Abstract
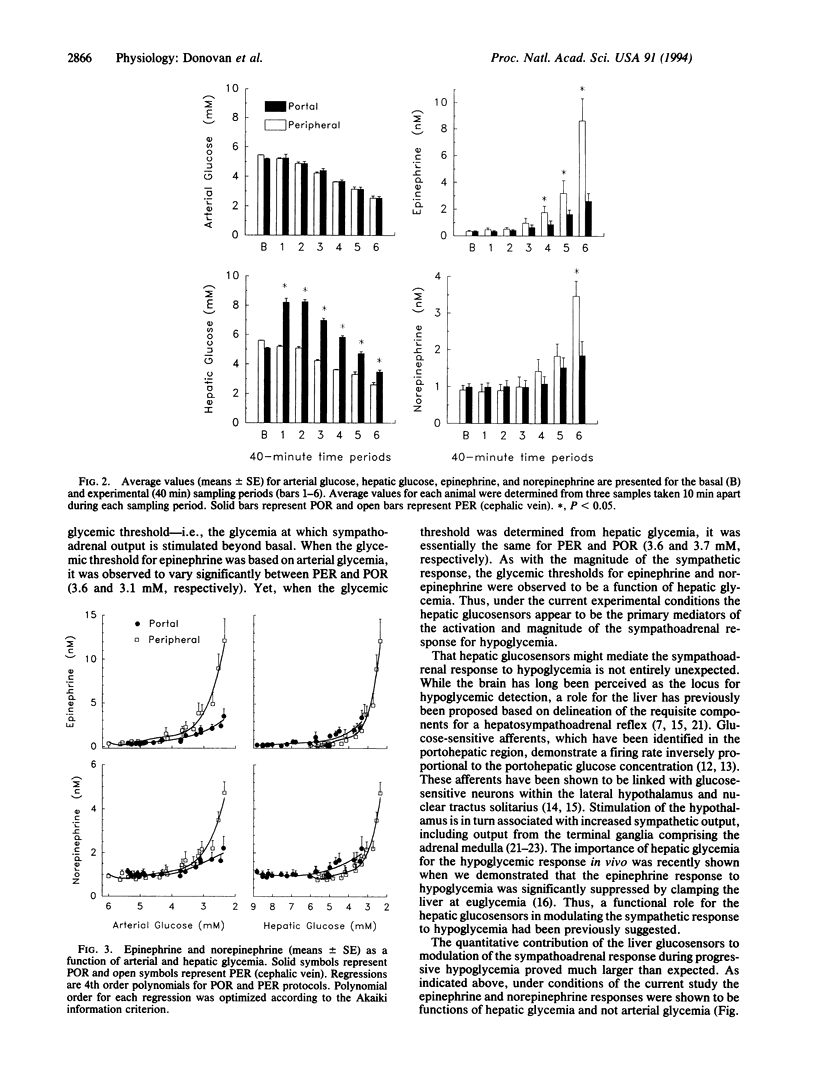
The impact of hepatic glucose concentration on the sympathetic response to progressive hypoglycemia was examined in chronically cannulated conscious male dogs (n = 6). Graded hypoglycemia was induced via peripheral insulin infusion (30 pmol.kg-1.min-1) with either peripheral (PER) or portal (POR) glucose infusion. Over the 260-min experimental period, arterial glycemia was adjusted from 5.2 +/- 0.1 to 2.5 +/- 0.1 mM in decrements of approximately 0.5 mM every 40 min. Arterial glycemias were not significantly different between PER and POR at any measured level. However, hepatic glycemia was significantly elevated at all times during POR (8.4 +/- 0.8 to 3.4 +/- 0.2 mM) when compared to PER (5.2 +/- 0.2 to 2.5 +/- 0.1 mM). Plasma epinephrine values were significantly greater during PER vs. POR at all arterial glycemias below 4.0 mM. At the lowest level of arterial glycemia studied (2.5 +/- 0.2 mM) the epinephrine response above basal was 3-fold greater for PER (8.7 +/- 1.7 nM) when compared to POR (2.6 +/- 0.6 nM) (P < 0.01). Plasma norepinephrine results were similar for the two protocols, with PER demonstrating a 3-fold greater response above basal when compared to POR at 2.5 mM arterial glycemia (P < 0.05). While the sympathetic response was markedly different between protocols when expressed as a function of arterial glycemia, when expressed as a function of hepatic glycemia this discrepancy was largely eliminated. This latter observation supports the liver as the primary locus for glycemic detection relevant to the sympathoadrenal response when hypoglycemia develops slowly--i.e., over a period of 2-3 h. A comparison of the current findings with our previous observations suggests that the hepatic glucosensors may play a greater role in hypoglycemic counterregulation as the rate of fall in glycemia is less.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiel S. A., Simonson D. C., Tamborlane W. V., DeFronzo R. A., Sherwin R. S. Rate of glucose fall does not affect counterregulatory hormone responses to hypoglycemia in normal and diabetic humans. Diabetes. 1987 Apr;36(4):518–522. doi: 10.2337/diab.36.4.518. [DOI] [PubMed] [Google Scholar]
- Benzo C. A. Minireview. The hypothalamus and blood glucose regulation. Life Sci. 1983 May 30;32(22):2509–2515. doi: 10.1016/0024-3205(83)90231-x. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Beir J. R., Hourigan P. M. Intraportal glucose infusion matched to oral glucose absorption. Lack of evidence for "gut factor" involvement in hepatic glucose storage. Diabetes. 1982 Jan;31(1):27–35. doi: 10.2337/diab.31.1.27. [DOI] [PubMed] [Google Scholar]
- Biggers D. W., Myers S. R., Neal D., Stinson R., Cooper N. B., Jaspan J. B., Williams P. E., Cherrington A. D., Frizzell R. T. Role of brain in counterregulation of insulin-induced hypoglycemia in dogs. Diabetes. 1989 Jan;38(1):7–16. doi: 10.2337/diab.38.1.7. [DOI] [PubMed] [Google Scholar]
- Cane P., Artal R., Bergman R. N. Putative hypothalamic glucoreceptors play no essential role in the response to moderate hypoglycemia. Diabetes. 1986 Mar;35(3):268–277. doi: 10.2337/diab.35.3.268. [DOI] [PubMed] [Google Scholar]
- Cane P., Haun C. K., Evered J., Youn J. H., Bergman R. N. Response to deep hypoglycemia does not involve glucoreceptors in carotid perfused tissue. Am J Physiol. 1988 Nov;255(5 Pt 1):E680–E687. doi: 10.1152/ajpendo.1988.255.5.E680. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Andres R., Bedsoe T. A., Boden G., Faloona G. A., Tobin J. D. A test of the hypothesis that the rate of fall in glucose concentration triggers counterregulatory hormonal responses in man. Diabetes. 1977 May;26(5):445–452. doi: 10.2337/diab.26.5.445. [DOI] [PubMed] [Google Scholar]
- Donovan C. M., Halter J. B., Bergman R. N. Importance of hepatic glucoreceptors in sympathoadrenal response to hypoglycemia. Diabetes. 1991 Jan;40(1):155–158. doi: 10.2337/diab.40.1.155. [DOI] [PubMed] [Google Scholar]
- Evans M. I., Halter J. B., Porte D., Jr Comparison of double- and single-isotope enzymatic derivative methods for measuring catecholamines in human plasma. Clin Chem. 1978 Apr;24(4):567–570. [PubMed] [Google Scholar]
- Gerich J. E., Campbell P. J. Overview of counterregulation and its abnormalities in diabetes mellitus and other conditions. Diabetes Metab Rev. 1988 Mar;4(2):93–111. doi: 10.1002/dmr.5610040202. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- MARSHALL N. B., MAYER J. Specificity of gold thioglucose for ventromedial hypothalamic lesions and hyperphagia. Nature. 1956 Dec 22;178(4547):1399–1400. doi: 10.1038/1781399a0. [DOI] [PubMed] [Google Scholar]
- Mitrakou A., Ryan C., Veneman T., Mokan M., Jenssen T., Kiss I., Durrant J., Cryer P., Gerich J. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991 Jan;260(1 Pt 1):E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- Niijima A. Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Ann N Y Acad Sci. 1969 May 15;157(2):690–700. doi: 10.1111/j.1749-6632.1969.tb12914.x. [DOI] [PubMed] [Google Scholar]
- Niijima A. Glucose-sensitive afferent nerve fibers in the liver and their role in food intake and blood glucose regulation. J Auton Nerv Syst. 1983 Oct;9(1):207–220. doi: 10.1016/0165-1838(83)90142-x. [DOI] [PubMed] [Google Scholar]
- Schwartz N. S., Clutter W. E., Shah S. D., Cryer P. E. Glycemic thresholds for activation of glucose counterregulatory systems are higher than the threshold for symptoms. J Clin Invest. 1987 Mar;79(3):777–781. doi: 10.1172/JCI112884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T. Central nervous system regulation of liver and adipose tissue metabolism. Diabetologia. 1981 Mar;20 (Suppl):343–356. [PubMed] [Google Scholar]
- Shimazu T., Matsushita H., Ishikawa K. Hypothalamic control of liver glycogen metabolism in adult and aged rats. Brain Res. 1978 Apr 14;144(2):343–352. doi: 10.1016/0006-8993(78)90159-2. [DOI] [PubMed] [Google Scholar]
- Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev. 1987 Jan;3(1):185–206. doi: 10.1002/dmr.5610030109. [DOI] [PubMed] [Google Scholar]
- Shimazu T., Ogasawara S. Effects of hypothalamic stimulation on gluconeogenesis and glycolysis in rat liver. Am J Physiol. 1975 Jun;228(6):1787–1793. doi: 10.1152/ajplegacy.1975.228.6.1787. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Oomura Y., Novin D., Grijalva C. V., Cooper P. H. Functional correlations between lateral hypothalamic glucose-sensitive neurons and hepatic portal glucose-sensitive units in rat. Brain Res. 1983 Apr 11;265(1):49–54. doi: 10.1016/0006-8993(83)91332-x. [DOI] [PubMed] [Google Scholar]



