Abstract
Scrub typhus usually presents as acute undifferentiated fever. This cross-sectional study included adult patients presenting with acute undifferentiated fever defined as any febrile illness for ≤ 14 days without evidence of localized infection. Scrub typhus cases were defined by an antibody titer of a ≥ fourfold increase in paired sera, a ≥ 1:160 in a single serum using indirect immunofluorescence assay, or a positive result of the immunochromatographic test. Multiple regression analysis identified predictors associated with scrub typhus to develop a prediction rule. Of 250 cases with known etiology of acute undifferentiated fever, influenza (28.0%), hepatitis A (25.2%), and scrub typhus (16.4%) were major causes. A prediction rule for identifying suspected cases of scrub typhus consisted of age ≥ 65 years (two points), recent fieldwork/outdoor activities (one point), onset of illness during an outbreak period (two points), myalgia (one point), and eschar (two points). The c statistic was 0.977 (95% confidence interval = 0.960–0.994). At a cutoff value ≥ 4, the sensitivity and specificity were 92.7% (79.0–98.1%) and 90.9% (86.0–94.3%), respectively. Scrub typhus, the third leading cause of acute undifferentiated fever in our region, can be identified early using the prediction rule.
Introduction
A variety of etiologies have been reported in patients presenting with acute undifferentiated fever in tropical areas.1–3 Malaria, dengue fever, scrub typhus, other rickettsioses, leptospirosis, and enteric fever are common causes of acute undifferentiated fever, causing considerable morbidity, mortality, and economic burden. However, the etiologic spectrum of acute undifferentiated fever has been poorly characterized in non-tropical areas.
The incidence of scrub typhus, one of the potentially life-threatening causes of acute undifferentiated fever, has continuously increased in Korea, a non-tropical endemic area.4 Delayed treatment in patients with scrub typhus might increase morbidity and mortality.5–7 Therefore, early treatment with doxycycline or azithromycin is warranted in patients who are suspected of having scrub typhus. However, clinical suspicion of scrub typhus is often difficult in patients presenting with acute undifferentiated fever in acute healthcare settings, leading to misdiagnosis or overdiagnosis of scrub typhus.3
The diagnosis of scrub typhus is based on history of exposure, clinical features, and results of serologic testing. Other than eschar, which is characteristic but not always detectable, clinical features of scrub typhus are notoriously non-specific.8 Based on clinical features (except eschar), it is almost impossible for clinicians to differentiate scrub typhus from other etiologies of acute undifferentiated fever. Currently, indirect immunofluorescence assay (IFA) is the gold standard reference test for the diagnosis of scrub typhus.9 However, IFA requires acute and convalescent paired sera as well as technical expertise and equipment. As a result, IFA is not useful in the early diagnosis of scrub typhus in the acute healthcare setting, such as emergency departments or outpatient clinics. For rapid diagnosis of scrub typhus, several point-of-care tests have been developed, albeit with lower sensitivity.10,11 For these reasons, we sought to develop a clinical prediction rule consisting of demographic, clinical, or laboratory parameters at the time of initial presentation to facilitate early detection of suspected cases of scrub typhus in patients with acute undifferentiated fever.
In this study, we investigated the etiologic spectrum in adult patients presenting with acute undifferentiated fever at the emergency department in a non-tropical scrub typhus-endemic area. In addition, we tried to develop a prediction rule to identify suspected cases of scrub typhus in patients with acute undifferentiated fever using predictors derived from a multiple regression model.
Methods
Design.
This cross-sectional study was conducted at a 550-bed suburban university hospital located in the western part of Kangwon Province, Republic of Korea. On average, 30,000 patients present at our emergency department each year. Of these patients, 11.7% have a fever or history of a fever.
Subjects.
Information from all patients who presented with fever or history of a fever at the emergency department from January of 2009 to December of 2013 was retrieved from the administrative registry. From these data, adult patients (≥ 18 years old) who were hospitalized with acute undifferentiated fever were included in this study. Acute undifferentiated fever was defined as any febrile illness (tympanic membrane temperature ≥ 37.8°C) with a duration of ≤ 14 days without evidence of localized infection by history, physical examination, complete blood count, chemistry profile, urinalysis, or chest radiography at the time of initial presentation at the emergency department.2 Patients who were receiving cancer chemotherapy or immunosuppressive therapy, had human immunodeficiency virus (HIV) infection, had been hospitalized for ≥ 72 hours within the preceding 30 days, or had incomplete medical records were excluded. This study was approved by the institutional review board of our hospital (approval no. 201405011001) with an exemption of informed consent.
Measurements.
Demographic, clinical, and laboratory data at the time of initial presentation were retrospectively collected by review of medical records. Demographic data included gender, age, residence, and recent history (within the preceding 30 days) of fieldwork/outdoor activities, insect bite, and overseas travel.12 Comorbidity included chronic pulmonary, hepatic, or renal disorders, cardiovascular disorders, diabetes mellitus, or malignancy. Clinical features at the time of initial presentation included time of onset of illness, headache, myalgia, vomiting, abdominal pain, cough, dyspnea, hypotension (systolic blood pressure < 90 mmHg), relative bradycardia, altered mental status, conjunctival injection, eschar, maculopapular rash, lymphadenopathy, hepatomegaly, and abdominal tenderness.8,13 A relative bradycardia was defined as a heart rate of < 110 beats per minute (bpm) with temperature ≥ 38.9°C, < 120 bpm with a temperature of ≥ 39.4°C, < 130 bpm with a temperature ≥ 40.0°C, < 140 bpm with a temperature ≥ 40.6°C, or < 150 bpm with a temperature ≥ 41.1°C.14 Laboratory data included the presence or absence of leukopenia (white blood cell count < 5,000 cells/mm3), anemia (hemoglobin < 12.0 g/dL), thrombocytopenia (platelet count < 150,000 cells/mm3), prolonged prothrombin time (international normalized ratio > 1.2), hypoalbuminemia (albumin < 3.5 g/dL), hyperbilirubinemia (total bilirubin > 1.2 mg/dL), elevated transaminase (aspartate transaminase or alanine transaminase > 50 U/L), or azotemia (blood urea nitrogen > 20 mg/dL or serum creatinine > 1.3 mg/dL) within 24 hours before or after admission.15,16 To evaluate the etiology of acute undifferentiated fever, IFA for Orientia tsutsugamushi or hantavirus; microscopic agglutination test for Leptospira species; immunochromatographic test for O. tsutsugamushi, hantavirus, or Leptospira species; peripheral blood smear or antigen test for malaria; rapid antigen test or reverse transcription polymerase chain reaction (RT-PCR) for influenza virus; serology for hepatitis A, B, or C; enzyme-linked immunosorbent assay (ELISA) for HIV antibody, blood cultures, and other testing have been performed according to the clinical judgment of the physicians caring for the patients. Consequently, final diagnosis of cases with acute undifferentiated fever was estimated based on the etiologic evaluation. Patients who showed IFA antibody titer with a ≥ fourfold increase in paired sera were defined as having definite scrub typhus. Patients who showed either an IFA antibody titer of ≥ 1:160 in a single serum or a positive result of an immunochromatographic test for O. tsutsugamushi were defined as possibly having scrub typhus.17,18 In Korea, IFA was mostly performed in a commercial laboratory to measure the levels of total antibodies (immunoglobulin G [IgG], IgM, and IgA) against the standard O. tsutsugamushi antigens from the Gilliam, Karp, and Boryong strains. Immunochromatographic test was performed in each hospital to detect antibodies against 56-kDa major surface protein antigens from representative strains using commercial kits (SD Bioline Tsutsugamushi Assay; Standard Diagnostics, Yongin, Korea). Cases that showed serological cross-reactivity between O. tsutsugamushi and other pathogens were assigned to unknown etiology of acute undifferentiated fever. After excluding cases with unknown etiology of acute undifferentiated fever, patients having either definite or possible scrub typhus were assigned to the scrub typhus group, whereas those having other causes of acute undifferentiated fever were assigned to the non-scrub typhus group. To eliminate information bias, all data were collected without the knowledge of final diagnosis.
Analyses.
In univariate analysis, baseline characteristics of the scrub typhus group were compared with those of the non-scrub typhus group. Pearson's χ2 test was used for categorical variables. Student's t test was used for continuous variables, and the results were represented as means ± SDs. Thereafter, all variables of P < 0.20 identified in univariate analysis were included in a multiple logistic regression model using forward conditional stepwise selection. Level of significance of P < 0.10 was used for inclusion, and P > 0.05 was used for exclusion. The goodness of fit of the model was evaluated using the Hosmer–Lemeshow test. Results of multivariable analyses were presented as the odds ratios (ORs) and 95% confidence intervals (95% CIs). A prediction rule was developed by assigning the nearest whole-number points to all chosen variables derived from a multiple logistic regression model in proportion to their regression coefficients. With this rule applied to the entire dataset, the optimal cutoff value was determined to approach the positive likelihood ratio of ≥ 5 by drawing a receiver-operating characteristic (ROC) curve. As a surrogate for internal validation while adjusting the model parameters for potential overfitting, 95% CI of the area under the ROC curve (c statistic) was calculated by bootstrapping with 1,000 replications.
The IBM SPSS Statistics 20 (IBM Corporation, Somers, NY) and R (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) programs with the POCR, pROC, and verification packages (http://cran.r-project.org) were used for the statistical analyses. A two-tailed P value of < 0.05 was considered to be statistically significant.
Results
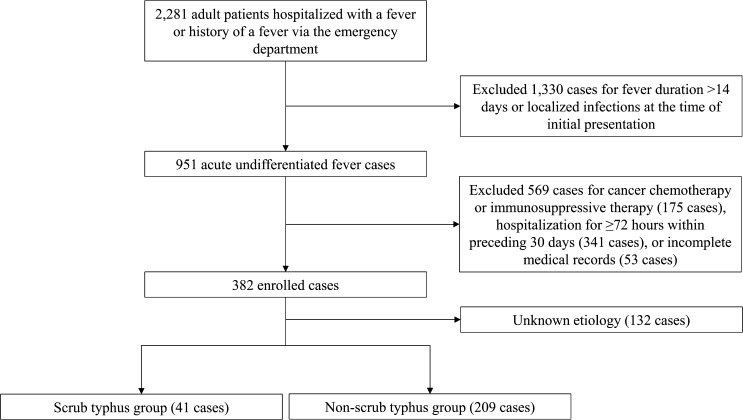
From 2009 to 2013, a total of 142,556 patients visited our emergency department, including 2,281 adult patients who were hospitalized with fever or history of a fever. Of these patients, 951 cases met the definition of acute undifferentiated fever at the time of initial presentation, of which 569 cases were excluded because of cancer chemotherapy or immunosuppressive therapy (175 cases), hospitalization for ≥ 72 hours within the preceding 30 days (341 cases), or incomplete medical records (53 cases). Therefore, in total, 382 patients were included in this study, with etiology of acute undifferentiated fever determined in 250 (65.4%) cases (Figure 1).
Figure 1.
Flow diagram for eligibility of cases with acute undifferentiated fever.
Of 250 cases with known etiology, influenza (28.0%) was the most common cause of acute undifferentiated fever followed by acute hepatitis A (25.2%), scrub typhus (16.4%), hemorrhagic fever with renal syndrome (4.8%), primary bacteremia (4.4%), and vivax malaria (3.6%) (Table 1). Of 41 cases in the scrub typhus group, 3 (7.3%) cases were in the definite scrub typhus group, and 38 (92.7%) cases were in the possible scrub typhus group.
Table 1.
Etiology of acute undifferentiated febrile illness (N = 250)
| No. of cases (%) | |
|---|---|
| Infectious etiologies | |
| Influenza | 70 (28.0) |
| Acute hepatitis A | 63 (25.2) |
| Scrub typhus | 41 (16.4) |
| HFRS | 12 (4.8) |
| Primary bacteremia | 11 (4.4) |
| Vivax malaria | 9 (3.6) |
| Infective endocarditis | 7 (2.8) |
| Acute hepatitis B | 2 (0.8) |
| Dengue fever | 2 (0.8)* |
| SFTS | 2 (0.8) |
| HGA | 1 (0.4) |
| Chickenpox | 1 (0.4) |
| Cytomegalovirus infection | 1 (0.4) |
| Epstein–Barr virus infection | 1 (0.4) |
| Total | 223 (89.2) |
| Non-infectious etiologies | |
| Drug fever or eruption | 7 (2.8) |
| Heat stroke | 5 (2.0) |
| Delirium tremens | 4 (1.6) |
| Panniculitis | 4 (1.6) |
| Systemic lupus erythematosus | 2 (0.8) |
| Adult-onset Still disease | 1 (0.4) |
| Kikuchi's disease | 1 (0.4) |
| Acute myeloid leukemia | 1 (0.4) |
| Non-Hodgkin lymphoma | 1 (0.4) |
| Thyrotoxicosis | 1 (0.4) |
| Total | 27 (10.8) |
HFRS = hemorrhagic fever with renal syndrome; HGA = human granulocytic anaplasmosis; SFTS = severe fever with thrombocytopenia syndrome.
Includes two cases imported from Cambodia and Myanmar.
Comparison of baseline characteristics of the scrub typhus and non-scrub typhus groups.
Baseline characteristics of the scrub typhus and non-scrub typhus groups are summarized in Table 2. Demographically, the mean age (mean ± SD; 69.2 ± 10.5 years) of the scrub typhus group was significantly higher than that (52.7 ± 22.2 years) of the non-scrub typhus group (P < 0.001). In addition, female gender or recent history of fieldwork/outdoor activities or insect bite was more common in the scrub typhus group than in the non-scrub typhus group (P < 0.05). Of clinical features, myalgia was more frequent in the scrub typhus group (P = 0.001). In addition, the onset of illness during an outbreak period of scrub typhus (from September to December in Korea) was more common in the scrub typhus group (P < 0.001). Hypotension, altered mental status, and eschar were more frequent in the scrub typhus group (P < 0.05), whereas relative bradycardia was more common in the non-scrub typhus group (P = 0.046). In the scrub typhus group, eschar was observed in 23 (56.1%) of 41 cases. In laboratory findings, anemia, hypoalbuminemia, elevated transaminase, and azotemia were more frequent in the scrub typhus group (P < 0.05), whereas leukopenia, prolonged prothrombin time, and hyperbilirubinemia were more common in the non-scrub typhus group (P < 0.05).
Table 2.
Comparison of baseline characteristics between the scrub typhus and the non-scrub typhus groups (N = 250)
| Baseline characteristics | No. (%) of cases | P value | |
|---|---|---|---|
| Scrub typhus group (N = 41) | Non-scrub typhus group (N = 209) | ||
| Demography | |||
| Female gender | 27 (65.9) | 91 (43.5) | 0.010 |
| Age ≥ 65 years old | 30 (73.2) | 76 (36.4) | < 0.001 |
| Residence in a suburban area | 40 (97.6) | 206 (98.6) | 0.514 |
| Recent history of fieldwork/outdoor activities | 31 (75.6) | 33 (15.8) | < 0.001 |
| Recent history of insect bite | 4 (9.8) | 3 (1.4) | 0.015 |
| Recent history of overseas travel | 0 | 5 (2.4) | 1.000 |
| Comorbid conditions | |||
| Cardiovascular diseases | 17 (41.5) | 61 (29.2) | 0.141 |
| Chronic pulmonary diseases | 1 (2.4) | 24 (11.5) | 0.091 |
| Chronic liver diseases | 0 | 10 (4.8) | 0.375 |
| Chronic renal diseases | 0 | 6 (2.9) | 0.593 |
| Diabetes mellitus | 8 (19.5) | 31 (14.8) | 0.481 |
| Malignancy | 1 (2.4) | 6 (2.9) | 1.000 |
| Clinical features | |||
| Onset during outbreak period of scrub typhus* | 39 (95.1) | 51 (24.4) | < 0.001 |
| Headache | 17 (41.5) | 54 (24.4) | 0.057 |
| Myalgia | 27 (65.9) | 76 (36.4) | 0.001 |
| Vomiting | 9 (22.0) | 42 (20.1) | 0.833 |
| Abdominal pain | 4 (9.8) | 33 (15.8) | 0.470 |
| Cough | 14 (34.1) | 77 (36.8) | 0.860 |
| Dyspnea | 12 (29.3) | 46 (22.0) | 0.317 |
| Systolic blood pressure < 90 mmHg | 8 (19.5) | 7 (3.3) | 0.003 |
| Relative bradycardia† | 8 (19.5) | 76 (36.4) | 0.046 |
| Altered mental status | 11 (26.8) | 27 (12.9) | 0.032 |
| Conjunctival injection | 2 (4.9) | 2 (1.0) | 0.127 |
| Eschar | 23 (56.1) | 4 (1.9) | < 0.001 |
| Maculopapular rash | 7 (17.1) | 19 (9.1) | 0.158 |
| Lymphadenopathy | 3 (7.3) | 4 (1.9) | 0.089 |
| Hepatomegaly | 0 | 7 (3.3) | 0.603 |
| Abdominal tenderness | 6 (14.6) | 29 (13.9) | 1.000 |
| Laboratory findings | |||
| WBC < 5,000 cells/mm3 | 5 (12.2) | 79 (37.8) | 0.001 |
| Hemoglobin < 12.0 g/dL | 18 (43.9) | 46 (22.0) | 0.006 |
| Platelet < 150,000 cells/mm3 | 31 (75.6) | 124 (59.3) | 0.077 |
| Prothrombin time (INR) > 1.2 | 3 (7.3) | 45 (21.5) | 0.028 |
| Albumin < 3.5 g/dL | 28 (68.3) | 84 (40.2) | < 0.001 |
| Total bilirubin > 1.2 mg/dL | 9 (22.0) | 103 (49.3) | 0.001 |
| AST or ALT > 50 U/L | 38 (92.7) | 119 (56.9) | < 0.001 |
| BUN > 20 mg/dL or serum creatinine > 1.3 mg/dL | 20 (48.8) | 56 (26.8) | 0.009 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; INR = international normalized ratio; WBC = white blood cell.
From September to December in Korea.
Defined as a heart rate of < 110 bpm with a temperature ≥ 38.9°C, < 120 bpm with a temperature ≥ 39.4°C, < 130 bpm with a temperature ≥ 40.0°C, < 140 bpm with a temperature ≥ 40.6°C, or < 150 bpm with a temperature ≥ 41.1°C.
Predictors associated with scrub typhus in patients with acute undifferentiated fever.
A multiple logistic regression model showed that age ≥ 65 years (OR = 26.75; 95% CI = 2.526–283.3; P = 0.006), recent history of fieldwork/outdoor activities (OR = 8.579; 95% CI = 1.648–44.66; P = 0.011), onset of illness during an outbreak period of scrub typhus (OR = 164.6; 95% CI = 10.76–2518; P < 0.001), myalgia (OR = 22.84; 95% CI = 1.838–283.7; P = 0.015), and eschar (OR = 29.10; 95% CI = 2.008–421.7; P = 0.013) were predictors associated with scrub typhus (Table 3). The Hosmer–Lemeshow test revealed that the goodness of fit of this model was appropriate (χ2 = 3.702; P = 0.717).
Table 3.
Predictors associated with scrub typhus in patients with acute undifferentiated fever (N = 250)
| Variables | OR (95% CI) | β-coefficients | Points |
|---|---|---|---|
| Age ≥ 65 years old | 26.75 (2.526–283.3) | 3.286 | 2 |
| Recent history of fieldwork/outdoor activities | 8.579 (1.648–44.66) | 2.149 | 1 |
| Onset of the illness during an outbreak period of scrub typhus* | 164.6 (10.76–2518) | 5.104 | 2 |
| Myalgia | 22.84 (1.838–283.7) | 3.128 | 1 |
| Eschar | 29.10 (2.008–421.7) | 3.371 | 2 |
From September to December in Korea.
Derivation of the prediction rule to identify scrub typhus in patients with acute undifferentiated fever.
The prediction rule represented the sum of the points for the following five predictors (total score ranging from zero to eight points): age ≥ 65 years (two points), recent history of fieldwork/outdoor activities (one point), onset of illness during an outbreak period of scrub typhus (two points), myalgia (one point), and eschar (two points).
The c statistic was 0.977 (95% CI = 0.960–0.994). The corrected c statistic derived from bootstrapping for 1,000 repetitions was 0.977 (95% CI = 0.961–0.993). Performance characteristics of the prediction rule are summarized in Table 4. When a cutoff value was set at ≥ 4, the rule had a sensitivity of 92.7% (95% CI = 79.0–98.1%), a specificity of 90.9% (95% CI = 86.0–94.3%), a positive predictive value of 66.7% (95% CI = 52.8–78.3%), a negative predictive value of 98.4% (95% CI = 95.2–99.6%), a positive likelihood ratio of 10.20 (95% CI = 6.584–15.79), and a negative likelihood ratio of 0.080 (95% CI = 0.027–0.230). When a cutoff value was set at ≥ 5, the rule had a sensitivity of 78.0% (95% CI = 62.0–88.9%), a specificity of 98.1% (95% CI = 94.9–99.4%), a positive predictive value of 88.9% (95% CI = 73.0–96.4%), a negative predictive value of 95.8% (95% CI = 91.9–97.9%), a positive likelihood ratio of 40.74 (95% CI = 15.24–109.1), and a negative likelihood ratio of 0.224 (95% CI = 0.126–0.399).
Table 4.
Performance of a prediction rule to identify suspected cases of scrub typhus in patients with acute undifferentiated fever
| Cutoff points | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) |
|---|---|---|---|---|---|---|
| ≥ 3 | 1.000 (0.893–1.000) | 0.770 (0.706–0.824) | 0.461 (0.336–0.569) | 1.000 (0.971–1.000) | 4.354 (3.397–5.581) | 0 |
| ≥ 4 | 0.927 (0.790–0.981) | 0.909 (0.860–0.943) | 0.667 (0.528–0.783) | 0.984 (0.952–0.996) | 10.20 (6.584–15.79) | 0.080 (0.027–0.239) |
| ≥ 5 | 0.780 (0.620–0.889) | 0.981 (0.949–0.994) | 0.889 (0.730–0.964) | 0.958 (0.919–0.979) | 40.78 (15.24–109.1) | 0.224 (0.126–0.399) |
The prevalence was presumed to be 16.4%.
Discussion
Our data showed that the etiologic spectrum of acute undifferentiated fever was widely distributed in our region. Scrub typhus was the major cause of acute undifferentiated fever next to influenza and acute hepatitis A. Using a prediction rule comprising five predictors, scrub typhus cases could be early identified in adult patients presenting with acute undifferentiated fever at the emergency department with excellent discriminatory performance.
Acute undifferentiated fever has been used to characterize many febrile illnesses without any evidence of localized infection in tropical areas.1–3 Korea, a non-tropical area, is also an endemic area of several undifferentiated febrile illnesses, such as scrub typhus, hemorrhagic fever with renal syndrome, leptospirosis, and vivax malaria.19 In addition, the incidence of imported febrile illnesses, such as falciparum malaria and dengue fever, has been rising because of increased travel to tropical areas.20 Recently, tick-borne illnesses, such as severe fever with thrombocytopenia syndrome and human granulocytic anaplasmosis, have been reported in our country.21,22 For these reasons, it has become increasingly challenging for Korean clinicians in acute healthcare settings to differentiate scrub typhus from other etiologies of acute undifferentiated fever. This study revealed that the etiologic spectrum of acute undifferentiated fever in Korea was diverse, although the major causes differed somewhat from those in tropical areas.
This study derived and internally validated a prediction rule to identify scrub typhus that consists of five predictors (total score ranges from zero to eight): age ≥ 65 years (two points), recent history of fieldwork/outdoor activities (one point), onset of illness during an outbreak period of scrub typhus (two points), myalgia (one point), and eschar (two points). These predictors have been well-known features of scrub typhus, with the exception of age and onset of illness during an outbreak period.4,12,23,24 The susceptibility of the elderly, especially in rural or suburban areas in Korea, might be explained by their increased history of fieldwork and decreased numbers of young farmers in those areas.25 In addition, the outbreak period of scrub typhus in Korea was from September to December, with more than 95% of cases reported during this period.26
For a prediction rule to be useful, a cutoff value is important. We suggested two cutoff values. First, a cutoff value ≥ 4 was a good screening tool for early detection of scrub typhus because of its high sensitivity (92.7%) and specificity (90.9%) as well as its good negative predictive value (98.4%). Second, we suggested a cutoff value of ≥ 5 as a near-confirmatory tool for the detection of scrub typhus because of its high positive predictive value (88.9%), albeit with slightly compromised sensitivity (78.0%).
Our prediction rule has several merits. First, its discriminatory performance was excellent, with a c statistic of 0.977. Second, it only requires patient history and physical examination without the need for laboratory tests, thus providing a potential benefit of reduction in the rates of unnecessary testing and antibiotic use.27 Third, all information using our prediction rule can be easily obtained at the time of initial presentation, assuring a prompt detection of scrub typhus without the necessity to wait for the results of expensive high-technology testing.
This study has several limitations. First, because only uncomplicated scrub typhus cases were in this study, our prediction rule should not be used to identify complicated cases accompanied by pneumonia, meningitis, or cholecystitis. Second, the possibility of selection bias could not be ruled out, because acute undifferentiated febrile cases with etiology that was not established were excluded from the final analysis (132 [34.6%] of 382 cases). This could have led to under- or overestimation of the prevalence of scrub typhus. Third, many possible cases showing either an IFA antibody titer of ≥ 1:160 in a single serum or a positive result of an immunochromatographic test were included in this study. IFA antibody titers obtained from single acute-phase specimens may be clinically informative based on local data for determination of the seroprevalence levels in the healthy population of endemic areas.28 However, inclusion of these possible cases in the final analysis could result in an overestimation of the prevalence of scrub typhus and the discriminatory power of our prediction rule. Therefore, our prediction rule should be used as a high index of clinical suspicion for early detection and empirical treatment of suspected cases with scrub typhus in patients with acute undifferentiated fever and should not be used as a surrogate for confirmatory testing for O. tsutsugamushi. Fourth, external validation of our prediction rule is required in other non-tropical endemic areas.
In summary, the etiologic spectrum of acute undifferentiated fever is highly diverse in our region, and scrub typhus is one of its leading causes. Our prediction rule can help clinicians identify suspected cases of scrub typhus in patients with acute undifferentiated fever because of its high sensitivity (92.7%) and specificity (90.9%) at a cutoff score of ≥ 4 and its even higher positive predictive value (88.9%) at a cutoff value of ≥ 5.
Footnotes
Authors' addresses: Ho-Chul Jung, Won Sup Oh, Dong-Hyun Lee, and Ho-Jin Lee, Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Republic of Korea, E-mails: jhc0563@hanmail.net, onesbi@gmail.com, superhigh1@daum.net, and kobestman@hotmail.com. Sung-Bin Chon, Department of Emergency Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea, E-mail: 1tim4ezra7@gmail.com.
References
- 1.Acestor N, Cooksey R, Newton PN, Ménard D, Guerin PJ, Nakagawa J, Christophel E, González IJ, Bell D. Mapping the aetiology of non-malarial febrile illness in Southeast Asia through a systematic review–terra incognita impairing treatment policies. PLoS ONE. 2012;7:e44269. doi: 10.1371/journal.pone.0044269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leelarasamee A, Chupaprawan C, Chenchittikul M, Udompanthurat S. Etiologies of acute undifferentiated febrile illness in Thailand. J Med Assoc Thai. 2004;87:464–472. [PubMed] [Google Scholar]
- 3.Chrispal A, Boorugu H, Gopinath KG, Chandy S, Prakash JA, Thomas EM, Abraham AM, Abraham OC, Thomas K. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors—an experience from a tertiary care hospital in south India. Trop Doct. 2010;40:230–234. doi: 10.1258/td.2010.100132. [DOI] [PubMed] [Google Scholar]
- 4.Korea Centers for Disease Control and Prevention . Infectious Diseases Surveillance Yearbook 2012. Seoul, Korea: Korea Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 5.Yasunaga H, Horiguchi H, Kuwabara K, Hashimoto H, Matsuda S. Delay in tetracycline treatment increases the risk of complications in Tsutsugamushi disease: data from the Japanese Diagnosis Procedure Combination database. Intern Med. 2011;50:37–42. doi: 10.2169/internalmedicine.50.4220. [DOI] [PubMed] [Google Scholar]
- 6.Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis. 2003;16:429–436. doi: 10.1097/00001432-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Rapsang AG, Bhattacharyya P. Scrub typhus. Indian J Anaesth. 2013;57:127–134. doi: 10.4103/0019-5049.111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silpapojakul K, Varachit B, Silpapojakul K. Paediatric scrub typhus in Thailand: a study of 73 confirmed cases. Trans R Soc Trop Med Hyg. 2004;98:354–359. doi: 10.1016/j.trstmh.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–307. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–370. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KD, Moon C, Oh WS, Sohn KM, Kim BN. Diagnosis of scrub typhus: introduction of the immunochromatographic test in Korea. Korean J Intern Med. 2014;29:253–255. doi: 10.3904/kjim.2014.29.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyu Y, Tian L, Zhang L, Dou X, Wang X, Li W, Zhang X, Sun Y, Guan Z, Li X, Wang Q. A case-control study of risk factors associated with scrub typhus infection in Beijing, China. PLoS ONE. 2013;8:e63668. doi: 10.1371/journal.pone.0063668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathi NB, Rathi AN, Goodman MH, Aghai ZH. Rickettsial diseases in central India: proposed clinical scoring system for early detection of spotted fever. Indian Pediatr. 2011;48:867–872. doi: 10.1007/s13312-011-0141-7. [DOI] [PubMed] [Google Scholar]
- 14.Cunha BA. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect. 2000;6:633–634. doi: 10.1046/j.1469-0691.2000.0194f.x. [DOI] [PubMed] [Google Scholar]
- 15.Varghese GM, Abraham OC, Mathai D, Thomas K, Aaron R, Kavitha ML, Mathai E. Scrub typhus among hospitalised patients with febrile illness in South India: magnitude and clinical predictors. J Infect. 2006;52:56–60. doi: 10.1016/j.jinf.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Chen NY, Huang PY, Leu HS, Chiang PC, Huang CT. Clinical prediction of endemic rickettsioses in northern Taiwan-relevance of peripheral blood atypical lymphocytes. J Microbiol Immunol Infect. 2008;41:362–368. [PubMed] [Google Scholar]
- 17.Kim DM, Lee YM, Back JH, Yang TY, Lee JH, Song HJ, Shim SK, Hwang KJ, Park MY. A serosurvey of Orientia tsutsugamushi from patients with scrub typhus. Clin Microbiol Infect. 2010;16:447–451. doi: 10.1111/j.1469-0691.2009.02865.x. [DOI] [PubMed] [Google Scholar]
- 18.Silpasakorn S, Waywa D, Hoontrakul S, Suttinont C, Losuwanaluk K, Suputtamongkol Y. Performance of SD Bioline Tsutsugamushi assays for the diagnosis of scrub typhus in Thailand. J Med Assoc Thai. 2012;5((Suppl 2)):S18–S22. [PubMed] [Google Scholar]
- 19.Lee HW. Hemorrhagic fever with renal syndrome in Korea. Rev Infect Dis. 1989;11:S864–S876. [PubMed] [Google Scholar]
- 20.Park SH, Lee MJ, Baek JH, Lee WC. Epidemiological aspects of exotic malaria and dengue fever in travelers in Korea. J Clin Med Res. 2011;3:139–142. doi: 10.4021/jocmr571e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013;19:1892–1894. doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KH, Yi J, Oh WS, Kim NH, Choi SJ, Choe PG, Kim NJ, Lee JK, Oh MD. Human granulocytic anaplasmosis, South Korea, 2013. Emerg Infect Dis. 2014;20:1708–1711. doi: 10.3201/eid2010.131680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashinkunti MD, Gundikeri SK, Dhananjaya M. Acute undifferentiated febrile illness-clinical spectrum and outcome from a tertiary care teaching hospital of north Karnataka. Int J Biol Med Res. 2013;4:3399–3402. [Google Scholar]
- 24.Berman SJ, Kundin WD. Scrub typhus in South Vietnam. A study of 87 cases. Ann Intern Med. 1973;79:26–30. doi: 10.7326/0003-4819-79-1-26. [DOI] [PubMed] [Google Scholar]
- 25.Bang HA, Lee MJ, Lee WC. Comparative research on epidemiological aspects of tsutsugamushi disease (scrub typhus) between Korea and Japan. Jpn J Infect Dis. 2008;61:148–150. [PubMed] [Google Scholar]
- 26.Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY, Ryu SY, Yoo HS, Park O. Rapid increase of scrub typhus, South Korea, 2001–2006. Emerg Infect Dis. 2009;15:1127–1129. doi: 10.3201/eid1507.080399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thangarasu S, Natarajan P, Rajavelu P, Rajagopalan A, Seelinger Devey JS. A protocol for the emergency department management of acute undifferentiated febrile illness in India. Int J Emerg Med. 2011;4:57. doi: 10.1186/1865-1380-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blacksell SD, Bryant NJ, Paris DH, Doust JA, Sakoda Y, Day NP. Scrub typhus serologic testing with the indirect immunofluorescence method as a diagnostic gold standard: a lack of consensus leads to a lot of confusion. Clin Infect Dis. 2007;44:391–401. doi: 10.1086/510585. [DOI] [PubMed] [Google Scholar]