Abstract
Hematopoietic stem cells (HSCs) are highly susceptible to ionizing radiation–mediated death via induction of ROS, DNA double-strand breaks, and apoptotic pathways. The development of therapeutics capable of mitigating ionizing radiation–induced hematopoietic toxicity could benefit both victims of acute radiation sickness and patients undergoing hematopoietic cell transplantation. Unfortunately, therapies capable of accelerating hematopoietic reconstitution following lethal radiation exposure have remained elusive. Here, we found that systemic administration of pleiotrophin (PTN), a protein that is secreted by BM-derived endothelial cells, substantially increased the survival of mice following radiation exposure and after myeloablative BM transplantation. In both models, PTN increased survival by accelerating the recovery of BM hematopoietic stem and progenitor cells in vivo. PTN treatment promoted HSC regeneration via activation of the RAS pathway in mice that expressed protein tyrosine phosphatase receptor-zeta (PTPRZ), whereas PTN treatment did not induce RAS signaling in PTPRZ-deficient mice, suggesting that PTN-mediated activation of RAS was dependent upon signaling through PTPRZ. PTN strongly inhibited HSC cycling following irradiation, whereas RAS inhibition abrogated PTN-mediated induction of HSC quiescence, blocked PTN-mediated recovery of hematopoietic stem and progenitor cells, and abolished PTN-mediated survival of irradiated mice. These studies demonstrate the therapeutic potential of PTN to improve survival after myeloablation and suggest that PTN-mediated hematopoietic regeneration occurs in a RAS-dependent manner.
Introduction
Total body irradiation (TBI) is successfully used in the conditioning of patients for hematopoietic cell transplantation (1). Radiation causes toxicity to hematopoietic stem cells (HSCs) through the generation of ROS, induction of DNA strand breaks and apoptosis, and damage to the BM microenvironment (2–4). Despite an understanding of mechanisms through which ionizing radiation causes hematopoietic toxicity, few effective mitigators of radiation-induced hematopoietic injury have been developed (5–9). The lack of effective mitigators for acute radiation sickness (ARS) has become a public health concern, as the risk of terrorism using radiological or nuclear devices has escalated (10, 11). Elucidation of novel mechanisms through which HSCs respond to radiation and the development of therapeutics targeting such mechanisms could potentially benefit not only victims of ARS but also patients receiving TBI for hematopoietic cell transplantation.
HSCs reside in specialized niches within the BM, and distinct cells within these niches regulate HSC maintenance in vivo (12–20). We and others have shown that BM-derived endothelial cells regulate the response of HSCs to genotoxic stressors such as ionizing radiation (3, 4, 16, 21). However, the precise mechanisms through which BM niche cells promote HSC regeneration after injury remain poorly understood. We recently described the hematopoietic function of pleiotrophin (PTN), a protein which is secreted by BM endothelial cells and which promotes the expansion of long-term HSCs in culture (22). Deletion of Ptn in the BM microenvironment significantly decreased long-term HSCs in mice (23). However, the therapeutic potential and mechanism of action of PTN remained undefined. Here, we demonstrate that systemic administration of PTN increases the survival of mice in the settings of ARS and BM transplantation. PTN mediates these effects by induction of RAS/MEK/ERK signaling in HSCs and by promotion of HSC quiescence after irradiation.
Results and Discussion
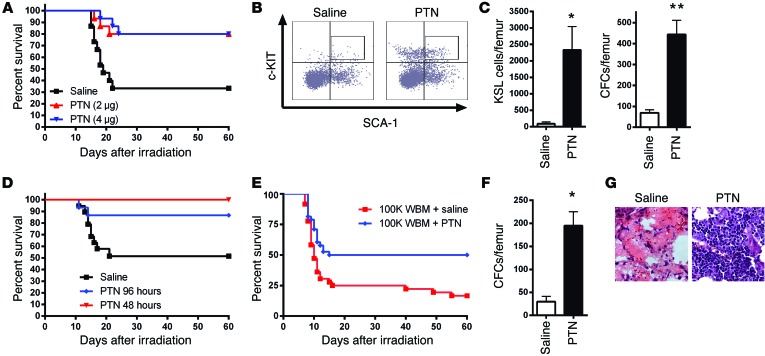
We tested whether systemic administration of PTN could mitigate hematopoietic injury and improve the survival of lethally irradiated mice. When administered beginning +24 hours following 700 cGy TBI, 80% of PTN-treated mice survived, compared with 33% of irradiated controls (Figure 1A). Irradiated PTN-treated mice displayed increased BM cellularity; increased BM cKIT+SCA-1+LIN– (KSL) cells, which are enriched for hematopoietic stem and progenitor cells (HSPCs) (22); and increased colony-forming cells (CFCs) compared with irradiated controls at day +10 (Figure 1, B and C, and Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI76838DS1). These data suggested that PTN improved survival by mitigating radiation damage to HSPCs.
Figure 1. PTN treatment improves the survival of irradiated mice and hematopoietic cell transplant recipients.
(A) Survival of 700 cGy–irradiated mice treated intraperitoneally with 2 or 4 μg PTN or saline administered at +24 hours and every other day through day +14 (PTN-treated groups: 12 of 15 for both; saline-treated group: 5 of 15 mice; P = 0.002 for PTN 4 μg vs. saline, P = 0.004 for PTN 2 μg vs. saline). (B) Flow cytometric analysis of BM KSL cells from irradiated mice at day +10 treated with saline or PTN. (C) BM KSL cells and CFCs per femur (n = 6, *P = 0.02, **P = 0.0003). (D) Survival of irradiated mice treated subcutaneously, beginning at +48 hours and +96 hours, with PTN or saline (PTN 48 hours: 15 of 15 mice and PTN 96 hours: 13 of 15 mice; saline: 10 of 19 mice; P = 0.002 for PTN 48 hours vs. saline, P = 0.04 for PTN 96 hours vs. saline.). (E) Survival of irradiated mice transplanted with BM cells and treated with PTN or saline (PTN, 19 of 38 mice, 50% vs. saline, 6 of 36 mice, 17%; P = 0.003). (F) CFCs per femur at day +14 following transplantation and treatment with PTN or saline (n = 6, *P = 0.005). (G) H&E images (original magnification, ×63) of femurs at day +14 from transplanted mice treated with PTN or saline.
Historically, radiation mitigators have shown little efficacy when administered >24 hours after TBI (24, 25). Initiation of PTN treatment at +48 hours or +96 hours after 700 cGy significantly improved the survival of irradiated mice compared with that of irradiated controls (Figure 1D). These results suggested a unique therapeutic potential for PTN to improve survival in ARS.
We next tested whether PTN administration could accelerate hematopoietic reconstitution and improve the survival of mice receiving myeloablative (850 cGy) hematopoietic cell transplantation (14). Fifty percent of mice transplanted with 1 × 105 BM cells and treated with PTN survived, compared with 17% of transplanted control mice (Figure 1E). PTN-treated mice showed increased CFCs and BM cellularity at day +14 compared with controls (Figure 1, F and G), suggesting that PTN treatment accelerated hematopoietic reconstitution following BM transplantation.
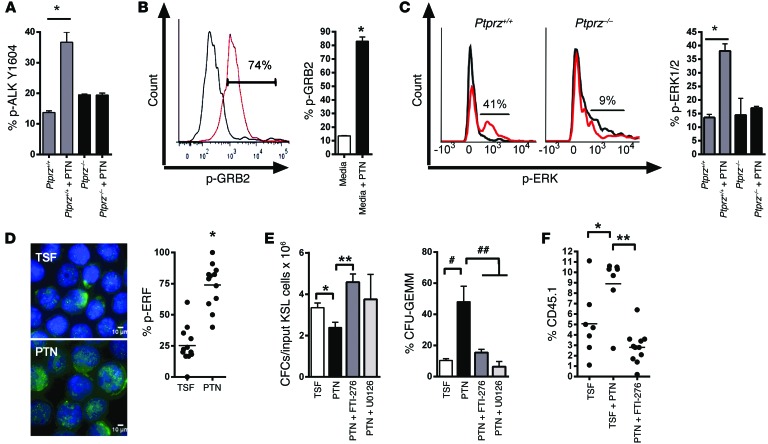
PTN has been shown to inactivate the phosphatase domain of protein tyrosine phosphatase receptor-zeta (PTPRZ) on neural cells, thereby activating kinases, including anaplastic lymphoma kinase (ALK), which promote neurite outgrowth (26, 27). PTN treatment significantly increased p-ALK levels in HSCs from Ptprz+/+ mice but had no effect on HSCs from Ptprz–/– mice, suggesting that PTPRZ was required for PTN-mediated activation of ALK (Figure 2A). p-ALK can phosphorylate GRB2, which, in cooperation with son of sevenless (SOS), activates the RAS/MEK/ERK pathway (28). PTN treatment increased p-GRB2 levels and p-ERK1/2 levels in KSL cells from Ptprz+/+ mice but had no effect on p-ERK1/2 levels in KSL cells from Ptprz–/– mice (Figure 2, B and C). p-ERK1/2 levels were also significantly decreased in KSL cells from Ptn–/– mice compared with p-ERK1/2 levels in KSL cells from Ptn+/+ mice (Supplemental Figure 2). Expression of p-ERF, a transcriptional repressor that is regulated by ERK1/2 (29), also increased in PTN-treated KSL cells compared with that in control cells (Figure 2D). These data suggested that PTN activated the RAS/MEK/ERK pathway in HSCs in a PTPRZ-dependent manner.
Figure 2. RAS signaling is necessary for PTN-mediated HSPC expansion.
(A) p-ALK expression in BM KSL cells from the represented groups (n = 3, *P = 0.003). (B) p-GRB2 expression in BM KSL cells treated with media alone (gray curve) or PTN (red curve), with mean percentage p-GRB2 levels shown (n = 3, *P < 0.0001). (C) Representative p-ERK1/2 expression in KSL cells treated with media alone (gray curve) or PTN (red curve), with mean percentage p-ERK1/2 levels shown (n = 5, *P < 0.001). (D) p-ERF expression (green) in BM KSL cells cultured with thrombopoietin, SCF, and FLT-3 ligand (TSF), with or without PTN, and scatter plot of p-ERF levels in KSL cells (horizontal bars represent means; n = 12, *P < 0.0001). Scale bar: 10 μm. (E) CFCs per input KSL cells and percentage CFU-GEMMs at day +7 of the represented cultures (n = 3, *P = 0.04, **P = 0.01, #P = 0.02, ##P = 0.03). (F) CD45.1+ donor cell engraftment at 8 weeks following competitive transplantation of the progeny of 10 CD34–KSL cells cultured in the conditions shown (n = 7–11 per group, *P = 0.04, **P < 0.0001).
Functionally, PTN treatment of BM KSL cells in cytokine cultures (thrombopoietin, SCF, and FLT-3 ligand) caused a decrease in total CFC output but increased the frequency of granulocyte-erythroid-macrophage-megakaryocyte–CFUs (CFU-GEMMs) (Figure 2E). Coupled with RAS or MEK inhibition (30), PTN-mediated maintenance of CFU-GEMMs was abolished. RAS inhibition also blocked PTN-mediated expansion of HSCs in culture, as measured with a competitive repopulation assay (Figure 2F). These results suggested that PTN-mediated expansion of HSPCs was dependent upon RAS activation.
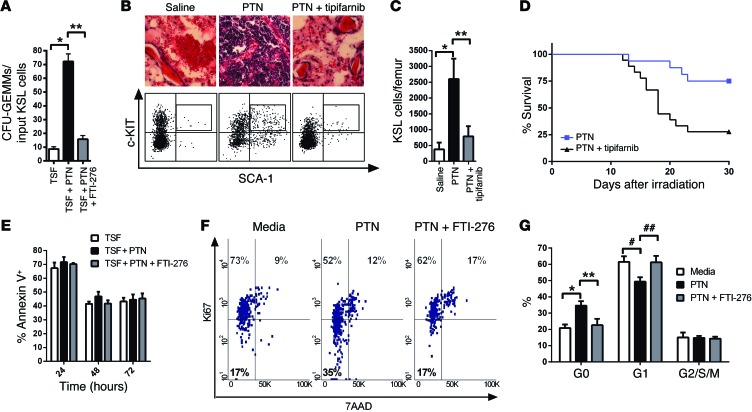
PTN-treatment also significantly increased the number of CFU-GEMMs recovered from culture of irradiated BM KSL cells compared with cytokines alone, whereas RAS inhibition blocked this effect (Figure 3A). Furthermore, systemic administration of PTN promoted the recovery of BM KSL cells in irradiated mice, whereas the administration of tipifarnib, a RAS inhibitor (31), blocked PTN-mediated regeneration of KSL cells (Figure 3, B and C). Importantly, tipifarnib treatment also blocked PTN-mediated improvement in the survival of irradiated mice (Figure 3D). Whereas 75% of PTN-treated mice (12 of 16) survived 800 cGy TBI, 28% of mice (5 of 18) treated with PTN and tipifarnib survived. These data suggested that PTN-mediated improvement in survival of irradiated mice was dependent on RAS activation.
Figure 3. PTN promotes hematopoietic regeneration and survival following irradiation in a RAS-dependent manner.
(A) CFU-GEMMs at day +3 of culture of KSL cells following 300 cGy (n = 6, *P < 0.001, **P < 0.001). (B) H&E-stained femurs (original magnification, ×63) and flow cytometric analysis of KSL cells from mice at day +10 following 700 cGy and the treatments shown. (C) KSL cells per femur at day +10 following 700 cGy and the treatments shown (n = 3, *P = 0.004, **P = 0.01). (D) Survival curves of 800 cGy–irradiated mice treated with 4 μg PTN every other day, with and without 1 mg tipifarnib, through day +21 (P = 0.004, log-rank test). (E) Percentage annexin-positive KSL cells following 300 cGy (n = 5). (F) Representative percentages of KSL cells in G0 (Ki67–7AAD–), G1 (Ki67+7AAD–), and G2/SM phase (Ki67+7AAD+) at 6 hours following 300 cGy and cultured as shown. (G) Mean G0, G1, and G2/SM levels for each group (n = 5, *P = 0.004, **P = 0.04, #P = 0.02, ##P = 0.01).
Interestingly, PTN treatment did not alter the percentage of apoptotic BM KSL cells early after 300 cGy irradiation in vitro (Figure 3E). However, PTN treatment significantly increased the percentage of KSL cells remaining in the G0 phase of cell cycle after irradiation compared with that in control cells (Figure 3, F and G). Of note, RAS inhibition blocked PTN-mediated inhibition of HSC cycling following irradiation (Figure 3, F and G). These data suggest that PTN may promote HSC regeneration after irradiation via induction of HSC quiescence and that this effect occurs in a RAS-dependent manner.
Our findings have significant implications for the treatment of ARS. Recently, novel mechanisms have been described that may be targeted to mitigate radiation injury to the hematopoietic system (5, 6, 8, 32, 33). To our knowledge, PTN represents the first therapeutic demonstrated to improve survival when administered more than 24 hours after exposure. This could be advantageous in a mass casualty radiation disaster, in which medical care may be delayed for several days. Therefore, PTN has unique therapeutic potential to improve the survival of victims of ARS. Going forward, we will generate cell-specific genetic models to discern whether the in vivo effects of PTN treatment are HSC autonomous or also reflect indirect effects on the BM microenvironment.
Our results also suggest that PTN has therapeutic potential for patients undergoing limiting dose hematopoietic cell transplantation, such as adult cord blood transplantation, which can be complicated by delayed engraftment, graft failure, and death (34). While ex vivo CB expansion is currently being tested in clinical trials to augment hematopoietic recovery (35–37), an alternative strategy would be to administer systemic therapeutics to accelerate hematopoietic reconstitution in transplant recipients. Our results suggest that systemic PTN has therapeutic potential to accelerate hematopoietic reconstitution in such a setting.
Mechanistically, our data suggest that PTN-mediated expansion of HSPCs, in steady state or following irradiation, is dependent upon RAS activation. While overexpression of oncogenic RAS in hematopoietic cells causes a myeloproliferative disorder (38), the effects of physiologic RAS activation in HSCs are less well understood. Overexpression of oncogenic H-RAS in human HSCs, coupled with pharmacologic Ras inhibition, was previously suggested to promote HSC expansion (39). We postulate that PTN promotes HSC expansion via activation of physiologic RAS signaling. Our data also suggest that PTN activates MEK/ERK signaling downstream of RAS in HSCs. In a prior study, we observed that PI3K inhibition abrogated PTN-mediated expansion of KSL cells in culture (22). Since RAS proteins can activate both PI3K/AKT and MEK/ERK signaling, it is possible that PTN activates both arms of RAS signaling. Going forward, we will use Mx1-Cre Erk1–/– Erk2fl/fl mice (40) to determine whether PTN-mediated HSC regeneration is dependent upon RAS/MEK/ERK signaling.
Activation of the RAS pathway has not been previously shown to radioprotect HSCs. Here, we observed that PTN treatment promoted HSC quiescence after injury in a RAS-dependent manner. The effects of RAS/MEK/ERK signaling on cell cycle status are context dependent and may be related to the effects of cooperating oncogenes or inactivation of tumor suppressor genes (41–43). Going forward, we will perform RNA sequencing analysis of PTN-treated KSL cells to identify downstream effectors that may be responsible for RAS-dependent inhibition of HSC cycling. One candidate is p21, since prolonged activation of RAF/MEK/ERK signaling in fibroblasts induced p21-mediated cell cycle arrest (41, 44). The cyclin D binding myb-like transcription factor 1 (DMTF1) is also responsive to the RAS/MEK/ERK pathway and has been shown to promote HSC quiescence (45).
Broadly, our results suggest that the pharmacologic induction of HSC quiescence after irradiation can mitigate radiation injury to the hematopoietic system. Several studies have shown the lack of efficacy of cell cycle–inducing cytokines in promoting survival when administered to mice after irradiation (24, 25). However, when administered prior to TBI, these same cytokines can radioprotect, perhaps by promoting the synchronized entry of HSCs into late S phase, a radioresistant phase of the cell cycle (24, 46). Conversely, administration of a CDK4/6 inhibitor within the first +20 hours after TBI improved the survival of lethally irradiated mice (7). Our results are most consistent with these findings and those of Cheng et al. (47), who showed that cycling HSCs from p21–/– mice displayed increased sensitivity to 5-FU chemotherapy and poor serial transplant capability compared with more quiescent p21+/+ HSCs. Our studies suggest that PTN, a BM niche–derived protein, promotes HSC quiescence early after irradiation and powerfully mitigates radiation injury to the hematopoietic system.
Methods
For more detailed information, see the Supplemental Methods.
Mice.
We used PTN-deficient (Ptn–/–) mice and PTPRZ-deficient (Ptprz–/–) mice as previously described (23). RAS and MEK inhibitors were provided by Christopher Counter and Donita Brady (Duke University).
Statistics.
Survival analyses were performed using the log-rank test. Data are presented as mean ± SEM throughout, and the Student’s 2-tailed t test was used for comparisons. P < 0.05 was considered significant.
Study approval.
Animal procedures followed protocols approved by the Duke University and UCLA animal care committees.
Supplementary Material
Acknowledgments
These studies were supported by NIH grants AI-067798 (to J.P. Chute) and HL-086998 (to J.P. Chute).
Footnotes
Note regarding evaluation of this manuscript: Manuscripts authored by scientists associated with Duke University, The University of North Carolina at Chapel Hill, Duke-NUS, and the Sanford-Burnham Medical Research Institute are handled not by members of the editorial board but rather by the science editors, who consult with selected external editors and reviewers.
Conflict of interest: John P. Chute and Nelson J. Chao are cofounders of C2Regenerate, a private company that holds licenses for the development of pleiotrophin.
Reference information:J Clin Invest. 2014;124(11):4753–4758. doi:10.1172/JCI76838.
References
- 1.Nagler A, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen-a report from the acute leukemia working party of the European group for blood and marrow transplantation. J Clin Oncol. 2013;31(28):3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 2.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30(6):513–528. doi: 10.1016/S0301-472X(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 3.Doan P, et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31(2):327–337. doi: 10.1002/stem.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4(3):263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geiger H, et al. Pharmacologic targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18(7):1123–1129. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinan EC, et al. Bactericidal/permeability-increasing protein (rBPI21) and fluoroquinolone mitigate radiation-induced bone marrow aplasia and death. Sci Transl Med. 2011;3(110):1–11. doi: 10.1126/scitranslmed.3003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SM, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120(7):2528–2536. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doan PL, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19(3):295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farese A, et al. The ability of filgrastim to mitigate mortality following LD50/60 total-body irradiation is administration time-dependent. Health Phys. 2014;106(1):39–47. doi: 10.1097/HP.0b013e3182a4dd2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mettler FA, Voelz GL. Major radiation exposure-what to expect and how to respond. N Engl J Med. 2002;346(20):1554–1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 11.Waselenko J, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140(12):1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 15.Méndez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salter AB, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113(9):2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chute JP, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109(6):2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himburg HA, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16(4):475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himburg HA, et al. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2012;2(4):964–975. doi: 10.1016/j.celrep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zsebo K, et al. Radioprotection of mice by recombinant stem cell factor. Proc Natl Acad Sci U S A. 1992;89(20):9464–9468. doi: 10.1073/pnas.89.20.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouthon M, et al. Thrombopoietin protects mice from mortality and myelosuppression following high dose irradiation: importance of time scheduling. Can J Physiol Pharmacol. 2002;80(7):717–721. doi: 10.1139/y02-090. [DOI] [PubMed] [Google Scholar]
- 26.Meng K, et al. Pleiotrophin signals increased tyrosine phosphorylation of beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci U S A. 2000;97(6):2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Pinera P, Zhang W, Chang Y, Vega J, Deuel TF. Anaplastic lymphoma kinase is activated through the pleiotrophin/receptor protein-tyrosine phosphatase beta/zeta signaling pathway: an alternative mechanism of receptor tyrosine kinase activation. J Biol Chem. 2007;282(39):28683–28690. doi: 10.1074/jbc.M704505200. [DOI] [PubMed] [Google Scholar]
- 28.Riera L, Lasorsa E, Ambrogio C, Surrenti N, Voena C, Chiarle R. Involvement of Grb2 adaptor protein in nucleophosmin-anaplastic lymphoma kinase (NPM-ALK)-mediated signaling and anaplastic large cell lymphoma growth. J Biol Chem. 2010;285(34):26441–26450. doi: 10.1074/jbc.M110.116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twigg SR, et al. Reduced dosage of ERF causes complex craniosynostosis in humans and mice and links ERK1/2 signaling to regulation of osteogenesis. Nat Genet. 2013;45(3):308–313. doi: 10.1038/ng.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan M, Dolp O, Reuter CW. Cell-cycle dependent activation of mitogen-activated protein kinase kinase (MEK-1/2) in myeloid leukemia cell lines and induction of growth inhibition and apoptosis by inhibitors of Ras signaling. Blood. 2001;97(6):1823–1834. doi: 10.1182/blood.V97.6.1823. [DOI] [PubMed] [Google Scholar]
- 31.Kuzrock R, et al. Phase I study of alternate-week administration of tipifarnib in patients with myelodysplastic syndrome. Clin Cancer Res. 2008;14(2):509–514. doi: 10.1158/1078-0432.CCR-07-1532. [DOI] [PubMed] [Google Scholar]
- 32.Burdelya L, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320(5873):226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, et al. Nrf2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest. 2014;124(2):730–741. doi: 10.1172/JCI70812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigues CA, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27(2):256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 35.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Lima M, et al. Cord blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutler C, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122(17):3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun BS, et al. Somatic activation of oncogenic KRas in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101(2):597–602. doi: 10.1073/pnas.0307203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorrell C, Takenaka K, Minden MD, Hawley RG, Dick JE. Hematopoietic cell fate and the initiation of leukemic properties in primitive primary human cells are influenced by Ras activity and fernesyltransferase inhibition. Mol Cell Biol. 2004;24(16):6993–7002. doi: 10.1128/MCB.24.16.6993-7002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staser K, et al. Normal hematopoiesis and neurofibromin-deficient myeloproliferative disease require Erk. J Clin Invest. 2013;123(1):329–334. doi: 10.1172/JCI66167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang F, et al. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway. Int J Oncol. 2003;22(3):469–480. [PubMed] [Google Scholar]
- 42.Hirakawa T, Ruley H. Rescue of cells from ras oncogene-induced growth arrest by a second complementing oncogene. Proc Natl Acad Sci U S A. 1988;85(5):1519–1523. doi: 10.1073/pnas.85.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serrano M, et al. Oncogenic Ras provokes premature cell senescence associated with accumulation of p53 and p16ink4a. Cell. 1997;88(5):593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 44.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip. Mol Cell Biol. 1997;17(9):5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi M, Srour E. Regulation of murine hematopoietic stem cell quiescence by Dmtf1. Blood. 2011;118(25):6562–6571. doi: 10.1182/blood-2011-05-349084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mouthon M, et al. Single administration of thrombopoietin to lethally irradiated mice prevents infectious and thrombotic events leading to mortality. Exp Hematol. 2001;29(1):30–40. doi: 10.1016/S0301-472X(00)00624-X. [DOI] [PubMed] [Google Scholar]
- 47.Cheng T, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.