Abstract
Optimizing management of patients with heart failure remains quite challenging despite many significant advances in drug and device therapy for this syndrome. Although a large body of evidence from robust clinical trials supports multiple thera-pies, utilization of these well-established treatments remains inconsistent and outcomes suboptimal in “real-world” patients with heart failure. Disease management programs may be effective, but are difficult to implement due to cost and logistical issues. Another approach to optimizing therapy is to utilize biomarkers to guide therapeutic choices. Natriuretic peptides pro-vide additional information of significant clinical value in the diagnosis and estimation of risk inpatients with heart failure. Ongoing research suggests a potential important added role for natriuretic peptides in heart failure. Guiding therapy based on serial changes in these biomarkers may be an effective strategy to optimize treatment and achieve better outcomes in this syn-drome. Initial, innovative, proof-of-concept studies have provided encouraging results and important insights into key as-pects of this strategy, but well designed, large-scale, multicenter, randomized, outcome trials are needed to definitively estab-lish this novel approach to management. Given the immense and growing public health burden of heart failure, identification of cost-effective ways to decrease the morbidity and mortality due to this syndrome is critical.
Keywords: Biomarker, guided, heart failure, natriuretic peptides.
INTRODUCTION
Pharmacological and device therapy for heart failure with reduced ejection fraction (HFrEF) have evolved significantly over the past two decades with several interventions well documented to improve outcomes [1, 2]. However, heart failure continues to be characterized by high morbidity and mortality especially in those hospitalized for decompensation [3]. The economic impact associated with hospitalization for this condition is immense and has created a major public health problem [4]. Despite extensive results from clinical trials that are clearly positive, meaningful gaps persist in the use of evidence-based therapy [5-7]. These realities have recently intensified interest in developing more effective strategies to optimize the care of patients with heart failure at reduced cost [8-12]. Disease management strategies have received a lot of attention, but these approaches are often labor intensive and widespread application has been limited by variable results and concerns about cost [13-15].
Cardiac biomarkers are emerging as a novel strategy for management of patients with heart failure [16, 17]. A number of molecular markers are now well established to be of diagnostic and prognostic value in heart failure. This strong association led to the idea that serial monitoring of these biomarkers could guide therapy to improve patient outcomes [18-22]. Initial studies provide a strong signal that this strategy may be effective. This review is intended to be a contemporary update of current findings in this field. After a brief introduction concerning the rationale for a biomarker-guided approach to heart failure management, key issues raised by initial clinical trial results will be carefully reviewed. Potential reasons for the heterogeneous results of these trials will be discussed in the context of study design and the actual application of the strategy in the studies conducted to date.
GUIDING BY BIOMARKERS
There are two important characteristics that successful biomarkers for guiding therapy for heart failure will share. First, useful biomarkers must reflect the severity of the pathophysiology of heart failure. Effective markers for guiding therapy may not assist in diagnosis, but should accurately predict future risk. Second, biomarkers effective for guiding therapy must improve in response to proper application of evidence-based therapies for heart failure. Thus, serial monitoring to achieve a reduced target level of that biomarker should be related to optimization of evidence-based therapy. In this way, biomarkers useful in guiding therapy in heart failure may merely be monitors of disease activity, like N-terminal pro-B type natriuretic peptide(NT-proBNP), rather than biotargets closely linked to the pathophysiological process causing disease progression, like norepinephrine or angiotensin II.
CHOICE OF BIOMARKER
The natriuretic peptides, B-type natriuretic peptide (BNP) and (NT-proBNP), are the most extensively studied biomarkers for guiding therapy of heart failure [19]. These markers have been studied for over a decade in a variety of cardiovascular diseases and are well established as aids in the diagnosis and prognosis of heart failure [23-26]. Activation of natriuretic peptides are closely correlated with many forms of underlying structural heart disease including degree of mitral regurgitation, right ventricular function, and importantly, left ventricular function, size and wall stress [27-29]. This supports their successful use in the diagnosis of heart failure and why they are able to effectively predict risk in this syndrome [30-32]. Although there are a number of other biomarkers that reliably identify high-risk patients like cardiac specific troponins, ST2, and galectin-3, to date, these markers have not been studied as aids to guiding therapy [33-36].
Although natriuretic peptides are the current choice for biomarker guidance, promising new evidence suggests that combining novel markers with natriuretic peptides may be even more effective in a so-called multi-marker based strategy. The rationale for multiple markers is straight-forward. The pathophysiology of heart failure involves multiple, interrelated but distinct pathways that cause myocardial damage and circulatory failure. Combinations of carefully selected markers can better reflect the activity of multiple pathological pathways in heart failure and allow therapeutic adjustments based on a comprehensive assessment of this syndrome. Support for this concept is provided by prognostic studies that show multiple markers, including natriuretic peptides, cardiac troponin, ST2, and galectin-3, have independent predictive value for adverse outcomes [37-39].
EFFICACY OF BIOMARKER-GUIDED THERAPY IN THE ELDERLY
One point of controversy that has emerged from the pilot studies of biomarker-guided heart failure therapy concerns the ability of this strategy to improve heart failure outcomes in elderly patients. Four studies (BATTLESCARRED, TIME-CHF, PROTECT, and UPSTEP) have reported detailed analysis of the effectiveness of this strategy specifically in the elderly (typically age ≥75years old) heart failure population [40-44].
The first of these studies, BATTLESCARRED, reported a significant interaction between age and the treatment benefit of NT-proBNP biomarker-guided therapy. Patients< 75 years of age showed a significant reduction in all-cause mortality during the study while patients ≥ 75 years of age had no improvement in mortality with biomarker guidance. Biomarker-guided therapy did not reduce the risk of hospitalization in the overall patient population or any age group. In analyzing their data, the study investigators found a number of potential explanations for differential effectiveness by age group. There was significantly less medication titration in the older patients and they were significantly less likely to reach target doses (all p<0.001), especially for beta-blockers. At the end of 12 months, the dose of beta-blocker was 50% less for patients ≥ 75 years of age compared to younger patients and only 12% of older patients achieved target doses of beta-blockers (versus 27% of younger patients). Dose of ACE-Inhibitor was likewise reduced with older patients who received 79% of the dose of younger patients. Only 30% of older patients reached target doses for ACE-Inhibitor compared to 50% for younger patients. Finally, this trial included patients with heart failure and preserved left ventricular ejection fraction (HFpEF), defined as LVEF ≥ 40% in this study. Fully 53% of study patients aged 75 years or older had preserved ejection fraction. At present there are no therapies proven to reduce mortality, the primary end point in this trial, in patients with HFpEF [45]. Taken together, these age-related differences would be expected to reduce the effectiveness of biomarker-guided therapy compared to standard of care in the elderly patients in this study.
Even though the main TIME-CHF trial was restricted to patients with HFrEF, study results still suggested a difference in the effectiveness of NT-proBNP-guided therapy by age. Younger but not older patients showed improved outcomes with this strategy (Table 1). Overall, medication utilization was similar in older and younger patients in this study, but the dose titration of beta-blockade was less in older patients. The older patients in TIME-CHF did have significantly more comorbidities (e.g., cancer and kidney disease) than younger patients. Subgroup analysis stratified on the frequency of baseline comorbidity suggested that hospitalization-free-survival and all-cause mortality were reduced by guided therapy when comorbidity burden was low.
Table 1.
Treatment effect on main outcomes in TIME-CHF (overall and by age group).
| Group | Overall Survival | All-Cause Hospital-Free Survival | HF Hospital-Free Survival | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Overall | 0.68 (0.45 - 1.02) | 0.06 | 0.91 (0.72 - 1.14) | 0.39 | 0.68 (0.50 - 0.92) | 0.01 |
| <75 years | 0.41 (0.19 - 0.87) | 0.02 | 0.70 (0.49 - 1.01) | 0.05 | 0.42 (0.24 - 0.75) | 0.002 |
| ≥ 75 years | 0.88 (0.54 - 1.44) | 0.61 | 1.10 (0.82 - 1.47) | 0.54 | 0.87 (0.60 - 1.26) | 0.45 |
CI=confidence interval, HF=heart failure HR=hazard ratio.p-values from Log-rank test comparing biomarker-guided to standard of care.Table results are adapted from Figure 6 in Pfisterer et al. [41].
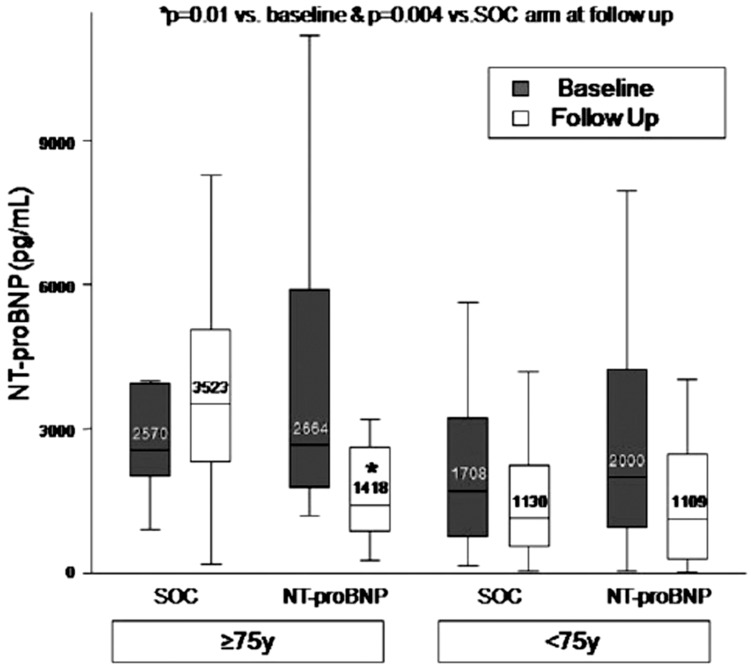
In contrast to these two earlier trials, the PROTECT study reported a beneficial effect from the strategy of NT-proBNP-guided therapy in elderly patients. Elderly patients in the standard of care arm of the trial had a higher rate of cardiovascular events compared to older patients in the NT-proBNP-guided arm (1.76 events per patient versus 0.71 events per patient, p=0.03). The adjusted logistic odds ratio for cardiovascular events (NT-proBNP-guided care versus standard of care) in the elderly study patients (n=38) was 0.24 (p<0.008). In study patients<75 years (n=113), the adjusted logistic odds ratio for events was 0.61 (p=0.10). Of interest, overall there was no difference in intensification of pharmacological therapy by age group. However, among elderly patients there was a greater intensification of therapy in the biomarker-guided arm than in the standard of care arm. Consistent with the finding of better outcomes, NT-proBNP values increased in elderly patients in the standard therapy arm, but declined in the NT-proBNP-guided arm (Fig. 1). Some caveats about the positive findings in PROTECT among older patients need to be recognized. The number of patients with advanced age in this study was relatively small (n=38) especially compared to the TIME-CHF trial (n=289). Also, there were too few deaths in the PROTECT trial to make any assessment of the effect of guided therapy on mortality in elderly patients. In the UPSTEP trial there was no overall benefit of natriuretic peptide-guided therapy on adverse outcomes in HFrEF patients. However, there was no interaction between age and efficacy of biomarker-guided therapy, with no observed benefit from this strategy in younger or older patients [44].
Fig. (1).
Change in NT-proBNP by age group in the study arms. In the older patients there was an increase in NT-proBNP in the standard of care (SOC) group, while there was a decline in NT-proBNP in the guided arm of the study. Boxes indicate interquartile ranges, with a median cross line; the upper and lower whiskers extend to the 5th and 95th percentiles. Figure from Gaggin et al. [43].
Careful analysis of available results suggests that elderly patients with HFrEF may benefit from a biomarker-guided strategy if intensification of medical therapy is possible and competing risk from comorbidities does not override the beneficial effects of biomarker monitoring. Ongoing analysis of trial results with regard to age related differences in the benefit of natriuretic peptide-guided therapy in patients with heart failure due to systolic dysfunction is warranted.
MECHANISTIC RATIONALE FOR POSITIVE GUIDED TRIALS
A recent substudy of the PROTECT trial reported the effect of NT-proBNP-guided treatment compared to standard of care on echocardiographic assessments of cardiac function [46, 47]. Serial echocardiographic studies demonstrated a greater improvement in left ventricular ejection fraction in the biomarker-guided arm than in standard therapy alone. There were also trends for reduction in left ventricular end diastolic and end systolic volume with biomarker guidance. Additionally, there was evidence for improvement in other key echocardiographic assessments of cardiac structure and function in the biomarker-guided arm compared to the standard therapy arm. Patients managed by NT-proBNP-guidance demonstrated significant improvement in measures of right ventricular size and function and significant decreases in the ratio of early transmitral peak velocity to early diastolic peak annular velocity (E/E’), right ventricular systolic pressure, and severity of mitral regurgitation compared to standard therapy. These findings from the PROTECT echocardiographic substudy provide an important mechanistic rationale for the improvement in outcome observed in patients managed by natriuretic peptide guidance versus standard of care.
DISEASE MANAGEMENT VERSUS BIOMARKER MONITORING
Trials comparing heart failure disease management with standard care have mixed results, but this strategy remains of interest to many. The recent study of Berger et al. provides a direct test of whether the addition of natriuretic peptide monitoring produces incremental benefit compared to heart failure disease management alone versus usual care [48]. These investigators compared outcomes in a three-arm, prospective, randomized trial comparing standard of care, heart failure disease management, and heart failure disease management plus biomarker-guided therapy. Natriuretic peptide levels decreased from baseline to 12 month follow-up in the biomarker-guided group more than in the disease management arm. In contrast, there was no decrease in NT-proBNP level observed in the usual care group. This study found that NT-proBNP-guided therapy reduced the days of heart failure hospitalization when compared to both disease management and usual care cohorts. The combined endpoint of death or heart failure rehospitalization was lower in the biomarker-guided group than in the disease management arm. Both the biomarker guided arm and the disease management arms had improved outcomes compared to the usual care group.
HEALTH ECONOMICS OF BIOMARKER-GUIDED THERAPY
One rationale for investigation of biomarker-guided therapy is the hope that this strategy will help reduce the high cost of care associated with heart failure. Recurrent hospitalization accounts for the vast majority of the economic burden associated with this syndrome. Given the expected marked cost differential between serial biomarker monitoring and hospitalization for heart failure, even a modest reduction in admissions due to biomarker guiding could actually result in a net cost savings. Only rarely can life years be prolonged by medical therapy while the cost of care is simultaneously reduced.
Recently published results from the TIME-CHF study provide important, novel data in support of this concept in the case of natriuretic peptide-guided therapy for HFrEF patients [49]. Analysis of health care costs in this trial found that biomarker-guided therapy had a high probability of being cost-effective. Biomarker-guided therapy resulted in an incremental cost-effectiveness ratio of $5,870 per life-year gained which is well within the range of commonly accepted cost for medical treatments (up to $50,000 per year gained). Cost effectiveness was especially evident in patients 60 to 75 years old and in patients with less than two co-morbidities. Of interest, in patients ≥ 75 years of age, biomarker-guided therapy did not reduce hospitalization cost but was associated with the ability to remain in the home as opposed to requiring care in assisted living or a nursing home. When the additional cost of an alternative residence was taken into account, biomarker-guided therapy was associated with an actual cost savings of $2,979 per life year gained.
CRITICAL ASPECTS OF NATRIURETIC PEPTIDE-GUIDED THERAPY
Careful consideration of the rationale for biomarker-guided therapy with natriuretic peptides helps identify some features that will likely be critical to the success of this strategy. Foremost, serial determination of natriuretic peptides must drive intensification of heart failure therapy based on changes in BNP or NT-proBNP concentration. Clearly, if serial elevations of biomarker measurements do not result in a change in the pattern of treatment, the strategy is unlikely to alter outcomes. Certainly, knowledge of risk provided by the initial measurement of the biomarker may also drive beneficial behaviors on the part of the patient and the physician as well as other health care providers that could result in better outcomes on existing therapy. This knowledge may lead to more careful follow-up and general monitoring in the clinic, better compliance with sodium restriction and medication use by the patient, and greater willingness of the patient to seek additional evaluation and treatment at an earlier point if they worsen.
Monitoring diuretic use with natriuretic peptides may improve the utilization of these agents. Diuretic therapy is generally regarded as of secondary value, due to inability to change or possibly even worsen the underlying natural history of heart failure. However, congestion remains the overwhelming reason for hospital admission and diuretics remain the mainstay for treating volume overload. Monitoring for early, marked elevation or increasing natriuretic peptides concentration, can lead to prompt, aggressive use of diuretics that could reduce hospitalization for congestion. In addition, improving heart failure status related to monitoring natriuretic peptide levels may allow reduction in diuretic dose, thus making hypotension less of an issue for medication initiation or up-titration.
As noted above, since the biomarker-guided strategy is dependent on the ability to favorably alter evidenced-based therapy, the type of heart failure may influence the success rate of this approach. There is a relative wealth of effective therapy for HFrEF versus HFpEF. Currently the paucity of proven therapies for HFpEF makes these patients unlikely to experience a mortality benefit from serial monitoring of natriuretic peptides [50]. In contrast, it is possible that serial natriuretic peptide monitoring in selected patients with HFpEF that focuses on optimizing volume status and decreasing hospital admissions due to congestion could be effective.
Another key aspect of the biomarker-guided strategy is the approach to patient follow-up. Clearly there must be a balance between visit frequency and patient burden. However, there must be adequate opportunity to adjust therapy in patients with high initial or persistently elevated natriuretic peptide levels. Individualizing the number of encounters is appropriate but the protocol must allow for potential recurrent and timely visits for therapy adjustment.
INSIGHTS INTO VARIABLE RESULTS OF PILOT BIOMARKER-GUIDED TRIALS
The strategy of monitoring natriuretic peptides as an adjunct to standard care has been investigated in a number of randomized clinical trials to date [47]. Assessed from the perspective of classical randomized trial design for mortality and morbidity investigation, these studies, in most cases, would be regarded as hypothesis generating due to their choice of primary endpoint, small sample size and limited number of events. Nevertheless, they provide critical insights into characteristics likely to be part of the optimal design for natriuretic peptide-guided trials, and meta-analysis of their results show a very promising suggestion of mortality reduction [51, 52]. As discussed in detail in this review, there are a number of design and performance characteristics likely to help distinguish positive versus neutral studies of the biomarker-guided strategy. These are discussed in further detail below and their close correlation with trial results is presented in summary form in Table 2.
Table 2.
Design and application aspects of natriuretic peptide-guided heart failure trials.
| Study | N | Primary Endpoint | Event/ Deaths |
Mean Age | PEF | Target NT-pro BNP or BNP* (pg/mL) | Low Target NP Reached | Inc Rx Guided Arm > SOC | NP Reduced in Guided Arm > SOC |
|---|---|---|---|---|---|---|---|---|---|
| Positive | |||||||||
| PROTECT | 151 | CV Events | 158/10 CV | 63 | N | 1000 | Y | Y | Y |
| STARS-BNP | 220 | HF Death + HF Hosp | 82/ 18 | 65 | N | 100* | Y | Y | NM |
| Troughton et al. | 69 | CV Events | 73/ 8 | 70 | N | 1735 | Y | Y | Y |
| Berger et al. | 278 | Survival Free HF Hosp |
201/ 76 | 71 | N | 2200 | Y | Y | Y |
| Equivocal | |||||||||
| TIME-CHF | 499 | Death + All Cause Hosp |
202/ 95 | 77 | N | 400 < 75y/o 800 ≥ 75y/o |
N | Y | N |
| BATTLE-SCARRED | 364 | Death + HF Hosp | 196/ NM | 76 | Y | 1270 | N | N | N |
| Neutral | |||||||||
| STAR-BRITE | 130 | Days Alive Out of Hospital, 90 days | N/A/ 4 | 60 | N | <450* at discharge | N | N | N |
| SIGNAL-HF | 252 | Days Alive Out of Hospital, 9 months | N/A/ 14 | 78 | N | 50% below trial entry | N | N | N |
| PRIMA | 345 | Days Alive Out of Hospital | N/A/ 103 | 72 | Y | Level at discharge | N | N | N |
| NorthStar | 407 | Death or CV Hosp | 175/ 84 | 73 | N | 1000 | N | N | N |
| UPSTEP | 279 | Death or Hosp or Worsening HF | NM/ 60 | 71 | N | 150* ≤ 75y/o 300* > 75y/o |
N | N | NM |
BNP = B-type natriuretic peptide, CV = Cardiovascular, HF=heart failure,Hosp = hospitalizations, Inc = increased,N/A = not applicable, NM = no mention, NP=natriuretic peptide, NT-proBNP = N-terminal pro–B-type natriuretic peptide, PEF=preserved ejection fraction, Rx=treatment, SOC= standard of care, y/o = years old. Table results modifiedfrom Januzzi [47].
DESIGN ISSUES
The ideal primary endpoint for a biomarker-guided therapy trial can be debated. In retrospect, questions can be raised about the primary endpoint used in the TIME-CHF trial, survival free of all-cause hospitalizations. A more targeted, disease specific endpoint such as survival free of hospitalization for heart failure may be more likely to be improved by a biomarker-guided strategy. In fact, this secondary endpoint in the TIME-CHF study was significantly reduced in the overall study population (Table 1). The study of Berger et al. also showed a signification reduction in the risk in this endpoint [48]. In contrast, the PROTECT study was positive even though the primary endpoint was not disease specific. However, the primary endpoint was cardiovascular events and the treatment difference was driven by reduction in worsening heart failure and heart failure hospitalization. Although the baseline characteristics of patients in the various trials are difficult to compare directly, the frequency of significant comorbidities appears to be greater TIME-CHF than PROTECT. These comorbidities would not be expected to improve with biomarker guidance and could contribute to death and hospitalization even if biomarker guidance reduced the risk of heart failure outcomes. In most drug development trials, the study selection process results in a patient population with a low burden of comorbidity where cardiovascular events represent the great majority of adverse outcomes that occur. This degree of concordance between all-cause hospitalization and cardiovascular hospitalization helps to minimize the difference in results by end point.
APPLICATION ISSUES
Mandating intensification of therapy in the trial design does not guarantee this will happen in the conduct of the study. So, it is important to determine what actions were taken in response to elevated biomarker concentrations. As pointed out by the investigators of the BATTLESCARRED trial, availability of NT-proBNP results did not drive greater intensification of therapy in the biomarker-guided arm of the study (Table 3). This may have contributed to neutral nature of this study’s outcome results.
Table 3.
Medication titration in different arms of the BATTLESCARRED trial.
| Drug | Treatment group | 0 mos | 3 mos | 6 mos | 12 mos | 24 mos |
|---|---|---|---|---|---|---|
| Furosemide, mg/day | NT-proBNP* Clinically Guided* Usual Care† |
128±23 149±23 124±22 |
138±20 144±21 121±21 |
140±22 134±21 119±21 |
182±22 166±23 123±22 |
200±27 197±28 140±25 |
| ACE-I, mg/day | NT-proBNP Clinically Guided Usual Care |
12.7±6 13.3±6 10.3±6 |
13.0±6 14.7±6 11.3±6 |
13.3±6 14.6±6 11.0±6 |
13.1±6 14.2±6 11.0±6 |
12.4±7 14.0±7 10.8±6 |
| Beta-blocker, mg/day | NT-proBNP† Clinically Guided† Usual Care† |
76±11 80±11 73±10 |
83±9 91±9 74±9 |
95±9 95±9 75±9 |
95±10 99±10 73±10 |
94±11 99±12 72±10 |
| Spironolactone, mg/day | NT-proBNP § Clinically Guided Usual Care |
20±6 21±6 20±2 |
22±4 22±5 20±2 |
22±4 24±5 21±2 |
20±5 23±5 21±2 |
16±7 20±6 21±3 |
Data are shown as mean±SD. Mean doses are for patients receiving drug. Angiotensin-converting enzyme inhibitor (ACE-I) doses are given in enalapril equivalents. Betablockerdoses are given in metoprolol equivalents. *Dose increased over follow-up, p <0.001. †No significant change in dose, and either average dose or increment in dose over follow-up is less than in the N-terminal pro–B-type natriuretic peptide (NT-proBNP) group and clinically-guided group, p<0.05. ‡Beta-blocker doses rise in first 6 months, p <0.001. §Significant falls over 24 months, p <0.001. Table results from Lainchbury et al. [40].
Another way to assess likelihood of effective medication titration in biomarker-guided trials is to examine changes in natriuretic peptide levels in the guided therapy and standard therapy arms. Routinely recommended therapies for heart failure, beta-blockers, ACE-Inhibitors, ARBs, and aldosterone antagonists, all reduce natriuretic peptide levels during sustained therapy [53-58]. Non-pharmacologic treatments, like exercise and cardiac resynchronization therapy, also reduce natriuretic peptide concentrations [47, 59, 60]. Reduction in natriuretic peptide levels in the biomarker-guided arm relative to standard therapy is a characteristic finding of positive trials. In contrast, neutral trials show no change in natriuretic peptides in either the biomarker guided arm or the standard therapy arm. Interestingly in the TIME-CHF trial, a neutral effect on the study’s primary endpoint was associated with a decline in NT-proBNP level in both the biomarker-guided and standard therapy arms. This suggests that a differential effect on natriuretic peptide concentrations between biomarker-guided and standard therapy arms is likely an even better marker of success of natriuretic peptide monitoring.
APPROPRIATE CONTROL GROUP FOR BIOMARKER-GUIDED THERAPY TRIALS
The appropriate comparison group for biomarker-guided therapy remains an important consideration. From a purely scientific perspective, there is an understandable desire to separate other effects of the strategy, like visit frequency, from measurement of the biomarker itself. Although often referred to as standard of care, this has led some trials to develop a comparison arm to biomarker guidance that is similar to disease management in intensity. However, in the end, some increase in the frequency of follow-up visits over usual care is essential to the biomarker-guided strategy. At least in the biomarker strategy, these added visits will be targeted to patients with elevated natriuretic peptide levels, unlike in a disease management strategy where all patients have an intensified visit schedule.
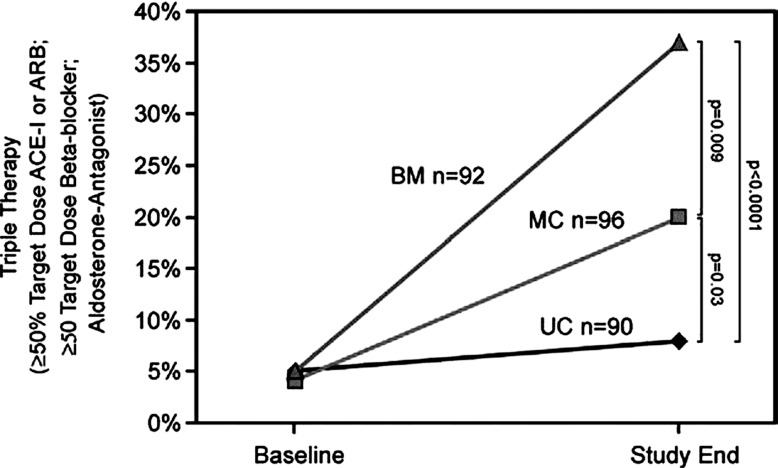
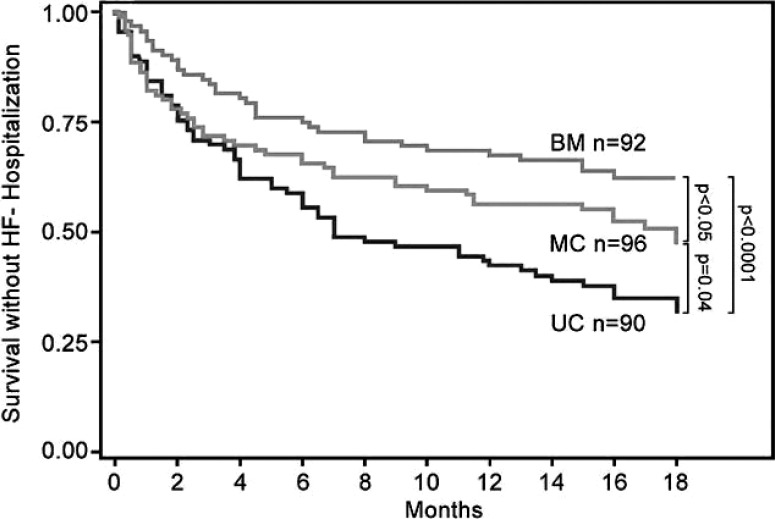
An additional argument against a disease management style control arm in biomarker-guided trials is that this approach may give a false indication of true heart failure patient risk in usual care environments. As the ultimate goal is to apply this approach to heart failure patients managed in primary care and general cardiology, the true potential benefit of the guided strategy may be more accurately reflected by comparing outcomes with true usual care approaches. Review of previous trial results inpatients followed in the course of usual care (outside the structure of the trial) show the strikingly poor outcome of patients managed in this way, especially in high-risk populations defined by a recent hospitalization and/or elevated natriuretic peptide levels (Fig. 2). Rather than representing a “Straw Man”, poor results in usual care are simply a reflection of the reality of heart failure management in the absence of risk stratification and monitoring of the results of therapy over time. At a minimum, future studies should consider collecting data on patients managed by usual care approaches to complement results from a comparison group treated by “standard of care” that mimics disease management (Fig. 3).
Fig. (2).
The proportion of patients on triple therapy at adequate dose defined as on spironolactone and at ≥ 50% of the target dose of an angiotensin- converting enzyme inhibitor/angiotensin receptor blocker and a beta-blocker. Proportions on triple therapy were similar among randomized groups at baseline but differed significantly by study end. This proportion was higher in the BM group versus the MC group, and higher in the MC versus the UC group at end of follow-up. BM=biomarker group, MC=multidisciplinary care, UC=usual care. Figure from Berger et al. [48].
Fig. (3).
The combined end point of death or heart failure hospitalization was lower in the BM (37%) versus MC group (50%; p < 0.05) and in the MC versus UC group (65%; p = 0.04). HF=heart failure. Other abbreviations are as in Fig. (2). Figure from Berger et al. [48].
FUTURE DIRECTIONS
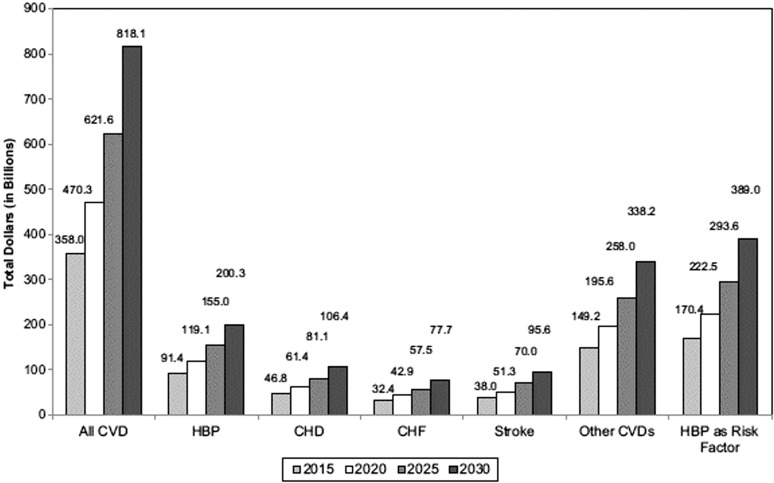
Forecasts concerning the long-term economic burden related to cardiovascular disease, including heart failure, in the United States remain bleak (Fig. 4) [61, 62]. Ongoing evaluation of novel clinical strategies for optimizing treatment, improving outcomes, and reducing the cost of cardiovascular disease is sorely needed. Using biomarkers to optimize cardiovascular therapy represents a major new, potentially effective approach to achieve these goals.
Fig. (4).
The projected total costs of cardiovascular diseases from 2015–2030 (2010 $ in billions) in the United States are shown. HBP=hypertension, CHD= coronary heart disease, CHF=congestive heart failure. Unpublished data tabulated by the American Heart Association using methods described in Heidenreich et al. Figure from Go et al. [61, 62].
New biomarker guided studies in heart failure need to apply the rigorous methodology evolved for prospective, randomized, controlled clinical trials and build carefully on the lessons learned from the pioneering work to date in this field. This requirement made it apparent that a prospective, large-scale outcomes study was needed to definitively test this innovative strategy. This led to the development of the GUIDing Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) trial, funded by the National Heart Lung and Blood Institute, which is currently enrolling (www.clinicaltrials.gov identifier NCT01685840).
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The following authors have no conflicts: Amanda E. Pruett, DO, Amanda K. Lee, BA, J. Herbert Patterson, PharmD, Todd A. Schwartz, DrPH, and Jana M. Glotzer C-ANP. Kirkwood F. Adams, Jr., MD has received fees for serving as a consultant and an advisory board member for BG Medicine, Roche Diagnostics and Critical Diagnostics. He has received research funding from Roche Diagnostics and Critical Diagnostics. He has no conflicts of interest to report regarding service on Speaker’s Bureau, employment, ownership of stock or patents related to this publication.
REFERENCES
- 1.Adams K, Lindenfeld J, Arnold JMO , et al. Executive summary HFSA 2006 comprehensive heart failure practice guideline. J Card Fail. 2006;12:10–38. doi: 10.1016/j.cardfail.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B , et al. 2013 ACCF/AHA guideline for the management of heart failure A report of the American College of Cardiology Foundation/American Heart As-sociation task force on practice guidelines. J Am Coll Cardiol. 2013;Jun 5. [Epub ahead of print] doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Curtis LH, Greiner MA, Hammill BG , et al. Early and long-term outcomes of heart failure in elderly persons, 2001-2005. Arch Intern Med. 2008;168:2481–8. doi: 10.1001/archinte.168.22.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM , et al. Heart dis-ease and stroke statistics--2010 update A report from the American-Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Lenzen MJ, Boersma E, Reimer WJ , et al. Under-utilization of evidence-based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials a report from the Euro Heart Survey on heart fail-ure. Eur Heart J. 2005;26:2706–13. doi: 10.1093/eurheartj/ehi499. [DOI] [PubMed] [Google Scholar]
- 6.Lainscak M, Cleland JG, Lenzen MJ, Follath F, Ko-majda M, Swedberg K. International variations in the treatment and co-morbidity of left ventricular systolic dysfunction data from the EuroHeart Failure Survey. Eur J Heart Fail. 2007;9:292–9. doi: 10.1016/j.ejheart.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.de Groote P, Isnard R, Assyag P , et al. Is the gap be-tween guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur J Heart Fail. 2007;9:1205–11. doi: 10.1016/j.ejheart.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Chan PS, Oetgen WJ, Buchanan D , et al. Cardiac per-formance measure compliance in outpatients The American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (Practice Innovation and Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal im-plementation of evidence-based heart failure therapies on mortality. AmHeart J. 2011;161:1024–30 e1023. doi: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson LW. Projecting heart failure into bankruptcy in 2012?. Am Heart J. 2011;161:1007–11. doi: 10.1016/j.ahj.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Kass DA. Rescuing a failing heart Putting on the squeeze. Nat Med. 2009;15:24–5. doi: 10.1038/nm0109-24. [DOI] [PubMed] [Google Scholar]
- 12.Anderson ME, Mohler PJ. Rescuing a failing heart Think globally, treat locally. Nat Med. 2009;15:25–6. doi: 10.1038/nm0109-25. [DOI] [PubMed] [Google Scholar]
- 13.Peters-Klimm F, Muller-Tasch T, Remppis A, Szecsenyi J, Schellberg D. Improved guideline adherence to phar-macotherapy of chronic systolic heart failure in general practice--results from a cluster-randomized controlled trial of implementation of a clinical practice guideline. J Eval Clin Pract. 2008;14:823–9. doi: 10.1111/j.1365-2753.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 14.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death The Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–64. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 15.Clark RA, Inglis SC, McAlister FA, Cleland JG, Stew-art S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure Systematic review and meta-analysis. BMJ (Clinical research ed.). 2007;334:942. doi: 10.1136/bmj.39156.536968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasan RS. Biomarkers of cardiovascular disease Molecu-lar basis and practical considerations. Circulation. 2006;113:2335–62. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 17.Tang WH, Francis GS, Morrow DA , et al. National academy of clinical biochemistry laboratory medicine practice guide-lines Clinical utilization of cardiac biomarker testing in heart failure. Circulation. 2007;116:e99–109. doi: 10.1161/CIRCULATIONAHA.107.185267. [DOI] [PubMed] [Google Scholar]
- 18.Braunwald E. Biomarkers in heart failure. N EnglJMed. 2008;358:2148–59. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 19.Januzzi JL, Troughton R. Are serial BNP measurements useful in heart failure management? Serial natriuretic peptide meas-urements are useful in heart failure management. Circulation. 2013;127:500–7; discussion 508. doi: 10.1161/CIRCULATIONAHA.112.120485. [DOI] [PubMed] [Google Scholar]
- 20.Maisel A, Mueller C, Adams K , Jr, Anker SD , et al. State of the art Using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–39. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Mueller C, Breidthardt T, Laule-Kilian K, Christ M, Perruchoud AP. The integration of BNP and NT-proBNP into clinical medicine. Swiss Med Wkly. 2007;137:4–12. doi: 10.4414/smw.2007.11614. [DOI] [PubMed] [Google Scholar]
- 22.Adams KF , Jr, Felker GM, Fraij G, Patterson JH, O'Connor CM. Biomarker guided therapy for heart failure Focus on natriuretic peptides. Heart Fail Rev. 2010;15:351–70. doi: 10.1007/s10741-009-9139-9. [DOI] [PubMed] [Google Scholar]
- 23.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Rehman SU, Januzzi JL ., Jr Natriuretic peptide testing in clinical medicine. Card Rev. 2008;16:240–49. doi: 10.1097/CRD.0b013e3181815333. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Rumayor A, Richards AM, Burnett JC, Januz-zi JL ., Jr Biology of the natriuretic peptides. The Am J Cardiol. 2008;101:3–8. doi: 10.1016/j.amjcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Lee CY, Burnett JC ., Jr Natriuretic peptides and therapeu-tic applications. Heart Fail Rev. 2007;12:131–42. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 27.Sutton TM, Stewart RA, Gerber IL , et al. Plasma natriu-retic peptide levels increase with symptoms and severity of mitral regurgitation. J Am Coll Cardiol. 2003;41:2280–7. doi: 10.1016/s0735-1097(03)00486-8. [DOI] [PubMed] [Google Scholar]
- 28.Krittayaphong R, Boonyasirinant T, Saiviroonporn P, Thanapiboonpol P, Nakyen S, Udompunturak S. Correlation be-tween NT-pro BNP levels and left ventricular wall stress, sphericity index and extent of myocardial damage A magnetic resonance imaging study. J Card Fail. 2008;14:687–94. doi: 10.1016/j.cardfail.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Iwanaga Y, Nishi I, Furuichi S , et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure Comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–8. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Morrison LK, Harrison A, Krishnaswamy P, Kazanegra R, Clopton P, Maisel A. Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol. 2002;39:202–9. doi: 10.1016/s0735-1097(01)01744-2. [DOI] [PubMed] [Google Scholar]
- 31.Januzzi JL , Jr, Camargo CA, Anwaruddin S , et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency de-partment (PRIDE) study. Am J Cardiol. 2005;95:948–54. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Maisel AS, Krishnaswamy P, Nowak RM , et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 33.Babuin L, Jaffe AS. Troponin The biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173:1191–1202. doi: 10.1503/cmaj.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peacock WFt, De Marco T, Fonarow GC , et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–26. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 35.Januzzi JL ., Jr ST2 as a cardiovascular risk biomarker From the bench to the bedside. J Cardiovasc Transl Res. 2013;Apr 5.[Epub ahead of print] doi: 10.1007/s12265-013-9459-y. [DOI] [PubMed] [Google Scholar]
- 36.de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. 2010;7:1–8. doi: 10.1007/s11897-010-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Antonio M, Lupon J, Galan A, Vila J, Urrutia A, Bayes-Genis A. Combined use of high-sensitivity cardiac troponin T and N-terminal pro-B type natriuretic peptide improves measurements of performance over established mortality risk factors in chronic heart failure. Am Heart J. 2012;163:821–8. doi: 10.1016/j.ahj.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Pascual-Figal DA, Manzano-Fernandez S, Boronat M , et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide Complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13:718–25. doi: 10.1093/eurjhf/hfr047. [DOI] [PubMed] [Google Scholar]
- 39.Ky B, French B, McCloskey K , et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–7. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lainchbury JG, Troughton RW, Strangman KM , et al. N-terminal pro-b-type natriuretic peptide-guided treatment for chronic heart failure Results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol. 2009;55:53–60. doi: 10.1016/j.jacc.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 41.Pfisterer M, Buser P, Rickli H , et al. Bnp-guided vs symptom-guided heart failure therapy The Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301:383–92. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 42.Januzzi JL , Jr, Rehman SU, Mohammed AA , et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58:1881–9. doi: 10.1016/j.jacc.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 43.Gaggin HK, Mohammed AA, Bhardwaj A , et al. Heart failure outcomes and benefits of NT-proBNP-guided management in the elderly results from the prospective, randomized ProBNP outpa-tient tailored chronic heart failure therapy (PROTECT) study. J Card Fail. 2012;18:626–34. doi: 10.1016/j.cardfail.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Karlstrom P, Alehagen U, Boman K, Dahlstrom U. Brain natriuretic peptide-guided treatment does not improve morbidity and mortality in extensively treated patients with chronic heart failure Responders to treatment have a significantly better outcome. Eur J Heart Fail. 2011;13:1096–103. doi: 10.1093/eurjhf/hfr078. [DOI] [PubMed] [Google Scholar]
- 45.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction Pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–79. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiner RB, Baggish AL, Chen-Tournoux A , et al. Im-provement in structural and functional echocardiographic parameters during chronic heart failure therapy guided by natriuretic peptides Mechanistic insights from the ProBNP Outpatient Tailored Chronic Heart Failure (PROTECT) study. Eur J Heart Fail. 2013;15:342–51. doi: 10.1093/eurjhf/hfs180. [DOI] [PubMed] [Google Scholar]
- 47.Januzzi JL ., Jr The role of natriuretic peptide testing in guiding chronic heart failure management Review of available data and recommendations for use. Arch Cardiovasc Dis. 2012;105:40–50. doi: 10.1016/j.acvd.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Berger R, Moertl D, Peter S , et al. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 2010;55:645–53. doi: 10.1016/j.jacc.2009.08.078. [DOI] [PubMed] [Google Scholar]
- 49.Sanders-van Wijk S, van Asselt ADI, Rickli H , et al. Cost-effectiveness of N-Terminal Pro-B-Type natriuretic-guided ther-apy in elderly heart failure patients Results from TIME-CHF (Trial of Intensified versus Standard Medical therapy in Elderly patients with Congestive Heart Failure). J Am Coll Cardiol HF. 2013;1:64–71. doi: 10.1016/j.jchf.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Maeder MT, Rickenbacher P, Rickli H , et al. N-terminal pro brain natriuretic peptide-guided management in patients with heart failure and preserved ejection fraction findings from the Trial of In-tensified versus Standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF). Eur J Heart Fail. 2013;May 8. [Epub ahead of print] doi: 10.1093/eurjhf/hft076. [DOI] [PubMed] [Google Scholar]
- 51.Felker GM, Hasselblad V, Hernandez AF, O'Connor CM. Biomarker-guided therapy in chronic heart failure A meta-analysis of randomized controlled trials. Am Heart J. 2009;158:422–30. doi: 10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 52.Porapakkham P, Porapakkham P, Zimmet H, Billah B, Krum H. B-type natriuretic peptide-guided heart failure therapy A meta-analysis. Arch Intern Med. 2010;170:507–14. doi: 10.1001/archinternmed.2010.35. [DOI] [PubMed] [Google Scholar]
- 53.Fung JW, Yu CM, Yip G , et al. Effect of beta blockade (carvedilol or metoprolol) on activation of the renin-angiotensin-aldosterone system and natriuretic peptides in chronic heart failure. Am J Cardiol. 2003;92:406–10. doi: 10.1016/s0002-9149(03)00658-1. [DOI] [PubMed] [Google Scholar]
- 54.Olsson LG, Swedberg K, Cleland JG , et al. Prognostic importance of plasma nt-pro bnp in chronic heart failure in patients treated with a beta-blocker Results from the Carvedilol Or Metoprolol European Trial (COMET) trial. Eur J Heart Fail. 2007;9:795–801. doi: 10.1016/j.ejheart.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Davis ME, Richards AM, Nicholls MG, Yandle TG, Frampton CM, Troughton RW. Introduction of metoprolol increas-es plasma B-type cardiac natriuretic peptides in mild, stable heart failure. Circulation. 2006;113:977–85. doi: 10.1161/CIRCULATIONAHA.105.567727. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg J, Gustafsson F, Remme WJ, Riegger GA, Hildebrandt PR. Effect of beta-blockade and ACE inhibition on B-type natriuretic peptides in stable patients with systolic heart failure. CardiovascDrugs Ther. 2008;22:305–11. doi: 10.1007/s10557-008-6099-6. [DOI] [PubMed] [Google Scholar]
- 57.Latini R, Masson S, Anand I , et al. Effects of Valsartan on circulating brain natriuretic peptide and norepinephrine in symp-tomatic chronic heart failure The Valsartan Heart Failure Trial (VAL-Heft). Circulation. 2002;106:2454–8. doi: 10.1161/01.cir.0000036747.68104.ac. [DOI] [PubMed] [Google Scholar]
- 58.Berry C, Murphy NF, De Vito G , et al. Effects of aldos-terone receptor blockade in patients with mild-moderate heart failure taking a beta-blocker. Eur J Heart Fail. 2007;9:429–34. doi: 10.1016/j.ejheart.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Smart NA, Meyer T, Butterfield JA , et al. Individual patient meta-analysis of exercise training effects on systemic brain natriuretic peptide expression in heart failure. Eur J Prev Cardiol. 2012;19:428–35. doi: 10.1177/1741826711409171. [DOI] [PubMed] [Google Scholar]
- 60.Fruhwald FM, Fahrleitner-Pammer A, Berger R , et al. Early and sustained effects of cardiac resynchronization therapy on N-terminal pro-B-type natriuretic peptide in patients with moderate to severe heart failure and cardiac dyssynchrony. Eur Heart J. 2007;28:1592–7. doi: 10.1093/eurheartj/ehl505. [DOI] [PubMed] [Google Scholar]
- 61.Go AS, Mozaffarian D, Roger VL , et al. Executive summary Heart disease and stroke statistics--2013, update A report from the AmericanHeart Association. Circulation. 2013;127:143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 62.Heidenreich PA, Albert NM, Allen LA , et al. Forecasting the impact of heart failure in the united states A policy statement from the AmericanHeart Association. Circ Heart Fail. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]