Abstract
Pulmonary arterial hypertension (PAH) is a panvasculopathy that affects the distal pulmonary arteries and leads to restricted blood flow. This increased afterload leads to adaptive mechanisms of the right ventricle, with eventual failure once it can no longer compensate. Pulmonary hypertension from associated conditions, most importantly left heart disease, i.e. heart failure, can also lead to the same sequela. Patients often experience early vague symptoms of dyspnea and exercise intolerance, and thus PH can elude clinicians until right heart failure symptoms predominate. Evidence-based treatment options with pulmo-nary vasodilators are available for those with PAH and should be employed early. It is essential that patients be accurately categorized by their etiology of PH, as treatment strategies differ, and can potentially be dangerous if employed in the wrong clinical scenario.
Keywords: Pulmonary arterial hypertension, pulmonary hypertension, pulmonary vascular disease, pulmonary venous hypertension, right ventricular failure.
INTRODUCTION
Pulmonary hypertension (PH), defined as mean pulmonary artery pressure > 25mm Hg, is being increasingly recognized and diagnosed. This complex disorder has an estimated prevalence of 10-20% within the general population [1]. The observation of PH, whether actively sought or incidentally uncovered on echocardiogram or right heart catheterization, deserves a search for the etiology, an understanding of the pathophysiology, and an appreciation of treatment options. Left heart disease complicated by PH has an estimated prevalence of 3-4 million in the United States and results in a two-fold increased risk of death when right ventricular (RV) failure ensues [2, 3]. While right-sided heart failure symptoms often prompt many clinicians to initiate treatment or referral, the aim of this review is to enhance early accurate classification of PH and an improved understanding of the different therapeutic approaches so they may be employed earlier in the disease course.
CATEGORIZATION AND PATHOPHYSIOLOGY
The World Health Organization-sponsored 2008 Dana Point Clinical Classification schema categorizes PH into five groups based on etiologies resulting in similar histopathologic changes.
WHO Group 1: Pulmonary Arterial Hypertension (PAH)
With an estimated 15 to 25 cases per million, true PAHencompasses a multitude of etiologies and portends a 1-year mortality of approximately 15% on traditional therapy [4, 5]. It is hemodynamically defined as a mean pulmonary artery (PAP) of ≥ 25 mm Hg plus a pulmonary capillary wedge pressure (PCWP) or left ventricular end-diastolic pressure (LVEDP) ≤ 15 mm Hg with a pulmonary vascular resistance (PVR) of > 3 woods units [6]. While it is the least common form of PH, Group 1 PAH is the most widely studied.
Formerly known as Primary PH, this group includes idiopathic, familial predominately due to mutations in the BMPR2 gene, which encodes bone morphogenic protein receptor, anorexigen induced PH, portal hypertension, HIV, connective tissue disease, congenital heart disease, myleoproliferative disorders, and schistosomiasis. PAH is generally caused by a vasculopathy primarily affecting the distal pulmonary arteries. The vascular abnormalities are characterized by intimal proliferation and fibrosis, coupled with medial hypertrophy and enhanced pulmonary artery vasoconstriction [7]. Over time, complex plexiform lesions develop and the vasculopathy may be further complicated by thrombus-in-situ. These processes are driven by an imbalance in the production of endothelin and thromboxane A2, both potent vasoconstrictors and initiators of cellular proliferation [8]. Vasoconstriction is further enhanced by rapid hydrolysis of cyclic GMP to inactive GMP by phosphodiesterase-5, thus impairing the vasodilatory endothelial response to nitric oxide.
WHO Group 2: Pulmonary Venous Hypertension (PVH) Due to Left Heart Disease
Pulmonary Venous Hypertension (PVH) due to left heart disease is the most commonly encountered secondary form of PH [9]. It results from chronically elevated left atrial pressure as a consequence of left heart disease: left ventricular (LV) systolic or diastolic dysfunction, and valvular disease. It is hemodynamically defined as a mean pulmonary artery (PAP) of ≥ 25 mm Hg plus a pulmonary capillary wedge pressure (PCWP) or left ventricular end-diastolic pressure (LVEDP) ≤ 15 mm Hg.
Relatively little is known about the initial pulmonary vascular changes that underlie PVH. It is postulated that elevated pulmonary artery (PA) pressures result from a “back-up” of venous pressure secondary to left atrial hypertension. In a subset of these patients, the resultant venous hypertension may be complicated by an additional “reactive” phenomenon of vasoconstriction and remodeling in the distal pulmonary arteries [10]. This vascular remodeling has been identified in autopsy specimens from patients with mitral stenosis that reveal typical pulmonary arterial vasculopathic changes like those encountered in Group 1 [11].
WHO Group 3: Pulmonary HTN Due to Lung Disease/Hypoxemia
WHO Group 3 is most commonly seen in the setting of chronic obstructive pulmonary disease (COPD), Interstitial lung disease (ILD), and Sleep Disordered Breathing. The exact frequency of PH in COPD and ILD is unknown; however in one series of patients with severe COPD, the incidence of PH exceeded 50% [12].
WHO Group 4: Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
WHO Group 4 represents PH that is a result of chronic pulmonary thromboembolic events. Pengo et al. have estimated the cumulative incidence of CTEPH in patients presenting with acute PE and no other venous thromboembolism history to be 3.8% after 2 years in a prospective, longitudinal study [13]. Pulmonary arterial hypertensive arteriopathyappears to be the driving force behind the microvasular changes seen [1]. Intraluminal thrombus leads to classic arteriopathy of both small muscular arteries and arterioles just distal to the partially or fully occluded vessel. This also occurs in the non-obstructed vessels that are spared, likely as a result of high shear stress [14].
WHO Group 5: PH Due to Unclear Multifactorial Mechanisms
WHO Group 5 is a category of miscellaneous causes of pulmonary hypertension with unclear pathogenesis. Sarcoid lung disease, glycogen storage disease, and thyroid disorders are just a few examples that fall within this class.
PRESENTATION
Dyspnea is the most common presenting symptom at presentation of PH, and PH should always be included in the differential diagnosis for breathlessness and exercise intolerance. Lower extremity edema and syncope are encountered, though less frequently and typically in more advanced stages of the disease [15]. Chest pain is often overlooked as a consequence of progressive PH. Angina due to PH results from increased right ventricular (RV) myocardial oxygen demand due to high wall stress, as well as a drop in systolic blood flow in coronary branches to the RV, and occasionally compression of the left main coronary artery by a dilated main pulmonary artery.
Symptom severity is classified according to WHO functional class, a modified form of the New York Heart Association classification of heart failure [16]. WHO functional Class 1 indicates patients with PH who have no limitation in physical activity, whereas Class II indicates a slight limitation. Such patients experience dyspnea, fatigue, chest pain, or near syncope with ordinary activity but are comfortable at rest. Marked limitation defines Class III with less than ordinary activity precipitating symptoms. Class IV patients often have symptoms even at rest and exhibit signs and symptoms of right heart failure. Syncope is considered to be a Class IV symptom. WHO functional classification predicts prognosis and directs treatment.
Physical examination and radiography can also suggest a possible diagnosis of PH. In advanced PH, signs of right-sided heart failure may be present. These include an elevated jugular venous pressure, hepatojugular reflux, ascites, hepatomegaly, or lower extremity edema. Auscultation can reveal a tricuspid regurgitation murmur, an increased pulmonic component of the second heart sound (P2), or a right ventricular gallop. Palpation of the precordium may reveal an RV lift as well. Review of chest radiography can uncover dilated pulmonary arteries, enlarged right atria and RV contour, and peripheral pruning.
DIAGNOSTIC EVALUATION
Echocardiography: Transthoracic echocardiography is the initial diagnostic tool if PH is suspected after the history and physical exam. A screening echocardiogram is also recommended for clinical conditions associated with a high pretest probability of PAH, including known BMPR2 mutation and systemic sclerosis. Conditions with known PAH risk including portal hypertension and congenital heart disease should also have periodic echocardiograms, especially if symptoms suggestive of PH arise [1]. Elevated estimated pulmonary artery systolic pressures are often detected incidentally on echocardiograms and a rational approach to such patients is a focus of this review.
The echocardiogram is central to the diagnosis and management of PH. It provides an estimate of the pulmonary artery systolic pressure and assessment of right atrial (RA) and right ventricular (RV) size and function. The use of agitated saline contrast can exclude the presence of an intracardiac shunt, and is recommended as part of a PH evaluation [17]. Of equal importance, the echocardiogram also may alert the clinician to other common PH associated conditions - most frequently, left heart disease.
Several distinct echo findings can help to distinguish a Group 2 PH (PVH) from other categories. Echocardiographic characteristics of PVH include a dilated left atrium (LA) or ventricle (LV) and left ventricular hypertrophy [18]. Importantly, left ventricular ejection fraction (LVEF) can either be reduced or preserved. Mitral regurgitation will often be visualized and transmitral Doppler inflow reveals rapid early diastolic filing (E wave velocity > A wave velocity). In contrast, findings more supportive of PAH include a normal LA and LV size with a normal to high LVEF. The RV: LV size is generally greater than 1.0 and the RV “takes over” the apex [19]. The transmitral inflow pattern is E<A and there is generally no mitral regurgitation present [20]. As PH progresses and the right ventricle dilates over time, interventricularseptal flattening can become pronounced consistent with right ventricular volume and pressure overload.
Additional non-invasive testing: Pulmonary artery systolic pressure (PASP) can be elevated for multiple reasons other than an increased pulmonary vascular resistance. Thus, initial testing should focus on identification of underlying etiology. Left atrial hypertension caused by left sided heart disease (valvular, systolic or diastolic dysfunction/heart failure) can often be detected on echocardiogram. High output cardiac states need to be excluded including anemia, pregnancy, cirrhosis, AV fistulas, or hyperthyroidism. Increased systemic blood pressure (i.e. chronic kidney disease) can also cause increase pulmonary blood flow leading to increased pulmonary pressures. If these common causes of elevated PASP are ruled out than additional testing is warranted.
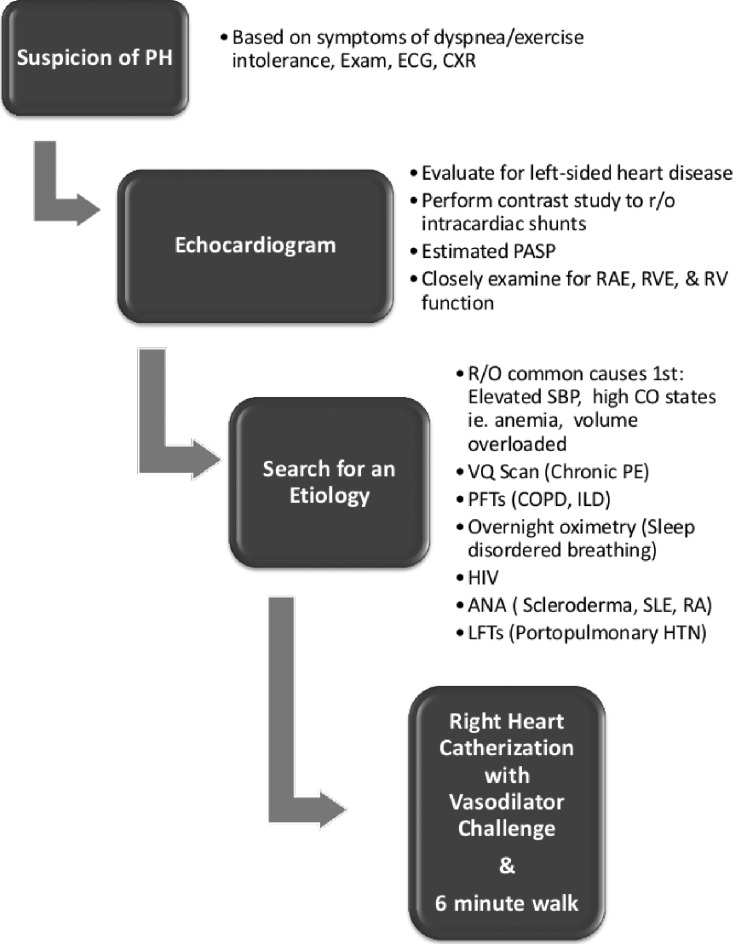
A 6 minute walk test is an easy way to evaluate for hypoxemia and is strongly predictive of survival in the PAH population [21]. Laboratory evaluation with antinuclear antibody, HIV, and liver function test should also be obtained. Pulmonary function tests, overnight oximetry, and Ventilation/Perfusion scans can also aid in the determination of etiology. Spiral computed tomography (CT) is not recommended in place of VQ scans because of the possibility that thromboembolic disease in the smaller, more distal vessels might be missed [1]. (see Fig. 1 for algorithm).
Fig. (1).
Pulmonary Hypertension Initial Diagnostic Approach Algorithm. Adapted from 2009 ACCF/AHA Pulmonary Hypertension Consensus Statement.
Invasive hemodynamic testing is the gold standard in diagnosis of PH. PH is a broad term that purely reflects elevated PA pressures, specifically defined as a mean PAP > 25. Right heart catheterization (RHC) is the next step in the diagnostic algorithm for patients who are considered to have likely PAH, a form of PH, to reveal if true vascular obstruction is present. The key feature is an elevated pulmonary vascular resistance (PVR) > 3 woods units [1]. The RHC is also vital for distinguishing PAH (PCWP ≤ 15) from PVH (PCWP>15) [22]. The essential measurements include oxygen saturations (in the superior and inferior vena cava, as well as the PA and a systemic artery), RA and RV pressure, systolic/diastolic/mean PAP, PCWP, cardiac output, and PVR. Quality standards during measuring of hemodynamics should be employed [23]. Pressure wave forms should be measured at end-expiration. If PCWP is at all uncertain, LV end diastolic pressure should be measured [24]. RHC is generally very well tolerated with a reported 1.1% risk of adverse events including arrhythmias, hematomas, and pneumothorax [25]. In a large multicenter registry of 7,218 procedures, the overall procedure-related mortality was 0.055%.
The RHC not only provides a confirmatory diagnosis, but aids in determination of prognosis and allows for an assessment of pulmonary vasoreactivity. Vasodilator challenges are an essential component of the invasive hemodynamics for individuals with Group 1 PAH. A positive response to pulmonary vasodilator challenge is defined as a decrease in mean PA pressure of at least 10mmHg to an absolute mean of less than 40 mmHg [26] without a decrease in cardiac output. A positive test predicts safety of response to calcium channel blockers, more commonly observed in the Idiopathic PAH group. Patients without evidence of a “reactive” pulmonary vasculature generally have more advanced disease and strong consideration should be given for more aggressive treatment [27].
Inhaled nitric oxide is generally the agent of choice for acute vasodilator testing given the ease of use and limited adverse effects. Intravenous epoprostenol and adenosine are alternatives though they are more cumbersome to administer and may have additional adverse effects. Caution should be undertaken in the administration of these agents in patients with coexistent left heart disease, as pulmonary edema can be triggered possibly by selective arterial vasodilation leading to flooding of the capillary bed from elevated left atrial pressures [28].
PH: A Progressive Disease
As PH progresses, the PVR continues to rise, reflecting the on-going abnormal structural changes in the pulmonary arterial bed. Elevated PVR increases after load to the RV, which is a thin-walled structure better suited to a much lower-pressure system. Initially, pulmonary artery pressures rise until the RV starts to fail, leading to decreased right ventricular stroke volume. PA pressure may then begin to decline and RA pressure may rise, both of which are ominous prognostic signs [29].
Hemodynamic evaluation is essential for risk-stratifying patients with PH, in large part because RV failure dramatically worsens prognosis. In the REVEAL registry, a contemporary PAH observational study, mean right atrial pressure (RAP) was inversely related to survival [30]. A right atrial pressure less than 10 mmHg with a cardiac index of greater than 2.5 L/min/m2 confers low risk. On the contrary, a right atrial pressure of greater than 20 mmHg and a cardiac index of less than 1.0 L/min/m2 place a patient at higher risk [31]. Clinical evidence of right-sided heart failure, a significantly elevated BNP, WHO functional Class IV, and rapid progression of symptoms indicate the need for aggressive treatment [1]. A 6 minute walk test of less than 300 meters should raise concern. Cardiopulmonary exercise testing can be useful prognostically, and a peak VO2 of greater than 10.4 ml/kg/min suggests milder disease [32].
Echocardiographic assessment of the RV helps to classify patients as well. Minimal RV dysfunction is consonant with low risk, whereas significant enlargement and dysfunction implies a higher risk patient [33]. As PH progresses, RV geometry changes as a compensatory mechanism to continued increases in afterload. The RV initially elongates longitudinally and then in its cross-sectional diameter. Eventually, the latter causes bowing into the LV as the RV takes on a more oval shape [32]. Identification of right ventricular failure is imperative, as it is a major determinant of morbidity and mortality [33].
THERAPEUTIC OPTIONS
WHO Group 1: PAH
Calcium channel blockers (CCBs), including amlodopine, diltiazem, and long acting nifedipine, may be used in patients who exhibit an acute response to a vasodilator challenge. True responders are generally uncommon. In one study, only 6.8% of the total IPAH had a positive challenge and maintained their long-term response to CCB [34]. Systemic hypotension is generally the most commonly encountered adverse effect. Patients initiated on CCBs should be followed closely to monitor for sustained efficacy.
WHO Group 1 PAH is the only category with large randomized controlled data to support the use of pulmonary artery vasodilators: phosphodiesterase-5 (PDE-5) inhibitors, endothelin receptor antagonists (ERAs), and prostacyclin analogues. Lower risk patients are often started on oral PDE-5 inhibitors or ERAs. Currently approved PDE-5 inhibitors include sildenafil and tadalafil. Sildenafil is started at 20 mg three times daily. Tadalafil is a once daily administered dose of 40 mg. Studies have shown improved 6 minute walk test (MWT) distance, cardiopulmonary hemodynamics, and reduced symptoms in PAH functional class I-IV patients with both of the PDE-5 inhibitors [35, 36]. Flushing, nausea, and headache are the most commonly reported side effects. Bosentan and ambrisentan are endothelin receptor antagonists. Bosentan has been shown to improve 6MWT, cardiopulmonary hemodynamics, and delay clinical worsening [37, 38] in WHO class II-IV patients. Dosing is twice daily administration of 62.5 mg for the first month and then a target dose of 125 mg. Hepatotoxicity has been encountered in about 10% of patients prescribed bosentan, which requires monthly liver function monitoring. Ambrisentan improves 6MWT and delayed clinical worsening in functional class II and III patients [39]. Dosing is 5 or 10 mg once daily. Pregnancy is contraindicated while on these medications, and monthly pregnancy tests are required. These drugs are generally well tolerated and side effects are typically dose dependent. Fluid retention occurs at rate of about 1.7% with bosentan as compared to placebo and 6% with ambrisentan. Hemoglobin should be checked prior to initiating either drug, and after 1 month of therapy, and periodically as anemia has been reported [40, 41].
Intravenous epoprostenol was the first agent to increase survival in addition to improving hemodynamics and symptoms in patients with severe PAH [42]. The typical starting dose of epoprostenol is 2 ng/kg/min with titration upwards to 20-40 ng/kg/min. Over the last several years, other prostacyclin analogues have been approved as well. Iloprost is inhaled six to nine times daily at either the 2.5 or 5 µg/dose. Treprostinil comes in multiple formulations including intravenous, subcutaneous, and inhaled (3 to 9 inhalations four times daily). The use of prostacyclins is typically reserved for the sickest PAH patients who are unresponsive to other drug therapies. Adverse effects include headache, flushing, rash, diarrhea, and jaw pain. Caution must be taken, because the administration of these agents to patients with elevated left sided filling pressures can result in flash pulmonary edema [43]. If PAH-specific therapies are deemed warranted in patients with underlying lung disease, care must be taken as increased blood flow to non-ventilated alveoli can worsen dyspnea and hypoxemia via this V/Q mismatch [1].
Lung transplantation remains the only definitive cure for PAH, and is considered in those who do not improve despite optimal medical therapy. Atrial septostomy can be used palliatively in end-stage PAH patients who are not transplant candidates, or in those awaiting transplantation [44, 45].
WHO Groups 2-4
The primary aim in treatment with WHO Group 2-4, or PH associated disease, is to first and foremost aggressively treat the underlying causative condition. This often will lead to an improvement in pulmonary artery pressures. In Group 2 (PVH), seen primarily in patients with left heart disease, that generally means strict volume control and titration of neurohormonal modulators in systolic heart failure, strict volume and blood pressure control in diastolic heart failure (see special section below), or surgical repair/replacement of the left sided valvular dysfunction. In WHO group 3, anti-inflammatory therapies and bronchodilators are the mainstays of COPD treatments and continuous positive pressure at night is essential for patients with sleep disordered breathing. Supplemental oxygen should not be overlooked in the hypoxic patient, as hypoxemia is a potent driver of vasoconstriction. CTEPH, or WHO Group 4 PH, can potentially be cured, and these patients should always be referred to surgical centers with experience in pulmonary thromboendarterectomy. If a patient’s transpulmonary gradient and PVR remain elevated after optimal treatment of the underlying condition, pulmonary vasodilators may be considered on an off label basis [1]. However, there are no randomized controlled trials to support the benefit of Group 1 PAH specific therapies in Group 2 and 3 PH, and there are potential risks including worsening pulmonary edema in Group 2 disease and worsening V-Q mismatch in Group 3 disease.
A SPECIAL FOCUS: PATIENTS WITH HEART FAILURE
Pulmonary venous hypertension occurs in an estimated 19-35% of patients with left heart disease [46, 47]. Treatment of PVH is aimed at the underlying disease process. Hypervolemia in both diastolic and systolic heart failure is treated with diuretics, salt and fluid restriction. Optimization of therapy is patients with systolic heart failure include administration of an ACE-I or ARB, beta blocker, aldosterone antagonist and in some patients the combination of a nitrate and hydralazine. Blood pressure control in patients with diastolic heart failure is essential. However, elevated pulmonary artery pressure may persist after augmenting diuresis and optimizing medical therapy. An additional “reactive” phenomenon with superimposed pulmonary arterial disease may exist in a subset of patients with left-sided heart failure. Generally, this reactive vasculopathy is diagnosed when the mean PA pressure is > than 25 mm Hg, PVR> 3 woods units, and the transpulmonary gradient (TPG) is >20 mmHg [1]. It is postulated that this “out of proportion” PH is triggered by chronically elevated pulmonary venous pressures leading to pre-capillary vasoconstriction and remodeling. However, it remains unclear what degree and duration of PVH is required to trigger this phenomenon. Also consider evaluation for COPD, sleep disordered breathing and thromboembolic disease in appropriate patients.
The 2009 PH Consensus document notes that if after optimal treatment of the etiology of PVH, the TPG and PVR remain elevated in the setting of a normal or only minimally elevated PCWP, that PAH specific therapy may offer clinical benefit [1]. However, traditional PAH therapies have been employed in the PVH population with variable success. ERA trials in systolic heart failure have reported early exacerbations of heart failure secondary to fluid retention [48]. Prostacyclin has been shown to improve cardiac index and decrease PVR in advanced heart failure, but create a strong trend toward increased mortality [49]. PDE-5 inhibitors may be a potential therapy for PVH. A growing body of evidence, in both experimental models and human studies, has shown that NO-dependent pulmonary vasodilatation is impaired in heart failure [50, 51]. Sildenafil (50 mg three times daily) administered in a randomized controlled trial of 44 patients with heart failure with preserved ejection fraction and PH demonstrated a substantial improvement in pulmonary pressures , RV function, LV diastolic function, and quality of life over a one year follow up [52]. However, the recently published RELAX (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure) trial failed to demonstrate a significant benefit of sildenafil on clinical outcomes or exercise capacity [53]. Of note, this study did not require the presence of PH. Small studies of patients with systolic heart failure and PVH have demonstrated safety, improved hemodynamics, and increased exercise capacity with PDE-5 inhibitor use [54, 55]. Sildenafil 50 mg three times daily randomized to 45 NYHA class II/III patients with systolic heart failure on a stable heart failure regimen resulted in a significant increase in ejection fraction, 29.5% to 36.3%, p< 0.01, LV end diastolic dimensions, and LV mass index at 1 year compared to placebo [55]. The effect of PDE-5 inhibitors on cardiovascular outcomes including mortality will be the focus of the multicenter PITCH-HF trial (PDE5 Inhibition with Tadalafil Changes Outcomes in Heart Failure) in patients with LVEF< 40% and secondary PH. The body of evidence for PDE-5 inhibitor use is growing, but further research is needed.
TAKE HOME POINTS
Pulmonary hypertension should always be included in the differential diagnosis for breathlessness and exercise intolerance.
Transthoracic echocardiography is the initial diagnostic tool if PH is suspected after the history and physical exam.
Right heart catheterization is the gold standard in the diagnosis of PH.
Pulmonary venous hypertension is common in patients with heart failure. Strict volume control, blood pressure control and titration of neurohormonal therapy are initial treatment strategies. PDE-5 inhibitors may provide additional benefit and studies are on-going.
Referral to specialists in pulmonary hypertension for hemodynamic evaluation is recommended.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
Conflicts: Dr. Rose-Jones has no documented conflicts. Dr McLaughlin has consulted for Actelion, Bayer, Gilead, and United Therapeutics; was a member of the speaker's bureau for Actelion (ending October 2011) and is a member of the speaker's bureau for Gilead and United Therapeutics; and has provided research support for Actelion, Bayer, and Novartis.
REFERENCES
- 1.McLaughlin VV, Archer SL, Badesch DB , et al. ACCF/AHA.ACCF/AHA expert consensus document on pulmonary hyper-tension. . J AM Coll Cardiol. 2009; 119(16):2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Thenappan T, Shah SJ, Rich S , et al. A USA-based registry for pulmonary arterial hypertension 1982,-2006,. EurRespir J. 2007;30:1103–20. doi: 10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 3.Ghio S, Gavazzi A, Campana C , et al. Independent and additive prognostic value of right ventricular systolic function and pulmo-nary artery pressure in patients with chronic heart failure. J Am CollCardiol. 2001;37:183–8. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A , et al. Pulmonary arterial hypertension in France results from a national registry. Am J RespirCrit Care Med. 2006;173:1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 5.Peacock AJ, Murphy NF, McMurray JJV , et al. An epidemiological study of pulmonary arterial hypertension. EurRespir J. 2007;30:104–9. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 6.McGoon M, Guetterman D, Steen V , et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension ACCP evi-dence-based clinical practice guidelines. Chest. 2004;126:14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 7.Archer S, Rich S. Primary pulmonary hypertension a vascular biology and translation research "Work in progress". Circulation. 2000;102:2781–91. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- 8.Christmas BW, McPherson CD, Newman JH , et al. An imbalance between the excretion of thromboxane and prostacyclin metabo-lites in pulmonary hypertension. N Engl J Med. 1992;327:70–5. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- 9.Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med. 2007;28:233–41. doi: 10.1016/j.ccm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Guglin M, Khan H. Pulmonary hypertension in heart failure. J Card Fail. 2010;16(6):461–74. doi: 10.1016/j.cardfail.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Tryka AF, Godleski JJ, Shoen FJ, Vandevater SH. Pulmonary vascular disease and hypertension after valve surgery for mitral stenosis. Hum Pathol. 1985;16(1):65–71. doi: 10.1016/s0046-8177(85)80215-x. [DOI] [PubMed] [Google Scholar]
- 12.Thabut G, Dauriat G, Stern JB , et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–6. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 13.Pengo V, Lensing AW, Prins MG , et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 14.Hoeper MM, Mayer E, Simmonneau G , et al. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113:2011,–20. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- 15.Rich S, Dantzker DR, Ayres SM , et al. Primary pulmonary hypertension a national prospective study. Ann Intern Med. 1987;107:216–23. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 16.Simonneau G, Robbins IM, Beghetti M , et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(1):S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Barst RJ, McGoon M, Torbicki A , et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J AM Coll Cardi-ol. 2004;43:40S–7S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Thenappan T, Shah SJ, Gomberg-Maitland M , et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–65. doi: 10.1161/CIRCHEARTFAILURE.110.958801. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Candales A, Dohi K, Iliescu A , et al. An abnormal right ventricular apical angle is indiciative of global right ventricular im-pairment. Echocardiography. 2006;23:361–8. doi: 10.1111/j.1540-8175.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 20.Forfia PR. Separating the 'right from the left' in patients with pulmonary hypertension. 2013 Feb Available from: http //mdprimer. com/opinioupdate1.htm . 2010 [Google Scholar]
- 21.Miyamoto S, Nagaya N, Satoh T , et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med. 2000;161:487–92. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 22.Mathier MA. The nuts and bolts of interpreting hemodynamics in pulmonary hypertension associated with diastolic heart failure. Adv Pulmonary Hypertens. 2011;10:33049. [Google Scholar]
- 23.Hemnes AR, Forfia PR, Champion HC. Assessment of pulmonary vasculature and right heart by invasive haemodynamics and echocardiography. Int J Clin Pract. 2009;63(Suppl. ):4–19. doi: 10.1111/j.1742-1241.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 24.Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136:37–43. doi: 10.1378/chest.08-2784. [DOI] [PubMed] [Google Scholar]
- 25.Hoeper MM, Lee S, Voswinckel R , et al. Complications of Right Heart Catherization Procedures in Patients with Pulmonary Hyper-tension in Experienced Centers. J Am Coll Cardiol. 2006;48:2546–52. doi: 10.1016/j.jacc.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 26.Badesch DB, Abman SH, Simonneau G , et al. Medical therapy for pulmonary arterial hypertension updated ACCP evidenced-based clinical practice guidelines. Chest. 2007;131:1917–28. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 27.Sitbon O, Humbert M, Jagot J-L , et al. Inhaled nitric oxide as a screening agent for safely identifying responders to oral calcium-channel blockers in primary pulmonary hypertension. Eur Respir J. 1998;12:265–70. doi: 10.1183/09031936.98.12020265. [DOI] [PubMed] [Google Scholar]
- 28.Bocchi EA, Bacal F, Auler Junior JO , et al. Inhaled nitric oxide leading to pulmonary edema in stable severe heart failure. Am J Cardiol. 1994;74:70–2. doi: 10.1016/0002-9149(94)90496-0. [DOI] [PubMed] [Google Scholar]
- 29.15th edition. 2001. Rich In Harrison's Principles of Internal Medicine. pp. 1506–7. [Google Scholar]
- 30.McGoon M, Frost A, Miller D , et al. Four-year outcomes of patients with pulmonary arterial hypertension risk, prognosis, and the disease duration continuum. Chest October. 2011;140(4_Meeting Abstracts):724A–724A. [Google Scholar]
- 31.McLaughlin VV, McGoon M. Pulmonary arterial hypertension. Circulation. 2006;114:1417–31. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 32.Paul R. Forfia and Jean-Luc Vachiery.Echocardiography in pulmonary arterial hypertension. Am J Card. 2012;6:S16–S24. doi: 10.1016/j.amjcard.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Maruitz GJ, Kind T, Marcus JT , et al. Progressive changes in right ventricular geometric shortening and long-term survival in pul-monary arterial hypertension. Chest. 2012;141(4):935–43. doi: 10.1378/chest.10-3277. [DOI] [PubMed] [Google Scholar]
- 34.Sitbon O, Humbert M, Jais X , et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111:3105–11. doi: 10.1161/CIRCULATIONAHA.104.488486. [DOI] [PubMed] [Google Scholar]
- 35.Gaile N, Ghofrani H, Torbicki A , et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 36.Gaile N, Brundage BH, Ghofrani H , et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 37.Rubin LJ, Badesch DB, Barst RJ , et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 38.Gaile N, Ribin LJ, Hoeper M , et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 39.Gaile N, Olschewski H, Oudiz RJ , et al. Ambrisentan for the treatment of pulmonary arterial hypertension results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 40.United States Food and Drug Administration drug labeling Medication Guide-Tracleer. Available from http: //www.fda.gov/ downloads/ Drugs/DrugSafety/UCM089801.pdf. [Google Scholar]
- 41.United States Food and Drug Administration drug labeling Medication Guide - Letaris. Available from http: //www.fda.gov/ downloads/Drugs/DrugSafety/ucm088617.pdf. [Google Scholar]
- 42.Barst RJ, Rubin LJ, Long WA , et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. New Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 43.Dadfarmay S, Berkowitz R, Kim B , et al. Differentiating pulmonary arterial and pulmonary venous hypertension and the implica-tions for therapy. Congest Heart Fail. 2010;16:287–91. doi: 10.1111/j.1751-7133.2010.00192.x. [DOI] [PubMed] [Google Scholar]
- 44.Reichenberger F, Pepke-Zaba J, McNeil K , et al. Atrial septostomy in the treatment of severe pulmonary arterial hypertension. Thor-ax. 2003;58:797–800. doi: 10.1136/thorax.58.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman A, Sklansky MS, Lucas VW , et al. Atrial septostomy as a bridge to lung transplantation in patients with severe pulmonary hypertension. Am J Cardiol. 1999;84:682–6. doi: 10.1016/s0002-9149(99)00416-6. [DOI] [PubMed] [Google Scholar]
- 46.Simonneau G, Robbins IM, Beghetti M , et al. Updated clinical classification of pulmonary hypertension. J AM Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med. 2007;28:233–41. doi: 10.1016/j.ccm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Teerlink Jr. Recent heart failure trials of neurohormonal modulation (OVERTURE and ENABLE): approaching the asymptome of effi-cacy?. J Card Fail. 2003;8:124–7. doi: 10.1054/jcaf.2002.126486. [DOI] [PubMed] [Google Scholar]
- 49.Califf RM, Adams KF, McKenna WJ , et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 50.Ontkean M, Gay R, Greenberg B. Diminished endothelium-derived relaxing factor activity in an experimental model of chronic heart failure. Circ Res. 1991;69:1088–96. doi: 10.1161/01.res.69.4.1088. [DOI] [PubMed] [Google Scholar]
- 51.Porter TR, Taylor DO, Cycan A , et al. Endothelium-dependent pulmonary artery responses in chronic heart failure influence of pulmonary hypertension. J Am Coll Cardiol. 1993;22:1418–24. doi: 10.1016/0735-1097(93)90552-c. [DOI] [PubMed] [Google Scholar]
- 52.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction. Circulation. 2011;124:164–74. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 53.MM Redfield, HH Chen, BA Borlaug , et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction a randomized clinical trial. JAMA. 2013;309(12):1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guazzi M, Samaja M, Arena R , et al. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–44. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 55.Lewis GD, Lachmann J, Camuso J , et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115:59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]