test, P<0.05.
Values are presented as number or mean±standard deviation.
IV. Discussion
Angiogenesis is an indispensable requisite for tumor growth, infiltration, and metastasis1. Although early-stage tumors are avascular, the cells in tumors 1 to 2 mm or larger or infiltrated fibroblasts around tumor cells secrete substances that stimulate angiogenesis to proliferate new micro-vessels. The proliferated micro-vessels supply nutrients to tumor cells, and vascular endothelial cells secrete growth factors such as basic fibroblast growth factor (bFGF), insulin-like growth factor-2, and PDGF to help tumor growth. In addition, these factors produce breakdown enzymes such as urokinase, collagenase, and plasminogen activator that contribute to infiltration into surrounding tissues4,18,19,20,21.
Many factors are involved in angiogenesis. VEGF is secreted by diverse cells and has specificity to vascular endothelial cells. VEGF receptors such as VEGF-1 and VEGF-2 are known to play a role in this specificity. These factors are located in the cell membranes of endothelial cells and are activated after binding to other factors in the extracellular matrix. They are known to promote cell nucleus division and contribute to angiogenesis through extracellular matrix dissolution and endothelial cell movement5,22.
The genes of human VEGFs are composed of eight exons separated into seven introns and are located on chromosome 6p21.3. Four different isoforms, VEGF121, VEGF165, VEGF189, and VEGF206, exist due to diverse exon splicing; of these, VEGF165 has been reported as the most functionally important isoform23,24. Ferrara and Davis-Smyth25 reported that factors that control the expression of VEGF genes include tissue oxygen tension, growth factors, hormones, and oncogenes, and that the expression increases when tissue pO2 concentration is low due to the effects of growth control factors such as TGF-α, TGF-β, and FGF or adrenal cortical hormones. That is, the low oxygen states in the environment around a tumor characterized by fat growth produce reversible increases in VEGF mRNA transcription, leading to increases in expression within the tumor. As tumor sizes increases, the distances between the nearest blood vessels increase, causing cells in expanding tumors to experience oxygen deficiency and producing low-oxygen areas within the tumor. In response to this, tumor cells produce endothelial growth factors. Through this mechanism, VEGF expression increases within tumors, in particular, in low-oxygen areas in necrotic regions. Therefore, VEGF overexpression due to low oxygen can be thought of as a compensatory mechanism that enables tumor tissues to increase oxygen through vascular proliferation26.
Many studies have reported that VEGF expression increases with micro-blood vessel density in diverse tumors such as colon cancer, ovarian cancer, hepatocellular carcinoma, gastrointestinal malignancy, renal cancer, breast cancer, and head and neck cancer, and that tumor cells express VEGF mRNA and secrete VEGF-like proteins27,28,29,30. In addition, study results indicate that tumors with rich VEGFs recur in much shorter periods of time after operation than do those with insufficient VEGFs, supporting the fact that VEGFs contribute to tumor occurrence and angiogenesis and affect prognosis. For the same reason, studies have reported that, in many tumors, increases in expression of VEGF receptor appear in proportion to increases in expression of vascular endothelial cell growth factor31,32. In a study of the correlation between diverse clinicopathological profiles and VEGF expression in breast cancer, Maeda et al.33 stated that prognosis worsened as the expression increased. Smith et al.34 reported that VEGF overexpression was the most influential factor on poor prognosis of oral squamous cell carcinoma. As such, studies conducted to examine the relationship between VEGF expression and carcinoma prognosis have noted that the relationship may be an ancillary measure for determining carcinoma prognosis with limitations that had been following existing histological classification or clinical TNM classification.
According to the present results of VEGF immunohistochemical tests, very little expression of VEGF was observed in normal oral squamous cell tissues. This is consistent with the results of other studies indicating that, when VEGF is normal, its expression will be limited in endothelial cells25,35. In addition, based on histopathological findings, little VEGF expression was observed in well-differentiated and less-invasive intraepithelial carcinoma tissues or highly differentiated oral squamous cell carcinoma than in normal cells. On the other hand, strong VEGF expression was observed in less-differentiated invasive oral squamous cell carcinoma. These results suggest that the degree of VEGF expression is correlated with the degree of differentiation or invasiveness of carcinoma; this was supported by the statistical analysis conducted with VEGF expression levels based on the results of immunohistochemical staining and clinical and histological profiles of carcinomas. The correlation coefficient r of the Pearson correlation analysis, which was the statistical method used to that end, can be interpreted as indicating a very high correlation when its value is 0.5 or higher, moderate correlation when its value is between 0.4 and 0.5, and very low correlation when its value is lower than 0.4. Consequently, in the present study, the correlation between the degree of histological differentiation and VEGF expression was significant, as was the correlation between classification according to tumor size and VEGF expression.
In addition, VEGF qRT-PCR was conducted to quantify relative VEGF gene expression; based on the analysis of the relationship between relative level of VEGF mRNA (VEGF/GAPDH) and the clinical and pathological profiles of the carcinoma, VEGF expression increased with tumor size, leading to increased oxygen demand compared to cases with small tumors (T1). In addition, compared to intraepithelial carcinoma tissues in the early stage, the amount of VEGF expression increased in oral squamous cell carcinoma tissues with progression of tumor cell invasion into connective tissues, and the difference was shown to be statistically significant. This result is similar to the results of immunohistochemical tests, suggesting that VEGF expression is activated with tumor growth, and it can be assumed that increases in VEGF expression are involved in the angiogenesis of tumors.
In conclusion, VEGF gene expression was more highly increased in progressed oral squamous cell carcinoma than in normal tissue cells or intraepithelial carcinoma, and the correlation between VEGF expression and the degree of histological differentiation of oral squamous cell carcinoma according to tumor size was significant. These results lead to the inference that VEGF expression in carcinomas is involved in angiogenesis and progression and affects the prognosis. However, since the number of samples tested was small, a study with a larger number of samples is needed to support the correlation between VEGF gene expression and clinicopathological factors in oral squamous cell carcinoma.
V. Conclusion
VEGF binds to VEGFR-1 and VEGFR-2, which are receptors in vascular endothelial cells, and is involved in endothelial cell differentiation and migration as well as vascular proliferation, and plays important roles in the angiogenesis of tumors. In addition, VEGF produces plasma fibers outside of blood vessels, causing changes in the extracellular matrix through cellulose deposition. The matrix then promotes the growth of macrophages, fibroblasts, and endothelial cells in tumors, in particular, promoting vascular endothelial cell proliferation, and are thereby involved in the angiogenesis of tumors.
In this study, we used 20 tissue slices excised after surgery from 20 patients diagnosed with oral squamous cell carcinoma. To identify VEGF expression in each tissue slice, we performed immunohistochemical tests, conducted VEGF gene qRT-PCR analysis, and statistically analyzed the results.
1. Immunohistochemical test: In normal oral squamous cells, VEGF expression was observed only in the vascular endothelial cells in the mesenchymal tissues. On the other hand, greatly increased VEGF expression was observed in weakly differentiated oral squamous cell carcinoma.
2. VEGF gene expression: VEGF gene expression was observed in all 20 tumor tissue slices, with only weak VEGF gene expression observed in intraepithelial carcinoma tissues.
3. Differences in VEGF expression among different tumor sizes were significant, and VEGFs were overexpressed in highly invasive carcinomas compared to intraepithelial carcinoma tissues.
In conclusion, VEGF expression was increased in insufficiently differentiated invasive carcinomas and was overexpressed in invasive oral squamous cell carcinoma but not in intraepithelial carcinoma tissues. These findings suggest that VEGF likely plays a role in angiogenesis of oral squamous cell carcinoma.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 4.Kwon SK, Choi YS, Park YH, Jang HK. Meanings of expression of vascular endothelial growth factor in thyroid tumors. J Korean Soc Endocrinol. 2005;20:134–141. [Google Scholar]
- 5.Han SJ, Lee JH. Anti-tumor effects of vascular endothelial growth factor inhibitor on oral squamous cell carcinoma cell lines. J Korean Assoc Oral Maxillofac Surg. 2009;35:66–73. [Google Scholar]
- 6.Connolly DT. Vascular permeability factor: a unique regulator of blood vessel function. J Cell Biochem. 1991;47:219–223. doi: 10.1002/jcb.240470306. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa F, Miyazono K, Hellman U, Drexler H, Wernstedt C, Hagiwara K, et al. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989;338:557–562. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- 8.Tischer E, Gospodarowicz D, Mitchell R, Silva M, Schilling J, Lau K, et al. Vascular endothelial growth factor: a new member of the platelet-derived growth factor gene family. Biochem Biophys Res Commun. 1989;165:1198–1206. doi: 10.1016/0006-291x(89)92729-0. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 10.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci U S A. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 13.Plouët J, Moukadiri H. Characterization of the receptor to vasculotropin on bovine adrenal cortex-derived capillary endothelial cells. J Biol Chem. 1990;265:22071–22074. [PubMed] [Google Scholar]
- 14.Vaisman N, Gospodarowicz D, Neufeld G. Characterization of the receptors for vascular endothelial growth factor. J Biol Chem. 1990;265:19461–19466. [PubMed] [Google Scholar]
- 15.Jakeman LB, Winer J, Bennett GL, Altar CA, Ferrara N. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest. 1992;89:244–253. doi: 10.1172/JCI115568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–753. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 17.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987;8:95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- 19.Thomas KA, Rios-Candelore M, Giménez-Gallego G, DiSalvo J, Bennett C, Rodkey J, et al. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A. 1985;82:6409–6413. doi: 10.1073/pnas.82.19.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esch F, Baird A, Ling N, Ueno N, Hill F, Denoroy L, et al. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985;82:6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaye M, Howk R, Burgess W, Ricca GA, Chiu IM, Ravera MW, et al. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986;233:541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- 22.Fontanini G, Vignati S, Boldrini L, Chinè S, Silvestri V, Lucchi M, et al. Vascular endothelial growth factor is associated with neovascularization and influences progression of non-small cell lung carcinoma. Clin Cancer Res. 1997;3:861–865. [PubMed] [Google Scholar]
- 23.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 24.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 25.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 26.Byun JH, Park BW, Chung IK, Kim JR, Kim UK, Park BS, et al. Correlation between vascular endothelial growth factor expression and malignancy grading in biopsy specimens of tongue cancers. J Korean Assoc Maxillofac Plast Reconstr Surg. 2005;27:528–534. [Google Scholar]
- 27.Lalla RV, Boisoneau DS, Spiro JD, Kreutzer DL. Expression of vascular endothelial growth factor receptors on tumor cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:882–888. doi: 10.1001/archotol.129.8.882. [DOI] [PubMed] [Google Scholar]
- 28.Volm M, Koomägi R, Mattern J, Stammler G. Angiogenic growth factors and their receptors in non-small cell lung carcinomas and their relationships to drug response in vitro. Anticancer Res. 1997;17:99–103. [PubMed] [Google Scholar]
- 29.Volm M, Koomägi R, Mattern J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int J Cancer. 1997;74:64–68. doi: 10.1002/(sici)1097-0215(19970220)74:1<64::aid-ijc11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 30.Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995;26:86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 31.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- 32.Suzuki K, Hayashi N, Miyamoto Y, Yamamoto M, Ohkawa K, Ito Y, et al. Expression of vascular permeability factor/vascular endothelial growth factor in human hepatocellular carcinoma. Cancer Res. 1996;56:3004–3009. [PubMed] [Google Scholar]
- 33.Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–863. doi: 10.1002/(sici)1097-0142(19960301)77:5<858::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 34.Smith BD, Smith GL, Carter D, Sasaki CT, Haffty BG. Prognostic significance of vascular endothelial growth factor protein levels in oral and oropharyngeal squamous cell carcinoma. J Clin Oncol. 2000;18:2046–2052. doi: 10.1200/JCO.2000.18.10.2046. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9(Suppl 1):2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]


