Abstract
Q fever is a worldwide zoonosis historically associated with exposure to infected livestock. This study summarizes cases of Q fever, a notifiable disease in the United States, reported to the Centers for Disease Control and Prevention through two national surveillance systems with onset during 2000–2012. The overall incidence rate during this time was 0.38 cases per million persons per year. The reported case fatality rate was 2.0%, and the reported hospitalization rate was 62%. Most cases (61%) did not report exposure to cattle, goats, or sheep, suggesting that clinicians should consider Q fever even in the absence of livestock exposure. The prevalence of drinking raw milk among reported cases of Q fever (8.4%) was more than twice the national prevalence for the practice. Passive surveillance systems for Q fever are likely impacted by underreporting and underdiagnosis because of the nonspecific presentation of Q fever.
Introduction
Coxiella burnetii is the etiologic agent of Q fever, a zoonotic illness historically associated with exposure to infected livestock, particularly sheep, cattle, and goats. The organism is highly infectious and shed in large numbers in milk and during parturition.1 It is resistant to desiccation, heat, and disinfection; and, it is capable of being transferred long distances by wind, which complicates diagnosis and control, and that causes concern that C. burnetii could be used as a tool for bioterrorism.2–9 Inhalation of infectious organisms is widely recognized as the most prevalent route of exposure; transmission by ingestion of unpasteurized milk, tick bite, sexual contact, and transfusion of infected blood products have rarely been implicated in rare human infections.10–14 Large outbreaks associated with inhalational exposure have occurred in slaughterhouses, auction yards, dairies, military units, laboratories, and households.15–21
In humans in the United States, acute Q fever most commonly presents as a flu-like illness, more rarely as hepatitis or pneumonia; asymptomatic infections have also been documented.22,23 In addition to the acute illness, Q fever can cause chronic infection. Chronic infection occurs rarely (< 5%) and may present months to years after an acute infection. Chronic Q fever typically manifests as blood-culture negative endocarditis or infection of a vascular aneurysm or vascular prosthesis.22,24–26 Doxycycline is recommended for patients with acute Q fever to shorten the duration of symptoms, but the recommended treatment of chronic Q fever is a long-term (≥ 18 months) combination of doxycycline and hydroxychloroquine.27,28
Q fever was added to the list of nationally notifiable infectious diseases in 1999 for the United States.29 The original case definition was updated in 2008, and the single reporting category of Q fever was divided into acute Q fever and chronic Q fever.30 Here, we summarize all cases of Q fever reported with onset during 2000–2007 and all cases reported under the newer case definition with onset during 2008–2012 to two national surveillance systems. Previously, we summarized reports from a single national surveillance system from 1978 to 2004; and, this past report overlaps with our report for 2000–2004.31
Methods
Case definition 2000–2007.29
“Acute Infection: A febrile illness usually accompanied by rigors, myalgia, malaise, and retrobulbar headache. Severe disease can include acute hepatitis, pneumonia, and meningoencephalitis. Clinical laboratory findings may include elevated liver enzyme levels and abnormal chest film findings. Asymptomatic infections may also occur.
Chronic Infection: Potentially fatal endocarditis may evolve months to years after acute infection, particularly in persons with underlying valvular disease. A chronic fatigue-like syndrome has been reported in some Q fever patients.”
Confirmed cases are clinically compatible or epidemiologically linked cases with a documented seroconversion, isolation of C. burnetii by culture, demonstration of C. burnetii by immunohistochemistry (IHC), or detection of nucleic acids by polymerase chain reaction (PCR). Probable cases are clinically compatible or epidemiologically linked cases with only a single, positive serological result (as defined by the testing laboratory).
Case definition 2008–2012.30
“Acute Q fever: Acute fever and one or more of the following: rigors, severe retrobulbar headache, acute hepatitis, pneumonia, or elevated liver enzyme levels.
Chronic Q fever: Newly recognized, culture-negative endocarditis, particularly in a patient with previous valvulopathy or compromised immune system, suspected infection of a vascular aneurysm or vascular prosthesis, or chronic hepatitis, osteomyelitis, osteoarthritis, or pneumonitis in the absence of other known etiology.”
Confirmed laboratory evidence for acute Q fever was changed from the 2000 to 2007 case definition: documenting a seroconversion is limited to indirect immunofluorescent assay (IFA) phase II immunoglobin G (IgG) titers. Supportive laboratory evidence of acute Q fever requires an IFA phase II IgG titer of at least 1:128 or a positive enzyme-linked immunosorbent assay (ELISA), dot-ELISA, or latex agglutination. Laboratory confirmation of a chronic Q fever case requires a single IFA phase I IgG titer of at least 1:800, a PCR positive result, isolation by culture, or demonstration by IHC. Supportive laboratory evidence of chronic Q fever requires a single IFA phase I IgG titer of at least 1:128 but < 1:800. To meet the case definition a case must fall into one of four categories:
A confirmed acute Q fever case must have laboratory-confirmed evidence and either must be clinically compatible or epidemiologically linked to a laboratory-confirmed case.
A probable acute Q fever case must have laboratory supportive evidence and must be clinically compatible.
A confirmed chronic Q fever case must have laboratory-confirmed evidence and be clinically compatible.
A probable chronic Q fever case must have laboratory supportive evidence and be clinically compatible.
Surveillance systems.
State and local public health departments report cases of Q fever to the Centers for Disease Control and Prevention (CDC) through the National Notifiable Disease Surveillance System (NNDSS). These data include reporting category, place of residence, date of onset, and demographics: sex, age, race, and ethnicity. Although California only reported confirmed and probable cases, whether a case was classified as confirmed or probable was not always transmitted to NNDSS by California. Therefore, some cases of Q fever reported through NNDSS have an unknown case classification and are not included in the number of probable or confirmed reports.
In addition to the data collected by NNDSS, state and local public health departments also report epidemiological data, clinical data, and laboratory data about cases by paper case report forms (CRFs). Additional data collected by CRFs include patient occupation, patient pregnancy status, history of animal contact, exposure to parturient animals and unpasteurized milk, clinical signs and syndromes, pre-existing medical conditions, whether hospitalized, whether the case survived or died, and results of serologic and other diagnostic testing.
Data analysis.
Hypothesis testing was performed at a significance level of 0.05. The exact binomial test was used to test equality of binomial proportions. The exact χ2 test was used to test equality of multinomial proportions. Fisher's exact test was used to assess independence of the row and column effects in contingency tables. The Cochran-Armitage test was used to assess trends in contingency tables. The Cochran-Armitage tests the null hypothesis of independence of row and column effects, similar to Fisher's exact test, in tables with two rows and multiple columns. However, the Cochran-Armitage test for trend uses a two-sided alternative hypothesis of increasing or decreasing column proportions with increasing column rank. To reduce computation time, estimated P values from Monte-Carlo simulations were used for Fisher's exact test with contingency tables larger than 2 × 2, for the exact χ2 test, and the Cochran-Armitage test for trend. Calculation of reported incidence rates (IRs) used the United States Census Bureau population estimates from 2000 to 2012.32,33 Because Q fever was not notifiable in some states during certain years, those populations were not considered at risk for calculating IR: Alaska (2000, 2005–2006), Arkansas (2000–2001), District of Columbia (2011–2012), Delaware (2000–2001), Illinois (2000), Indiana (2001), Iowa (2000–2002, 2005–2012), Louisiana (2005), Maryland (2000–2001), Massachusetts (2000–2001), Mississippi (2000–2001), New Hampshire (2007–2012), New York (2000), Oklahoma (2000, 2002–2003, 2007–2008), Ohio (2000–2001), Pennsylvania (2000–2001, 2003–2004), Rhode Island (2000–2001), Texas (2000–2001), Vermont (2000–2002, 2004–2012), Virginia (2000–2001), and West Virginia (2002–2005). Reported IRs were calculated as the number of Q fever cases per million persons per year (MPY). Because of the large proportion of missing data for race and ethnicity, IR were not calculated or compared for these demographics. All analyses were performed using SAS 9.3.34 Reported occupations were classified as agricultural occupations according to the U.S. Bureau of Labor Statistics'(BLS) Standard Occupational Classification system.35
Results
NNDSS.
A total of 1,366 confirmed and probable cases of Q fever were reported through NNDSS with year of onset from 2000 to 2012, and the overall reported IR was 0.38 cases per MPY. Broken down by reporting category, 732 cases of Q fever were reported from 2000 to 2007 (IR = 0.35). From 2008 to 2012, 635 cases of Q fever were reported (IR = 0.42), including 512 cases of acute Q fever (IR = 0.35) and 110 cases of chronic Q fever (IR = 0.07). The reported annual incidence rate of Q fever increased during 2000–2007 (P < 0.0001, Figure 1). However, the combined annual incidence rate of acute and chronic Q fever did not change during 2000–2008 (P = 0.13, Figure 1).
Figure 1.
Reported incidence rate of Q fever cases per million persons per year. Cases were reported to the National Notifiable Disease Surveillance System, and the population at risk was calculated from the Census Bureau population estimates.32,33 Cases were reported as Q fever from 2000 to 2007 and as acute or chronic Q fever for 2008–2012.
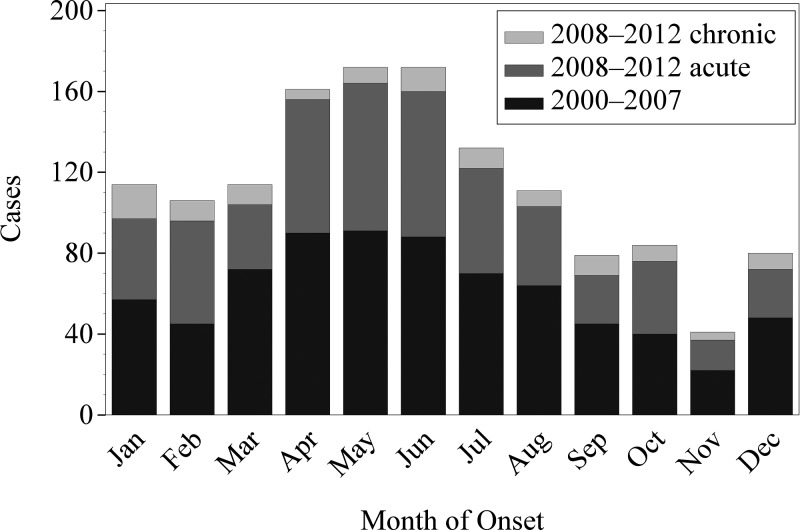
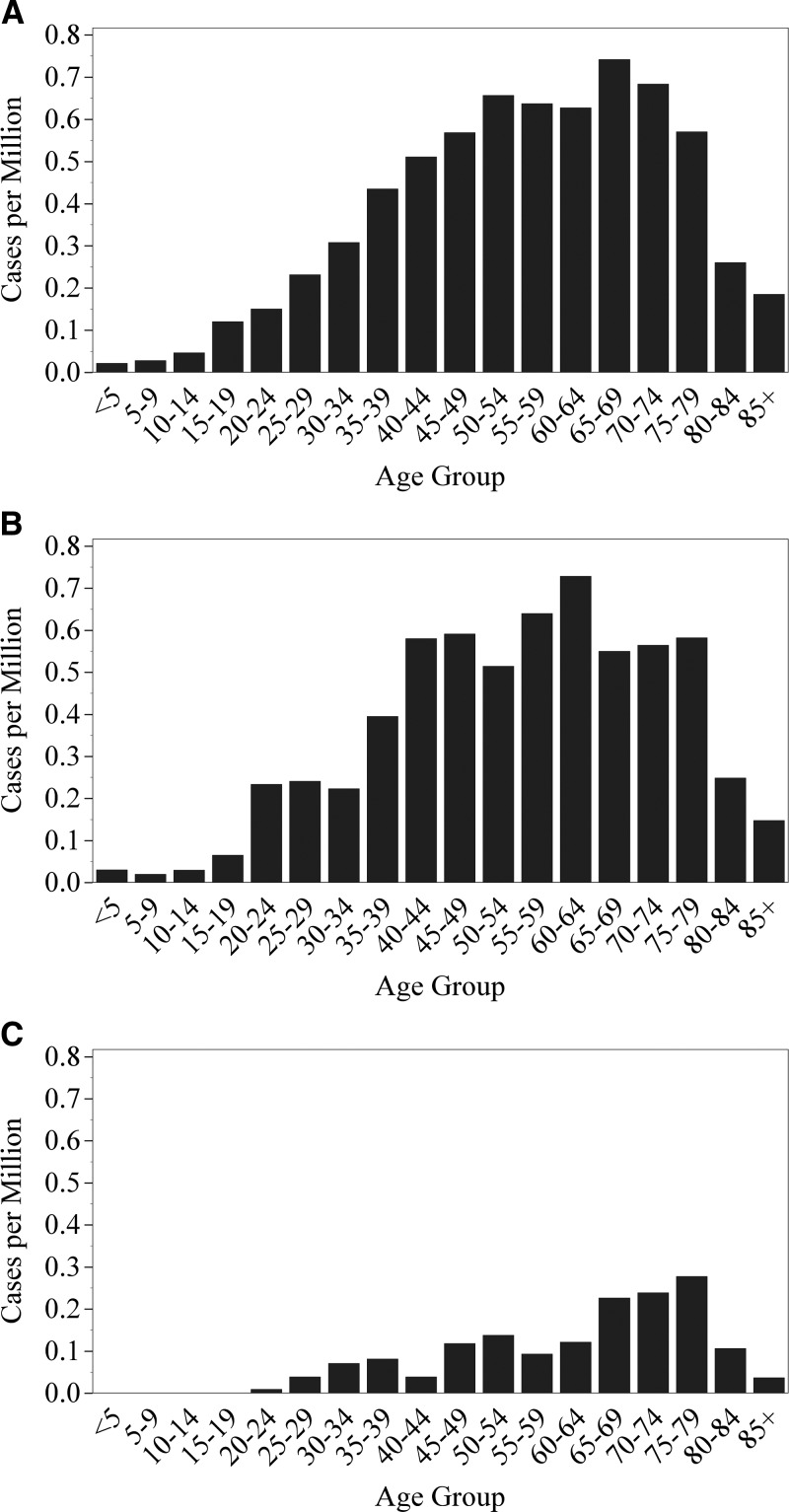
Across the 2000–2012 reporting period, the incidence of Q fever varied significantly by month (P < 0.0001) and peaked in May and June (Figure 2 ). When chronic Q fever cases were examined separately, however, the monthly incidence during 2008–2012 was not significantly different (P = 0.25, Figure 2). The IR of Q fever from 2000 to 2012 varied by U.S. census division, which are nine groups of geographically related states (P < 0.0001). The highest reported IR was from the Mountain division (IR = 0.88, 235 cases) and the West North Central division (IR = 0.72, 163 cases) (Table 1). The IR increased with age group to a maximum of 0.63 cases per MPY among 60–64 year olds, and then decreased with age group (P < 0.001, Figure 3 ).
Figure 2.
Frequency of reported cases of Q fever versus month of onset of symptoms. Cases were reported to the National Notifiable Disease Surveillance System. Cases were reported as Q fever from 2000 to 2007 and as acute or chronic Q fever for 2008–2012.
Table 1.
Division and state incidence rates (IR) of Q fever in cases per million persons per year, as reported through the Nationally Notifiable Disease Surveillance System, 2000–2012*
| Division state | Q fever, 2000–2007 IR (N) | Acute Q fever, 2008–2012 IR (N) | Chronic Q fever, 2008–2012 IR (N) | Q fever, 2000–2012 IR (N) |
|---|---|---|---|---|
| New England | 0.34 (31) | 0.05 (3) | 0.02 (1) | 0.23 (35) |
| Connecticut | 0.04 (1) | 0.02 (1) | ||
| Maine | 1.53 (16) | 0.15 (1) | 0.15 (1) | 1.05 (18) |
| Massachusetts | 0.36 (14) | 0.06 (2) | 0.22 (16) | |
| New Hampshire | ||||
| Rhode Island | ||||
| Vermont | ||||
| Mid Atlantic | 0.11 (28) | 0.27 (55) | 0.09 (19) | 0.22 (102) |
| New Jersey | 0.09 (6) | 0.41 (18) | 0.07 (3) | 0.24 (27) |
| New York | 0.10 (13) | 0.23 (22) | 0.14 (14) | 0.21 (49) |
| Pennsylvania | 0.18 (9) | 0.24 (15) | 0.03 (2) | 0.23 (26) |
| W.N. Central | 0.67 (94) | 0.60 (52) | 0.19 (17) | 0.72 (163) |
| Iowa | ||||
| Kansas | 0.41 (9) | 0.63 (9) | 0.07 (1) | 0.53 (19) |
| Minnesota | 0.35 (14) | 0.30 (8) | 0.33 (22) | |
| Missouri | 0.96 (44) | 0.40 (12) | 0.10 (3) | 0.78 (59) |
| Nebraska | 1.36 (19) | 0.88 (8) | 1.09 (10) | 1.60 (37) |
| North Dakota | 0.39 (2) | 0.30 (1) | 0.35 (3) | |
| South Dakota | 0.97 (6) | 3.68 (15) | 0.49 (2) | 2.25 (23) |
| E.N. Central | 0.34 (109) | 0.31 (73) | 0.05 (12) | 0.35 (194) |
| Illinois | 0.57 (50) | 0.19 (12) | 0.03 (2) | 0.42 (64) |
| Indiana | 0.18 (8) | 0.12 (4) | 0.03 (1) | 0.17 (13) |
| Michigan | 0.15 (12) | 0.34 (17) | 0.08 (4) | 0.25 (33) |
| Ohio | 0.33 (23) | 0.10 (6) | 0.03 (2) | 0.25 (31) |
| Wisconsin | 0.36 (16) | 1.20 (34) | 0.11 (3) | 0.73 (53) |
| S. Atlantic | 0.21 (83) | 0.17 (52) | 0.02 (6) | 0.20 (141) |
| Delaware | 0.22 (1) | 0.13 (1) | ||
| District of Columbia | 0.66 (3) | 1.69 (3) | 0.95 (6) | |
| Florida | 0.16 (22) | 0.10 (9) | 0.13 (31) | |
| Georgia | 0.10 (7) | 0.25 (12) | 0.16 (19) | |
| Maryland | 0.39 (13) | 0.14 (4) | 0.27 (17) | |
| North Carolina | 0.32 (22) | 0.38 (18) | 0.34 (40) | |
| South Carolina | 0.12 (4) | 0.04 (1) | 0.04 (1) | 0.11 (6) |
| Virginia | 0.24 (11) | 0.07 (3) | 0.10 (4) | 0.21 (18) |
| West Virginia | 0.14 (1) | 0.11 (1) | 0.11 (1) | 0.18 (3) |
| E.S. Central | 0.52 (70) | 0.10 (9) | 0.07 (6) | 0.38 (85) |
| Alabama | 0.13 (3) | 0.04 (1) | 0.07 (4) | |
| Kentucky | 1.03 (34) | 0.09 (2) | 0.23 (5) | 0.75 (41) |
| Mississippi | 0.17 (3) | 0.09 (3) | ||
| Tennessee | 0.70 (33) | 0.13 (4) | 0.47 (37) | |
| W.S. Central | 0.29 (57) | 0.45 (80) | 0.12 (22) | 0.42 (159) |
| Arkansas | 0.18 (3) | 0.96 (14) | 0.54 (17) | |
| Louisiana | 0.16 (5) | 0.09 (5) | ||
| Oklahoma | 0.28 (4) | 0.27 (4) | 0.20 (3) | 0.38 (11) |
| Texas | ||||
| Mountain | 0.89 (140) | 0.69 (76) | 0.17 (19) | 0.88 (235) |
| Arizona | 0.29 (13) | 0.37 (12) | 0.12 (4) | 0.38 (29) |
| Colorado | 1.78 (65) | 1.03 (26) | 0.20 (5) | 1.56 (96) |
| Idaho | 0.54 (6) | 0.13 (1) | 0.13 (1) | 0.42 (8) |
| Montana | 3.43 (17) | 0.61 (3) | 1.62 (20) | |
| Nevada | 1.25 (23) | 0.44 (6) | 0.91 (29) | |
| New Mexico | 1.38 (21) | 1.07 (11) | 0.10 (1) | 1.30 (33) |
| Utah | 0.05 (1) | 0.14 (2) | 0.29 (4) | 0.21 (7) |
| Wyoming | 2.70 (11) | 0.36 (1) | 0.36 (1) | 1.89 (13) |
| Pacific | 0.32 (120) | 0.50 (124) | 0.03 (8) | 0.40 (252) |
| Alaska | 0.28 (1) | 0.15 (1) | ||
| California | 0.37 (105) | 0.54 (100) | 0.01 (2) | 0.44 (207) |
| Hawaii | 0.59 (4) | 0.24 (4) | ||
| Oregon | 0.42 (12) | 0.47 (9) | 0.05 (1) | 0.46 (22) |
| Washington | 0.06 (3) | 0.30 (10) | 0.15 (5) | 0.22 (18) |
Figure 3.
Reported incidence rate of Q fever per million persons per year by age group. (A) Q fever, 2000–2007. (B) Acute Q Fever, 2008–2012. (C) Chronic Q fever, 2008–2012. Cases were reported to the National Notifiable Disease Surveillance System and the population at risk was calculated from the Census Bureau population estimates.32,33
The male-to-female ratio was 3.0:1 for cases reported during 2000–2007 (P < 0.0001). During 2008–2012, the male-to-female ratio was 2.9:1 for acute Q fever (P < 0.0001), and 3.7:1 for chronic Q fever (P < 0.0001, Table 2). During 2000–2012, the majority of cases (63%) were reported as white race, and 32% of cases were reported with unknown race (Table 2). Most cases (56%) were reported as non-Hispanic ethnicity, and 32% of cases were reported with unknown ethnicity (Table 2). Three hundred and thirteen cases (23%) were reported with neither race nor ethnicity.
Table 2.
Age, ethnicity, race, sex, and case definition of cases of Q fever as reported through case report forms (CRFs) and through the Nationally Notifiable Disease Surveillance System (NNDSS), by reporting category, 2000–2012
| Characteristic | CRFs | NNDSS | ||||
|---|---|---|---|---|---|---|
| Q fever, 2000–2007 | Acute Q fever, 2008–2012 | Chronic Q fever, 2008–2012 | Q fever, 2000–2007 | Acute Q fever, 2008–2012 | Chronic Q fever, 2008–2012 | |
| Age | ||||||
| < 4 | 1 (0.6%) | 1 (0.5%) | 3 (0.4%) | 3 (0.6%) | ||
| 5–9 | 1 (0.6%) | 4 (0.5%) | 2 (0.4%) | |||
| 10–19 | 4 (2.4%) | 7 (3.4%) | 1 (1%) | 25 (3.4%) | 10 (1.9%) | |
| 20–29 | 20 (11.8%) | 11 (5.4%) | 8 (8%) | 54 (7.4%) | 50 (9.5%) | 5 (4.5%) |
| 30–39 | 17 (10.1%) | 30 (14.6%) | 12 (12%) | 110 (15%) | 61 (11.6%) | 15 (13.6%) |
| 40–49 | 41 (24.3%) | 46 (22.4%) | 16 (16%) | 170 (23.2%) | 125 (23.9%) | 17 (15.5%) |
| 50–59 | 32 (18.9%) | 45 (22%) | 19 (19%) | 165 (22.5%) | 118 (22.5%) | 24 (21.8%) |
| 60–69 | 21 (12.4%) | 29 (14.1%) | 22 (22%) | 109 (14.9%) | 94 (17.9%) | 24 (21.8%) |
| 70+ | 27 (16%) | 16 (7.8%) | 15 (15%) | 88 (12%) | 58 (11.1%) | 25 (22.7%) |
| Unknown | 5 (3%) | 20 (9.8%) | 7 (7%) | 4 (0.5%) | 3 (0.6%) | |
| Ethnicity | ||||||
| Hispanic | 8 (4.7%) | 36 (17.6%) | 16 (16%) | 69 (9.4%) | 78 (14.9%) | 5 (4.5%) |
| Not Hispanic | 118 (69.8%) | 122 (59.5%) | 63 (63%) | 412 (56.3%) | 294 (56.1%) | 65 (59.1%) |
| Unknown | 43 (25.4%) | 47 (22.9%) | 21 (21%) | 251 (34.3%) | 152 (29%) | 40 (36.4%) |
| Race | ||||||
| American Indian | 1 (0.6%) | 4 (2%) | 2 (2%) | 6 (0.8%) | 6 (1.1%) | 2 (1.8%) |
| Asian | 2 (1.2%) | 3 (1.5%) | 1 (1%) | |||
| Black | 13 (7.7%) | 7 (3.4%) | 6 (6%) | 35 (4.8%) | 16 (3.1%) | 1 (0.9%) |
| White | 128 (75.7%) | 158 (77.1%) | 71 (71%) | 459 (62.7%) | 336 (64.1%) | 70 (63.6%) |
| Unknown | 25 (14.8%) | 33 (16.1%) | 20 (20%) | 232 (31.7%) | 166 (31.7%) | 37 (33.6%) |
| Sex | ||||||
| Female | 45 (26.6%) | 49 (23.9%) | 19 (19%) | 184 (25.1%) | 132 (25.2%) | 23 (20.9%) |
| Male | 121 (71.6%) | 154 (75.1%) | 81 (81%) | 545 (74.5%) | 384 (73.3%) | 86 (78.2%) |
| Unknown | 3 (1.8%) | 2 (1%) | 3 (0.4%) | 8 (1.5%) | 1 (0.9%) | |
| Classification | ||||||
| Confirmed | 41 (24.3%) | 52 (25.4%) | 48 (48%) | 290 (39.6%) | 153 (29.2%) | 64 (58.2%) |
| Probable | 128 (75.7%) | 153 (74.6%) | 52 (52%) | 337 (46%) | 305 (58.2%) | 44 (40%) |
| Unknown | 105 (14.3%) | 66 (12.6%) | 2 (1.8%) | |||
CRFs.
A total of 709 reports of Q fever were submitted to the CDC by CRF with onset during 2000–2012, and 474 of these reports (67%) were unique cases—not duplicates of another report—that met the Council of State and Territorial Epidemiologists (CSTE) case definition. The most frequently reported symptoms among the 474 cases included fever (95%), malaise (70%), headache (60%), myalgia (50%), and cough (37%), although symptoms varied by acute versus chronic case status (Table 3). Of the 440 cases reported during 2000–2012 with information on hospitalization, 271 cases reported being hospitalized, yielding a reported hospitalization rate (HR) of 62%. The HR was not the same for Q fever during 2000–2007, acute Q fever from 2008 to 2012, and chronic Q fever during 2008–2012 (P = 0.0001); the HR for chronic Q fever cases during 2008–2012 was 80%. During 2000–2012, the HR increased with age (P = 0.0003). There was no association with HR and reported race category (P = 0.18). However, the HR of blacks, Asians, and American Indians grouped together (78%) was higher than the HR of whites alone (59%, P = 0.04). The HR among Hispanics (76%) was greater than the HR among non-Hispanics (57%, P = 0.01). The HR was not significantly different by gender (P = 0.16).
Table 3.
Frequency of symptoms among cases of Q fever as reported through case report forms, by reporting category, 2000–2012*
| Symptom | Q fever, 2000–2007 | Acute Q fever, 2008–2012 | Chronic Q fever, 2008–2012 | Q fever, 2000–2012 |
|---|---|---|---|---|
| Fever | 154 (91.1%) | 205 (100%) | 70 (70%) | 429 (94.9%) |
| Malaise | 120 (71%) | 142 (69.3%) | 55 (55%) | 317 (70.1%) |
| Headache | 90 (53.3%) | 147 (71.7%) | 36 (36%) | 273 (60.4%) |
| Myalgia | 83 (49.1%) | 105 (51.2%) | 39 (39%) | 227 (50.2%) |
| Cough | 52 (30.8%) | 75 (36.6%) | 38 (38%) | 165 (36.5%) |
| Chills | 26 (15.4%) | 72 (35.1%) | 14 (14%) | 112 (24.8%) |
| Pneumonia | 25 (14.8%) | 25 (12.2%) | 38 (38%) | 88 (19.5%) |
| Endocarditis | 34 (20.1%) | 5 (2.4%) | 35 (35%) | 74 (16.4%) |
| Sweats | 18 (10.7%) | 46 (22.4%) | 10 (10%) | 74 (16.4%) |
| Retro orbital pain | 11 (6.5%) | 41 (20%) | 14 (14%) | 66 (14.6%) |
| Rash | 26 (15.4%) | 22 (10.7%) | 11 (11%) | 59 (13.1%) |
| Hepatitis | 15 (8.9%) | 18 (8.8%) | 24 (24%) | 57 (12.6%) |
| Anorexia | 18 (10.7%) | 25 (12.2%) | 11 (11%) | 54 (11.9%) |
| Fatigue | 3 (1.8%) | 23 (11.2%) | 10 (10%) | 36 (8%) |
| Nausea | 5 (3%) | 18 (8.8%) | 7 (7%) | 30 (6.6%) |
| Hepatomegaly | 8 (4.7%) | 15 (7.3%) | 4 (4%) | 27 (6%) |
| Weakness | 10 (5.9%) | 14 (6.8%) | 2 (2%) | 26 (5.8%) |
| Vomiting | 3 (1.8%) | 13 (6.3%) | 6 (6%) | 22 (4.9%) |
| Splenomegaly | 9 (5.3%) | 8 (3.9%) | 4 (4%) | 21 (4.6%) |
| Arthralgia | 8 (4.7%) | 9 (4.4%) | 3 (3%) | 20 (4.4%) |
| Diarrhea | 1 (0.6%) | 12 (5.9%) | 6 (6%) | 19 (4.2%) |
| Elevated liver enzymes | 4 (2.4%) | 14 (6.8%) | 18 (4%) | |
| Shortness of breath | 1 (0.6%) | 9 (4.4%) | 5 (5%) | 15 (3.3%) |
| Abdominal pain | 4 (2.4%) | 4 (2%) | 5 (5%) | 13 (2.9%) |
| Altered mental status | 7 (3.4%) | 4 (4%) | 11 (2.4%) |
Only categories with at least 11 reports are presented here.
A total of nine fatal Q fever cases were reported among 428 cases with known outcome, yielding a case fatality rate (CFR) of 2.10%. An additional five fatal reports did not meet the CSTE case definition: four reports were not clinically compatible and a fifth report was submitted without sufficient laboratory evidence. Fatal cases were reported with endocarditis, pneumonia, or encephalitis (Table 4). During 2008–2012, the CFR for acute Q fever (0.5%) was different than the CFR of chronic Q fever (4.5%, P = 0.03). The reported CFR was similar among males and females (P = 0.24). The CFR increased with age group (P = 0.047), and fatal cases were not reported among those younger than 40. The CFR was not different among race groups (P = 0.23). There were no fatal cases reported among Asians or American Indians. The difference in the CFR among blacks (8.0%) compared with the CFR among whites was not statistically significant (1.8%, P = 0.10). The CFR among Hispanics (1.8%) was not significantly different than among non-Hispanics (2.4%, P = 1).
Table 4.
Demographics, whether hospitalized, and clinical syndrome for the nine fatal cases of Q fever reported through case report forms during 2000–2012
| Age | Sex | Hospitalized | Endocarditis | Pneumonia | Hepatitis | Encephalitis |
|---|---|---|---|---|---|---|
| 40–49 | Male | + | + | |||
| 60–69 | Female | + | + | |||
| 70+ | Female | + | + | |||
| 70+ | Female | + | ||||
| 49–49 | Male | + | + | |||
| 50–59 | Male | + | + | |||
| 70+ | Male | + | + | |||
| 50–59 | Female | + | + | + | ||
| 50–59 | Male | + | + |
No cases of Q fever were reported among pregnant woman during 2000–2012. Pre-existing medical conditions were reported for 167 cases (35%) during the overall study period. During 2000–2007, 24 cases (14%) reported valvular heart disease or vascular graft. During 2008–2012, 4 acute cases (2.0%) reported valvular heart disease or vascular graft, and 30 chronic cases (30%) reported valvular heart disease or vascular graft.
Among all reports, the most common occupation reported for 2000–2012 was rancher (17%), followed by working in the military (8%) (Table 5). Of the 292 reports listing an occupation, 106 reports (36%) were categorized as agricultural workers as defined by the BLS. Of the 38 reported cases in the military, 36 cases (95%) reported travel to a foreign country: 32 to Iraq (84%), 2 to Afghanistan (5%), 1 to Israel (3%), 1 to Kuwait (3%), and 1 to Germany (3%). In contrast, only 9.2% of cases not in the military reported travel to a foreign country (P < 0.0001). The proportion of cases reporting hepatitis as a clinical syndrome was higher for those in the military (29%) than among those not in the military (11%, P = 0.003); whereas, the proportion of cases reporting pneumonia was not significantly different between those in the military (21%) and those not in the military (18%, P = 0.67).
Table 5.
Occupation and occupational setting of cases of Q fever as reported through case report forms, by reporting category, 2000–2012*
| Occupation or occupational setting | Q fever, 2000–2007 | Acute Q fever, 2008–2012 | Chronic Q fever, 2008–2012 | Q fever, 2000–2012 |
|---|---|---|---|---|
| Unknown | 39 (23.1%) | 74 (36.1%) | 40 (40%) | 153 (33.8%) |
| Rancher | 32 (18.9%) | 39 (19%) | 9 (9%) | 80 (17.7%) |
| Military | 15 (8.9%) | 17 (8.3%) | 6 (6%) | 38 (8.4%) |
| Retired | 10 (5.9%) | 15 (7.3%) | 13 (13%) | 38 (8.4%) |
| Farm | 7 (4.1%) | 11 (5.4%) | 3 (3%) | 21 (4.6%) |
| Construction | 5 (3%) | 7 (3.4%) | 5 (5%) | 17 (3.8%) |
| Dairy | 2 (1.2%) | 10 (4.9%) | 3 (3%) | 15 (3.3%) |
| Office | 8 (4.7%) | 3 (1.5%) | 2 (2%) | 13 (2.9%) |
| Unemployed | 6 (3.6%) | 3 (1.5%) | 3 (3%) | 12 (2.7%) |
| Disabled | 1 (0.6%) | 5 (2.4%) | 4 (4%) | 10 (2.2%) |
| Driver | 1 (0.6%) | 4 (2%) | 3 (3%) | 8 (1.8%) |
| Student | 4 (2.4%) | 2 (1%) | 1 (1%) | 7 (1.5%) |
| Education | 4 (2.4%) | 1 (0.5%) | 5 (1.1%) | |
| Retail | 3 (1.8%) | 2 (1%) | 5 (1.1%) | |
| Veterinarian | 2 (1.2%) | 3 (1.5%) | 5 (1.1%) | |
| Custodian | 3 (1.8%) | 1 (1%) | 4 (0.9%) | |
| Laboratorian | 2 (1.2%) | 2 (2%) | 4 (0.9%) | |
| Nurse | 2 (1.2%) | 1 (0.5%) | 1 (1%) | 4 (0.9%) |
| Slaughterhouse | 3 (1.5%) | 1 (1%) | 4 (0.9%) |
Only categories with at least four reports are presented here.
A total of 314 reported cases (66%) noted exposure to any animal, and 185 reported cases (39%) had exposure to cattle, goats, or sheep. The most commonly reported animal contacts were cattle (25%), cats (22%), dogs (21%), goats (20%), and sheep (17%) during 2000–2012 (Table 6); 101 cases (22%) reported exposure to birthing animals. Thirty-eight cases (8.4%) reported exposure to unpasteurized milk, with 26 cases reporting the source: 16 reported exposure to cow's milk, 6 to goat's milk, and 4 were exposed to both cow and goat's milk. The reported prevalence of drinking raw milk among those reporting work in a dairy (53%) was significantly greater than the prevalence among those not reporting work in a dairy (6.5%, P < 0.0001). The prevalence of drinking raw milk was significantly greater among those reporting exposure to cattle (15%) than those not (5.8%, P = 0.002). Similarly, the reported prevalence of drinking raw milk among those working in agriculture (15%) was greater than those not (6%, P = 0.003). Among reported cases with exposure to unpasteurized milk, 4 cases (11%) reported no other risk factors for Q fever.
Table 6.
Animal contact among cases of Q fever reported through case report forms by reporting category, 2000–2012*
| Animal | Q fever, 2000–2007 | Acute Q fever, 2008–2012 | Chronic Q fever, 2008–2012 | Q fever, 2000–2012 |
|---|---|---|---|---|
| Cattle | 33 (19.5%) | 61 (29.8%) | 19 (19%) | 113 (25.0%) |
| Cats | 39 (23.1%) | 45 (22%) | 17 (17%) | 101 (22.3%) |
| Dogs | 30 (17.8%) | 45 (22%) | 20 (20%) | 95 (21.0%) |
| Goats | 28 (16.6%) | 50 (24.4%) | 14 (14%) | 92 (20.4%) |
| Sheep | 18 (10.7%) | 42 (20.5%) | 17 (17%) | 77 (17.0%) |
| Equine | 14 (8.3%) | 24 (11.7%) | 4 (4%) | 42 (9.3%) |
| Birds | 12 (7.1%) | 14 (6.8%) | 3 (3%) | 29 (6.4%) |
| Rabbits | 10 (5.9%) | 10 (4.9%) | 4 (4%) | 24 (5.3%) |
| Rodents | 5 (3%) | 7 (3.4%) | 2 (2%) | 14 (3.1%) |
| Pigs | 3 (1.8%) | 7 (3.4%) | 1 (1%) | 11 (2.4%) |
| Ticks | 4 (2.4%) | 5 (2.4%) | 1 (1%) | 10 (2.2%) |
| Deer | 4 (2.4%) | 2 (1%) | 3 (3%) | 9 (2.0%) |
Only categories with at least four reports are presented here.
Because the clinical, epidemiological, and laboratory data required to apply the case definition are reported through the CRFs, whether a report met the case definition as confirmed or probable was known for all reports. During 2000–2007, 128 cases (76%) reported through CRF met the probable case definition, and 41 cases (24%) met the confirmed case definition. Among confirmed cases reported during this time period, a seroconversion was documented in 39 cases (95%); 3 cases (7.3%) were PCR positive, 2 cases (4.9%) were positive by IHC, and C. burnetii was isolated by culture in 1 case (2.4%). During 2008–2012, 153 acute cases (75%) met the probable case definition. Fifty-two acute cases (25%) met the confirmed case definition: a seroconversion was documented in 51 acute cases (98%), and 1 acute case (2%) was PCR positive. Fifty-two chronic cases (52%) met the probable case definition, and 48 chronic cases (48%) were laboratory confirmed. Of the cases reported during 2000–2007, 3 cases (1.8%) did not meet the new case definition for either chronic or acute Q fever.
Discussion
The reported incidence of Q fever remains low in the United States compared with most nationally notifiable diseases, at 0.38 cases per MPY for 2000–2012. Historically, Q fever has been associated with occupational exposure to livestock, especially exposure to parturient animals.23 In this study, 36% of employed cases reported an occupation related to agriculture, and 22% of cases reported exposure to birthing animals. However, < 0.5% of employed people in the United States work in agriculture.36 The higher reported incidence rates in states from the Mountain division and the West North Central division may reflect increased exposure risk in these regions where livestock are more prevalent.37,38 The observed seasonality of reported Q fever cases (especially acute Q fever), which peaks during May and June (Figure 2), corresponds with expected calving, lambing, and kidding seasons in the United States.39–42 Although many cases of acute Q fever in the United States are likely attributed to direct animal exposure, windborne transmission of C. burnetii is also a likely mode of exposure, as one-third of cases reported no exposure to any animal. Coxiella burnetii is easily spread long distances by wind, and a 1–2 mile proximity to a livestock reservoir may be sufficient exposure for infection.3,4,8 The largest documented epidemic of Q fever was primarily caused by airborne transmission of C. burnetii from infected goat farms to more highly populated areas in the Netherlands from 2007 through 2010.43 A similar increase in incidence of Q fever during spring, attributed to windborne transmission following lambing season, has also been documented in France.4 Clinicians should consider Q fever when appropriate despite an absence of direct exposure to livestock, including cases of community acquired pneumonia and other flu-like illnesses, especially when a potential risk factor for chronic Q fever is present.
In our data, 8% of cases reported a military occupation, yet military personnel account for only about 1% of the total United States population.44 Eighty-four percent of the cases of Q fever among military personnel had traveled to Iraq; whereas, foreign travel was only 9% in other reported cases. In our data, military personnel were three times as likely as civilians to have reported hepatitis; yet, pneumonia was noted at similar proportions among military and civilian occupations. Differences in mode of transmission or variations in geographic strain virulence may be responsible for the higher rate of hepatitis observed among military service members.22,45,46
A higher proportion of reported cases were hospitalized among Hispanics than non-Hispanics. Furthermore, cases among blacks, Asians, and American Indians together were more likely to be hospitalized versus whites alone. This finding may represent an artifact of surveillance where cases of less serious disease among whites and non-Hispanics are more likely to receive laboratory diagnostics.
The prevalence of consuming raw milk among reported cases of Q fever from 2000 to 2012 was 8.4%, well above the national estimate of 3.0% for the prevalence of consuming raw milk.47 This suggests that consuming raw milk may be an important risk for Q fever in the United States, and four of the cases reported through CRFs had no other known risk factor for Q fever. Coxiella burnetii is frequently detected in dairy products by PCR testing, and consuming unpasteurized dairy products may increase the risk of Q fever.13,48–52 However, the strong association between drinking raw milk with working on a dairy, with working in agriculture, and with exposure to cattle indicates exposure to livestock and employment in an agricultural setting potentially confounds the association between raw milk consumption and Q fever in the general population.
These results are based upon passive surveillance at the national level, and the individual practices of state and local public health departments, laboratories, and clinics are diverse. Because infection with C. burnetii can be asymptomatic, mild, or easily mistaken for more common etiologies, reported cases of Q fever likely represent only a fraction of actual infections, and less severe cases and cases among people with inadequate access to healthcare are unlikely to be captured by passive reporting systems. Therefore, Q fever cases reported through passive surveillance often reflect a more severe clinical presentation, which is indicated by the high overall hospitalization rate of 62% and the 2.0% case fatality rate reported here. Seroprevalence of Q fever in the United States is estimated to be 3.1%.53 Not all seropositive people have a history of Q fever, some may simply have exposure not leading to infection or have asymptomatic infections. The seroprevalence dwarves the reported incidence rate in the United States, suggesting the possibility of underreporting through national surveillance. In our concurrent report, we estimate that for every case of Q fever reported through CRFs there are at least 13 cases that go unreported.54 The large proportion of missing data for race and ethnicity among reports from both surveillance systems precludes more meaningful comparison of race and ethnic groups. Similarly, the number of reported fatal cases is small, and the actual association between severe and fatal disease with sex, race, and ethnicity may be different than what has been reported. Despite these limitations, the data presented herein represent the most comprehensive summary for the trends in Q fever in the United States during 2000–2012.
ACKNOWLEDGMENTS
We thank everyone in the clinic, laboratory, and public health departments who diagnose and investigate cases of Q fever. The work here is possible because of their continued efforts. We also thank Naomi Drexler, John Krebs, Eric Mandel, and Lindsey Pool for their help in building the surveillance data used in this analysis.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Footnotes
Authors' addresses: F. Scott Dahlgren, Jennifer H. McQuiston, Robert F. Massung, and Alicia D. Anderson, Centers for Disease Control and Prevention, Rickettsial Zoonoses Branch, Atlanta, GA, E-mails: iot0@cdc.gov, fzh7@cdc.gov, rmassung@cdc.gov, and aha5@cdc.gov.
References
- 1.Roest HJ, van Gelderen B, Dinkla A, Frangoulidis D, van Zijderveld F, Rebel J, van Keulen L. Q Fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii. PLoS ONE. 2012;7:e48949. doi: 10.1371/journal.pone.0048949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott GH, Williams JC. Susceptibility of Coxiella burnetii to chemical disinfectants. Ann N Y Acad Sci. 1990;590:291–296. doi: 10.1111/j.1749-6632.1990.tb42235.x. [DOI] [PubMed] [Google Scholar]
- 3.Hawker JI, Ayres JG, Blair I, Evans MR, Smith DL, Smith EG, Burge PS, Carpenter MJ, Caul EO, Coupland B, Desselberger U, Farrell ID, Saunders PJ, Wood MJ. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187. [PubMed] [Google Scholar]
- 4.Tissot-Dupont H, Torres S, Nezri M, Raoult D. Hyperendemic focus of Q fever related to sheep and wind. Am J Epidemiol. 1999;150:67–74. doi: 10.1093/oxfordjournals.aje.a009920. [DOI] [PubMed] [Google Scholar]
- 5.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noah DL, Noah DL, Crowder HR. Biological terrorism against animals and humans: a brief review and primer for action. J Am Vet Med Assoc. 2002;221:40–43. doi: 10.2460/javma.2002.221.40. [DOI] [PubMed] [Google Scholar]
- 7.Madariaga MG, Rezai K, Trenholme GM, Weinstein RA. Q fever: a biological weapon in your backyard. Lancet Infect Dis. 2003;3:709–721. doi: 10.1016/s1473-3099(03)00804-1. [DOI] [PubMed] [Google Scholar]
- 8.Tissot-Dupont H, Amadei MA, Nezri M, Raoult D. Wind in November, Q fever in December. Emerg Infect Dis. 2004;10:1264–1269. doi: 10.3201/eid1007.030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyston PC, Davies C. Q fever: the neglected biothreat agent. J Med Microbiol. 2011;60:9–21. doi: 10.1099/jmm.0.024778-0. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous Q fever transmitted by blood transfusion—United States. Can Dis Wkly Rep. 1977;3:210. [Google Scholar]
- 11.Kruszewska D, Lembowicz K, Tylewska-Wierzbanowska S. Possible sexual transmission of Q fever among humans. Clin Infect Dis. 1996;22:1087–1088. doi: 10.1093/clinids/22.6.1087. [DOI] [PubMed] [Google Scholar]
- 12.Milazzo A, Hall R, Storm PA, Harris RJ, Winslow W, Marmion BP. Sexually transmitted Q fever. Clin Infect Dis. 2001;33:399–402. doi: 10.1086/321878. [DOI] [PubMed] [Google Scholar]
- 13.Signs KA, Stobierski MG, Gandhi TN. Q Fever cluster among raw milk drinkers in Michigan, 2011. Clin Infect Dis. 2012;55:1387–1389. doi: 10.1093/cid/cis690. [DOI] [PubMed] [Google Scholar]
- 14.Janbon F, Raoult D, Reynes J, Bertrand A. Concomitant human infection due to Rickettsia conorii and Coxiella burnetii. J Infect Dis. 1989;160:354–355. doi: 10.1093/infdis/160.2.354. [DOI] [PubMed] [Google Scholar]
- 15.Robbins FC, Gauld RL, Warner FB. Q fever in the Mediterranean area; report of its occurrence in allied troops; epidemiology. Am J Hyg. 1946;44:23–50. doi: 10.1093/oxfordjournals.aje.a119082. [DOI] [PubMed] [Google Scholar]
- 16.Spicknall CG, Huebner RJ, Finger JA, Blocker WP. Report on an outbreak of Q fever at the National Institute of Health; clinical features. Ann Intern Med. 1947;27:28–40. doi: 10.7326/0003-4819-27-1-28. [DOI] [PubMed] [Google Scholar]
- 17.Topping NH, Shepard CC, Irons JV. Q fever in the United States; epidemiologic studies of an outbreak among stock handlers and slaughterhouse workers. J Am Med Assoc. 1947;133:813–815. doi: 10.1001/jama.1947.02880120001001. [DOI] [PubMed] [Google Scholar]
- 18.Shepard CC. An outbreak of Q fever in a Chicago packing house. Am J Hyg. 1947;46:185–192. doi: 10.1093/oxfordjournals.aje.a119162. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control Q fever among slaughterhouse workers—California. MMWR Morb Mortal Wkly Rep. 1986;35:223–226. [PubMed] [Google Scholar]
- 20.Graham CJ, Yamauchi T, Rountree P. Q fever in animal laboratory workers: an outbreak and its investigation. Am J Infect Control. 1989;17:345–348. doi: 10.1016/0196-6553(89)90004-7. [DOI] [PubMed] [Google Scholar]
- 21.Faix DJ, Harrison DJ, Riddle MS, Vaughn AF, Yingst SL, Earhart K, Thibault G. Outbreak of Q fever among US military in western Iraq, June–July 2005. Clin Infect Dis. 2008;46:e65–e68. doi: 10.1086/528866. [DOI] [PubMed] [Google Scholar]
- 22.Raoult D, Tissot-Dupont H, Foucault C, Gouvernet J, Fournier PE, Bernit E, Stein A, Nesri M, Harle JR, Weiller PJ. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore) 2000;79:109–123. doi: 10.1097/00005792-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 23.McQuiston JH, Childs JE. Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis. 2002;2:179–191. doi: 10.1089/15303660260613747. [DOI] [PubMed] [Google Scholar]
- 24.Marmion BP. Subacute rickettsial endocarditis: an unusual complication of Q fever. J Hyg Epidemiol Microbiol Immunol. 1962;6:79–84. [PubMed] [Google Scholar]
- 25.Turck WP, Howitt G, Turnberg LA, Fox H, Longson M, Matthews MB, Das Gupta R. Chronic Q fever. Q J Med. 1976;45:193–217. [PubMed] [Google Scholar]
- 26.Fergusson RJ, Shaw TR, Kitchin AH, Matthews MB, Inglis JM, Peutherer JF. Subclinical chronic Q fever. Q J Med. 1985;57:669–676. [PubMed] [Google Scholar]
- 27.Raoult D, Marrie T. Q fever. Clin Infect Dis. 1995;20:489–495. doi: 10.1093/clinids/20.3.489. [DOI] [PubMed] [Google Scholar]
- 28.Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH, Nicholson WL, Paddock CD, Sexton DJ. Diagnosis and management of Q fever–United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62:1–30. [PubMed] [Google Scholar]
- 29.Council of State and Territorial Epidemiologists Placing Q Fever (Coxiella burnetii) Under National Surveillance in the United States Under the National Public Health Surveillance System (NPHSS). Position Statement 1999 [Google Scholar]
- 30.Council of State and Territorial Epidemiologists Revision of the Surveillance Case Definition for Q Fever. Position Statement 2007 [Google Scholar]
- 31.McQuiston JH, Holman RC, McCall CL, Childs JE, Swerdlow DL, Thompson HA. National surveillance and the epidemiology of human Q fever in the United States, 1978–2004. Am J Trop Hyg. 2006;75:36–40. doi: 10.4269/ajtmh.2006.75.1.0750036. [DOI] [PubMed] [Google Scholar]
- 32.United States Census Bureau Intercensal estimates of the resident population by single year of age and sex for states and the United States: April 1, 2000 to July 1, 2010 2011 [Google Scholar]
- 33.United States Census Bureau Annual state resident population estimates for 6 race groups by age, sex, and Hispanic origin: April 1, 2010 to July 1, 2012 2013 [Google Scholar]
- 34.SAS Institute . Cary, NC: SAS Institute; 2012. SAS System for Windows [computer program]. Version 9.3. [Google Scholar]
- 35.Bureau of Labor Statistics, United States Department of Labor 2010 Standard Occupational Classification Definitions 2013 [Google Scholar]
- 36.Bureau of Labor Statistics, United States Department of Labor Occupational Employment Statistics 2010 [Google Scholar]
- 37.United States Department of Agriculture . Cattle. Washington, DC: National Agricultural Statistics Service, Agricultural Statistics Board, United States Department of Agriculture; 2010. [Google Scholar]
- 38.United States Department of Agriculture . Sheep and Goats. Washington, DC: National Agricultural Statistics Service, Agriculture Statistics Board, United States Department of Agriculture; 2011. [Google Scholar]
- 39.United States Department of Agriculture . Beef 1997. In: USDA, editor. Part I: Reference of 1997 Beef Cow-Calf Management Practices. Fort Collins, CO: Veterinary Services' Centers for Epidemiology and Animal Health, Animal and Plant Inspection Service, United States Department of Agriculture; 1997. pp. 49–52. [Google Scholar]
- 40.Dargatz DA, Dewell GA, Mortimer RG. Calving and calving management of beef cows and heifers on cow-calf operations in the United States. Theriogenology. 2004;61:997–1007. doi: 10.1016/S0093-691X(03)00145-6. [DOI] [PubMed] [Google Scholar]
- 41.United States Department of Agriculture . Goat 2009. In: USDA, editor. Part I: Reference of Goat Management Practices in the United States. Fort Collins, CO: Veterinary Services' Centers for Epidemiology and Animal Health, Animal and Plant Inspection Service, United States Department of Agriculture; 2010. pp. 63–64. [Google Scholar]
- 42.United States Department of Agriculture . Sheep 2011. In: USDA, editor. Part I: Reference of Sheep Management Practices in the United States, 2011. Fort Collins, CO: Veterinary Services' Centers for Epidemiology and Animal Health, Animal and Plant Inspection Service, United States Department of Agriculture; 2012. pp. 87–90. [Google Scholar]
- 43.Dijkstra F, van der Hoek W, Wijers N, Schimmer B, Rietveld A, Wijkmans CJ, Vellema P, Schneeberger PM. The 2007–2010 Q fever epidemic in The Netherlands: characteristics of notified acute Q fever patients and the association with dairy goat farming. FEMS Immunol Med Microbiol. 2012;64:3–12. doi: 10.1111/j.1574-695X.2011.00876.x. [DOI] [PubMed] [Google Scholar]
- 44.Office of the Deputy Under Secretary of Defense (Military Community and Family Policy) under contract with ICF International, Demographics . Profile of the Military Community. 2010. [Google Scholar]
- 45.Tissot Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller PJ, Chicheportiche C, Nezri M, Poirier R. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am J Med. 1992;93:427–434. doi: 10.1016/0002-9343(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 46.Angelakis E, Million M, D'Amato F, Rouli L, Richet H, Stein A, Rolain JM, Raoult D. Q fever and pregnancy: disease, prevention, and strain specificity. Eur J Clin Microbiol Infect Dis. 2013;32:361–368. doi: 10.1007/s10096-012-1750-3. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention . Foodborne Active Surveillance Network (FoodNet) Population Survey Atlas of Exposures. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2006–2007. [Google Scholar]
- 48.Clark WH, Lennette EH, Romer MS. Q fever in California. XI. An epidemiologic summary of 350 cases occurring in northern California during 1948–1949. Am J Hyg. 1951;54:319–330. [PubMed] [Google Scholar]
- 49.Fishbein DB, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40. doi: 10.4269/ajtmh.1992.47.35. [DOI] [PubMed] [Google Scholar]
- 50.Kim SG, Kim EH, Lafferty CJ, Dubovi E. Coxiella burnetii in bulk tank milk samples, United States. Emerg Infect Dis. 2005;11:619–621. doi: 10.3201/eid1104.041036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loftis AD, Priestley RA, Massung RF. Detection of Coxiella burnetii in commercially available raw milk from the United States. Foodborne Pathog Dis. 2010;7:1453–1456. doi: 10.1089/fpd.2010.0579. [DOI] [PubMed] [Google Scholar]
- 52.Eldin C, Angelakis E, Renvoise A, Raoult D. Coxiella burnetii DNA, but not viable bacteria, in dairy products in France. Am J Trop Med Hyg. 2013;88:765–769. doi: 10.4269/ajtmh.12-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson AD, Kruszon-Moran D, Loftis AD, McQuillan G, Nicholson WL, Priestley RA, Candee AJ, Patterson NE, Massung RF. Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg. 2009;81:691–694. doi: 10.4269/ajtmh.2009.09-0168. [DOI] [PubMed] [Google Scholar]
- 54.Dahlgren FS, Haberling DL, McQuiston JH. Q fever is underestimated in the United States: a comparison of fatal Q fever cases from two national reporting systems. Am J Trop Med Hyg. 2015;92:244–246. doi: 10.4269/ajtmh.14-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]