Abstract
This review will outline the management of patients with symptomatic systolic heart failure or heart failure with reduced ejec-tion fraction (HFrEF), i.e., those with structural heart disease and previous or current symptoms. Determination of volume status and appropriate diuretic administration is important in heart failure management. Inhibition of the renin-angiotensin-aldosterone and sympathetic nervous systems improves survival and decreases hospitalizations in patients with systolic or reduced ejection fraction HF (HFrEF). Beta blockers and aldosterone antagonists improve ejection fraction. Indications for additional agents including nitrates plus hydralazine, digoxin, statins, omega 3 polyunsaturated fatty acids, anticoagulants, and antiarrhythmics will be discussed. Choice of agents, dose-related effects, strategies to minimize adverse effects, and medications to avoid will be presented.
Keywords: ACE inhibitor, aldosterone antagonist, angiotensin receptor blocker, beta blocker, heart failure with reduced ejection fraction, systolic heart failure.
INTRODUCTION
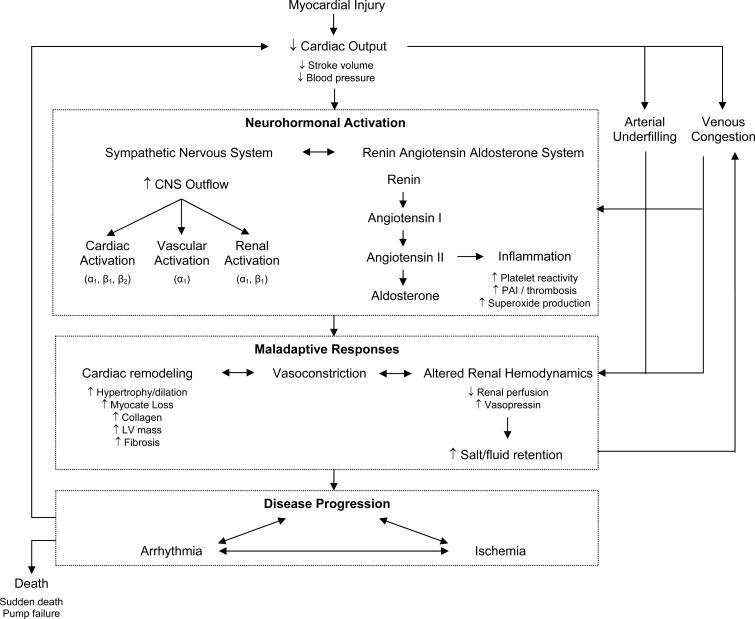
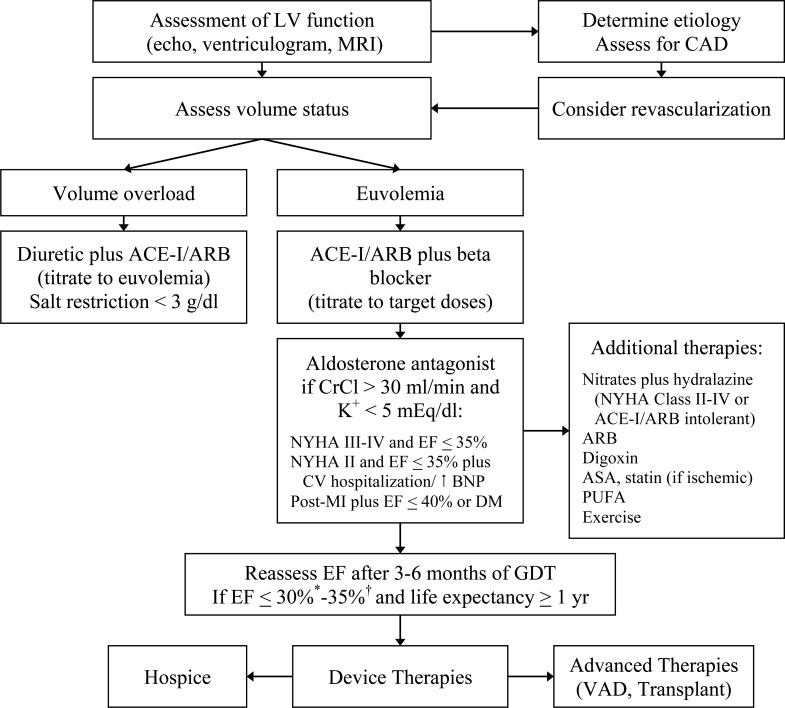
Stage C and D heart failure comprises patients with known structural heart disease and current or previous symptoms and signs of heart failure (HF), New York Heart Association (NYHA) Classes II-IV. The cornerstone for pharmacologic management of symptomatic systolic heart failure or heart failure with reduced ejection fraction (HFrEF) is inhibition of the renin-angiotensin-aldosterone (RAAS) and sympathetic nervous systems (SNS) (Fig. 1). Pharmacotherapies that have resulted in reverse cardiac remodeling have been associated with improved outcomes in patients with HFrEF. This review will provide a practical stepwise approach to managing Stage C and D patients (Fig. 2).
Fig. (1).
Neurohormonal Activation in Heart Failure.
Fig. (2).
Algorithm for management of patients with symptomatic HFrEF.
*NYHA Class I and previous myocardial infarction
† NYHA Class II-IV
ACE-I = ACE inhibitor, ARB = angiotensin receptor blocker, ASA = aspirin, BNP = brain natriuretic peptide, CAD = coronary artery disease, CrCL = creatinine clearance, DM = diabetes mellitus, EF = ejection fraction, GDT = goal-directed therapy, LV = left ventricular, MI = myocardial infarction, MRI = magnetic resonance imaging, NYHA = New York Heart Association, PUFA = polyunsaturated fatty acid, VAD = ventricular assist device.
EVALUATION OF VOLUME STATUS
After confirming the diagnosis and etiology of HF, the first decision is to determine the volume status of the patient.
Hypervolemia or fluid overload is most often manifested by increasing weight and dyspnea, peripheral edema, jugular venous distension, rales, and elevated brain natriuretic peptide. Chest pressure can also be a manifestation of fluid overload. Hepatomegaly, ascites, and anasarca may occur in more severe cases. Management includes initiation or augmentation of diuretics and salt and fluid restriction. If outpatient management fails, hospitalization may be necessary.
Patients may feel as unwell from hypovolemia as they do from volume overload. Symptoms and signs of dehydration include decreased weight, skin turgor, and urine output;thirst; and orthostatic blood pressure and heart rate. An increase in pulse ≥ 20 bpm from a supine to standing position indicates mild dehydration. A drop in systolic blood pressure ≥ 20 mmHg is consistent with more severe hypovolemia. Waiting at least 3 minutes after the patient stands before repeating measurements ensures an accurate assessment. Heart rate response will be blunted in patients taking beta blockers. Patients with long-standing diabetes may exhibit autonomic dysfunction even when euvolemic, which will manifest as a drop in blood pressure with no change in pulse. Management of hypovolemia includes decreasing or discontinuing diuretics, liberalizing fluid restriction, and in severe cases, careful administration of intravenous fluids. Volume status should be assessed at each visit including symptoms and signs with biomarker evaluation as needed. Patient education should include daily weight monitoring instructions for managing significant changes (i.e., > 3 lbs. in 1 day or > 5 lbs. in 1 week).
DIURETICS, SODIUM AND FLUID RESTRICTION
Although diuretics do not improve mortality, they do relieve symptoms in patients with volume overload. The lowest effective dose should be used, as diuretics adversely activate the RAAS and SNS systems. Loop diuretics (e.g., furosemide, bumetanide, torsemide) are the most widely used. A common regimen is twice daily with the second dose being administered in the afternoon to minimize nocturnal diuresis. Bumetanide and torsemide may have improved absorption compared to furosemide. Equivalent doses are listed in Table 1. Thiazide-like diuretics (e.g., hydrochlorothiazide, chlorothiazide, metolazone) are weak diuretics when used alone, although they have greater impact on blood pressure secondary to direct vasodilating effects. With the exception of metolazone, thiazide-like diuretics are less effective in renal impairment, i.e., creatinine clearance (CrCl) < 30 mL/min.
Table 1.
Drug Therapy for HFrEF.
| Diuretics | Starting dose | Maximum Daily Dose | |
|---|---|---|---|
| Loop | |||
| Furosemide | 40 mg | 600 mg | |
| Torsemide | 20 mg | 200 mg | |
| Bumetanide | 1 mg | 10 mg | |
| Thiazide | |||
| Hydrochlorothaizide | 25 mg | 200 mg | |
| Chlorthalidone | 12.5-25 mg | 100 mg | |
| Metolazone | 2.5 mg once daily | 20 mg | |
| ACE-Inhibitors | Starting daily dose | Target (max) | |
| Enalapril | 2.5 mg twice | 10 mg (20 mg) twice | |
| Lisinopril | 2.5-5 mg | 20 mg (40 mg) | |
| Ramipril | 1.25-2.5 mg | 10 mg | |
| Trandolapril | 1 mg | 4 mg | |
| Quinapril | 5 mg | 80 mg | |
| Fosinopril | 5-10 mg | 80 mg | |
| Captopril | 6.25 mg 3 times | 50 mg 3 times | |
| Perindopril | 2 mg | 8-16 mg | |
| Angiotensin receptor blockers | |||
| Losartan | 12.5 -25 mg | 150 mg | |
| Valsartan | 40 mg twice | 160 mg twice | |
| Candesartan | 4-8 mg | 32 mg | |
| Beta blockers | |||
| Bisoprolol* | 2.5 mg | 10 mg | |
| Carvedilol* | 3.125-6.25 mg twice | 25-50 mg twice | |
| Metoprolol succinate* | 12.5-25 mg | 200 mg | |
| Metoprolol tartrate | 12.5-25 mg twice | 100 mg twice | |
| Carvedilol controlled-release (Coreg CR) | 10 mg | 80 mg | |
| Aldosterone antagonists | |||
| Spironolactone | GFR > 50 | 12.5-25 mg | 25 mg (50 mg) |
| GFR 31-49 | 12.5 mg once or every other day | ||
| Eplerenone | GFR > 50 | 25 mg | 50 mg (100 mg) |
| GFR 31-49 | 25 mg every other day | ||
| Isosorbide dinitrate plus hydralazine | |||
| Without ACE-I/ARB | |||
| Isosorbide dinitrate | 10-20 mg 4 times | 40 mg 4 times | |
| Hydralazine | 10-25 mg 4 times | 75 mg 4 times | |
| With ACE-I/ARB | |||
| Isosorbide dinitrate | 10-20 mg 3 times | 40 mg 3 times | |
| Hydralazine | 10-25 mg 3 times | 75 mg 3 times | |
| Digoxin | 0.125 mg | serum level < 1.0 ng/ml |
Preferred, ACE-I = angiotensin converting-enzyme inhibitor, ARB = angiotensin receptor blocker, GFR = glomerular filtration rate.
In patients with signs of diuretic resistance (i.e., poor diuresis despite high doses of diuretics), the addition of a thiazide-like diuretic may provide a synergistic effect. If used in combination with loop diuretics, thiazide-like diuretics should be used in low doses and for short durations to reduce the risk of over-diuresis and renal impairment. Adding an aldosterone antagonist (e.g., spironolactone, eplerenone) may provide additional diuretic effect. Table 1 details diuretics and dosing.
Sodium restriction is recommended if serum concentrations are < 130 mEq/L or if diuresis is difficult. Although guideline-recommended sodium restriction is < 2-3 g/day, many patients experience difficulty with restriction < 2 g but can be managed with a < 3 g daily sodium restriction. Severe sodium restriction during hospitalization may contribute to readmission rates as patients resume their normal diet. If patients are acutely decompensated or euvolemia is difficult to maintain, a < 2 liter or 68 fl oz daily fluid restriction is recommended.
ANGIOTENSIN CONVERTING ENZYME INHIBITORS / ANGIOTENSIN RECEPTOR BLOCKERS: INITIATE FIRST, AIM FOR TARGET DOSE
An angiotensin converting enzyme inhibitor (ACE-I) or angiotensin receptor blocker (ARB) is generally started first in euvolemic patients or in combination with a diuretic when patients are hypervolemic.
ACE-Inhibitors
➣ Decrease mortality and hospitalizations in chronic symptomatic HFrEF [1, 2].
➣ Decrease mortality including sudden death following acute myocardial infarction (AMI) with symptomatic HF [3].
ARBs are generally used in ACE-Inhibitor intolerant patients and are:
➣ Not superior to ACE-Is in reducing mortality or HF hospitalizations in chronic HFrEF [4-6].
ACE-Inhibitor + ARB Combination
➣ No added total mortality benefit in chronic HFrEF [9, 10].
➣ Further decreases cardiovascular deaths and HF hospitalizations in chronic HFrEF [9, 10].
➣ No added mortality benefit in AMI [8].
➣ More symptomatic hypotension and worsening renal function in AMI [8].
➣ Not recommended in patients receiving aldosterone antagonists due to increased risk of hyperkalemia.
Trial details are shown in Table 2 including the number needed to treat (NNT). Documentation of ACE-I or ARB administration or the reason that an ACE-I or an ARB was not prescribed in patients hospitalized with HFrEF or sustaining an AMI with reduced EF are nationally reported quality measures.
Table 2.
Landmark Clinical Trials in HFrEF.
| Drug | Trial | Patients | NYHA | LVEF | Outcome | NNT | Follow Up (years) |
|---|---|---|---|---|---|---|---|
| Enalapril | CONSENSUS | Chronic | IV | Mortality | 6 | 0.5 | |
| Enalapril | SOLVD | Chronic | II-III | ≤ 40 | Mortality | 22 | 3.5 |
| HF Hospitalization | 4.5 | 3.5 | |||||
| Candesartan | CHARM-Alt | Chronic | II-IV | < 40 | CV Death/HF Hospitalization | 14 | 2.8 |
| HF Hospitalization | 13 | 2.8 | |||||
| Losartan | ELITE II | Chronic | II-IV | ≤ 40 | Mortality Non-inferior to captopril | ||
| Ramipril | AIRE | AMI | HF | Mortality | 17 | 1.25 | |
| Trandolapril | TRACE | AMI | < 35 | Mortality | 13 | 2 to 4 | |
| Losartan | OPTIMAAL | AMI | HF or ≤ 35 | Mortality Non-inferior to captopril | |||
| Valsartan | VALIANT | AMI | ≤ 40 | Mortality Non-inferior to captopril | |||
| Bisoprolol | CIBIS II | Chronic | III-IV | ≤ 35 | Mortality | 18 | 1.3 |
| Hospitalization | 17 | 1.3 | |||||
| HF Hospitalization | 17 | 1.3 | |||||
| Metoprolol | MERIT-HF | Chronic | II-III | ≤ 40 | Mortality | 26 | 1 |
| Hospitalization | 24 | 1 | |||||
| HF Hospitalization | 21 | 1 | |||||
| Carvedilol | COPERNICUS | Chronic | III-IV | < 25 | Mortality | 14 | 0.9 |
| Hospitalization | 17 | 0.9 | |||||
| HF Hospitalization | 15 | 0.9 | |||||
| Carvedilol | CAPRICORN | AMI | ≤ 40 | Mortality | 33 | 1.3 | |
| Spironolactone | RALES | Chronic | (III)/IV | ≤ 35 | Mortality | 9 | 2 |
| HF Hospitalization | 11 | 2 | |||||
| Eplerenone | EMPHASIS | Chronic | II | ≤ 30 | CV Death/HF Hospitalization | 13 | 1.8 |
| ≤ 35 + QRS | Mortality | 33 | 1.8 | ||||
| >130 msec | HF Hospitalization | 16 | 1.8 | ||||
| Eplerenone | EPHESUS | AMI | ≤40 | Mortality | 43 | 1.3 | |
| HF Hospitalization | 19 | 1.3 | |||||
AMI = Acute myocardial infarction, CV = Cardiovascular, LVEF left ventricular ejection fraction, HF = Heart Failure, NYHA New York Association, NNT = number needed to treat.
Therapy with an ACE-I/ARB provides rapid hemodynamic benefit and is generally well-tolerated. Start with a low dose and titrate every 1-2 weeks (or every 1-2 days as tolerated in hospitalized patients). Serum potassium and renal function should be monitored at baseline and 1-2 weeks after initiation of therapy. An increase in serum creatinine (SCr) should be expected following initiation of an ACE-I or ARB due to reductions in circulating angiotensin II, which normally increases glomerular filtrate rate as a result of vasoconstriction of the efferent arterioles. Although an exact threshold for discontinuing therapy has not been established, up to a 30% increase in SCr is generally acceptable; an increase in potassium not exceeding 5.5 mEq/L is also acceptable. If renal function significantly worsens, bilateral renal artery stenosis should be suspected and the ACE-I/ARB discontinued. ACE-Is and ARBs may, and should, be initiated in patients with chronic kidney disease, even in those with advanced stages, as long as renal function is stable.
Side Effects
ACE-I and ARB therapy are associated with similar rates of renal dysfunction, hyperkalemia, and hypotension, while a lower incidence of cough and angioedema is observed among ARBs. If patients experience cough with an ACE-I, efforts should be made to distinguish between a true drug effect and other potential etiologies (i.e., pulmonary disease, worsening fluid overload). ACE-Is are contraindicated in patients with history of angioedema and in patients with bilateral renal artery stenosis. Angioedema in patients with a history of ACE-I induced angioedema has also been reported with ARBs. ARBs should be used with caution in this population and patients should be educated on the risks, as angioedema may not occur with the first dose.
Dosing
Two trials have evaluated dose-related effects of ACEI/ARB therapy. In the Assessment of Treatment with Lisinopril and Survival (ATLAS) trial, higher doses of lisinopril (32.5-35 mg/day) were associated with a significant decrease in hospitalizations but mortality when compared to lower doses (2.5-5 mg/day) [11]. Similar results were observed in the Heart failure Endpoint evaluation of Angiotensin II Antagonists Losartan (HEAAL) trial among those receiving losartan 150 mg daily versus 50 mg daily [12]. Therefore, up-titration to target doses is recommended. Table 1 lists ACE-I and ARB agents, including initiation and target doses. Losartan appears to have the weakest antihypertensive effect of ACE-I/ARBs. All ACE-Is and these ARBs are generic, although no ARB is inexpensive.
ADDITION OF BETA BLOCKERS: ADD EARLY TO ACE-INHIBITOR/ARB, AIM FOR TARGET DOSE
➣ Further reduce mortality, including sudden death in chronic HFrEF [13-16].
➣ Improve survival even in NYHA class IV when administered to euvolemic HFrEF patients [17].
➣ Reduce all cause and HF hospitalizations in chronic HFrEF [15-18].
➣ Increase LVEF in chronic HFrEF [19-21].
➣ Further reduce mortality after AMI with reduced LVEF [22].
Beta blockers should be added early before full up-titration of ACE-I or ARB therapy given the impressive additional mortality and morbidity benefit. Documentation of beta blocker administration in patients hospitalized with HFrEF or AMI or the reason they are not prescribed are nationally reported quality measures.
Patients should be hemodynamically stable and euvolemic (i.e., no rales and no more than minimal edema) before initiation of a beta blocker. Because of potential negative inotropic effects, initiating doses are low. Signs and symptoms of worsening heart failure (e.g., fatigue, fluid overload, increased body weight) should be assessed at baseline and 1-2 weeks after initiation. Doses are generally doubled at 2-week intervals in the ambulatory setting until the target dose is reached or the maximally tolerated dose is achieved. Patients should be educated to contact their provider should weight gain or worsening signs or symptoms of HF occur following initiation or up-titration of therapy. If these occur, the diuretic dose may be increased, or the dose of beta blocker may be decreased temporarily, but abrupt discontinuation should be avoided due to increased mortality risk. If dose-titration is difficult, consider referral to a heart failure specialist. Beta blockers may be initiated safely during hospitalization for acute decompensation once the patient is euvolemic [23], and should not be stopped unless patients are in cardiogenic shock. If discontinued or reduced, beta blockers should be restarted prior to discharge, as observational data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE) Registry suggest that patients discharged without beta blockers have the poorest prognosis [24]. In general, doses should be up-titrated to the previous dose as soon as safely possible. If patients do not tolerate beta blocker therapy initially or after chronic therapy, referral to a heart failure specialist is recommended.
Side Effects
Fatigue and fluid retention are the most common side effects of beta blockers. Fatigue generally resolves after several days, and may be minimized by lengthening the time between dose titrations, increasing doses by smaller increments, administration of once daily drugs at night, or switching to another beta blocker. Fluid retention may be minimized by ensuring patients are euvolemic at the time of initiation, instructing them to weigh daily and self-titrate their diuretic or call their provider if weight significantly increases.
Choice of Beta Blocker
Carvedilol, metoprolol succinate, and bisoprolol are supported by randomized controlled trials. [13-18, 22] and recommended by HF guidelines [25-27]. Trial details and NNT are provided in Table 2. However, a recent meta-analysis reported no obvious differences in morbidity and mortality among six different beta blockers including atenolol and nebivolol; improvements in LVEF (mean 4.1%) were also similar [21]. Overall, beta blockers were associated with a 31% reduction in mortality in comparison with placebo or standard treatment after a median of 12 months (odds ratio 0.69, 0.56 to 0.80).
The choice of beta blocker depends on patient-specific characteristics. Adherence is increased by once daily dosing. Beta blockers can be considered in patients with reactive airway disease but should not be initiated in the setting of active bronchospasm. Patients with reactive airway disease tolerate the beta-1 selective agents metoprolol and bisoprolol better than carvedilol, which is non-selective. However, beta selectivity may be lost at higher doses. Carvedilol has a more potent antihypertensive effect, while metoprolol succinate is better tolerated in patients with borderline hypotension but has more effect on heart rate. Bisoprolol appears to be the best tolerated beta blocker.
Dosing, Heart Rate and Outcomes
Several studies have investigated the dose-related effects of beta blockers. The Multicenter Oral Carvedilol Heart Failure Assessment (MOCHA) trial found both a survival and hospitalization benefit with 6.25, 12.5, or 25 mg twice daily of carvedilol compared to placebo. [19] Post-hoc analyses of the Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF) (100 mg/day, 200 mg/day) and the Cardiac Insufficiency BIsoprolol Study II (CIBIS II) trials (low dose:1.25, 2.5 or 3.75 mg/day, moderate dose: 5 or 7.5 mg/day and high dose: 10 mg/day) confirmed that while all doses reduced mortality compared to placebo, hospitalizations were reduced only in the moderate/high dose groups [28, 29].
A recent meta-analysis investigated whether the survival benefit of beta blockers in HF was related to degree of reduction in heart rate [30]. For every 5 beats/min reduction with beta blocker treatment, a commensurate 18% reduction (CI, 6 - 29%) in the risk for death occurred. There was no significant relationship between all-cause mortality and dose (risk ratio for death, 0.74 [CI, 0.64 -0.86]) in high-dose beta blocker trials vs. 0.78 [CI, 0.63 -0.96] in low-dose beta blocker trials.
Improvement in Ejection Fraction
Left ventricular ejection fraction has been reported to increase significantly in every beta blocker trial conducted with duration of at least 3 months. The MOCHA trial reported that higher doses of carvedilol resulted in greater improvements in LVEF [19]. Another study enrolled 171 patients with chronic HF who were assessed before and after 9-12 months of metoprolol tartrate or carvedilol. Patients with a ≥ 15 unit change in LVEF received higher doses of carvedilol and had a greater reduction in heart rate [20]. A recent meta-analysis reported similar improvements in LVEF (mean 4.1%) irrespective of the beta blocker administered [21]. Reverse remodeling and improvement in LVEF can be expected to take at least 3 months [31].
Guidelines recommend up-titration of beta blockers to doses used in clinical trials [25-27]. However, if the patient does not tolerate target doses, lower doses still confer a mortality benefit. Table 1 lists beta blockers, initiation and target doses.
Before proceeding with device therapy, patients should be treated with ACE-I/ARB plus beta blocker therapy for at least 3-6 months. Reassessment of LVEF should then be performed. If LVEF remains ≤ 35%, referral for device therapy is recommended.
ADDITION OF ALDOSTERONE ANTAGONISTS TO ACE-I/ARB PLUS BETA BLOCKER THERAPY
Aldosterone antagonists are guideline recommended therapy in patients with a serum creatinine<2 .5 mg/dL or an estimated glomerular filtration rate >30 mL/min/1.73 m2 and a stable serum potassium<5.0 mEq/L at initiation of therapy [25-27]. Clinical trials have shown that aldosterone antagonists:
➣ Further reduce mortality and hospitalization in chronic HFrEF (NYHA class II-IV) [32, 33].
➣ Reduce heart failure readmissions in patients with mild chronic NYHA class II HFrEF [34].
➣ Further reduce total mortality, including sudden death, and HF hospitalization in AMI and LVEF < 40% or in patients with diabetes [35].
➣ Further increase LVEF [36].
Reductions in mortality and hospitalizations with spironolactone were first demonstrated in patients with advanced (NYHA class III/IV) disease in the Randomized Aldactone Evaluation Study (RALES), while the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure (EMPHASIS-HF) demonstrated that eplerenone improved these same endpoints as well as readmission for HF among less symptomatic patients (NYHA class II with LVEF ≤ 30% or LVEF ≤ 35% plus QRS duration > 130 msec) who were already receiving ACE-Inhibitor/ARB plus beta blocker therapy [32-34] . A recent meta-analysis of 1,575 patients enrolled in fourteen studies reported a weighted mean improvement in LVEF of 3.2% and a significant improvement in NYHA class among subjects treated with aldosterone antagonists (p<0.001) [36]. These improvements were independent of baseline LVEF or NYHA class.
Aldosterone antagonists are usually started at the doses studied in clinical trials although lower doses may be considered in patients with renal impairment (Table 2). While these agents at low doses have a relatively weak antihypertensive and diuretic effect, higher doses may result in additional blood pressure control and diuresis. Initiation should be avoided in hypovolemic patients. Potassium and serum creatinine should be monitored at baseline, within 1 week, at 1 month and then every 3 months depending on renal function. Potassium levels increase with the administration of aldosterone antagonists and should not exceed 5.5 mEq/L. Patients experiencing hyperkalemia should have dietary potassium intake evaluated, including the use of potassium sparing salt substitutes. Patients experiencing dehydration should be evaluated immediately given the risk of subsequent renal impairment and hyperkalemia.
Both spironolactone and eplerenone are generic although the latter is not inexpensive. Eplerenone has less androgenic activity, thus minimal incidence of gynecomastia. Initiating and target doses are in listed Table 1.
NITRATES PLUS HYDRALAZINE
Nitrates reduce preload and afterload, inhibit remodeling, and have anti-ischemic effects. Hydralazine reduces afterload, exhibits antioxidant effects, and prevents nitrate tolerance so that a nitrate-free interval is not necessary when the two are used in combination. Hydralazine plus nitrate therapy improves survival, reduces hospitalizations, and improves symptoms in NYHA class III-IV African American patients already receiving ACE-Inhibitor/ARB and beta blocker therapy [37]. Guidelines recommend this combination as standard therapy in HFrEF among African Americans with NYHA class II-IV HF, as an alternative in non-African Americans (particularly those intolerant to beta blockers), and as an alternative in ACE-Inhibitor/ARB intolerant patients (i.e., hyperkalemia, acute kidney injury, allergies) [25-27].
Therapy with nitrates/hydralazine should be started at low doses (12.5-25 mg hydralazine and 10-20 mg isosorbide dinitrate three times daily) and titrated every 1-2 weeks (or every 1-2 days as tolerated in hospitalized patients). Higher doses may be initiated (e.g. 37.5 mg hydralazine and 20 mg isosorbide in normo- or hypertensive patients. Patients with low blood pressure at baseline derive similar benefit as normotensive patients. Dizziness and headache are common but tend to improve over time. Similar to ACE-I or ARB therapy, the risk of first-dose hypotension is greatest in patients who are hypovolemic. Initiating and target doses are in listed Table 1.
DIGOXIN
The administration of digoxin reduces hospitalization but does not improve survival in patients with HFrEF [38]. In fact, serum concentrations of > 1.2 are associated with increased mortality, especially among women [39]. Digoxin should be added only after maximally-tolerated doses of a beta blocker. Serum digoxin concentrations should be evaluated at steady state (usually 5-7 days of therapy, longer in renal impairment) and should ideally remain between 0.5 – 0.8 ng/mL (< 1 ng/mL). Serum concentrations should be obtained no earlier than 6 hours after the dose. Because benefits occur at lower serum concentrations, low maintenance doses (i.e. 125 mcg per day or every other day) are typically used. In patients with renal impairment, digoxin may be given as half doses or several days per week (i.e., Mondays, Wednesdays, and Fridays). Doses should be decreased when patients are also taking amiodarone. Additional drug-drug interactions exist.
ADDITIONAL MEDICATIONS
Aspirin and statins are recommended in patients with ischemic etiology.
Omega 3 polyunsaturated fatty acids (PUFAs) (1 g daily) added to optimal medical therapy reduced total mortality by 9% in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico- Heart Failure (GISSI-HF) trial [40]. An echocardiographic substudy also found a small but significant improvement in LVEF [41]. A small randomized trial of 133 patients with nonischemic cardiomyopathy randomized to 2 g daily of PUFAs demonstrated an improved LVEF, exercise tolerance, and symptoms [42].
Antiarrhythmics
Amiodarone is the most effective agent for maintaining normal sinus rhythm without increasing mortality in patients with atrial fibrillation and HF [43, 44]. Amiodarone is also the drug of choice for ventricular arrhythmias in most HF patients [44, 45]. Due to potential toxicities, thyroid, liver, and pulmonary function should be evaluated at baseline and periodically; baseline vision and dermatologic exams are also recommended. Dofetilide is an effective alternative for the maintenance of normal sinus rhythm in patients with atrial fibrillation [46]. Given the risk of torsade de pointes associated with its use, patients must be hospitalized for monitoring (i.e., QTc interval, renal function, electrolytes) during initiation. Mexiletine may be required as adjunct therapy for the short-term management of refractory ventricular arrhythmias, but long-term use should be avoided.
Anticoagulants
Until recently, vitamin K antagonists (VKA) (i.e., warfarin) have been the mainstay of anticoagulation therapy in HF patients. Vitamin K antagonists are characterized by wide inter-patient variability, a feature that can be further complicated in patients with advanced HF as a result of changes in end-organ perfusion, absorption, metabolic function, dietary and drug interactions, and other factors. Newer oral anticoagulants have not been studied exclusively in patients with HF, although significant percentages have been enrolled in trials comparing them to warfarin – approximately 35%, 32%, and 62% of patients enrolled in trials of apixaban, dabigatran, and rivaroxaban, respectively [47-49]. In comparison to warfarin, apixaban and dabigatran were demonstrated as being superior for preventing stroke or systemic embolism while rivaroxaban was non-inferior. In terms of bleeding, apixaban was shown to be safer than warfarin while dabigatran and rivaroxaban were equally as safe; all three had a lower incidence of intracranial bleeding compared to warfarin. Dabigatran is heavily dependent on renal function for systemic clearance while rivaroxaban and apixaban primarily undergo hepatic metabolism with some renal clearance. Selection of an appropriate anticoagulant in patients with HF is patient-specific, taking into consideration both clinical and socioeconomic factors. Based on the neutral results of two trials [50, 51], oral anticoagulation is not routinely recommended for patients with HFrEF who remain in normal sinus rhythm.
MEDICATIONS TO AVOID OR USE WITH CAUTION
Verapamil and diltiazem are contraindicated in patients with HFrEF because of their negative effect on contractility. The newer generation agents, felodipine and amlodipine, do not exhibit negative inotropy, have no effect on mortality in HFrEF [52, 53], and can be used in patients with angina or refractory hypertension after dose optimization of guideline recommended HF therapies.
With the exception of amiodarone and dofetilide, most Vaughn-William Class I and Class III antiarrhythmic drugs should be avoided. Dronedarone, a congener of amiodarone increases mortality in patients with HFrEF [54], and was recently shown to worsen cardiovascular outcomes in patients with preserved ejection fraction [55].
Medications associated with QT prolongation or other proarrhythmic effects should be avoided in advanced disease or in those with known conduction abnormalities. Prolongation of the QT interval has been observed with many common medications, including antibiotics (e.g., macrolides, fluoroquinolones), antidepressants (e.g., tricyclic antidepressants, certain selective serotonin reuptake inhibitors), antiemetics (e.g., promethazine, prochlorperazine, ondansetron), antifungals (e.g., ketoconazole, voriconazole), and antipsychotics (e.g., haloperidol, quetiapine, risperiodone).
Non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided given the risk of increased sodium and fluid retention and systemic vascular resistance. All NSAIDs, including COX-2 selective inhibitors also increase the risk of cardiovascular events, so they should be avoided in patients with ischemic disease. When used at higher doses (i.e., 650 mg), aspirin has clinical effects similar to traditional NSAIDs.
Corticosteroids increase sodium and fluid retention and should only be used when reasonable alternatives do not exist. If required, corticosteroids should be administered at the lowest doses for the shortest duration possible.
Thiazolidinediones (ie., pioglitazone) should be avoided based on evidence indicating that these agents increase weight gain and may exacerbate heart failure symptoms.
Phosphodiesterase-5 (PDE-5) inhibitors (sildenafil, tadalafil, vardenafil) should be avoided in patients receiving nitrates (including long-acting forms such as isosorbide dinitrate) but are being studied in ongoing trials in patients not receiving nitrates. TNF-alpha inhibitors (e.g., infliximab) should be avoided. Amphetamines and other stimulants should also be avoided, as they have been associated with an increased risk of cardiovascular events.
EXERCISE
Exercise is safe in patients with chronic HFrEF and should be encouraged. However, a mortality or morbidity benefit has not been demonstrated [55, 56].
CONCLUSIONS
Initiate ACE-I ± diuretic therapy.
Add beta blocker early to ACE-I/ARB and diuretic therapy, then alternate up-titration to target doses.
If uptitration is difficult, refer to a heart failure specialist.
Add aldosterone antagonists and nitrates/hydralazine in appropriate patients.
Reassess LVEF after a minimum of 3-6 months on guideline directed medical therapy before proceeding with device therapy, as LVEF may have improved.
If patients remain symptomatic, consider additional therapies: digoxin, omega 3 polyunsaturated fatty acids.
Ischemic patients should receive aspirin and statin therapy.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure Results of the Co-operative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 2.The SOLVD Investigators.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993;342:821–28. [PubMed] [Google Scholar]
- 4.Pitt B, Poole-Wilson PA, Segal R , et al. Effect of losartan compared with captopril on mortality in patients with symp-tomatic heart failure randomised trial - the Losartan Heart Failure Survival Study, ELITE, II. Lancet. 2000;355:1582–87. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, McMurray JJ, Yusuf S , et al. Effects of can-desartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors the CHARM-Alternative trial. Lancet. 2003;362:772–76. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 6.Heran BS, Musini VM, Bassett K , et al. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;4:CD003040. doi: 10.1002/14651858.CD003040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickstein K, Kjekshus J. OPTIMAAL Steering Committee of the OPTIMAAL Study Group.Effects of losartan and capto-pril on mortality and morbidity in high-risk patients after acute myocardial infarction the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. . Lancet. 2002;360:752–60. doi: 10.1016/s0140-6736(02)09895-1. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, McMurray JJ, Velazquez EJ , et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 9.Cohn JN, Tognoni G. Valsartan Heart Failure Trial Investiga-tors.A randomized trial of the angiotensin-receptor blocker valsartan in chron-ic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 10.McMurray JJ, Ostergren J, Swedberg K , et al. Effects of can-desartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors the CHARM-Added trial. Lan-cet. 2003;362:767–71. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 11.Packer M, Poole-Wilson PA, Armstrong PW , et al. Compara-tive effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Cir-culation. 1999;100:2312–18. doi: 10.1161/01.cir.100.23.2312. [DOI] [PubMed] [Google Scholar]
- 12.Konstam MA, Neaton JD, Dickstein K , et al. Effects of high-dose versus low-dose losartan on clinical outcomes in pa-tients with heart failure (HEAAL study): a randomised, dou-ble-blind trial. Lancet. 2009;374:1840–48. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Bristow MR, Cohn JN , et al. The effect of carve-dilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 14.Metoprolol CR/XL Randomised Intervention Trial in Conges-tive Heart Failure (MERIT-HF) Study Group.Effect of metoprolol CR/XL in chronic heart failure. Lancet. 1999;353:2001,–07. [PubMed] [Google Scholar]
- 15.Hjalmarson A, Goldstein S, Fagerberg B , et al. Effects of controlled-release metoprolol on total mortality, hospitaliza-tions, and well-being in patients with heart failure the Metoprolol CR/XL Randomized Intervention Trial in conges-tive heart failure (MERIT-HF). JAMA. 2000;283:1295–302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 16.abid CIBIS II The Cardiac Insufficiency Bisoprolol Study II (CI-BIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 17.Packer M, Coats AJ, Fowler MB , et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–58. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 18.Fowler MB, Vera-Llonch M, Oster G , et al. Influence of car-vedilol on hospitalizations in heart failure incidence, resource utilization and costs. U.S.; Carvedilol Heart Failure Study Group. J Am Coll Cardiol. 2001;37:1692–99. doi: 10.1016/s0735-1097(01)01190-1. [DOI] [PubMed] [Google Scholar]
- 19.Bristow MR, Gilbert EM, Abraham WT , et al. Carvedilol pro-duces dose-related improvements in left ventricular function and sur-vival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94:2807–16. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 20.Metra M, Nodari S, Parrinello G , et al. Marked improvement in left ventricular ejection fraction during long-term ?-blockade in pa-tients with chronic heart failure Clinical corre-lates and prognostic significance. Am Heart J. 2003;145:292–99. doi: 10.1067/mhj.2003.105. [DOI] [PubMed] [Google Scholar]
- 21.Chattergee S, Biondi-Zoccai G, Abbate A , et al. Benefits of ? blockers in patients with heart failure and reduced ejection fraction network meta-analysis. Br Med J. 2013;346:f 55. doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction the CAPRICORN randomised trial. Lancet. 2001;357:1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 23.Gattis WA, O'Connor CM, Gallup DS , et al. Predischarge initiation of carvedilol in patients hospitalized for decompen-sated heart failure results of the Initiation Management Pre-discharge Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol. 2004;43:1534–41. doi: 10.1016/j.jacc.2003.12.040. [DOI] [PubMed] [Google Scholar]
- 24.Fonarow GC, Abraham WT, Albert NM , et al. Influence of beta-blocker continuation or withdrawal on outcomes in pa-tients hospi-talized with heart failure findings from the OP-TIMIZE-HF program. J Am Coll Cardiol. 2008;52:190–99. doi: 10.1016/j.jacc.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Lindenfeld J, Albert NM, Boehmer JP , et al. Executive sum-mary HFSA 2010, comprehensive heart failure practice guide-line. J Card Fail. 2010;16:475–539. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 26.McMurray JJ, Adamopoulos S, Anker SD , et al. ESC guide-lines for the diagnosis and treatment of acute and chronic heart failure 2012,: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012, of the Eu-ropean Society of Cardiology devel-oped in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 27.Yancy CW, Jessup M, Bozkurt B , et al. 2013, ACC/AHA guideline for the management of heart failure a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Wikstrand J, Hjalmarson A, Waagstein F , et al. Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure analy-sis of the experience in metoprolol CR/XL ran-domized intervention trial in chronic heart failure (MERIT-HF). J Am Coll Cardiol. 2002;40:491–98. doi: 10.1016/s0735-1097(02)01970-8. [DOI] [PubMed] [Google Scholar]
- 29.Simon T, Mary-Krause M, Funck-Brentano C. on behalf of CIBIS II investigators.Bisoprolol dose-response relationship in patients with congestive heart failure a subgroup analysis in the cardiac insufficiency bisoprolol study (CIBIS II). Eur Heart J. 2003;24:552–59. doi: 10.1016/s0195-668x(02)00743-1. [DOI] [PubMed] [Google Scholar]
- 30.McAlister FA. Meta-analysis ?-Blocker Dose, Heart Rate Reduction, and Death in Patients With Heart Failure. Ann In-tern Med. 2009;150:784–94. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 31.Eichorn EJ, Bristow MR. Medical therapy can improve the biological properties of the chronically failing heart. Circula-tion. 1996;94:2285–96. doi: 10.1161/01.cir.94.9.2285. [DOI] [PubMed] [Google Scholar]
- 32.Pitt B, Zannad F, Remme WJ , et al. for the Randomized Al-dactone Evaluation Study Investigators.The effect of spiro-nolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 33.Zannad F, McMurray JJ, Krum H , et al. Eplerenone in pa-tients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 34.Rogers JK, McMurray JJV, Pocock SJ , et al. Eplerenone in patients with systolic heart failure and mild symptoms.Analysis of Repeat Hospitalizations. Circulation. 2012;126:2317–23. doi: 10.1161/CIRCULATIONAHA.112.110536. [DOI] [PubMed] [Google Scholar]
- 35.Pitt B, Remme W, Zannad F , et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 36.Phelan D, Thavendiranathan P, Collier P , et al. Aldosterone antagonists improve ejection fraction and functional capacity inde-pendently of functional class. a meta-analysis of randomised controlled trials Heart. 2012;98:1693–700. doi: 10.1136/heartjnl-2012-302178. [DOI] [PubMed] [Google Scholar]
- 37.Taylor AL, Ziesche S, Yancy C , et al. African-American Heart Failure Trial, I. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 38.The Digitalis Investigation Group.The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 39.Adams KF , Jr, Patterson JH, Gattis WA , et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial a retrospective analysis. J Am Coll Cardiol. 2005;46:497–504. doi: 10.1016/j.jacc.2005.02.091. [DOI] [PubMed] [Google Scholar]
- 40.Tavazzi L., Magioni AP., et al. GISSI-HF Investigators, Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial), a randomised, double blind, placebo-controlled trial. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 41.Ghio S, Scelsi L, Latini R , et al. Effects of n-3 polyunsaturat-ed fatty acids and of rosuvastatin on left ventricular function in chronic heart failure a substudy of GISSI-HF trial. Eur J Heart Fail. 2010;12:1345–53. doi: 10.1093/eurjhf/hfq172. [DOI] [PubMed] [Google Scholar]
- 42.Nodari S, Triggiani M, Campia U , et al. Effects of n-3 poly-unsaturated fatty acids (PUFAs) on left ventricular function and func-tional capacity in patients with dilated cardiomyopa-thy. J Am Coll Cardiol. 2011;57:870–79. doi: 10.1016/j.jacc.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe con-gestive heart failure.Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet. 1994;344:493–98. doi: 10.1016/s0140-6736(94)91895-3. [DOI] [PubMed] [Google Scholar]
- 44.Singh SN, Fletcher RD, Fisher SG , et al. Amiodarone in pa-tients with congestive heart failure and asymptomatic ventric-ular ar-rhythmia.Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- 45.Moss AJ, Hall WJ, Cannom DS , et al. Improved Survival with an Implanted Defibrillator in Patients with Coronary Disease at High Risk for Ventricular Arrhythmia. N Engl J Med. 1996;335:1933,–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 46.M ller M, Torp-Pedersen CT, K ber L. Dofetilide in patients with congestive heart failure and left ventricular dysfunction safety aspects and effect on atrial fibrillation.The Danish In-vestigators of Arrhythmia and Mortality on Dofetilide (DIA-MOND) Study Group. Congest Heart Fail. 2001;7:146–50. doi: 10.1111/j.1527-5299.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- 47.Granger CB, Alexander JH, McMurray JJV , et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 48.Connolly SJ, Ezekowitz MD, Yusuf S , et al. Dabigatran ver-sus Warfarin in Patients with Atrial Fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 49.Patel MR, Mahaffey KW, Garg J , et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 50.Massie BM, Collins JF, Ammon SE , et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart fail-ure.The Warfarin and Antiplatelet Therapy in Chron-ic Heart Failure (WATCH) Trial. Circulation. 2009;119:1616–24. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 51.Homma S, Thompson JLP, Pullicino PM , et al. Warfarin and Aspirin in Patients with Heart Failure and Sinus Rhythm. N Engl J Med. 2012;366:1859–69. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Packer M, O'Connor CM, Ghali JK , et al. Effect of Amlodi-pine on Morbidity and Mortality in Severe Chronic Heart Failure. N Engl J Med. 1996;335:1107–14. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 53.Cohn JN, Ziesche S, Smith R , et al. Effect of the calcium an-tagonist felodipine as Supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril V-HeFT III.Vasodilator-Heart Failure Trial (V-HeFT) Study Group. Circulation. 1997;96:856–63. doi: 10.1161/01.cir.96.3.856. [DOI] [PubMed] [Google Scholar]
- 54.K ber L, Torp-Pedersen C, McMurray JJV , et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–87. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 55.Connolly SJ, Camm AJ, Halperin JL , et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–76. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- 56.O'Connor CM, Whellan DJ, Lee KL , et al. Efficacy and Safety of Exercise Training in Patients With Chronic Heart Failure HF-ACTION Randomized Controlled Trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]