Abstract
Objective
To compare the detection rates of the pyramidal lobe of the thyroid gland (TPL) using ultrasonography (US) and computed tomography (CT) in a prospective multi-center study.
Materials and Methods
We enrolled 582 patients who underwent neck CT at six institutions. Each radiologist prospectively evaluated the presence and features of TPLs on thyroid US. Radiologists were divided into two groups according to their previous experience in detecting TPL on US or CT. The same radiologist also retrospectively assessed CT findings, blinded to the corresponding US findings.
Results
The pyramidal lobe of the thyroid glands were detected in 230 cases (39.5%) on US and in 276 cases (47.6%) on CT. The TPL detection rate at the six institutions ranged from 22.0% to 59% for US and from 34.1% to 59% using CT. There were significant differences between US and CT in the detection rate, length, anteroposterior diameter, volume, and superior extent of TPL (p ≤ 0.027). The TPL detection rates on both US and CT (p < 0.001) differed significantly according to the experience level of the radiologists. When the CT result was used as a reference standard, the sensitivity, specificity, positive and negative predictive values, as well as the accuracy of US for TPL detection were 72.6%, 91.5%, 89.3%, 77.3%, and 82.1%, respectively.
Conclusion
Our prospective multicenter study revealed that US could detect TPL with relatively high diagnostic accuracy compared to CT. Because the detection rate of TPL varied significantly according to the radiologists' level of experience, careful inspection is necessary to avoid imaging pitfalls and ensure appropriate evaluation of TPL on both US and CT.
Keywords: Thyroid, Variation, Pyramidal lobe, Ultrasonography, Computed tomography, Anatomy
INTRODUCTION
The pyramidal lobe of the thyroid gland (TPL) is an embryological remnant of the thyroglossal duct, a longitudinally arranged thyroid tissue protruding from the upper margin of the thyroid gland. TPL is present in 15% to 75% of individuals in anatomic studies (1, 2, 3, 4, 5, 6, 7). Preoperative detection of TPL or accessory thyroid is of great clinical importance in patients who undergo total thyroidectomy because these structures can be undesirable thyroid remnants and can be a cause of disease recurrence not only with malignant thyroid tumors but also with benign diseases such as Graves' disease. In addition, remnant thyroid tissue can increase the serum thyroglobulin concentration postoperatively and decrease the potential benefit of radioactive iodine ablation by absorbing most, if not all, of the radioactive material (8).
Available preoperative methods for detecting TPL include scintigraphy, ultrasonography (US), and computed tomography (CT) (4, 9, 10, 11, 12). Scintigraphic detection of a TPL depends on the specific thyroid disorder and the experience of the operator (4). In recent single- and multi-center CT studies, the detection rate of TPL was 41.3% and 44.6%, respectively (9, 10). However, the detection rates of TPL on US and CT in a recent, single-center study were 50.6% and 59.3%, respectively (11). Thus, it is possible that the detection rate of TPL in US and CT differs according to the investigators. To date, there are no known multi-center studies comparing the detection rate for TPL between US and CT. The purpose of the present study was to compare the detection rate and imaging features of a TPL on US and CT by multiple investigators using prospective multi-center analysis.
MATERIALS AND METHODS
Patients
This study was approved by the Institutional Review Board of each of the six participating institutions (Seoul St. Mary's, Soonchunhyang, Daerim St. Mary's, Haeundae Paik, Busan Paik, and Severance Hospitals) before patient selection began. To share the study concept, investigation method, as well as the definition and imaging features of TPL, we had two offline meetings before the study started. All investigators attended the offline meeting at least once. From February 2013 to January 2014, successive patients who had already undergone neck CT scan underwent thyroid US at each institution. The interval between US examination and CT scan was 2 months or less. Exclusion criteria were patients aged less than 19 or over 70, those with previous thyroidectomy or other neck operation, inappropriate CT protocol, or poor image quality for thyroid evaluation. Ultimately, 582 patients (427 women, 155 men; age 19-70 years; median age, 49 years) were included.
Imaging Technique and Analysis
Thyroid US was performed from the submental region to the supraclavicular level by a single radiologist at each institution using a high-resolution US instrument (Aplio SSA-770A, Toshiba Medical System, Otawara, Japan; iU22, Philips Healthcare, Andover, MA, USA; LOGIQ E9, GE Healthcare, Milwaukee, WI, USA) equipped with a linear probe (5-15 MHz). The presence of a TPL on real-time US was defined as follows: a longitudinally arranged accessory thyroid tissue with echogenicity and vascularity identical to the main thyroid gland on gray-scale and color Doppler images and protruding from the superior margin of the isthmus or the medial aspect of a right or left thyroid lobe, regardless of continuity with the main thyroid gland (Fig. 1) (11, 12). To rule out glandular deformity and avoid interobserver variability, TPLs that were < 6 mm in length were excluded. The six investigators were divided into experienced (A-D as shown in Tables 2, 5) and less experienced (E and F as shown in Tables 2, 5) groups according to the presence or absence of study experience with detecting a TPL on US and CT.
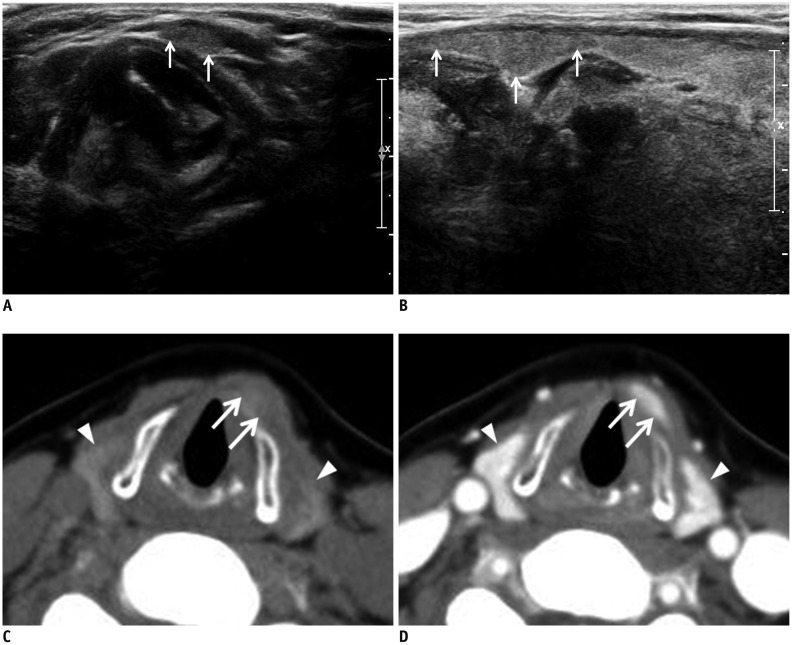
Fig. 1. Left pyramidal lobe detected on US and CT in 58-year-old woman.
Transverse (A) and longitudinal (B) gray-scale sonograms show left pyramidal lobe (arrows). Non-enhanced (C) and contrast-enhanced (D) axial CT images of left pyramidal lobe (arrows) show same attenuation and enhancement as main thyroid gland (arrowheads).
Table 2. Prevalence of Thyroid Pyramidal Lobes on US and CT in Six Institutions.
| Investigators | US | CT |
|---|---|---|
| A (n = 100) | 59 | 59 |
| B (n = 100) | 45 | 57 |
| C (n = 100) | 44 | 45 |
| D (n = 100) | 37 | 58 |
| E (n = 100) | 27 | 29 |
| F (n = 82) | 18 | 28 |
| Mean ± SD | 38.3 ± 14.5 | 46 ± 14.5 |
Note.- Significant difference in prevalence of thyroid pyramidal lobe between institutions was found (p < 0.001). CT = computed tomography, US = ultrasonography
Table 5. Diagnostic Index of Ultrasonography in Detection of Thyroid Pyramidal Lobes According to Radiologists.
| Institutions | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|
| Experienced | |||||
| A (n = 100) | 79.7 (67.2-89.0) | 82.9 (67.9-92.9) | 87.0 (75.1-94.6) | 73.9 (58.9-85.7) | 0.81 (0.72-0.88) |
| B (n = 100) | 78.0 (65.3-87.7) | 97.6 (87.2-99.9) | 97.9 (88.8-99.9) | 75.5 (61.7-86.2) | 0.88 (0.80-0.94) |
| C (n = 100) | 94.4 (81.3-99.3) | 100 (94.4-100.0) | 100 (89.7-100) | 97.0 (89.5-99.6) | 0.97 (0.92-0.99) |
| D (n = 100) | 58.3 (44.9-70.9) | 95.0 (83.1-99.4) | 94.6 (81.8-99.3) | 60.3 (47.2-72.4) | 0.77 (0.67-0.85) |
| Less experienced | |||||
| E (n = 100) | 66.7 (51.0-80.0) | 74.6 (61.0-85.3) | 68.2 (52.4-81.4) | 73.2 (59.7-84.2) | 0.71 (0.61-0.79) |
| F (n = 82) | 58.6 (38.9-76.5) | 96.2 (87.0-99.5) | 89.5 (66.9-98.7) | 81.0 (69.1-89.7) | 0.77 (0.67-0.86) |
Note.- There was significant difference of diagnostic indices between all institutions by receiver operating characteristic analysis using CT result as reference standard (p < 0.001). AUC = area under curve, CI = confidence interval, NPV = negative predictive value, PPV = positive predictive value
Neck CT was performed in the axial planes from the skull base to the upper mediastinum with intravenous administration of contrast medium. A 16-channel multidetector CT scanner (Sendation, Siemens Medical Solution, Erlangen, Germany; Lightspeed Ultra, GE Medical System, Milwaukee, WI, USA), a 64-channel multidetector CT scanner (Brilliance 64, Philips, Israel; Aquilion One; Toshiba Medical Systems, Otawara, Japan; Somatom Sensation 64, Siemens, Erlangen, Germany; Discovery CT750 HD, GE Medical System, Milwaukee, WI, USA), or a 128-channel multidetector CT scanner (Lightspeed, General Electric Medical Systems, Milwaukee, WI, USA; Somatom Definition AS, Siemens Medical Solution, Erlangen, Germany) was used. Non-enhanced axial, contrast-enhanced (2.0 mL/kg, maximum 100 mL; 3.0 mL/s, automatic venous injection with 20-30 mL saline flushing) axial, and contrast-enhanced coronal reformatted CT images were acquired in all cases (slice thickness, 2-3 mm; reconstruction increment, 2-3 mm). Contrast-enhanced sagittal reformatted CT images were also selectively acquired if necessary at each institution. Using a picture archiving and communication system, CT image analysis was performed independently by six radiologists with variable experience (range, 9-21 years) 1-5 months (mean, 2.1 months) after thyroid US.
The pyramidal lobe of the thyroid gland on CT was defined as follows: a longitudinally arranged accessory thyroid lobe protruding from the superior margin of the isthmus or the medial aspect of the right or left thyroid lobe, regardless of continuity with the main thyroid gland, with attenuation and enhancement identical to the main thyroid gland on non-enhanced and contrast enhanced CT images, in addition to appearing in three or more serial axial CT images (Fig. 1) (9, 10).
Information about the presence, location, length, largest anteroposterior (AP) and transverse diameter, superior extent, and separation or continuity with the main thyroid gland were recorded for each TPL on both US and CT. TPL origin was classified into four categories including right, left, midline, or bilateral. The volume of each TPL was calculated using an ellipsoid formula (volume = AP diameter × transverse diameter × length × 0.52). The TPLs were classified into one of four categories according to the location of the superior end: tongue base, hyoid bone, thyrohyoid membrane, or thyroid cartilage. Separation of TPLs was defined as a discontinuity with the thyroid proper on US and a lack of visualization of TPLs on one or more axial images between the TPL and the main thyroid gland on CT.
Statistical Analysis
Demographic data (patient age, gender, and indications for CT) were collected from each institution's electronic medical records. Categorical variables were presented as percentage and analyzed using chi-square and Fisher's exact tests. Continuous variables were checked for normality using the Shapiro-Wilk test, presented as mean and standard deviation (SD), then analyzed with the Wilcoxon rank sum test. Receiver operating characteristic analysis was used to calculate the diagnostic index of US for detecting TPL, using CT findings as the reference standard because CT was more accurate than US for detecting TPLs in previous studies (11, 13). Statistical analyses were performed using SAS software (version 9.3, SAS Institute Inc., Cary, NC, USA). The level of significance was defined as p < 0.05.
RESULTS
The patients underwent neck CT for the following reasons: old trauma (n = 4), known thyroid cancer (n = 317), cervical lymphadenopathy (n = 67), palpable neck mass (n = 126), oropharyngolaryngeal malignancy (n = 12), vocal cord palsy (n = 1), post-chemotherapeutic CT follow-up (n = 11), inflammatory or infectious neck lesion (n = 16), parathyroid abnormality (n = 1), and patient request (n = 27).
The detection rate, size, location, separation or continuity, and superior extent of TPLs in the 582 patients are summarized in Table 1. TPLs were observed in 230 (39.5%) patients (184 of 427 women [43.1%] and 46 of 155 men [29.7%]; age 19-84 years; mean age ± SD, 47.1 ± 13.6 years) on US and in 276 (47.6%) patients (224 women and 52 men; age 20-85 years; mean age ± SD, 46.5 ± 13.8 years) on CT, which was significantly different (p = 0.006). There was a significant difference in the detection rate, length, AP diameter, volume, and superior extent of TPL (p ≤ 0.027), but no statistical difference was found in the transverse diameter, location, and separation or continuity of TPLs between US and CT (p ≥ 0.297). The most common location of TPLs was the left side on both US (47.0%, 108/230) and CT (42.4%, 117/276). Complete separation of the TPL from the main thyroid was detected in 36 cases (15.7%) on US and in 42 cases (15.2%) on CT. The most frequent superior end of a TPL was a thyroid cartilage on both US and CT.
Table 1. Comparison in Detection Rate, Size, Location, Separation or Continuity, and Superior Extent of Thyroid Pyramidal Lobes on US and CT.
| Items | US | CT | P |
|---|---|---|---|
| Detection rate | 230 (39.5) | 276 (47.4) | 0.006 |
| Size | |||
| Length (mm) | 21.2 ± 10.5 | 23.5 ± 11.3 | 0.027 |
| AP diameter (mm) | 2.1 ± 1.6 | 2.4 ± 1.1 | < 0.001 |
| Transverse diameter (mm) | 6.1 ± 2.3 | 6.4 ± 2.5 | 0.297 |
| Volume (mm3) | 165.6 ± 211.5 | 221.6 ± 260.5 | < 0.001 |
| Location | 0.399 | ||
| Right | 61 (26.5) | 65 (23.6) | |
| Left | 108 (47.0) | 117 (42.4) | |
| Midline | 59 (25.7) | 90 (32.6) | |
| Bilateral | 2 (0.9) | 4 (1.4) | |
| Separation or continuity | 0.819 | ||
| Separation | 36 (15.7) | 42 (15.2) | |
| Continuity | 199 (84.3) | 246 (84.8) | |
| Superior extent | < 0.001 | ||
| Tongue base | 0 (0) | 3 (1.1) | |
| Hyoid bone | 20 (8.7) | 47 (17.0) | |
| Thyrohyoid membrane | 29 (12.6) | 52 (18.8) | |
| Thyroid cartilage | 186 (80.9) | 186 (67.4) |
Note.- Data are presented as mean ± standard deviation or as n (%). AP = anteroposterior, CT = computed tomography, US = ultrasonography
The pyramidal lobe of the thyroid gland detection according to investigator is summarized in Table 2. Among the institutions, the detection rate ranged from 22.0% to 59% on US and from 34.1% to 59% on CT. The detection rate, size, location, separation or continuity, and superior extent of TPLs on US for experienced and less experienced radiologists are summarized in Table 3. There was a significant difference between experienced and less experienced radiologists in the detection rate, length, volume, location, and separation or continuity of TPLs on US (p ≤ 0.027). However, no statistical difference was found in AP diameter, transverse diameter, and superior extent. The detection rate, size, location, separation or continuity, and superior extent of TPLs on CT for experienced and less experienced radiologists are summarized in Table 4. There was a significant difference between experienced and les experienced radiologists in the detection rate, length, volume, separation or continuity, and superior extent of TPLs on CT (p ≤ 0.002), but no statistical difference was found in AP diameter and transverse diameter.
Table 3. Comparison in Detection Rate, Size, Location, Separation or Continuity, and Superior Extent of Thyroid Pyramidal Lobes on US According to Experienced and Less Experienced Radiologists.
| Items | US | P | |
|---|---|---|---|
| Experienced | Less Experienced | ||
| Detection rate | 185 (80.4) | 45 (19.6) | < 0.001 |
| Size | |||
| Length (mm) | 22.7 ± 10.8 | 15.3 ± 6.7 | < 0.001 |
| AP diameter (mm) | 2.0 ± 0.9 | 2.5 ± 3.0 | 0.631 |
| Transverse diameter (mm) | 6.1 ± 2.2 | 5.8 ± 2.8 | 0.308 |
| Volume (mm3) | 168.9 ± 188.2 | 151.6 ± 290.7 | 0.012 |
| Location | 0.027 | ||
| Right | 53 (28.7) | 8 (17.8) | |
| Left | 90 (48.7) | 18 (40.0) | |
| Midline | 41 (22.2) | 18 (40.0) | |
| Bilateral | 1 (0.5) | 1 (2.2) | |
| Separation or continuity | 0.002 | ||
| Separation | 35 (18.9) | 0 | |
| Continuity | 150 (81.1) | 45 (100) | |
| Superior extent | 0.477 | ||
| Tongue base | 0 | 0 | |
| Hyoid bone | 19 (10.3) | 2 (4.4) | |
| Thyrohyoid membrane | 23 (12.4) | 6 (13.3) | |
| Thyroid cartilage | 143 (77.3) | 37 (82.2) | |
Note.- Data are presented as mean ± standard deviation or as n (%). AP = anteroposterior, US = ultrasonography
Table 4. Comparison in Detection Rate, Size, Location, Separation or Continuity, and Superior Extent of Thyroid Pyramidal Lobes on CT According to Experienced and Less Experienced Radiologists.
| Items | CT | P | |
|---|---|---|---|
| Experienced | Less Experienced | ||
| Detection rate | 219 (79.4) | 57 (20.6) | < 0.001 |
| Size | |||
| Length (mm) | 24.9 ± 11.8 | 18.3 ± 7.2 | < 0.001 |
| AP diameter (mm) | 2.5 ± 1.2 | 2.3 ± 1 | 0.526 |
| Transverse diameter (mm) | 6.5 ± 2.5 | 6 ± 2.8 | 0.087 |
| Volume (mm3) | 239.6 ± 277.7 | 152.3 ± 163.3 | 0.003 |
| Location | 0.099 | ||
| Right | 51 (23.3) | 14 (24.6) | |
| Left | 94 (42.9) | 23 (40.3) | |
| Midline | 73 (33.3) | 17 (29.8) | |
| Bilateral | 1 (0.5) | 3 (5.3) | |
| Separation or continuity | 0.002 | ||
| Separation | 39 (17.8) | 1 (1.8) | |
| Continuity | 180 (82.2) | 56 (98.2) | |
| Superior extent | 0.002 | ||
| Tongue base | 3 (1.4) | 0 | |
| Hyoid bone | 45 (20.5) | 2 (3.5) | |
| Thyrohyoid membrane | 44 (20.1) | 8 (14.0) | |
| Thyroid cartilage | 127 (58.0) | 47 (82.5) | |
Note.- Data are presented as mean ± standard deviation or as n (%). AP = anteroposterior
When the CT result was used as the reference standard for US diagnosis in detecting a TPL, the numbers of true-positive, false-positive, true-negative, and false-negative diagnoses were 209 (35.9%), 25 (4.3%), 269 (46.2%), and 79 (13.2%), respectively (Fig. 2). Among the false-negatives, TPLs located at the midline were the most common (n = 31, 5.3%). The diagnostic indices of US at each institution are listed in Table 5. In addition, the sensitivity, specificity, positive and negative predictive values, and the accuracy of US were 72.6%, 91.5%, 89.3%, and 77.3%, 82.1%, respectively. The diagnostic indices of US in the experienced group were higher than in the less experienced group (p < 0.001).
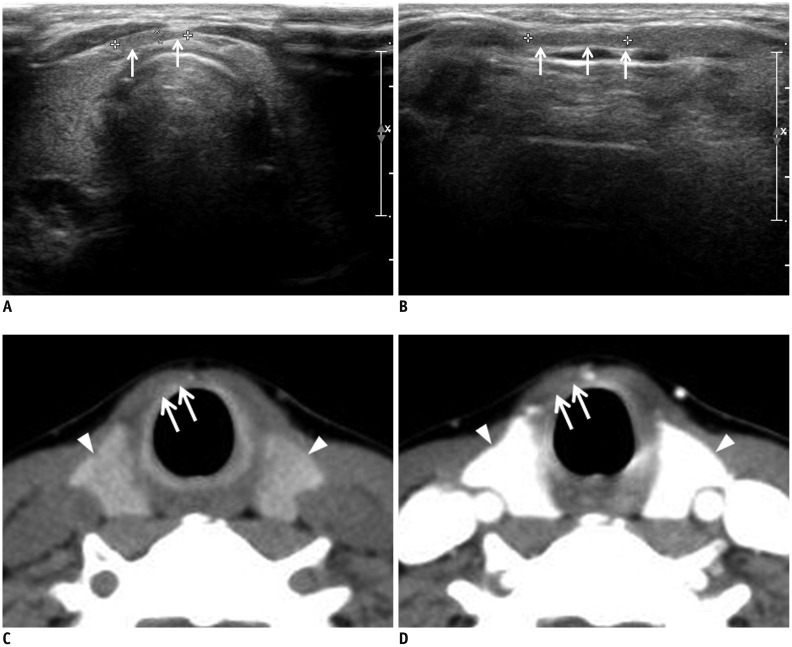
Fig. 2. Fatty tissue mimicking thyroid pyramidal lobe on ultrasonography in 49-year-old woman.
Transverse (A) and longitudinal (B) gray-scale sonograms show longitudinally arranged structure mimicking thyroid pyramidal lobe (arrows). Nonenhanced (C) and contrast-enhanced (D) axial CT images shows only fatty tissue in same position (arrows), and they show different attenuation and enhancement from main thyroid gland (arrowheads).
CONCLUSION
The thyroid gland has an attenuation of around 120 Hounsfield units on CT due to its high iodine concentration. It is also strongly enhanced by contrast agent due to its hypervascularity. Thyroid variants, such as a TPL and lingual thyroid, have pre-contrast attenuation and enhancement pattern identical to the thyroid gland, which makes them easily identifiable on CT (10, 14, 15). Single- and multicenter studies have reported that TPLs are present in 41.3% (135/327) and 44.6% (981/2200) of study patients, respectively (9, 10). These results are similar to cadaveric or surgical studies, suggesting that neck CT is a feasible diagnostic tool for detecting TPLs and other thyroid variants in patients undergoing total thyroidectomy (1, 8).
Thyroid US is established as the first-choice method for evaluating benign and malignant thyroid nodules (16, 17). Moreover, US is useful for detecting thyroid variants, thyroglossal duct cysts, or TPLs (18). Furthermore, US does not require an iodinated contrast medium and has no radiation hazard, unlike neck CT. In two recent studies using US, the TPL detection rates were 50.6% and 58.5% (12, 13), which were higher than the rates (41-44.6%) reported in previous CT studies (9, 10). Our study is the first to prospectively compare the detection rates of US and CT by investigators from multiple centers. Our study revealed that the mean TPL detection rates on US and CT were 39.5% and 47.4%, respectively. The low detection rate of TPLs on US might be related to the varying levels of experience among the investigators or a limitation in sonographic detection for thin TPLs.
In previous studies using surgical or CT findings as the reference standard, the sensitivity, specificity, positive predictive value, and negative predictive value of US diagnosis for detecting TPL were 81.0%, 79.2%, 85.3%, and 73.7%, and 82.3%, 95.3%, 93.3%, and 78.2%, respectively (11, 12). In the present study, the sensitivity, specificity, positive predictive value, and negative predictive value of US for detecting TPL were 72.6%, 91.5%, 89.3%, and 77.3%, respectively, similar to the previous studies. In particular, the diagnostic accuracy of thyroid US for detecting TPL was 82.1% in this study and is similar to the rates in previous single-center studies (83.7%, 87.5%) (12, 13). However, there was a significant difference in TPL detection between experienced and less experienced radiologists on both US and CT. The detection rate and diagnostic accuracy of US for experienced radiologists were higher than for less experienced radiologists. This suggests that accurate detection of TPL or other variants of the thyroid gland depends on the radiologists' levels of experience.
In this study, there were false-positive and false-negative diagnoses on US. All false positive cases on US were later identified as fibrofatty tissue when the images were compared to the CT findings. Among the false-negative cases, TPL located at the midline may be misidentified as normal muscular structure or fibrofatty tissue on US. Three cases of suprahyoid TPL were only detected on CT. The possible reason for this could be that sonographic evaluation of the suprahyoid neck is often limited by the poor sonic window. Thus, careful inspection is essential to avoid imaging pitfalls and to completely evaluate the presence of TPL or other variants of the thyroid gland.
This study has several limitations. First, CT findings were used as the reference standard of US in this study. Although surgical findings are more reliable as reference standards, the detection rate of TPL in previous studies using cadaver or surgery was similar to studies using CT (1, 2, 3, 4, 5, 6, 7, 9, 10, 11). Moreover, the diagnostic accuracy of CT for TPL detection was 92.6% in a previous CT study using surgical findings as the reference standard (18). Second, the patients underwent neck CT and US for various reasons, including head and neck abnormalities; thus, these patients may not represent the general population. Furthermore, patients aged < 19 years were not included in this study. Third, short pyramidal lobes were also not included in the study.
In conclusion, our prospective multi-center study demonstrates that US can detect TPL with relatively high diagnostic accuracy compared to CT modalities by comparing TPL detection by multiple investigators from different institutions. Because the detection rate of TPL differed significantly between experienced and less experienced radiologists, careful inspection is essential to avoid imaging pitfalls and for appropriate evaluation of TPL on both US and CT.
References
- 1.Braun EM, Windisch G, Wolf G, Hausleitner L, Anderhuber F. The pyramidal lobe: clinical anatomy and its importance in thyroid surgery. Surg Radiol Anat. 2007;29:21–27. doi: 10.1007/s00276-006-0165-1. [DOI] [PubMed] [Google Scholar]
- 2.Sultana S, Mannan S, Ahmed M, Rahman M, Khan M, Khalil M. An anatomical study on pyramidal lobe of thyroid gland in Bangladeshi people. Mymensingh Med J. 2008;17:8–13. [PubMed] [Google Scholar]
- 3.Ranade AV, Rai R, Pai MM, Nayak SR, Prakash, Krisnamurthy A, et al. Anatomical variations of the thyroid gland: possible surgical implications. Singapore Med J. 2008;49:831–834. [PubMed] [Google Scholar]
- 4.Geraci G, Pisello F, Li Volsi F, Modica G, Sciumè C. The importance of pyramidal lobe in thyroid surgery. G Chir. 2008;29:479–482. [PubMed] [Google Scholar]
- 5.Joshi SD, Joshi SS, Daimi SR, Athavale SA. The thyroid gland and its variations: a cadaveric study. Folia Morphol (Warsz) 2010;69:47–45. [PubMed] [Google Scholar]
- 6.Zivic R, Radovanovic D, Vekic B, Markovic I, Dzodic R, Zivaljevic V. Surgical anatomy of the pyramidal lobe and its significance in thyroid surgery. S Afr J Surg. 2011;49:110. [PubMed] [Google Scholar]
- 7.Ozgur Z, Celik S, Govsa F, Ozgur T. Anatomical and surgical aspects of the lobes of the thyroid glands. Eur Arch Otorhinolaryngol. 2011;268:1357–1363. doi: 10.1007/s00405-011-1502-5. [DOI] [PubMed] [Google Scholar]
- 8.Pacini F, Schlumberger M, Harmer C, Berg GG, Cohen O, Duntas L, et al. Post-surgical use of radioiodine (131I) in patients with papillary and follicular thyroid cancer and the issue of remnant ablation: a consensus report. Eur J Endocrinol. 2005;153:651–659. doi: 10.1530/eje.1.02014. [DOI] [PubMed] [Google Scholar]
- 9.Park JY, Kim DW, Park JS, Kang T, Kim YW. The prevalence and features of thyroid pyramidal lobes as assessed by computed tomography. Thyroid. 2012;22:173–177. doi: 10.1089/thy.2011.0397. [DOI] [PubMed] [Google Scholar]
- 10.Kim DW, Jung SL, Baek JH, Kim J, Ryu JH, Na DG, et al. The prevalence and features of thyroid pyramidal lobe, accessory thyroid, and ectopic thyroid as assessed by computed tomography: a multicenter study. Thyroid. 2013;23:84–91. doi: 10.1089/thy.2012.0253. [DOI] [PubMed] [Google Scholar]
- 11.Kim KS, Kim DW, Sung JY. Detection of thyroid pyramidal lobe by ultrasound versus computed tomography: a single-center study. J Comput Assist Tomogr. 2014;38:464–468. doi: 10.1097/RCT.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 12.Kim DW, Ha TK, Park HK, Kang T. Sonographic detection of thyroid pyramidal lobes before thyroid surgery: a prospective single-center study. J Ultrasound Med. 2014;33:239–244. doi: 10.7863/ultra.33.2.239. [DOI] [PubMed] [Google Scholar]
- 13.Ryu JH, Kim DW, Kang T. Pre-operative detection of thyroid pyramidal lobes by ultrasound and computed tomography. Ultrasound Med Biol. 2014;40:1442–1446. doi: 10.1016/j.ultrasmedbio.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Torlontano M, Attard M, Crocetti U, Tumino S, Bruno R, Costante G, et al. Follow-up of low risk patients with papillary thyroid cancer: role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab. 2004;89:3402–3407. doi: 10.1210/jc.2003-031521. [DOI] [PubMed] [Google Scholar]
- 15.Iida Y, Konishi J, Harioka T, Misaki T, Endo K, Torizuka K. Thyroid CT number and its relationship to iodine concentration. Radiology. 1983;147:793–795. doi: 10.1148/radiology.147.3.6844615. [DOI] [PubMed] [Google Scholar]
- 16.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 17.Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12:1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa C, Kammori M, Onose H, Yamada E, Shimizu K, Yamada T. Follicular carcinoma arising from the pyramidal lobe of the thyroid. J Nippon Med Sch. 2009;76:169–172. doi: 10.1272/jnms.76.169. [DOI] [PubMed] [Google Scholar]