Abstract
The pervasive nature of estrogenic industrial and dietary compounds is a growing health concern linked to cancer, obesity, and neurological disorders. Prior analyses of endocrine disruptor action have focused primarily on the short-term consequences of exposure. However, these studies are unlikely to reflect the consequences of constant exposures common to industrialized countries. Here we examined the global effects of long-term endocrine disruption on gene transcription and estrogen signaling. Estrogen-dependent breast cancer cell lines were chronically treated with physiologically relevant levels of bisphenol A or genistein for more than 70 passages. Microarray analysis demonstrated global reprogramming of the transcriptome when compared with a similarly cultured control cell line. Estrogen-responsive targets showed diminished expression in both the presence and absence of estrogen. Estrogen receptor recruitment, H3K4 monomethylation, and deoxyribonuclease accessibility were reduced at nearby response elements. Based on these observations, we investigated the potential of long-term endocrine disruptor exposure to initiate persistent transcriptional reprogramming. Culture of chronically exposed cell lines in the absence of the endocrine disruptors did not reverse many of the signaling defects that accumulated during treatment. Taken together, these data demonstrate that chronic exposure to endocrine disrupting compounds can permanently alter physiological hormone signaling.
Estrogen signaling is essential for normal development and physiology but, when deregulated, promotes pathological conditions such as cancer. Canonically, estrogens bind the estrogen receptor (ERα or ERβ), inducing dimerization, DNA binding, and the subsequent recruitment of coregulatory molecules responsible for target gene expression. Interaction of the ER with DNA can be either direct [through estrogen-responsive elements (EREs)] or indirect [via interaction with other transcription factors such as activator protein-1 (1)], serving to regulate defined gene ontologies.
Endocrine disruptors (EDCs) are industrial and dietary compounds that alter normal estrogen signaling and have been implicated in cancer, infertility, obesity, diabetes, and neurological disorders (2, 3). EDCs, like bisphenol A (BPA) and genistein (GEN), bind the ER directly and stimulate compound-specific transcriptional programs that are disparate from that of the estrogen-bound receptor (4, 5).
The ability of EDCs to stimulate aberrant ER signaling is a growing health concern. BPA is pervasive in industrialized countries where it is used in the manufacturing of plastics, dental sealants, and food packaging. As a result, BPA is detectable in the serum of greater than 95% of Americans (6). Serum BPA levels as high as 80 nM have been reported, but typical levels remain in the 1- to 10-nM range (7, 8).
Other estrogenic compounds, like GEN, are abundant in our diet. Although serum GEN levels typically range from 10 to 60 nM (10), individuals on a soy-rich diet can exhibit levels up to 140 nM (9, 10). The ER-binding affinities of BPA and GEN are approximately 10- to 10 000-fold less than 17β-estradiol (E2) (11, 12), but EDC levels within the range of human exposure are nonetheless capable of altering ER action (13, 14).
A specific fear surrounding EDCs has grown from the well-established link between life-long estrogen exposure and breast cancer (15, 16). Because EDCs are ubiquitous in industrialized countries, prolonged exposure to these compounds may increase breast cancer risk. To address this concern, prior studies have focused on defining the immediate effects of EDCs on ER signaling. However, these studies failed to address the persistent nature of real-life EDC exposures that may initiate distinct phenotypes compared with acute exposures (17–19). For example, although GEN acutely promotes estrogen-dependent growth, chronic exposure of human breast cancer cells (MCF7) inhibited estrogen-dependent cellular proliferation (20). In the setting of acute exposures, each EDC distinctly reprograms ER signaling. Therefore, the long-term consequences of EDC exposure are also likely to be compound specific.
Herein we investigated the impact of chronic BPA and GEN exposure on estrogen-dependent breast cancer models. To faithfully mimic human exposure, these EDCs were administered for more than 1 year in the presence of physiological hormones. Our work reveals that chronic EDC exposure initiates compound-specific transcriptional reprogramming. Specifically, ER target genes showed decreased expression and reduced receptor recruitment to nearby response elements. We show that decreases in ER binding are attributed to epigenetic changes (ie, the loss of histone 3 lysine 4 monomethylation (H3K4me1) and DNA accessibility at enhancer elements). These alterations in E2-induced ER activity are persistent, remaining long after the removal of EDCs from the culture. Together these data demonstrate the potential of chronic EDC exposure to permanently modify the normal estrogen signaling axis, suggesting that prolonged exposures result in lifetime health consequences.
Materials and Methods
Cell culture
MCF7 and T47D cells lines were acquired from American Type Culture Collection and maintained in DMEM supplemented with phenol red (Gibco by Life Technologies) 10% (vol/vol) fetal bovine serum (FBS), 1% (vol/vol) penicillin-streptomycin solution, and 2 mM L-glutamine. Chronic EDC-treated cells were cultured in the presence of no EDC (MCF7-F, T47D-F), 50 nM BPA; (Sigma; MKBF3852V; MCF7-B, T47D-B), or 50 nM GEN (Sigma G6649-5MG; MCF7-G, T47D-G) for more than 70 passages. Fresh medium was added to the cultures three times per week.
Western immunoblotting
Chronically treated MCF7 cells and low-passage (LP) MCF7 cells were plated at equal density and allowed to adhere overnight. The following day cells were harvested and lysed in radioimmunoprecipitation assay buffer (150 mM NaCL; 1% IGEPAL; 0.5% deoxycholate; 0.1% sodium dodecyl sulfate in 50 mM Tris, pH 8.0) with added protease inhibitors. Total protein was quantified (Bio-Rad Bradford protein assay) and subjected to SDS-PAGE. Gels were transferred to polyvinyl difluoride and probed with antibodies specific for ERα (GeneTex; number EPR4097) and β-actin (Cell Signaling; number 3700). Immunoblots were imaged on a LI-COR Odyssey.
RNA isolation, RT-PCR, and real-time PCR
Cells were initially seeded and then cultured for 72 hours in phenol red free DMEM supplemented with 5% charcoal dextran-treated FBS (CDT). Cells were then stimulated with ethanol, 10 nM E2, 100 nM BPA, or 100 nM GEN for 3 hours, and RNA was harvested using RiboZol (Amresco). Higher doses of BPA and GEN were used in the RT-PCR experiments as compared with the long-term exposure to maximize the ER response for these short time-point experiments. RNA was reverse transcribed using the Improm II reverse transcription system (Promega). The resulting cDNA was analyzed using (real-time) primers specific for the indicated genes (Supplemental Table 1). A minimum of three biological replicates, measured in triplicate, was performed for each target. Data were normalized to the vehicle-treated MCF7-F cell line and is depicted as the mean and SE. Statistical significance was determined using a two-tailed Student's t test.
cDNA microarray and real-time PCR validation
Cells were cultured and treated as described for RT-PCR analysis. RNA was isolated using the total RNA isolation Nucleospin RNA II kit (Macherey-Nagel). Microarray analysis was performed by the Microarray and Next Generation Sequencing Shared Resource at The Ohio State University Comprehensive Cancer Center using GeneChip human transcriptome arrays (version 2.0; Affymetrix). For the gene profiling microarray, we normalized the probe cell intensity with the Robust Multichip Average (RMA) method using Expression Console Software (Affymetrix). An ANOVA was performed to identify differentially expressed genes. Analysis was done using SAS version 9.3 software (SAS, Inc). Microarray intensity files can be accessed through Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE59345.
Chromatin immunoprecipitation (ChIP)
ChIP was performed as previously described (21). Briefly, cells were cultured in DMEM with 5% CDT for 72 hours followed by stimulation with vehicle control or 10 nM E2 for 45 minutes. Cells were cross-linked, sonicated, and immunoprecipitated with antibodies specific for ERα (GeneTex-EPR4097), IgG control (Cell Signaling), or H3K4me1 (Abcam; ab8895). Enriched DNA was quantified using the Qubit dsDNA High Sensitivity (HS) assay kit (Life Technologies). Real-time PCR was performed using 400 pg/well DNA with primers amplifying estrogen response elements for the target genes of interest (Supplemental Table 1). Data shown are representative of at least three independent biological replicates.
Deoxyribonuclease (DNase) sensitivity assays
Chronically treated MCF7 cells grown in DMEM/CDT for 72 hours were harvested, and DNase assays were performed as previously described (22). Data shown are representative of at least three independent biological replicates.
Results
Chronic EDC treatment reprograms global gene expression
Exposure to environmental estrogens is both constant and ubiquitous within industrialized countries. To generate an in vitro model of chronic EDC exposure, we supplemented the media of MCF7 cells with 50 nM BPA or GEN for greater than 70 passages (>1 y; Figure 1A). Two model cell lines, termed MCF7-B (BPA) and MCF7-G (GEN), were generated in addition to a control cell line, MCF7-F (FBS), grown in parallel without EDC addition. The concentrations of BPA and GEN chosen for these studies were within the documented ranges detected in human serum (6, 9, 10).
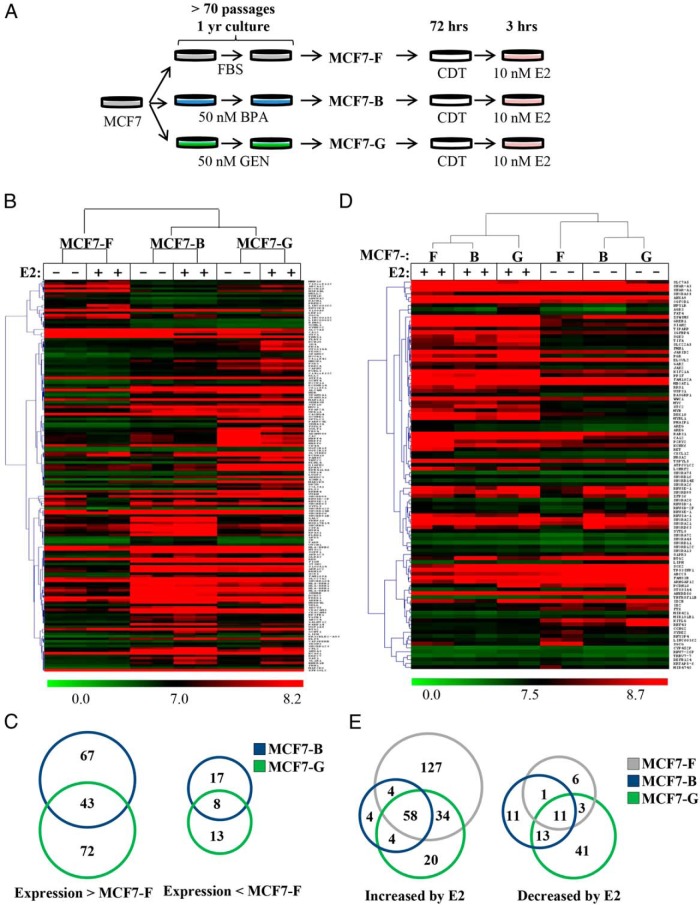
Figure 1.
Chronic EDC treatment reprograms global gene expression. A, A model of chronic EDC exposure was generated by passaging cells more than 70 passages under normal FBS conditions (MCF7-F) with an additional 50 nM BPA (MCF7-B) or GEN (MCF7-G). B, Microarray analyses were performed on the three generated cell lines and two-dimensional hierarchical clustering applied to significant, differentially expressed genes (≥ 2-fold). C, Genes whose expression was higher (> MCF7-F) or lower (< MCF7-F) by 2-fold or greater in the MCF7-B and MCF7-G lines as compared with MCF7-F cells are represented by Venn diagram. D, Two-dimensional hierarchical clustering of the three cell lines was performed using E2-responsive genes significantly regulated (≥ 1.5-fold) in the MCF7-F cell line between EtOH and E2. E, A Venn diagram depicts the overlap of genes with increased or decreased expression after E2 treatment for 3 hours.
Using microarray analysis, we examined whether chronic EDC exposure could induce transcriptional reprogramming. Cells were cultured in the absence of hormone for 72 hours and then stimulated for 3 hours with 10 nM E2 or ethanol vehicle (EtOH; Figure 1A). Microarray-based transcriptome analysis revealed genes that were statistically different in the chronically treated cell lines compared with the MCF7-F cells (Figure 1B). The heat map depicts the top 100 genes that demonstrated the highest fold changes (minimum > 2-fold, P < .05) between MFC7-F (EtOH) vs MFC7-B (EtOH) and MCF7-F vs. MFC7-G (EtOH) (200 total genes). Although many deregulated targets (up- or down-regulated ≥ 2-fold) in the MCF7-B and MCF7-G lines were distinct (Figure 1B), chronic EDC treatment was generally associated with increases in transcription (Figure 1C). There were 115 genes showing higher expression in the MCF7-G cell line compared with only 21 genes showing lower expression in the MCF7-G cell line when compared with the MCF7-F control (P < .05, > 2-fold). The MCF7-B cell line showed a similar ratio (110 to 25) of genes showing higher expression when compared with the control.
Chronic EDC treatment reprograms E2-regulated gene expression
We observed many established ER targets deregulated within the microarray data set. Therefore, we reanalyzed our microarray data, focusing on the top 150 ER target genes in the MCF7-F cell line that changed 1.5-fold or greater in response to E2. Hierarchical clustering revealed that although a large portion of E2-responsive genes remained similarly regulated, a unique signature representing specific deregulated genes was observed in the chronically treated cell lines (MCF7-B and MCF7-G) (Figure 1D). Moreover, there were differences in the genes responding to E2 between the three cell lines. Many genes showed altered expression in both the estrogen-depleted (CDT) and -stimulated state. However, EDC-dependent transcriptional reprogramming was not universal. Approximately 25% of E2-responsive genes were similarly regulated in all three cell lines plus or minus E2 (Figure E). In contrast to the clustering of genes performed in Figure 1, B and C, we found that most E2-responsive genes showed decreased expression in cell lines exposed to chronic BPA or GEN treatment (Figure 1E). Specifically, there were 127 genes induced greater than 1.5-fold by E2 in the MCF7-F cell line that were not induced to that extent in the MCF7-B and MCF7-G cell lines. Further, only 8 and 24 genes were more highly induced by E2 in the MCF7-B or MCF7-G cell lines, respectively. In relation to E2-mediated gene repression, the chronically treated cell lines had a larger number of repressed genes than the MCF7-F control (Figure 1E, right diagram). We observed only 21 genes repressed by E2 treatment in the MCF7-F cell line, whereas 36 and 68 genes were repressed (P < .05, > 1.5-fold) in the MCF7-B and MCF7-G lines, respectively. Taken together, there was a much larger percent of E2-regulated genes repressed in the chronically treated cell lines (MCF7-B/G) than the MCF7-F control (P < .0001, χ2 test). These data show that chronic EDC treatment causes the deregulation of E2 responses, frequently resulting in reduced target gene expression.
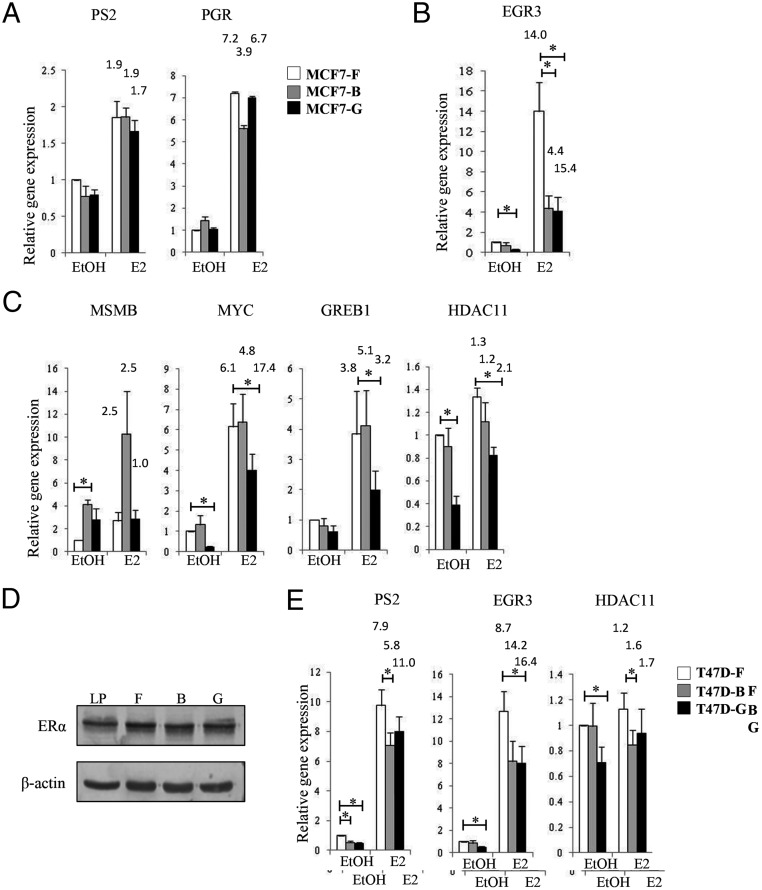
To further characterize the estrogen response in cell lines chronically treated with EDCs, we performed a more detailed analysis of known E2-responsive genes (Figure 2, A–C). These genes were selected based on a combination of either strong E2-mediated induction at 3 hours or the presence of a strong ERα ChIP peak proximal to the transcriptional start site. Each cell line was cultured for 72 hours in the absence of steroid hormones (CDT) and then stimulated with 10 nM E2 or vehicle for 3 hours (Figure 1A). As seen in our microarray analyses, a subset of estrogen-responsive genes responded similarly in all cell lines (eg, PS2, PGR; Figure 2A). Additionally, EGR3 showed significantly (P < .05) reduced expression in both the E2-stimulated MCF7-B and MCF7-G cell lines compared with control MCF7-F (Figure 2B). Finally, a third group of gene targets demonstrated an EDC-specific E2-response (eg, MSMB, MYC, GREB1, HDAC11; Figure 2C). For example, MSMB expression increased in the MCF7-B cell line yet had normal expression in MCF7-G cells compared with the control (Figure 2C). Interestingly, many targets with altered expression prior to E2 stimulation (EtOH) also had expression that differed from the MCF7-F control in the E2-treated samples (Figures 1E, 2B, and 2C). For example, the EGR3 gene in the MCF7-G cell line had much lower basal activity and E2-induced gene expression, but the fold change after E2 treatment was similar to the MCF7-F cell line. These data suggest that many of these targets can still respond to ER activation, but the overall transcriptional program is abnormal. Changes in gene expression in the absence of ER activation suggest that the mechanism driving deregulation is associated with alterations to the genomic loci.
Figure 2.
Chronic EDC treatment reprograms E2-regulated gene expression. A–C, Control, MCF7-F (white columns) and chronically treated, MCF7-B (gray columns) and MCF7-G (black columns) cells were induced with 10 nM E2 for 3 hours, and relative mRNA was measured by quantitative RT-PCR at genes known to rapidly respond to E2. An altered E2 response was gene-specific because E2-responsive targets showed no change (A), decreased expression in both chronically treated cell lines (B), or altered expression that was EDC-specific (C). D, An immunoblot of ERα protein expression was performed on the three cell lines to determine the effect of chronic EDC exposures on receptor expression. Low-passage (LP) MCF7 cells are presented for comparison. β-actin is depicted as a loading control. E, We performed a replicate experiment of chronic EDC exposure in another E2-responsive breast cancer cell line, T47D. The E2 response showed results similar to the MCF7 cell lines at multiple ER target genes. RT-PCR data are expressed relative to the mean of RPL13a mRNA and are presented as a mean ± SE (n = 5). *, P ≤ .05. Fold activation over basal (EtOH) for each cell line is represented above the E2-treated column.
To verify that decreased estrogen-targeted gene expression was not due to EDC-induced changes in ERα expression, protein was harvested from chronically treated cells and subjected to SDS-PAGE followed by immunoblotting. ERα was highly expressed across all three cell lines comparable with low-passage (LP) MCF7 cells (Figure 2D). Changes in estrogen-dependent signaling could also be influenced by stochastic events occurring during the long-term culture of each MCF7 derivative. Therefore, we compared the transcriptional response of early-passage MCF7 (LP) cells to the MCF7-F line. We looked at both E2-dependent and -independent targets that were differentially expressed in the chronically treated cell lines. At these targets, MCF7-F cells behaved similarly to low-passage MCF7 cells (Supplemental Figure 1).
To corroborate these findings, we generated chronically treated cultures of another estrogen-dependent breast cancer cell line, T47D. Similar to the MCF7-B and MCF7-G lines, chronic EDC-treated T47D cultures showed reduced expression of EGR3 and HDAC11 (Figure 2E). We also observed a significant (P < .05) decrease in PS2 (also known as TFF1) expression in the T47D-B cell line. At these three targets, transcriptional reprogramming in response to chronic EDC administration is conserved across the MCF7 and T47D cell lines.
Deregulated ER response is independent of the activating ligand
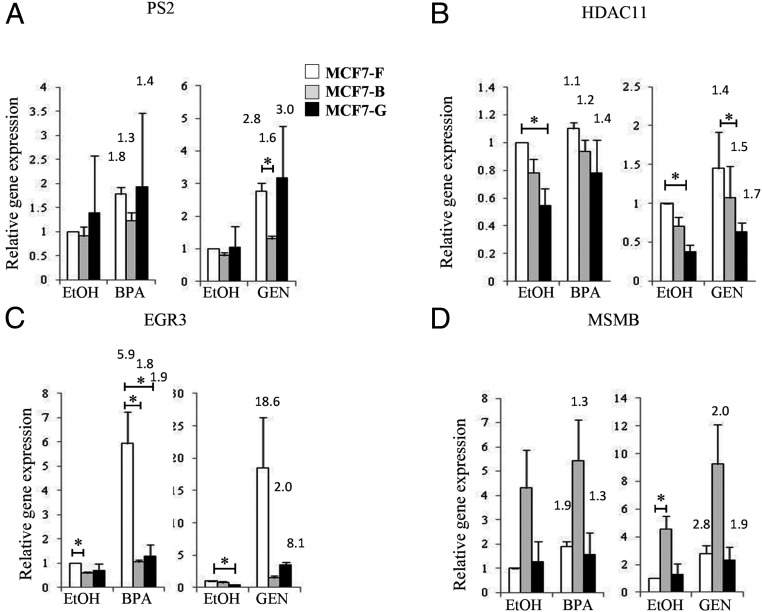
The ability of chronic EDC exposure to alter canonical estrogen-dependent transcriptional programs suggested that acute responses to noncanonical ligands might also be affected in these lines. Because these cells may have adapted to exposure of EDCs, a ligand-specific activation of the ER may be observed. To test this possibility, each MCF7 cell line was cultured in CDT for 72 hours and then stimulated with either 100 nM BPA or 100 nM GEN (Figure 3). Examination of classic estrogen-responsive genes revealed a statistically significant (P < .005) decrease in PS2 expression specific to the MCF7-B line when treated with GEN (Figure 3A). This difference was particularly interesting because E2-stimulated PS2 expression was nearly equivalent in the MCF7-F, MCF7-B, and MCF7-G cultures (Figure 2A). Although this difference may be a ligand-specific defect in ER signaling, it also is more likely indicative of a subtle deregulation only observed under conditions of minimal ER activation as is the case with low-affinity EDCs. This defect in signaling may have been masked in Figure 2 by the 10-nM E2 dose designed to maximize ER activation. Along those lines, the MCF7-B cell line showed reduced transcription of HDAC11 (Figure 3B) similar to MCF7-G under conditions of EDC activation. In this model, HDAC11 may be better grouped with EGR3 in that it has reduced sensitivity to estrogenic stimulation in both EDC-treated lines. When we looked at EGR3, the target showing the greatest reduction in gene expression (Figure 2B), a similar inability to respond to the noncanonical signaling of BPA or GEN was observed (Figure 3C). Finally, much of the altered gene expression in the chronically treated cells at ER-regulated genes (Figure 2C) showed identical deregulation in the presence of EDC ligands (Figure 3D and data not shown). These data demonstrate that defects in ER-target gene activation are ligand independent. The chronically treated cells did not adapt to the EDC exposure by generation mechanisms to respond to EDCs instead of canonical ligands.
Figure 3.
Deregulated ER response is independent of the activating ligand. A–D, MCF7-F (white columns), MCF7-B (gray columns), and MCF7-G (black columns) cells were induced with 100 nM BPA or 100 nM GEN, respectively, for 3 hours, and relative mRNA was measured by quantitative RT-PCR for expression of PS2 (A), HDAC11 (B), EGR3 (C), and MSMB (D). Data are expressed relative to the mean of RPL13a mRNA and are presented as a mean ± SE (n = 4). *, P ≤ .05. Fold activation over basal (EtOH) for each cell line is represented above the E2-treated column.
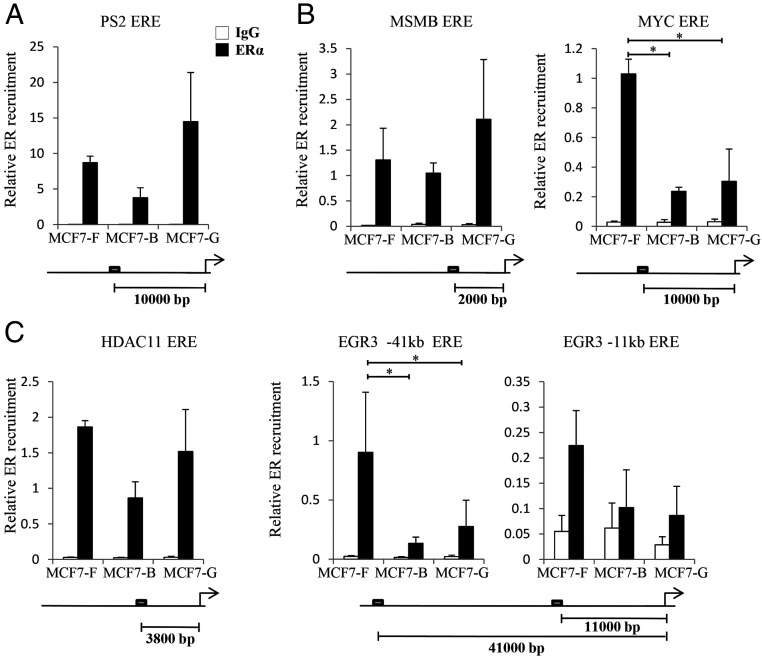
Chronic EDC treatment causes differential ER recruitment
To identify the molecular mechanisms driving deregulation of ER activity after chronic EDC exposure, we performed ChIP assays in the established MCF7 cell lines. Each culture was stimulated with 10 nM E2 for 45 minutes and then harvested for analysis. Real-time PCR primers were designed to assess EREs proximal to a set of well-characterized E2-responsive genes. In all three MCF7 lines (MCF7-F, MCF7-B, and MCF7-G), the ER was strongly recruited to an ERE near the PS2 gene (Figure 4A). This finding was not surprising, given that PS2 was equally expressed in the E2-stimulated control and EDC-exposed cell lines (Figure 2A). In general, alterations in gene expression observed in the MCF7-B and MCF7-G lines were paralleled by changes in ER recruitment (Figure 4, B and C). For example, reduced expression of MYC, EGR3, and HDAC11 in the MCF7-G line was accompanied by decreases in gene-specific ERE recruitment (compare Figure 2, B and C, with Figure 4, B and C). Although this correlation between ER binding activity and gene expression was consistent across most targets, it was not universal. The MCF7-B line demonstrated loss of ER recruitment at the MYC locus but no observable decrease in transcription. However, the MCF7-B cell line generally showed reduced ER recruitment at all analyzed sites, but that affect was most pronounced at the repressed targets (Figure 4, compare C with A).
Figure 4.
Chronic EDC treatment causes differential recruitment of ER. ChIP assays were performed to determine the level of receptor recruitment to EREs after activation with 10 nM E2. A–C, We examined genes unaltered (A), EDC-specifically altered (B), and mutually down-regulated by chronic EDC treatment (C) as depicted in Figure 2. The response elements' relative position to the transcriptional start site is depicted under each graph. Enriched ChIP DNA was measured by quantitative PCR and the data depicted is the mean ± SE (n = 3). *, P ≤ .05.
The EGR3 locus is epigenetically reprogrammed in EDC-treated cell lines
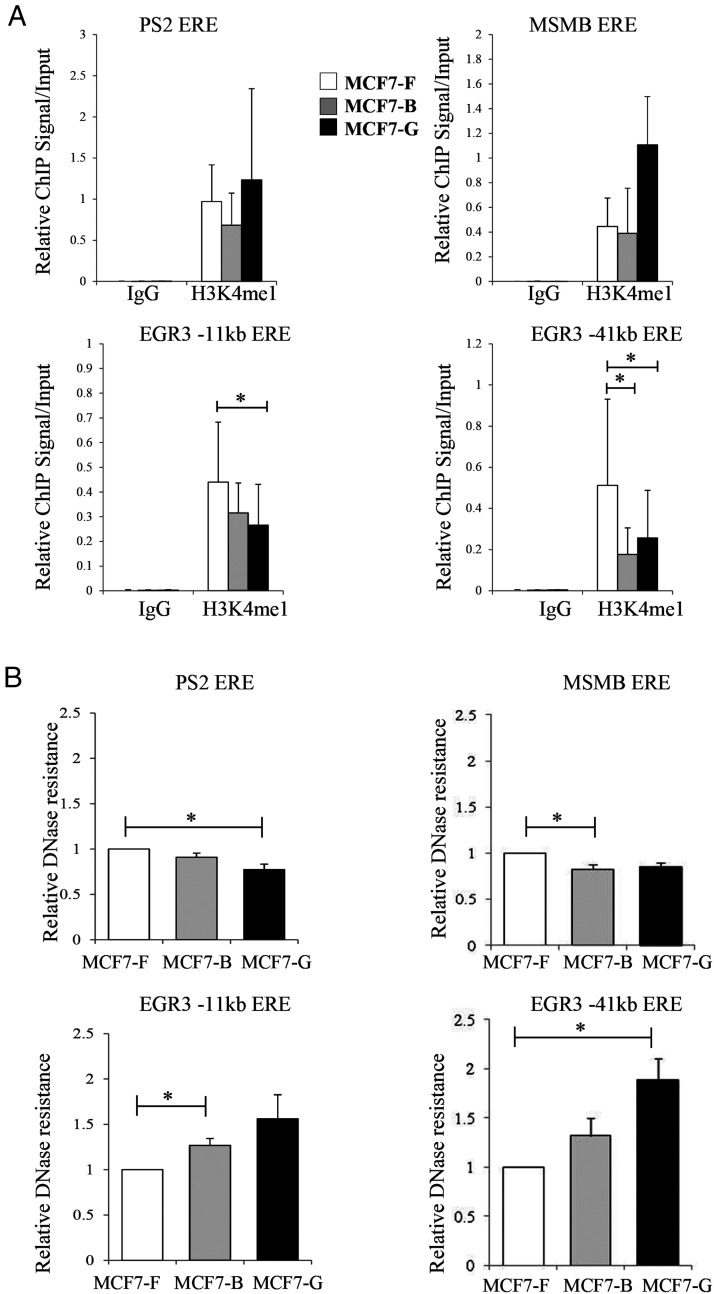
Many of the EREs that showed reduced ER recruitment were located in gene enhancers. To examine whether enhancer activity was altered in response to chronic EDC exposure, we assessed the H3K4me1 status of relevant EREs. H3K4me1 is a histone mark enriched at active enhancer elements and has been implicated in cell type-specific transcriptional responses (23). We postulated that a decrease in H3K4me1 might be responsible for the reduced ability of ER to locate and bind specific EREs in cell lines chronically treated with BPA or GEN. Therefore, we examined the status of H3K4me1 prior to estrogen stimulation. In the absence of hormone, MCF7-B and MCF7-G cells showed equal or greater levels of H3K4me1 at the PS2 ERE than those observed in the control line (MCF7-F). Specifically, H3K4me1 at this site was higher in the MCF7-G line than any other culture (Figure 5A, left panel), paralleling the enhanced recruitment of ER to the PS2 promoter observed in these cells (Figure 4A). The MSMB gene, which showed elevated expression in the MCF7-B line, demonstrated no increase in ER binding (Figure 4B) and no significant change in H3K4me1 (Figure 5A, right panel). MSMB did demonstrate a very high basal expression in the absence of hormone (see Figure 2C), suggesting that deregulation of this locus may not be ER dependent. However, at the two EGR3 response elements, we observed a decrease in H3K4me1 that was restricted to MCF7 cells chronically treated with EDCs (Figure 5A, see bottom panels). Again, these results paralleled the change in ER recruitment observed at the EGR promoter of the MCF7-B and MCF7-G cell lines (Figure 4C, left panel).
Figure 5.
Epigenetic reprogramming is seen at EGR3 response elements. A, ChIP assays were performed on the MCF7-F, MCF7-B, and MCF7-G cell lines prior to ER activation with antibodies specific for the enhancer mark H3K4me1. ChIP-enriched DNA was analyzed by quantitative PCR with real-time primers specific for response elements near the PS2, MSMB, and EGR3 genes. Data are depicted as the mean ± SE (n = 3). B, DNase accessibility assays were performed under identical conditions to the ChIP assays. Relative DNase resistance was measured by quantitative PCR at the response elements associated with the PS2, MSMB, and EGR3 genes. Data represent the DNA resistant to digestion relative to MCF7-F cells and is depicted as the mean ± SE (n = 3). *, P ≤ .05.
We further investigated the repressive histone marks H3K9me3 and H3K27me3 at these genomic loci but did not see changes in these modifications that would suggest active repression of the target genes (data not shown). These data show that chronic EDC exposure alters the chromatin landscape, leading to defects in the recruitment of activated ER to specific target gene promoters. We thus probed the microarray data for transcriptional changes in histone modifying enzymes and discovered only one transcript (MLL1/KMT2A) that was significantly altered greater than 1.5-fold in the chronically treated cell lines. MLL1 was down-regulated (1.53-fold MCF7-B, 1.35-fold MCF7-G, P < .05) and is a methyltransferase associated with the H3K4me2 and H3K4me3 marks. To ensure that global changes in H3K4 methylation were not influencing the ChIP signatures, we performed Western blots, demonstrating no changes in total histone H3, H3K4me1, and H3K4me3 for each cell line (Supplemental Figure 2).
DNA accessibility is known to control nuclear receptor recruitment to target genes (24, 25). Because EREs in the chronically treated cell lines showed a loss of H3K4me1 (Figure 5A, see EGR3 EREs), we investigated whether the chromatin architecture at these sites was concomitantly altered using DNase hypersensitivity assays. Here DNA was subjected to a limited DNase I digestion and then analyzed by real-time PCR to determine the relative resistance of key EREs to enzymatic cleavage. The PS2 and MSMB genes, both of which showed efficient binding by ER, showed either no change or a slight increase in accessibility (Figure 5B, upper panels). In the case of PS2, the MCF7-G cell line demonstrated a statistically significant decrease that corresponds to increased ER recruitment (Figure 4A) and H3K4me1 (Figure 5A), but not to transcriptional output (Figures 1, 2A). In contrast, EGR3 EREs were more resistant to DNase digestion in the GEN- and BPA-treated cell lines (Figure 5B, bottom panels). Therefore, chronic exposure to EDCs results in reduced accessibility of nearby enhancer elements and decreased recruitment of receptor to these EREs.
Estrogen signaling defects persist after EDC removal
Our data show that chronic EDC treatment causes gene-specific reprogramming of the estrogen response. Because chromatin alterations play a role in this process, the mechanism by which long-term EDC treatment alters hormone signaling may be epigenetic. By definition, epigenetic events are heritable changes to the chromatin structure that are sustained through multiple cell divisions.
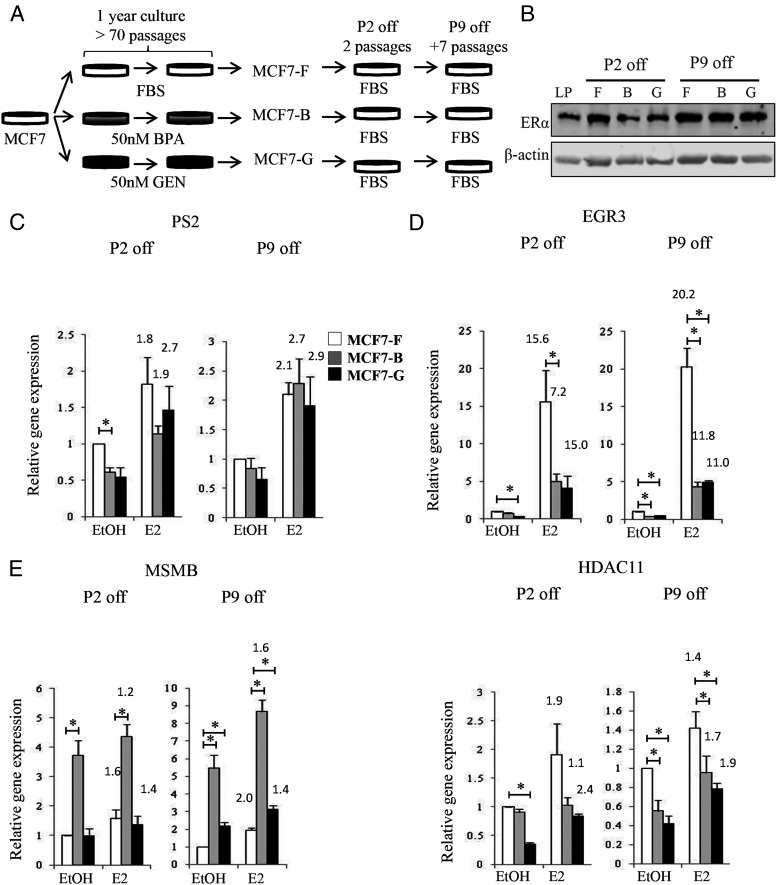
To test whether alterations in estrogen signaling caused by chronic EDC exposure were epigenetic, we removed BPA and GEN from our MCF7-B and MCF7-G lines for several passages (Figure 6A). First, we confirmed that EDC removal did not alter ERα expression levels in the MCF7 cell lines. Immunoblot demonstrated that ERα protein levels were unchanged after removal of the EDCs (compare Figures 2D and 6B). Next, we assessed E2-mediated gene expression in these same cell lines. As expected, genes that were initially unaffected by chronic BPA or GEN treatment (Figure 2A) remained unaffected in MCF7-B and MCF7-G lines cultured in the absence of EDC for two or nine passages (Figure 6C). However, the deregulation of EGR3 (Figures 2B and 3C) was sustained in the MCF7-B and MCF7-G cell lines for two to nine passages after EDC removal (Figure 6D). Interestingly, two genes, MSMB and HDAC11, which previously showed EDC-specific effects (Figures 2C), demonstrated enhanced deregulation in both EDC-treated cell lines (Figure 6E) after several passages without treatment. Such sustained alterations at the estrogen-responsive genes were typical but not universal. For example, MYC, a target deregulated solely in the MCF7-G cell line (Figure 2C), showed similar induction across all cell lines after removal of the EDCs (data not shown). Thus, although most of the genes deregulated by the estrogen response persisted, some had the ability to return to normal expression states after continued culture in the absence of EDCs. The observed changes in ER recruitment and DNA accessibility at nearby enhancer elements were also maintained in the chronically treated cell lines after the removal of the EDCs (Supplemental Figure 3).
Figure 6.
Changes in the E2 response persist after removal of the EDC. A, Diagram depicting the experimental model to test sustained E2 signaling defects. MCF7-F, MCF7-B, and MCF7-G cells were all placed under normal culturing conditions in the absence of BPA and GEN for a minimum of two passages followed by analysis of E2 signaling. B, After either two or nine passages off chronic EDC treatment, ERα protein expression was analyzed as described in Figure 2D. C–E, The response of each cell line to 10 nM E2 was measured by quantitative RT-PCR after two and nine passages of chronic EDC treatment at genes shown to be unaffected (C), mutually repressed (D), and EDC-specifically deregulated (E) in Figure 2. Data are expressed relative to the mean of RPL13a mRNA and are presented as a mean ± SE (n = 3). *, P ≤ .05. Fold activation over basal (EtOH) for each cell line is represented above the E2-treated column.
Together these data demonstrate that chronic EDC treatment can permanently alter the ability of estrogen to activate ER-target genes and further implicates the potential of these compounds to epigenetically reprogram hormone-responsive breast cancer cells.
Discussion
Detectable levels of EDCs in human sera and urine indicate that people in industrialized countries are constantly exposed to environmental hormones. However, prior work in the field has focused on acute exposures in an effort to demonstrate the ability of these compounds to activate ER signaling. Herein we describe sustained changes to the estrogen signaling program after chronic EDC exposure that cannot be represented in acute models. Utilizing a cell culture system to mimic chronic BPA and GEN exposure, we show reduced expression of many E2-responsive genes under both basal and E2-stimulated conditions (Figures 1D, 2B, and 2C). Although the acute EDC activation of ER stimulates expression (Figure 3, MCF7-F), chronic exposure led to reduced expression of many ER-responsive genes (Figures 1E, 2, and 3, MCF7-B, MCF7-G).
It is well established that EDCs can bind the ER, regulate gene expression, and drive estrogen-dependent proliferation (4, 5, 13, 14, 26–30), presumably dependent on the conformation of the ligand-bound ER and its ability to recruit distinct subsets of transcriptional coregulators (31, 32). After chronic EDC exposure, we found that canonical ER signaling is also deregulated in a compound- and gene-specific manner. For instance, MSMB expression was induced dramatically in MCF7-B cells but did not increase in the similarly treated MCF7-G line (Figure 2C). Likewise, MYC expression was decreased in MCF7-G cells alone (Figure 2C). Other genes were similarly deregulated in both the BPA and GEN treated cells (EGR3, HDAC11). Gene target specificity may be dependent upon the receptor conformation induced by each ligand, thus modulating coregulator recruitment. Also, BPA and GEN are known to induce different estrogenic activities. For example, BPA has been shown to have a better ability to induce the ER tethering activity at activator protein-1 response elements when compared with GEN (5). GEN has been shown to bind ERβ 20- to 30-fold stronger than ERα; however, in vitro assays suggest that GEN has only a weak preference for ERβ in gene transactivation (33). Finally, BPA and GEN are known to act through alternate pathways other than ER that may contribute to unique gene signatures (34, 35).
Interestingly, although many ER targets were deregulated, the receptor was still able to at least partially modulate gene expression. In fact, in some cases the alterations in gene expression are mostly attributed to basal activity and the relative fold induction after ER activation is approximately equivalent. Nonetheless, the transcriptional output at these genes in the chronically treated cells is substantially altered, even in the presence of E2. A potential caveat of these experiments is that CDT media retain a small amount of estrogenic compounds, and basal activity is not truly estrogen free. Thus, gene expression reductions in the EtOH-treated samples could be indicative of a loss in the residual estrogenic activity of CDT media. Moreover, ER could retain the ability to reorganize the gene to induce full activity but at some targets is not being effectively recruited. There is also the possibility that other factors synergize with ER at these loci. Transcription factors tend to bind in hot spots in which an enhancer may recruit multiple transcription factors (21). The changes we see in ER recruitment and enhancer chromatin architecture may also be affecting the recruitment of other transcription factors.
Many changes in gene expression and chromatin architecture persisted, even after removal of the EDCs (Figure 6 and Supplemental Figure 3). These data show that chronic EDC exposure may promote persistent and gene- and compound-specific defects. However, although some changes in transcription appeared permanent, MYC did not display evidence of epigenetic reprogramming, and normal expression patterns were restored after removal of the EDC. Clearly the complexity of ER signaling extends to chronic EDC action and the transcriptional changes observed may be dependent on the diverse set of molecular and estrogenic targets of these compounds. In particular, ligand-specific recruitment of coregulators may play a critical role in the gene specificity of epigenetic modulation. Coregulators often possess chromatin modifying enzymatic activities (36). Thus, the proposed role of coregulators in ligand-specific ER signaling is particularly relevant to the epigenetic modifications in cells chronically exposed to EDCs. The recruitment of chromatin modifying complexes to specific ER targets may drive the gene-specific epigenetic reprogramming observed in our cell lines as a result of chronic treatment. Alternatively, EDCs are known to selectively modulate the expression of chromatin readers, writers, and other modifying enzymes (37). It is possible that this process also influences the epigenetic landscape of cells chronically exposed to BPA or GEN. However, the deregulation of E2-signaling described herein is target specific, suggesting that general alterations in the expression of chromatin modifying enzymes is not solely responsible for the ERE reprogramming caused by chronic EDC exposures. Instead, our data put forth a model wherein differential coregulator recruitment results in transient changes in gene transcription. Upon chronic exposure, the repetitive activity of these cofactors induces epigenetic reprogramming, leading to long-term consequences on E2 signaling, even after subsequent removal of the EDC.
Our work demonstrates that chronic EDC exposures can permanently influence hormonal signaling. As such, the prevalence and persistence of compounds like BPA and GEN in the environment of industrialized countries may have unrealized and underappreciated health consequences. Unfortunately, epidemiological studies investigating the impact of EDCs on human health are often conflicting, making it difficult to assess whether a specific compound has beneficial or detrimental consequences. Overcoming the complexities of ER signaling through the study of EDCs and their mechanisms of hormonal disruption will begin to elucidate suspected and observed consequences of our chronic exposure.
Acknowledgments
We thank Dr C. E. Burd, X. Guan, and K. LaPak for critical reading and advice on the manuscript.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award R00ES019918.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- CDT
- charcoal dextran-treated FBS
- ChIP
- chromatin immunoprecipitation
- DNase
- deoxyribonuclease
- E2
- 17β-estradiol
- EDC
- endocrine disruptor
- ER
- estrogen receptor
- ERE
- estrogen-responsive element
- EtOH
- ethanol
- FBS
- fetal bovine serum
- GEN
- genistein
- H3K4me1
- histone 3 lysine 4 monomethylation.
References
- 1. Dahlman-Wright K, Qiao Y, Jonsson P, Gustafsson JA, Williams C, Zhao C. Interplay between AP-1 and estrogen receptor α in regulating gene expression and proliferation networks in breast cancer cells. Carcinogenesis. 2012;33:1684–1691. [DOI] [PubMed] [Google Scholar]
- 2. Palanza P, Morellini F, Parmigiani S, vom Saal FS. Prenatal exposure to endocrine disrupting chemicals: effects on behavioral development. Neurosci Biobehav Rev. 1999;23:1011–1027. [DOI] [PubMed] [Google Scholar]
- 3. Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong EJ, Park SH, Choi KC, Leung PC, Jeung EB. Identification of estrogen-regulated genes by microarray analysis of the uterus of immature rats exposed to endocrine disrupting chemicals. Reprod Biol Endocrinol. 2006;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Y, Luh CJ, Burns KA, et al. Endocrine-disrupting chemicals (EDCs): in vitro mechanism of estrogenic activation and differential effects on ER target genes. Environ Health Perspect. 2013;121:459–466, 466e451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol (Elmsford, NY). 2007;24:139–177. [DOI] [PubMed] [Google Scholar]
- 9. Grace PB, Taylor JI, Low YL, et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European Prospective Investigation of Cancer and Nutrition-Norfolk. Cancer Epidemiol Biomarkers Prev. 2004;13:698–708. [PubMed] [Google Scholar]
- 10. Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–5050. [PubMed] [Google Scholar]
- 11. Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. [DOI] [PubMed] [Google Scholar]
- 12. Gertz J, Reddy TE, Varley KE, Garabedian MJ, Myers RM. Genistein and bisphenol A exposure cause estrogen receptor 1 to bind thousands of sites in a cell type-specific manner. Genome Res. 2012;22:2153–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ricupito A, Del Pozzo G, Diano N, et al. Effect of bisphenol A with or without enzyme treatment on the proliferation and viability of MCF-7 cells. Environ Int. 2009;35:21–26. [DOI] [PubMed] [Google Scholar]
- 14. Han D, Tachibana H, Yamada K. Inhibition of environmental estrogen-induced proliferation of human breast carcinoma MCF-7 cells by flavonoids. In Vitro Cell Dev Biol Anim. 2001;37:275–282. [DOI] [PubMed] [Google Scholar]
- 15. Chlebowski RT. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–278. [DOI] [PubMed] [Google Scholar]
- 17. Clarke SD, Clarke IJ, Rao A, Evans RG, Henry BA. Differential effects of acute and chronic estrogen treatment on thermogenic and metabolic pathways in ovariectomized sheep. Endocrinology. 2013;154:184–192. [DOI] [PubMed] [Google Scholar]
- 18. Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J Neuroendocrinol. 2012;24:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuba T, Hunter D, Zhou L, Jenab S, Quinones-Jenab V. Acute and chronic estradiol replacements differentially alter corticosterone and COX-mediated responses to an inflammatory stimulus in female rats. Ethnic Dis 2010;20(1 suppl 1):S1–50-54. [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Jenkins S, Lamartiniere CA. Cell proliferation and apoptosis in rat mammary glands following combinational exposure to bisphenol A and genistein. BMC Cancer. 2014;14:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burd CJ, Ward JM, Crusselle-Davis VJ, et al. Analysis of chromatin dynamics during glucocorticoid receptor activation. Mol Cell Biol. 2012;32:1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burch JB, Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983;33:65–76. [DOI] [PubMed] [Google Scholar]
- 23. Hon G, Wang W, Ren B. Discovery and annotation of functional chromatin signatures in the human genome. PLoS Comput Biol. 2009;5(11):e1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burd CJ, Archer TK. Chromatin architecture defines the glucocorticoid response. Mol Cell Endocrinol. 2013;380:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He HH, Meyer CA, Chen MW, Jordan VC, Brown M, Liu XS. Differential DNase I hypersensitivity reveals factor-dependent chromatin dynamics. Genome Res. 2012;22:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. [DOI] [PubMed] [Google Scholar]
- 27. Maggiolini M, Bonofiglio D, Marsico S, et al. Estrogen receptor α mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol Pharmacol. 2001;60:595–602. [PubMed] [Google Scholar]
- 28. Korach KS, Sarver P, Chae K, McLachlan JA, McKinney JD. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988;33:120–126. [PubMed] [Google Scholar]
- 29. Soto AM, Justicia H, Wray JW, Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from “modified” polystyrene. Environ Health Perspectives. 1991;92:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu C, Duan W, Li R, et al. Exposure to bisphenol A disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity. Cell Death Dis. 2013;4:e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. [DOI] [PubMed] [Google Scholar]
- 32. vom Saal FS, Akingbemi BT, Belcher SM, et al. Chapel Hill Bisphenol A Expert Panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol (Elmsford, NY). 2007;24:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Henley DV, Korach KS. Physiological effects and mechanisms of action of endocrine disrupting chemicals that alter estrogen signaling. Hormones. 2010;9:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rezg R, El-Fazaa S, Gharbi N, Mornagui B. Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environ Int. 2014;64:83–90. [DOI] [PubMed] [Google Scholar]
- 36. Kato S, Yokoyama A, Fujiki R. Nuclear receptor coregulators merge transcriptional coregulation with epigenetic regulation. Trends Biochem Sci. 2011;36:272–281. [DOI] [PubMed] [Google Scholar]
- 37. Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010;1:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]