Abstract
Management of the advanced heart failure patient can be complex. Therapies include cardiac transplantation and mechanical circulatory support, as well inotropic agents for the short-term. Despite a growing armamentarium of resources, the clinician must carefully weigh the risks and benefits of each therapy to develop an optimal treatment strategy. While cardiac transplantation remains the only true “cure” for end-stage disease, this resource is limited and the demand continues to far outpace the supply. For patients who are transplant-ineligible or likely to succumb to their illness prior to transplant, ventricular assist device therapy has now become a viable option for improving morbidity and mortality. Particularly for the non-operative pa-tient, intravenous inotropes can be utilized for symptom control. Regardless of the treatments considered, care of the heart failure patient requires thoughtful dialogue, multidisciplinary collaboration, and individualized care. While survival is important, most patients covet quality of life above all outcomes. An often overlooked component is the patient’s control over the dying process. It is vital that clinicians make goals-of-care discussions a priority when seeing patients with advanced heart failure. The use of palliative care consultation is well-validated and facilitates these difficult conversations to ensure that all patient needs are ultimately met.
Keywords: Cardiac transplantation, end-stage heart failure, inotrope, palliative care, ventricular assist device.
INTRODUCTION
There are nearly half a million patients with end-stage chronic heart failure with reduced ejection fraction (HFrEF). For those individuals, average survival is less than six months [1]. Until recently, there was little available to support these patients, to extend life, and to improve functional status. However, advances in transplantation, emerging mechanical technologies, and novel, multidisciplinary approaches have all contributed substantially to the care of this growing patient population. Timely referral of eligible patients for advanced therapies is critical to an optimal outcome. Table 1 provides recommendations of when to refer and potential contraindications.
Table 1.
Indications and contraindications for referral and consideration of advanced heart failure therapies (heart transplantation, ventricular assist device implantation).
| Indications | Contraindications |
|---|---|
| NYHA Class III-IV despite optimal medical and device therapy 6-minute walk < 300 m (984 feet) and/or peak oxygen consumption < 12-14 cc/kg/min Intolerance or withdrawal of evidence-based HF medications (e.g., beta-blockers, ACE-inhibitors) due to hypotension Diuretic-refractory volume overload Worsening renal function Frequent acute HF hospitalizations not related to noncompliance Need for intravenous inotrope Refractory life-threatening ventricular arrhythmias or frequent ICD discharges Refractory debilitating angina despite revascularization and symptom-oriented therapies Heart Failure Survival Score (“high risk”) [7] Seattle Heart Failure Model (“high” score: 2-3) [12] |
Noncompliance with medical regimen Active substance abuse Severe symptomatic cerebrovascular disease Severe dysfunction of other organs (lung, kidney1, liver1, coagulopathy) Active infection Active mental illness Inadequate social support Fixed, severe pulmonary hypertension 1,2 Morbid obesity (BMI > 35 kg/m2) 2 Age > 70 years 2 Recent or uncured malignancy 2 HIV 2 Hepatitis C 2 Abdominal aortic aneurysm Inability to tolerate anticoagulation 3 |
Consider dual-organ transplant.
Relative or absolute contraindication for heart transplantation, but not for ventricular assist device (specific eligibility criteria vary by transplant center).
Contraindication for ventricular assist device support, but not for heart transplantation.
Abbreviations: ACE – angiotensin converting enzyme, BMI – body mass index, HF – heart failure, ICD – implantable cardioverter defibrillator, NYHA – New York Heart Association.
CARDIAC TRANSPLANTATION
The only true “cure” for refractory or end-stage HFrEF is cardiac transplantation. The first heart transplant using a cadaveric donor was performed December 3, 1967 by Dr. Christiaan Barnard in Cape Town, South Africa. This operation occurred one month before Dr. Norman Shumway performed the first case in the United States (US). The early years of transplantation were marked by difficulties balancing infection and rejection.
Medical immunosuppression was greatly advanced after Cyclosporine A became available in 1983, resulting in fewer complications. At present, over 104,000 heart transplantations have been performed in more than 200 hospitals across the world, with an estimated rate of approximately 3,800 per year, over half of which are performed in the US [2]. Unfortunately, organ shortages chronically limit this therapy. Approximately 2,800 patients are listed for heart transplant annually in the US, but the yearly wait-list mortality approaches 15% [3]. Thus, it has become paramount to choose transplant recipients judiciously, and to ensure that all other alternative therapies have been exhausted.
RECIPIENT SELECTION
The first transplant guideline was published in 1993 by the American Heart Association (AHA) and the American College of Cardiology (ACC) [4]. Subsequent guidelines have been sponsored by the American Society of Transplant Physicians (1998) [5] and the International Society of Heart and Lung Transplantation (ISHLT) in 2006 [6]. The pre-transplant evaluation consists of a combination of thorough medical assessment and an official evaluation by a multidisciplinary transplant team including physicians, nurses, social workers, psychologists, and financial counselors (Table 2).
Table 2.
Components of the pre-transplant evaluation .
| Testing | Rationale |
|---|---|
| Comprehensive history & physical examination | Rule out significant other organ dysfunction (lung, kidney, liver, coagulopathy) Potential contraindication: excessive obesity (>30% normal), major chronic disabling disease, active mental illness, substance abuse within 6 months |
| Cardiac tests: Coronary angiography or stress test Cardiopulmonary stress test Right heart catheterization |
Rule out reversible disease Rule out severe, fixed pulmonary hypertension (contraindication) |
| Pulmonary studies: chest x-ray ± CT, pulmonary function tests | Potential contraindication: severe lung disease (consider heart-lung transplantation if appropriate) |
| Vascular studies (as needed): ankle-brachial index, carotid ultrasound, brain imaging | Potential contraindication: severe peripheral vascular disease, severe cerebrovascular disease |
| Cancer screening, age-appropriate: colonoscopy, prostate specific antigen, mammography, PAP smear, chest and abdominal imaging | Potential contraindication: malignancy within previous 2 years |
| Consultations with transplant nurse coordinator, social worker, psychologist, dietician, financial counselor, others as needed (e.g., dental) | Potential contraindication: noncompliance, lack of social support, lack of insurance |
| Various blood studies: Serologies and antibody titers: viral hepatitis, HIV, RPR; toxoplasma, CMV, EBV, HSV, VZV Immunohistocompatibility: human leukocyte antigens, panel of reactive antibodies ABO blood type |
Potential contraindication: Active infection. Future need for immunizations and antimicrobial prophylaxis. Future risk of rejection For transplant listing |
Objective assessment of hemodynamics and functional status should include data from right heart catheterization as well as cardiopulmonary stress testing. The presence of systemic hypotension, low cardiac output, persistent tachycardia, and elevated cardiac filling pressures are all poor prognostic factors in this population. Cardiopulmonary stress testing measures peak exercise oxygen consumption (VO2). Results vary by age and gender, with normal-value ranges being lower in women and older patient populations. Based on a landmark study in 1991, a peak VO2 of ≤ 14 ml/kg/min is considered an indication for cardiac transplantation [8]. However, more recent studies have suggested that different thresholds should be considered for patients on chronic beta-blocker therapy (peak VO2 < 12 ml/kg/min), for women (peak VO2 ≤ 10 or 12 ml/kg/min) [9, 10] and for obese patients (peak VO2 ≤ 19 ml/kg/min adjusted for lean body mass) [11]. Risk stratification models, such as the Heart Failure Survival Score [6, 7] and the Seattle Heart Failure Model [12] can be used to assess prognosis in conjunction with peak VO2.
TRANSPLANT LISTING AND PATIENT SURVIVAL
Each US transplant center is part of the nationwide United Network of Organ Sharing (UNOS), which is divided into eleven regions, each with specific local organ procurement organizations (OPO) [13]. Patients are listed for transplantation by OPO, transplant center, and ABO blood type, and prioritized by medical urgency (UNOS Status). Since blood type O is most common, the number of blood type O patients awaiting transplant is greatest. Medical urgency codes are used to differentiate severity of illness (Table 3). In 2006-2011, 82% of transplant recipients required inotropic or mechanical circulatory support (Status 1A or 1B) at the time of transplantation, compared to 75% in 1992-2000 [2]. Because donor hearts have remained a scarce resource, some high-volume transplant centers have adopted a “marginal” or “alternate” waiting list for high-risk recipients. However, post-transplant survival may be worse for these patients [14].
Table 3.
United Network of Organ Sharing (UNOS) medical urgency codes.
| Status | Clinical Criteria |
|---|---|
| 1A | Mechanical circulatory support (MCS) (ventricular assist device [VAD] <30 days; total artificial heart; intraaortic balloon pump; extracorporeal membrane oxygenation) MCS with device-related complications Continuous mechanical ventilation Continuous infusion of multiple intravenous inotropic agents or a single agent at high-dose (dobutamine ≥ 7.5mcg/kg/min or milrinone ≥ 0.5 mcg/kg/min) with invasive hemodynamic monitoring (pulmonary artery catheterization) Life expectancy <7 days |
| 1B | VAD implanted Continuous intravenous inotrope infusion |
| 2 | Does not meet criteria for Status 1A/1B |
| 7 | Temporarily inactive (unsuitable) |
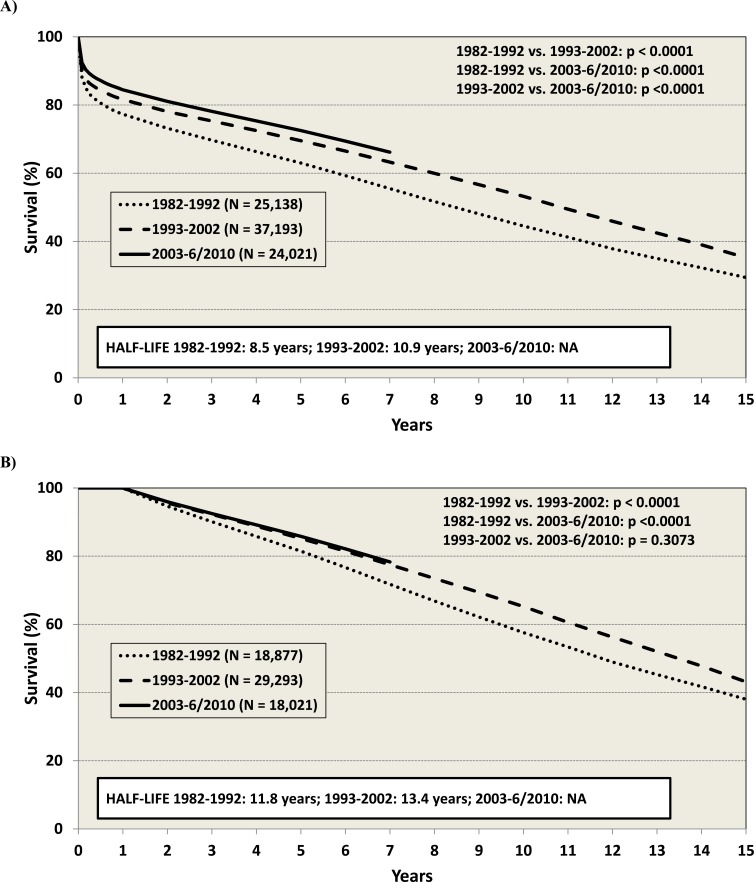
Survival, especially in the first year post-transplant has improved over time (Fig. 1) [2]. The median survival is now at least 10.9 years, and at least 13.4 years for those who have survived the first year. Survival is lower in patients with valvular cardiomyopathy, congenital heart disease, or following re-transplantation, and for those requiring pre-transplant mechanical circulatory support (MCS). Risk factors for long-term mortality (e.g., within 15 years) include older age (for both recipient and donor), history of prior transplant, ischemic HF etiology, HLA-mismatch, Hepatitis B-positive recipient, and female donor/ male recipient combinations [2]. In general, the sicker the patient is pre-transplant, the worse the survival – underscoring the importance of timely referral for transplantation in order to optimize outcomes (Table 1).
Fig. (1).
Survival after cardiac transplantation as reported by the International Society of Heart and Lung Transplantation (ISHLT). (A) Posttransplant survival by era for transplant recipients, January 1982-June 2010. (B) Conditional post-transplant survival by era for transplant recipients who survived to 1 year, January 1982-June 2010. Figures are obtained with permission from www.ishlt.org [2].
MEDICAL CARE FOLLOWING TRANSPLANTATION
Consistent improvements in post-transplantation survival are due to advances in anti-rejection therapies, infection prevention, and vigilance with regard to health maintenance. Recent guidelines for post-transplant care were published by the ISHLT in 2010 [15]. Medications include immunosuppressants, prophylactic antimicrobials, and agents to treat post-transplant complications and comorbidities. Risk factor modification is important. The key to optimal post-transplant care is the combined effort of the multidisciplinary transplant team, led by the transplant cardiologist, transplant nurse coordinator, and primary care provider.
The most feared post-transplant complication is cardiac rejection, which is mediated by infiltration of lymphocytes (acute cellular rejection, ACS), but can also be precipitated by pre-formed circulating antibodies (antibody-mediated rejection, AMR). Unlike other transplanted organs, such as the kidney or liver, there are no reliable serological markers to predict or diagnose rejection in cardiac transplant patients [16, 17]. Assessment of heart function by echocardiography is often minimally revealing in the absence of hemodynamic compromise. Since 1973, endomyocardial biopsy has been the gold standard for rejection surveillance. Fortunately, with improved immunosuppression, the incidence of acute rejection has declined over the years.
The severity of rejection is graded based upon an ISHLT classification [18, 19]. Only moderate or severe ACR (ISHLT grade 2R and 3R) typically requires treatment, but confirmed AMR (grade 1) should generally be treated as well. While systolic dysfunction from acute rejection is often a late finding, diastolic dysfunction can be an earlier, though less specific sign [20]. Recently, noninvasive blood-sampling techniques to assess leukocyte gene expression profiles for markers of both apoptosis and inflammation have evolved as tools for identifying rejection, and can reduce the frequency of biopsy (AlloMap( molecular expression testing) [21, 22]. Common complications post-transplant include hypertension, hyperlipidemia, renal dysfunction, and diabetes mellitus [23], often occurring as a side effect of immunosuppressants.. Thus, it is critical that clinicians and patients are aware of the multiple potential drug-drug interactions which can affect therapeutic immunosuppressant drug levels (Table 4) [24]. Other frequent post-transplant sequelae include steroid-related skin fragility, osteoporosis, cataracts, gout, and depression.
Table 4.
Common drug-drug interactions in cardiac transplantation.
| Drug Levels | Increased by | Decreased by |
|---|---|---|
| Cyclosporine A (CYA) Tacrolimus (TAC) |
Amiodarone Antidepressants: fluoxetine, fluvoxamine, nefazodone Antimicrobial (macrolides): clarithromycin, erythromycin Azole antifungal agents: fluconazole, ketoconazole, itraconazole, voriconazole Calcium channel blockers: diltiazem, verapamil, nifedipine, amlodipine, felodipine, nicardipine Statins: atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin Other lipid-lowering agents: ezetimibe Methylprednisolone (high dose) Metoclopramide Oral contraceptives Grapefruit juice |
Antacids Anticonvulsants: phenytoin, phenobarbital, carbamazepine Antimicrobial: rifampin, isoniazid Antiplatelets: clopidogrel, ticlopidine Fibrates: gemfibrozil, fenofribrate Omeprazole St. John’s Wort |
| Sirolimus Everolimus |
Diltiazem Azole antifungal agents: fluconazole, ketoconazole, itraconazole, voriconazole CYA |
|
| Mycophenolate Mofetil | CYA, TAC Cholestyramine Iron/antacids |
|
| Azathioprine | Allopurinol |
Coronary allograft vasculopathy (CAV, transplant coronary artery disease or accelerated graft arteriosclerosis) is the leading cause of long-term graft failure and mortality. Clinical presentation is variable, ranging from asymptomatic disease to sudden cardiac death. Angina is rare in the denervated heart whereas exertional dyspnea is more common. The mechanism of CAV is not entirely understood, but appears to be the result of a combination of endothelial injury from systemic atherosclerotic factors and a form of chronic rejection, related to cellular and/or antibody-mediated factors. Intravascular ultrasound (IVUS) can define the extent of CAV [25]. Therapies for CAV include percutaneous revascularization, HMGCoA reductase inhibitors (i.e. statins), and risk factor modification. In addition, diltiazem [26], sirolimus [27], and Vitamins C and E may decrease the burden of CAV [28].
Immunosuppressants predispose transplant recipients to both infection and neoplasm. The type and frequency of infection depends on the time following transplantation. Usual infections within the first month post-transplant are bacterial. At 1-4 months, non-bacterial opportunistic infections (e.g. cytomegalovirus and aspergillus) become more prevalent. After 4 months post-transplant, patients are susceptible to both opportunistic and non-opportunistic infections. Heart transplant recipients are also more prone to develop neoplastic complications than other solid organ transplant patients. Skin cancers and lymphomas (including post-transplant lymphoproliferative disorders, PTLD) are most common. Beyond 5 years post-transplant, non-skin cancers are responsible for >20% of deaths [2]. Therefore, annual dermatologic evaluation and vigilant cancer screening are crucial to long-term survival.
Although post-transplant survival has improved over time, the most common causes of death have not changed. In the immediate post-transplant period, deaths are often due to primary graft failure, technical problems with the operation, acute rejection, and infection. Later, CAV, malignancy, and other organ failure become the predominant causes of mortality.
The key to improving post-transplantation survival is the timely referral of eligible patients. It is important to remember that “healthier” pre-transplant patients do better following transplant.
VENTRICULAR ASSIST DEVICE THERAPY
Historically cardiac transplantation has been the only treatment for prolonging life in patients with advanced HFrEF. However, donor hearts remain a critically scarce resource and many end-stage HFrEF patients are transplant-ineligible, due to medical or psychosocial factors. MCS has now emerged as a treatment for enhancing quality-of-life and reducing mortality.
The origins of MCS date back to the 1950s with the early use of cardiopulmonary bypass for high-risk cardiac interventions [29]. In light of the obvious benefits of circulatory support in the operating room, these bypass machines soon were transitioned into the intensive care units where they were used to recover patients post-operatively and to buttress vital organ perfusion in individuals with refractory shock. Shortly thereafter, the National Institutes of Health established the Artificial Heart Program [30].
The ventricular assist device (VAD) has undergone substantial changes over the past decades. Beginning with Debakey’s use of a pneumatic left ventricular assist device (LVAD) [31], innovations in design have resulted in devices that are smaller, more durable, and more efficient. Figure 2 highlights monumental events which have shaped the evolution of VAD therapy.
LVAD PUMP DESIGN
First generation LVADs are often described as volume-displacement devices. They provided pulsatile flow as a blood-containing chamber would “fill-and-empty” in concert with the native heart’s contractile effort. Unfortunately, these devices were quite sizable, limiting their applicability particularly among smaller patients. In addition, numerous internal parts used to support device function were prone to wear, thus limiting their durability. Second-generation LVADs now employ continuous flow technology through the use of an axial rotor, which address constraints seen with its device predecessors. More recently, a third generation of LVADs now unload the left heart through a variety of centrifugal flow mechanisms and are currently being tested in ongoing clinical trials. Design features of the various LVAD devices are shown in Table 5.
Table 5.
Comparison of device characteristics and indications for currently approved first- through third-generation LVADs
| First-Generation | Second-Generation | Third-Generation | |
|---|---|---|---|
| Flow Profile | Pulsatile | Continuous (Axial) | Continuous (Centrifugal) |
| Device Example | HeartMate XVE | HeartMate II | HeartWare HVAD |
| Device Size | 1150 grams | 290 grams | 160 grams |
| Recommended Anticoagulation | Aspirin Only | Aspirin + Coumadin | Aspirin + Coumadin |
| Power Source | Pneumatic or Electric | Electric | Electric |
| Implant Site | Abdomen | Abdomen/Chest | Pericardium |
| Approved Indication | BTT, DT | BTT, DT | BTT |
Abbreviations: BTT – bridge-to-transplant, DT – destination therapy
THE EVIDENCE BASE FOR LVAD THERAPY
The landmark REMATCH (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) trial [32] investigated the impact of the pulsatile HeartMate XVE (HM XVE) device versus optimal medical management on survival in an extremely ill cohort of transplant-ineligible patients with advanced HFrEF. Patients randomized to LVAD support had a 48% reduction in mortality, with a 1-year survival rate of 52% compared to 25% with medical therapy alone. Based on this data, the FDA approved this device as permanent destination therapy (DT). Several other important LVAD trials are highlighted in Table 6.
Table 6.
Key completed and ongoing trials in LVAD patients.
| Study | Device | Indication | Patients | Trial Design | Results |
|---|---|---|---|---|---|
| REMATCH [32] | HeartMate XVE | DT, transplant-ineligible | NYHA class IV, LVEF ≤25%, VO2 <12 ml/kg-min or inotrope dependent | RCT, 1:1 to HM XVE vs. OMM | 48% reduction in death with LVAD compared to OMM (p=0.001) |
| HeartMate II BTT [51] | HeartMate II | BTT | NYHA class IV, status 1A or 1B for transplant | Non-randomized | 75% of patients were listed, eligible for listing or recovered at 180 days |
| HeartMate II DT [52] | HeartMate II | DT, transplant-ineligible | NYHA class IIIB or IV, LVEF ≤25%, VO2 ≤14 ml/kg-min or <50% predicted | RCT, 2:1 to HM II or HM XVE | 46% of HM II patients alive at 2 yrs free of disabling stroke or reoperation vs 11% for HM XVE (p<0.001) |
| ADVANCE [53] | HeartWare HVAD | BTT | NYHA class IV, status 1A or 1B for transplant | Non-randomized vs historical controls | 92% of HVAD vs 90% of controls alive or transplanted at 180 days (p<0.001) |
| *ROADMAP [54] | HeartMate II | DT | NYHA class IIIB/IV, LVEF ≤25%, not listed for transplant, no recent inotrope | HM II vs OMM | Primary EP = composite of survival with improvement in 6MWT from baseline at 1 yr |
| *REVIVE-IT [55] | HeartMate II | Chronic heart failure | Ambulatory, NYHA class III, LVEF ≤35%, no recent inotrope | RCT, 1:1 to HM II or OMM | Primary EP = composite of survival, freedom from stroke, improvement in 6MWT at 2 yrs |
| *Jarvik 2000 Heart BTT [56] | Jarvik 2000 Ventricular Assist System | BTT | Inotrope- or balloon pump-dependent UNOS status 1A or 1B | Open-label efficacy study | Primary EP = survival to transplant or survival and listed for transplant at 180 days |
Trials not yet completed or results not yet available
Abbreviations: BTT – bridge-to-transplant, DT – destination therapy, EP – endpoint, LVEF – left ventricular ejection fraction, NYHA – New York Heart Association, OMM – optimal medical management, RCT – randomized controlled trial, 6MWT – 6-minute walk test.
COMPLICATIONS AND KEY FACTORS RELATED TO PATIENT SELECTION
While revolutionizing the care of patients with advanced, end-stage HFrEF, the LVAD is not without its limitations. Complications may arise at device implantation or may develop during chronic support. Most, if not all, develop as a consequence of numerous factors attributable to the complex interplay between patient and device.
A recent analysis of LVAD implants from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) revealed a device-malfunction rate of 1.6 events per 100 patient months among patients with a continuous-flow LVAD [33]. Infection remains a common complication, with the driveline exit site often serving as the nidus for microbiologic invasion. Without careful attention to early infectious clues – and in many cases early surgical debridement – offending pathogens can migrate to the LVAD pump making eradication of infection nearly impossible.
Unlike the first generation LVADs where the blood-device interface was felt to have minimal thrombogenicity, current devices require systemic anticoagulation to avoid clot formation. Sequelae of thrombosis within the device can include pump failure, hemolysis, and embolic events. Novel strategies for avoiding these complications are evolving, and best-practice remains unclear. In most instances, a combination of aspirin in addition to Coumadin (with an International Normalized Ratio, INR target of 2-3) is the preferred prophylactic strategy. Patients are also at increased risk for bleeding. Antithrombotic and antiplatelet agents increase the risk for hemorrhage and recent data supports an association between axial-flow devices and the development of an acquired von Willebrand disorder [34]. Abnormal and excessive cleavage of von Willebrand factor (vWF) multimers can impair platelet aggregation and hence increase risk for bleeding, particularly involving mucosal sites.
In light of these risks, it is imperative that a number of patient factors be considered prior to device implantation. (Table 1).
FUTURE DIRECTIONS
There are no approved devices for long-term biventricular support. There are several that can be implanted in a biventricular configuration, or to support the right ventricle alone (i.e., Thoratec PVAD, Centrimag); however, these are currently available only for short-term in-hospital use. There is also growing interest in the use of the total artificial heart for patients who cannot be supported with univentricular devices alone, and trials are ongoing. Furthermore, earlier implementation of LVAD support is currently being studied in clinical trials, and totally implantable devices without external drivelines are undoubtedly on the horizon.
INOTROPE THERAPY
Some patients with end-stage HFrEF may tolerate little, if any, of the oral medications proven to reduce morbidity or prolong life. In other instances, patients may develop symptoms that are refractory to these oral therapies. Current HFrEF management guidelines recommend consideration of intravenous inotrope therapy for palliation of symptoms in Stage D patients [35]. Although two meta-analyses have reported that mortality is not reduced with the administration of inotropes [36, 37], inotropes can reduce hospitalizations and improve functional status and thus are often used as bridge to transplantation, DT, or as part of palliative care.
The use of chronic inotropic infusions to allow patients to be discharged home awaiting cardiac transplantation was shown to be a viable management option in 1997 [38]. Among 20 patients with continuous home infusions of dobutamine, dopamine, or both there were no sudden deaths, and mean duration of inotropic therapy was 5 months, with 70% of the time spent as an outpatient, leading to considerable cost savings. In a larger study, 73 patients received intermittent 4-hour inotrope infusions in a cardiovascular clinic two or three times per week [39]. Over a 49-month period, the time free of HF symptoms after the final infusion treatment ranged from 201 to 489 days, with no need for hospitalization or emergency room visits. However, randomized trials of intermittent dobutamine have consistently failed to demonstrate mortality or significant symptom benefit [40, 41]. One trial, however, reported a decrease in hospitalization [41].
Dobutamine and milrinone are most commonly used for chronic home infusion (see review Acute Decompensated Heart Failure Update). In a retrospective study of 112 patients considered ineligible for transplantation or LVAD implantation and discharged to home palliative care receiving continuous dobutamine or milrinone, the mean dose for dobutamine was 5.4 ± 2.5 mcg/kg/min and milrinone was 0.4 ± 0.2 mcg/kg/min [42]. There was no difference in the likelihood of death or hospitalization with the use of either inotrope.
Palliative inotropic therapy can be cost-effective as suggested in early studies [38]. In a randomly-derived cohort of 331 Medicare beneficiaries from a 17-state region who were inotrope-dependent and receiving home dobutamine or milrinone therapy, there were no differences in subsequent hospitalization rates at 30, 60, and 180 days with either agent, and no increase in admissions for arrhythmia [43]. Both inotropes were found to be cost-effective (defined as a decrease in overall Medicare expenditures) at 180 days.
Hemodynamic assessment with invasive monitoring in Stage D patients with refractory symptoms should be performed when chronic inotropic therapy is being considered. Reimbursement for inotrope therapy may require demonstration of hemodynamic improvement (20% decrease in pulmonary capillary wedge pressure and/or 20% increase in cardiac index) along with an improvement in dyspnea. Specific doses (e.g. milrinone 0.375 mcg/kg/min or dobutamine 3 mcg/kg/min) are also often required to be eligible for reimbursement.
PALLIATIVE CARE
Due to improving medical therapies and an aging population, the number of patients with end-stage HF is escalating. As a result, there is currently an unmet need to better prepare heart failure patients for end of life. The use of palliative care and hospice are well-validated systems for supporting the dying patient, but both have been underutilized among those with advanced HF. The updated ACC/AHA guidelines have added recommendations on prognostication, advanced directive planning, and palliation of symptoms as primary goals for the end-stage patient [35].
Despite these recommendations, there are still many barriers to facilitating the palliative care process. The natural course of HF is marked by acute exacerbations, usually requiring inpatient treatment, followed by periods of moderate symptoms, with gradual overall deterioration in health status. This pattern of stuttering decline often results in significant confusion among providers when considering the timing of palliative care and advanced directive discussions. For unclear reasons, there is discordance between a patient’s self-reported life expectancy and model-predicted outcomes. Patients with HF tend to see themselves living longer (closer to actuarial predictions based on age and sex alone) than the natural history of the disease would suggest [44]. Nevertheless, physicians treating patients with New York Heart Association (NYHA) class IV, Stage D HF should establish goals-of-care with their patients. If a patient is deemed to be ineligible for LVAD/transplant, a more focused discussion regarding palliative care options should be pursued.
Another barrier to end-of-life planning relates to the uncertainty of who should initiate these discussions. Liaison between the specialist palliative care and HF teams, or the primary care physician in a shared care approach, is encouraged to optimally address and coordinate the patient’s needs. These discussions often take many clinic visits, as goals of care frequently need to be re-addressed in light of changing patient expectations. Regardless of who initiates the conversation, communication between all of the care providers is pivotal in order to maintain a common dialogue.
Other issues to be addressed as part of end-of-life discussions include ICD deactivation, resuscitation status, appointing a healthcare decision maker, symptom management, and where the patient wishes to spend the end of their life. When asked, patients often are not aware that deactivating their ICD as an option and would welcome the opportunity to discuss this issue [45]. Providers, on the other hand, may hesitate to discuss ICD deactivation due to their own beliefs and fear of possible medico-legal consequences. Fortunately, resources are available to guide this discussion, including a sample ICD deactivation protocol [46]. In addition, a complete description of “Do Not Resuscitate (DNR)” is also important, as many patients do not understand the details of the resuscitation process. It is important to discuss options while the patient can still voice their opinions, and to clarify that these conversations will help alleviate much of the stress on their loved ones throughout the dying process [47].
Discussions about goals of care can be introduced at any point after the diagnosis of HF and it should be emphasized that the introduction of palliative care does not mean the patient is imminently dying. Palliative care focuses on symptom management and maximizing personal comfort. Palliative care consults in the inpatient setting can reduce procedures and interventions at the end of life, decrease length of stay in intensive care and inpatient units, and reduce overall hospital costs [47].
Any patient expected to live less than six months may be referred to hospice. The focus of hospice care is to offer medical, social, emotional and spiritual support through an interdisciplinary approach, either at home or in a home-like setting. Hospice care also reduces costs [48]. Most importantly, both palliative and hospice care have been shown to lessen symptoms and improve patient and family satisfaction [49]. On the other hand, late referrals to hospice have been associated with decreased patient satisfaction [50].
End-stage HFrEF has a poor prognosis, and providers for this population have a responsibility to discuss advanced directives, palliative care and eventually hospice with their patients. Having these conversations early in the disease process, and repeating them as needed during the progression of HF can offer patients peace and allow them to die with dignity in the manner that they choose.
CONCLUSIONS
Establish goals of care with ACC/AHA Stage D, NYHA Class IV patients to guide management.
Consider referral to a specialized center with expertise in heart transplantation and/or LVAD for those HFrEF patients who are not tolerating evidence-based medications, have frequent hospitalizations, or who are demonstrating worsening end-organ dysfunction.
Timely referral of potentially eligible patients for cardiac transplantation or LVAD is critical to an optimal outcome.
LVAD therapy can be used as a bridge to cardiac transplantation, as well as destination therapy for patients not eligible for transplant.
Consider intravenous inotrope therapy (dobutamine or milrinone) as palliative care in patients with refractory symptoms.
The use of palliative care and hospice are well-validated systems for supporting the dying HF patient and his/her family.
Fig. (2).
Important historical events in the evolution of LVAD technology for patients with advanced heart failure.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ABBREVIATIONS
- BTT
= bridge to transplant
- DT
= destination therapy
- FDA
= Federal Drug and Administration
- HM
= HeartMate
- LVAD
= left ventricular assist device
- NIH
= National Institutes of Health
REFERENCES
- 1.Stevenson LW, Rose EA. Left ventricular assist devices bridges to transplantation, recovery, and destination for whom?. Circulation. 2003;108:3059–63. doi: 10.1161/01.CIR.0000090961.53902.99. [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, Edwards LB, Kucheryavaya AY, et al. International Society of Heart and Lung Transplantation The Registry of the International Society for Heart and Lung Transplantation 29th official adult heart transplant report--2012. J Heart Lung Transplant. 2012;31:1052–64. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Colvin-Adams M, Smith JM, Heubner BM , et al. OPTN/SRTR 2011, Annual Data Report heart. Am J Transplant. 2013;13(Suppl 1 ):119–48. doi: 10.1111/ajt.12023. [DOI] [PubMed] [Google Scholar]
- 4.Mudge GH, Goldstein S, Addonizio LJ , et al. 24th Bethesda conference Cardiac transplanta-tion.Task Force 3: Recipient guidelines/prioritization. J Am Coll Cardiol. 1993;22:21–31. doi: 10.1016/0735-1097(93)90812-f. [DOI] [PubMed] [Google Scholar]
- 5.Miller LW. Listing criteria for cardiac transplantation results of an American Society of Trans-plant Physicians-National In-stitutes of Health conference. Transplantation. 1998;66:947–51. doi: 10.1097/00007890-199810150-00032. [DOI] [PubMed] [Google Scholar]
- 6.Mehra MR, Kobashigawa J, Starling R , et al. Listing criteria for heart transplantation Interna-tional Society for Heart and Lung Transplantation guidelines for the care of cardiac trans-plant candidates--2006. J Heart Lung Transplant. 2006;25:1024–42. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Aaronson KD, Schwartz JS, Chen TM , et al. Development and prospective validation of a clin-ical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95:2660–7. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 8.Mancini DM, Eisen H, Kussmaul W , et al. Value of peak exercise oxygen consumption for op-timal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation. 2005;111:2313–8. doi: 10.1161/01.CIR.0000164270.72123.18. [DOI] [PubMed] [Google Scholar]
- 10.Elmariah S, Goldberg LR, Allen MT, Kao A. Effects of gender on peak oxygen consumption and the timing of cardiac trans-plantation. J Am Coll Cardiol. 2006;47:2237–42. doi: 10.1016/j.jacc.2005.11.089. [DOI] [PubMed] [Google Scholar]
- 11.Osman AF, Mehra MR, Lavie CJ , et al. The incremental prog-nostic importance of body fat adjusted peak oxygen con-sumption in chronic heart failure. J Am Coll Cardiol. 2000;36:2126–31. doi: 10.1016/s0735-1097(00)00985-2. [DOI] [PubMed] [Google Scholar]
- 12.Levy WC, Aaronson KD, Dardas TF, Williams P, Haythe J, Mancini D. Prognostic impact of the addition of peak oxygen consumption to the Seattle Heart Failure Model in a transplant referral population. J Heart Lung Transplant. 2012;31(8):817–24. doi: 10.1016/j.healun.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 13.www.unos.org [Google Scholar]
- 14.Schulze PC, Jiang J, Yang J , et al. Preoperative Assessment of High Risk Candidates to Predict Survival Following Heart Transplantation. Circ Heart Fail. 2013;6:527–34. doi: 10.1161/CIRCHEARTFAILURE.112.000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costanzo MR, Dipchand A, Starling R et a. International Society of Heart and Lung Trans-plantation Guidelines.The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–56. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Mullen JC, Bentley MJ, Scherr KD , et al. Troponin T and I are not reliable markers of cardiac transplant rejection. Eur J Car-diothorac Surg. 2002;22:233–7. doi: 10.1016/s1010-7940(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill JO McRae AT, 3rd, Troughton RW , et al. Brain natriu-retic peptide levels do not corre-late with acute cellular rejec-tion in De Novo orthotopic heart transplant recipients. J Heart Lung Trans-plant. 2005;24:416–20. doi: 10.1016/j.healun.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Billingham ME, Cary NR, Hammond ME , et al. A working formulation for the standardization of nomenclature in the di-agnosis of heart and lung rejection Heart Rejection Study Group.The Interna-tional Society for Heart Transplantation. J Heart Transplant. 1990;9:587–93. [PubMed] [Google Scholar]
- 19.Stewart S, Winters GL, Fishbein MC , et al. Revision of the 1990, working formulation for the standardization of nomen-clature in the diagnosis of heart rejection. J Heart Lung Trans-plant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Mena C, Wencker D, Krumholz HM, McNamara RL. Detec-tion of heart transplant rejection in adults by echocardio-graphic diastolic indices a systematic review of the literature. J Am Soc Echocar-diogr. 2006;19:1295–300. doi: 10.1016/j.echo.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Deng MC, Eisen HJ, Mehra MR , et al. for the CARGO Inves-tigators.Noninvasive discrimina-tion of rejection in cardiac al-lograft recipients using gene expression profiling. Am J Transplant. 2006;6:150–60. doi: 10.1111/j.1600-6143.2005.01175.x. [DOI] [PubMed] [Google Scholar]
- 22.Pham MX, Teuteberg JJ, Kfoury AG et a. IMAGE Study Group.Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362:1890–900. doi: 10.1056/NEJMoa0912965. [DOI] [PubMed] [Google Scholar]
- 23.Lindenfeld J , et al. Page RL 2nd Zolty R Drug therapy in the heart transplant recipient Part III common medical problems. Circulation. 2005;111:113–7. doi: 10.1161/01.CIR.0000151609.60618.3C. [DOI] [PubMed] [Google Scholar]
- 24.Miller GG, Lindenfeld J. Page RL 2nd Drug therapy in the heart transplant recipient part IV drug-drug interactions. Cir-culation. 2005;111:230–9. doi: 10.1161/01.CIR.0000151805.86933.35. [DOI] [PubMed] [Google Scholar]
- 25.Mehra MR, Crespo-Leiro MG, Dipchand A , et al. Internation-al Society for Heart and Lung Transplantation working formu-lation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder JS, Gao SZ, Alderman EL , et al. A preliminary study of diltiazem in the prevention of coronary artery dis-ease in heart-transplant recipients. N Engl J Med. 1993;328:164–70. doi: 10.1056/NEJM199301213280303. [DOI] [PubMed] [Google Scholar]
- 27.Mancini D, Pinney S, Burkhoff D , et al. Use of rapamycin slows progression of cardiac trans-plantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]
- 28.Fang JC, Kinlay S, Beltrame J , et al. Effect of vitamins C and E on progression of transplant-associated arteriosclerosis a randomised trial. Lancet. 2002;359:1108–13. doi: 10.1016/S0140-6736(02)08154-0. [DOI] [PubMed] [Google Scholar]
- 29.Gibbon JH ., Jr Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–85. [PubMed] [Google Scholar]
- 30.Helman DN, Rose EA. History of mechanical circulatory support. Prog Cardiovasc Dis. 2000;43:1–4. doi: 10.1053/pcad.2000.7194. [DOI] [PubMed] [Google Scholar]
- 31.Debakey ME. Left ventricular bypass pump for cardiac assis-tance.Clinical experience. Am J Cardiol. 1971;27:3–11. doi: 10.1016/0002-9149(71)90076-2. [DOI] [PubMed] [Google Scholar]
- 32.Rose EA, Gelijns AC, Moskowitz AJ , et al. Long-term me-chanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 33.Kirklin JK, Naftel DC, Kormos RL , et al. Fifth INTERMACS annual report risk factor analysis from more than 6 000 me-chanical circulatory support patients. J Heart Lung Transpl. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Uriel N, Pak SW, Jorde UP , et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–13. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 35.2009 Focused Update Incorporated Into the ACC/AHA 2005, Guidelines for the Diagnosis and Management of Heart Fail-ure in Adults. JACC. 2009;53:e1–90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Thackray S, Easthaughb J, Freemantleb N, Cleland JGF. The effectiveness and relative effec-tiveness of intravenous ino-tropic drugs acting through the adrenergic pathway in patients with heart fail-ure-a meta-regression analysis. European Journal of Heart Failure. 2002;4:515–29. doi: 10.1016/s1388-9842(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 37.Tacon CL, McCaffrey J, Delaney A. Dobutamine for patients with severe heart failure a sys tematic review and meta analysis of randomised controlled trials. Intensive Care Med. 2012;38:359–67. doi: 10.1007/s00134-011-2435-6. [DOI] [PubMed] [Google Scholar]
- 38.Sindone AP , et al. Continuous Home Ambulatory Intravenous Inotropic Drug Therapy in Severe Heart Failure Safety and Cost Efficacy. Am Heart J. 1997;134:889–900. doi: 10.1016/s0002-8703(97)80012-9. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Candales A , et al. Need for hospice and palliative care services in patients with end-stage heart failure treated with intermittent infusion of inotropes. Clin Cardiol. 2004;27:23–48. doi: 10.1002/clc.4960270107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elis A, Bental T, Kimchi O , et al. Intermittent dobutamine treatment in patients with chronic refractory congestive heart failure a randomized, double-blind, placebo-controlled study. Clin Pharmacol Ther. 1998;63:682–5. doi: 10.1016/S0009-9236(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 41.Oliva F, Latini R, Politi A , et al. Intermittent 6-month low-dose dobutamine infusion in severe heart failure DICE multi-center trial. Am Heart J. 1999;138:247–53. doi: 10.1016/s0002-8703(99)70108-0. [DOI] [PubMed] [Google Scholar]
- 42.Gorodeski ED, Chu EC, Reese Jr , et al. Prognosis on Chronic Dobutamine or Milrinone Infu-sions for Stage D Heart Failure. Circ Heart Fail. 2009;2:320–324. doi: 10.1161/CIRCHEARTFAILURE.108.839076. [DOI] [PubMed] [Google Scholar]
- 43.Hauptman PJ, Mikolajczak P, George A , et al. Chronic ino-tropic therapy in end-stage heart failure. Am Heart J. 2006;152:1096.e121096.e8. doi: 10.1016/j.ahj.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen LA, Yager JE, Funk MJ , et al. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. J Am Med Assoc. 2008;299:2533–42. doi: 10.1001/jama.299.21.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dev S, Abernethy AP, Rogers JG, O'Connor CM. Preferences of people with advanced heart failure-a structured narrative literature review to inform decision making in the palliative care setting. American Heart J. 2012;164:313–19. doi: 10.1016/j.ahj.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Kini V, Kirkpatrick JN. Ethical challenges in advanced heart failure. Curr Opin Support Pall Care. 2013;7:21–28. doi: 10.1097/SPC.0b013e32835c4915. [DOI] [PubMed] [Google Scholar]
- 47.Habal MV, Micevski V, Greenwood S, Delgado DH, Ross HJ. How aware of Advanced Care Directives are Heart Failure Pa-tients, and are they using them?. Canadian J Card. 2011;27:376–81. doi: 10.1016/j.cjca.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 48.Lemond L, Allen LA. Palliative Care and Hospice in Ad-vanced Heart Failure. Prog Cardiovasc Dis. 2011;54:168–78. doi: 10.1016/j.pcad.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adler ED, Goldfinger JZ, Kalman J, Park ME, Meier DE. Palliative Care in the Treatment of Advanced Heart Failure. Circulation. 2009;120:2597–606. doi: 10.1161/CIRCULATIONAHA.109.869123. [DOI] [PubMed] [Google Scholar]
- 50.Goldfinger JZ, Adler ED. End-of-life Options for Patients with Advanced Heart Failure. Curr Heart Fail Rep. 2010;7:140–7. doi: 10.1007/s11897-010-0017-5. [DOI] [PubMed] [Google Scholar]
- 51.Miller LW, Pagani FD, Russell SD , et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 52.Slaughter MS, Rogers JG, Milano CA , et al. Advanced heart failure treated with continuous-flow left ventricular assist de-vice. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 53.Aaronson KD, Slaughter MS, Miller LW , et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;25:3191–200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 54.Risk assessment and comparative effectiveness of left ven-tricular assist device and medical management. ROADMAP. www.clinicaltrials.gov. Identifier # NCT01452802. Accessed March 1. 2013 [Google Scholar]
- 55.The evaluation of VAD intervention before inotropic therapy. REVIVE-IT. www.clinicaltrials.gov. Identifier # NCT01369407. Accessed March 1. 2013 [Google Scholar]
- 56.Jarvik 2000 Heart as a bridge to cardiac transplantation - Pivotal Trial. www.clinicaltrials.gov. Identifier # NCT00591799. Accessed April 30. 2013 [Google Scholar]