Abstract
Malaria has declined in recent years in countries of the American continents. In 2011, 12 of 21 endemic countries had already met their 2015 Millennium Development Goal. However, this declining trend has not been adequately evaluated. An analysis of the number of cases per 100,000 people (annual parasite index [API]) and the percentage of positive blood slides (slide positivity rate [SPR]) during the period of 1959–2011 in 21 endemic countries was done using the joinpoint regression methodology. During 1960–1979, API and SPR increased significantly and peaked in the 1980s. Since the 1990s, there have been significant declining trends in both API and SPR. Additionally, both Plasmodium vivax and P. falciparum species-specific incidence have declined. With the exception of two countries, such a collectively declining malaria trend was not observed in previous decades. This presents a unique opportunity for the Americas to seriously consider malaria elimination as a final goal.
Introduction
Malaria, a global health problem and one of the reasons for the establishment of the Pan American Health Organization (PAHO) in 1902, affects millions of people and has been associated with approximately 1 million deaths each year.1,2 The estimated disability-adjusted life years (DALYs) lost because of malaria was 111,000 DALYs in the Latin American countries.3 In the past decade, the burden of disease has been decreasing on the North and South American continents. At the end of the year 2011, 12 of 21 presently malaria-endemic countries in the continents had already met their 2015 Millennium Development Goal (MDG; a 75% reduction in malaria incidence compared with the year 2000), whereas another 5 countries were well on the way to achieve it.2 Six of the endemic countries transitioned into the pre-elimination phase in 2011, having been so classified by the World Health Organization (WHO), and a number of other countries are making similar progress toward the achievement of the pre-elimination criteria.2
PAHO adopted continental malaria eradication (now referred to as regional malaria elimination) as a Regional Program in 1954, a year before the Global Malaria Eradication Program (GMEP) was launched in Mexico in 1955; during its implementation, many previously endemic countries achieved malaria elimination.4,5 Increasing parasite resistance to the antimalarial drug chloroquine and vector resistance to insecticides coupled with waning focus on combatting malaria led to an increase in malaria morbidity and mortality globally in the 1970s and 1980s.6 The burden and need for continued focus on the disease led to the adoption of the Global Malaria Control Strategy in 1992 at a Ministerial Conference in Amsterdam.7 The magnitude of the disease as well as its high mortality among children in Africa resulted in a call to counter malaria with the formation of the Roll Back Malaria partnership between the WHO, the United Nations Development Program (UNDP), and the World Bank in 1998. The initiative was adopted in the Americas in 2000.8 The United Nations General Assembly declared 2001–2010 as the decade to roll back malaria, and in 2012, they called for accelerated efforts to control and eliminate malaria.9,10 To support the achievement of these global goals and facilitate the achievement of country targets, PAHO coordinated the multistakeholder development and implementation of the Regional Strategy for Malaria in the Americas 2006–2010 and the Strategy and Plan of Action for Malaria 2011–2015.11,12
The history of malaria throughout the last half century in the Americas has been documented, but temporal trends have not been adequately evaluated.5,13 Studies have assessed the impact of renewed efforts for malaria control over the last decade.14 Nevertheless, doubt remains, because a formal and systemic analysis of malaria trends in countries has not been done under claims of data paucity or unreliability. This study makes use of more than five decades of yearly incidence data for malaria-endemic countries in the Americas and systemically analyzes them to identify temporal trends and detect years of significant change (i.e., joinpoints or change points) in their trajectories over time.
Materials and Methods
Countries of the American continents annually report incident malaria cases to PAHO/WHO. These official country reports were used to compile annual data series for 21 endemic countries in the Americas from 1959 to 2011. Total number of slides examined, total confirmed cases, and cases by species type were analyzed. United Nations' mid-year annual population estimates for the corresponding year were used for standardization and allow comparison across time and countries.15
Annual data series for all countries were compiled. Analysis was done for four variables: annual parasite index (API) or the number of malaria cases per 100,000 inhabitants, slide positivity rate (SPR) or the number of cases per 100 blood slides examined, and Plasmodium falciparum and P. vivax rates or numbers of species-specific malaria cases per 100,000 inhabitants per year. For P. falciparum, all monoinfections and mixed infections were considered as P. falciparum infections, but only P. vivax monoinfections were included for calculating P. vivax rates. Data for mixed infections have been reported separately from P. falciparum infections since 1994, but in previous years, these two were consolidated as a single figure and so reported. Years of countries for which data for a variable were missing were removed from the analysis. API and species-specific rates were calculated using the total population of the country.
The population living at risk of malaria transmission should be used for calculation of API; however, there are vast differences in the manner in which countries estimate and report their at-risk population. The population in a locality or focus where malaria transmission occurs should be used to determine those at risk; nevertheless, it is logistically impractical in a highly endemic area. Population of the whole municipality where malaria is reported is considered at risk for reporting purposes, but not all municipalities have the same risk; therefore, the whole population may or may not be at risk. To avoid this bias, total country population instead of reported population at risk was used. API and SPR are not independent and may be expected to show similar trends given adequate quality of surveillance; thus, analysis of both indices allows detection of true trends and surveillance quality.
Joinpoint methodology has been used for trend analysis in cancer rates by the National Cancer Institute of the United States.16 The regression method tests for the possibility that two or more linear regression curves can be fitted for different ranges of the independent variables (i.e., in this case, one long period for a country can be possibly divided into two or more periods, each with a different trend). The slope of the linear curve or annual percentage change (APC) in disease incidence is different for each of the smaller periods; each time point (year) that divides the longer period into shorter periods where a significant change in APC is detected is considered to be a joinpoint.
SEs of the rates were calculated assuming Poisson distribution for incidence rates and binomial distribution for percentages. The data were analyzed using the Joinpoint program.17 A regression analysis using logarithmic rates as the dependent variable and years as the independent variable was carried out. A factor of 0.1 was added to all of the years in a data series of a variable for a country where a zero value was observed in 1 or more years. Heteroscedasticity was assumed, and the SEs calculated were used for the regression. An autocorrelated errors model was fitted for calculation of regression estimates. A minimum of 4 years between two subsequent joinpoints and 3 years before the first joinpoint were set. Permutation tests up to 4,999 were used to identify the best model. Results were considered to be significant at P value less than 0.05. Numbers of joinpoints, final selected model, APC for all years, and average APC (AAPC) for the period 2000–2011 were calculated and have been reported.
Results
Of 21 countries for which data were analyzed, annual malaria case series were available for all four variables over 53 years from 1959 to 2011 for 14 countries (Table 1). For Argentina, SPR data were not available for 2009. Information from Brazil was available for all variables during the entire period with the exception of the year 1959. Information on SPR for Colombia was unavailable for 1998, and information on SPR for French Guiana was unavailable for 1959 and 1998. Similarly, the rate was unavailable for Guatemala in 1998 and Peru in 1989 and 2011. Species-specific rates were unavailable for French Guiana in 1959. For Haiti, data were available since 1962, and information for all variables was missing for 1995, 2002, and 2003. Overall, these instances of data unavailability added up to 37 years per country per indicator, accounting for missing data of less than 1% (0.83%) of the possible total of 4,452 years per country per indicator.
Table 1.
Data availability by parameter evaluated and country, 1959–2011
| Country | Range of years | Number of data years available | |||
|---|---|---|---|---|---|
| API | SPR | PfIR | PvIR | ||
| Argentina | 1959–2011 | 53 | 52 | 53 | 53 |
| Belize | 1959–2011 | 53 | 53 | 53 | 53 |
| Bolivia | 1959–2011 | 53 | 53 | 53 | 53 |
| Brazil | 1960–2011 | 52 | 52 | 52 | 52 |
| Colombia | 1959–2011 | 53 | 52 | 53 | 53 |
| Costa Rica | 1959–2011 | 53 | 53 | 53 | 53 |
| Dominican Republic | 1959–2011 | 53 | 53 | 53 | 53 |
| Ecuador | 1959–2011 | 53 | 53 | 53 | 53 |
| El Salvador | 1959–2011 | 53 | 53 | 53 | 53 |
| French Guiana | 1959–2011 | 53 | 51 | 52 | 52 |
| Guatemala | 1959–2011 | 53 | 52 | 53 | 53 |
| Guyana | 1959–2011 | 53 | 53 | 53 | 53 |
| Haiti | 1962–2011 | 47 | 47 | 47 | 47 |
| Honduras | 1959–2011 | 53 | 53 | 53 | 53 |
| Mexico | 1959–2011 | 53 | 53 | 53 | 53 |
| Nicaragua | 1959–2011 | 53 | 53 | 53 | 53 |
| Panama | 1959–2011 | 53 | 53 | 53 | 53 |
| Paraguay | 1959–2011 | 53 | 53 | 53 | 53 |
| Peru | 1959–2011 | 53 | 51 | 53 | 53 |
| Suriname | 1959–2011 | 53 | 53 | 53 | 53 |
| Venezuela | 1959–2011 | 53 | 53 | 53 | 53 |
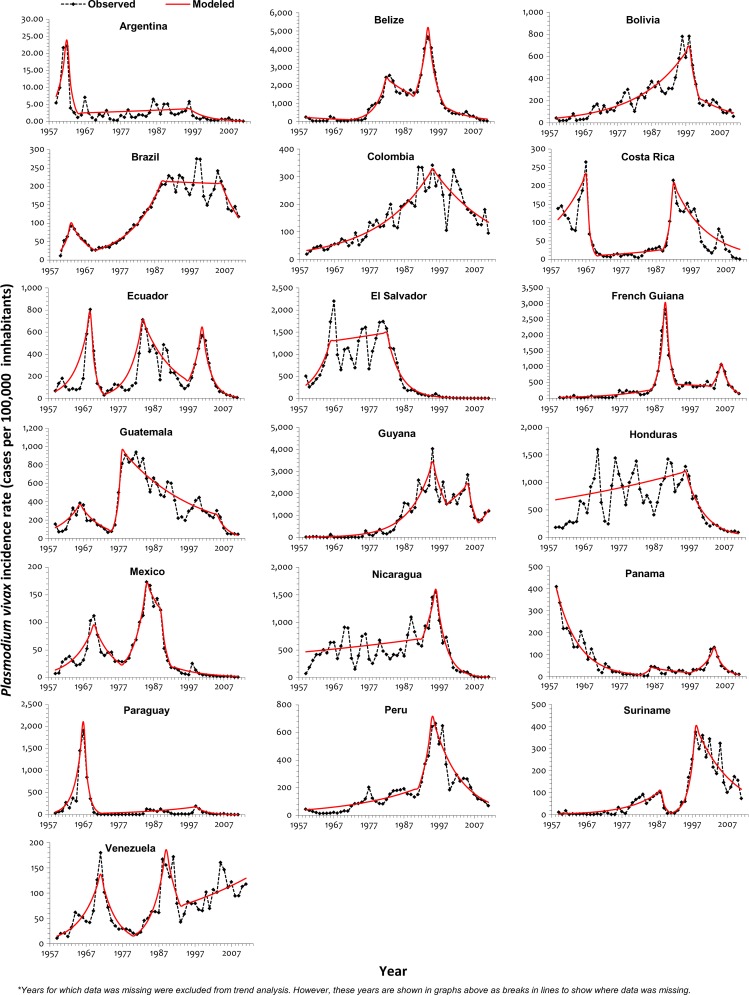
Trends in malaria incidence.
For the majority of countries, the trends of the principal indicators API and SPR were similar over most of the five decades studied, although they did vary in magnitude (Figures 1 and 2 ). In French Guiana and Guatemala, the trends for these two indicators were widely different and even in different directions. Specifically, in Guatemala, whereas API was declining significantly, SPR was increasing significantly between 1986 and 2005, pointing to a possible decrease in surveillance quality and coverage of diagnosis and treatment during those years. Mexico, El Salvador, and Paraguay were the only countries where the joinpoint years and trend directions for both SPR and API coincided for almost all segments, but different values were obtained for others (Table 2). For Guyana, no significant (P value < 0.05) SPR trend was seen for the entire period.
Figure 1.
Malaria trend in endemic countries of the Americas, 1959–2011.
Figure 2.
Trend of SPR for malaria in the Americas, 1959–2011.
Table 2.
APC in API (per 100,000 inhabitants) and SPR (per 100 blood slides examined) in the Americas, 1959–2011
| Country | API | SPR | ||||
|---|---|---|---|---|---|---|
| Number of joinpoints | Year range | APC (%; 95% confidence interval) | Number of joinpoints | Year range | APC (%; 95% confidence interval) | |
| Argentina | 3 | 1959–1962 | 48.14 (−3.74, 127.98) | 2 | 1959–1969 | −27.23 (−34.13, −19.60) |
| 1962–1965 | −53.25 (−88.38, 88.08) | 1969–1989 | 20.07 (13.82, 26.67) | |||
| 1965–1996 | 1.38 (−1.03, 3.85) | 1989–2011 | −2.67 (−8.23, 3.22) | |||
| 1996–2011 | −19.26 (−29.14, −8.00) | |||||
| Belize | 5 | 1959–1972 | −17.49 (−27.31, −6.34) | 3 | 1959–1973 | −17.11 (−26.32, −6.75) |
| 1972–1982 | 39.69 (19.76, 62.95) | 1973–1982 | 38.81 (16.58, 65.29) | |||
| 1982–1990 | −7.01 (−13.35, −0.21) | 1982–1995 | 5.02 (2.29, 7.83) | |||
| 1990–1994 | 37.96 (14.65, 66.00) | 1995–2011 | −15.56 (−19.02, −11.95) | |||
| 1994–2000 | −29.69 (−36.96, −21.58) | |||||
| 2000–2011 | −12.89 (−22.18, −2.49) | |||||
| Bolivia | 3 | 1959–1993 | 5.79 (4.22, 7.39) | 2 | 1959–1998 | 8.92 (7.59, 10.27) |
| 1993–1998 | 16.70 (2.32, 33.10) | 1998–2002 | −28.00 (−44.01, −7.41) | |||
| 1998–2001 | −37.21 (−58.28, −5.49) | 2002–2011 | −5.31 (−13.04, 3.12) | |||
| 2001–2011 | −7.46 (−13.15, −1.39) | |||||
| Brazil | 4 | 1960–1963 | 71.28 (−21.41, 273.27) | 5 | 1960–1974 | −8.59 (−11.27, −5.82) |
| 1963–1974 | −7.45 (−13.48, −1.01) | 1974–1987 | 14.64 (12.27, 17.06) | |||
| 1974–1987 | 15.23 (11.10, 19.50) | 1987–1999 | 3.58 (2.22, 4.96) | |||
| 1987–2006 | −2.20 (−3.36, −1.03) | 1999–2002 | −11.22 (−23.47, 2.98) | |||
| 2006–2011 | −12.16 (−20.19, −3.32) | 2002–2005 | 8.17 (−7.21, 26.10) | |||
| 2005–2011 | −12.82 (−16.02, −9.50) | |||||
| Colombia | 1 | 1959–2001 | 4.12 (3.26, 4.99) | 2 | 1959–1978 | 11.86 (9.61, 14.16) |
| 2001–2011 | −11.39 (−15.32, −7.28) | 1978–2000 | 4.11 (3.24, 4.99) | |||
| 2000–2011 | −5.02 (−6.68, −3.33) | |||||
| Costa Rica | 4 | 1959–1967 | 9.39 (−2.28, 22.46) | 4 | 1959–1963 | −38.97 (−55.81, −15.70) |
| 1967–1970 | −62.83 (−86.39, 1.52) | 1963–1967 | 49.47 (−1.13, 125.95) | |||
| 1970–1989 | 4.47 (−4.2, 13.93) | 1967–1970 | −67.22 (−88.08, −9.89) | |||
| 1989–1992 | 95.53 (−6.44, 308.61) | 1970–2005 | 16.42 (12.30, 20.70) | |||
| 1992–2011 | −9.82 (−13.53, −5.94) | 2005–2011 | −43.06 (−64.70, −8.14) | |||
| Dominican Republic | 3 | 1959–1968 | −38.86 (−49.09, −26.56) | 4 | 1959–1961 | −4.47 (−44.90, 65.62) |
| 1968–1982 | 31.35 (18.29, 45.86) | 1961–1967 | −70.99 (−81.70, −54.01) | |||
| 1982–1985 | −44.67 (−76.95, 32.79) | 1967–1982 | 41.92 (29.79, 55.18) | |||
| 1985–2011 | 2.00 (−0.49, 4.54) | 1982–1985 | −46.61 (−74.81, 13.14) | |||
| 1985–2011 | 3.25 (1.09, 5.46) | |||||
| Ecuador | 5 | 1959–1969 | 23.48 (4.78, 45.52) | 5 | 1959–1965 | −30.1 (−48.53, −5.07) |
| 1969–1974 | −43.68 (−65.18, −8.91) | 1965–1968 | 128.05 (−13.03, 497.99) | |||
| 1974–1984 | 34.53 (15.14, 57.19) | 1968–1980 | −13.78 (−20.23, −6.81) | |||
| 1984–1996 | −8.71 (−15.48, −1.40) | 1980–1984 | 72.02 (24.22, 138.22) | |||
| 1996–2001 | 25.73 (−3.5, 63.82) | 1984–2002 | 0.25 (−1.65, 2.19) | |||
| 2001–2011 | −36.89 (−48.55, −22.60) | 2002–2011 | −36.95 (−47.39, −24.45) | |||
| El Salvador | 1 | 1959–1982 | 3.29 (1.05, 5.58) | 1 | 1959–1984 | 4.68 (2.71, 6.68) |
| 1982–2011 | −24.98 (−30.08, −19.51) | 1984–2011 | −21.42 (−28.58, −13.54) | |||
| French Guiana | 3 | 1959–1986 | 8.85 (4.35, 13.55) | 4 | 1960–1989 | 11.30 (8.10, 14.70) |
| 1986–1989 | 44.01 (−9.05, 128.01) | 1989–1992 | −19.90 (−42.70, 12.10) | |||
| 1989–2009 | −5.35 (−6.61, −4.08) | 1992–2005 | 2.20 (−0.50, 5.00) | |||
| 2009–2011 | −44.61 (−70.69, 4.67) | 2005–2008 | 32.20 (−4.60, 83.20) | |||
| 2008–2011 | −29.70 (−46.70, −7.40) | |||||
| Guatemala | 4 | 1959–1966 | 15.26 (−4.35, 38.89) | 4 | 1959–1975 | −9.32 (−13.87, −4.53) |
| 1966–1975 | −17.33 (−29.42, −3.18) | 1975–1978 | 115.70 (2.02, 356.05) | |||
| 1975–1978 | 136.99 (4.20, 438.99) | 1978–1986 | −2.65 (−7.65, 2.61) | |||
| 1978–2005 | −4.95 (−6.19, −3.70) | 1986–2005 | 3.45 (1.78, 5.14) | |||
| 2005–2011 | −28.78 (−41.77, −12.89) | 2005–2011 | −31.06 (−40.88, −19.61) | |||
| Guyana | 4 | 1959–1984 | 9.32 (1.34, 17.92) | 4 | 1959–1982 | 1.68 (−6.92, 11.07) |
| 1984–1987 | 104.49 (−22.2, 437.45) | 1982–1985 | 93.97 (−78.52, 1651.59) | |||
| 1987–1995 | 5.24 (−1.82, 12.80) | 1985–1992 | 6.99 (−2.13, 16.96) | |||
| 1995–2009 | −6.78 (−9.69, −3.77) | 1992–1997 | −11.66 (−24.74, 3.69) | |||
| 2009–2011 | 31.50 (−29.43, 145.01) | 1997–2011 | −1.01 (−3.68, 1.72) | |||
| Haiti | 3 | 1962–1983 | 10.20 (7.40, 13.10) | 2 | 1962–1999 | 11.61 (9.98, 13.26) |
| 1983–1986 | −40.88 (−82.8, 102.9) | 1999–2004 | −37.55 (−96.36, 971.81) | |||
| 1986–2004 | −1.47 (−6.50, 3.80) | 2004–2011 | 20.22 (−0.65, 45.47) | |||
| 2004–2011 | 21.75 (9.60, 35.30) | |||||
| Honduras | 1 | 1959–1996 | 1.56 (0.02, 3.13) | 3 | 1959–1981 | 9.76 (5.89, 13.78) |
| 1996–2011 | −17.86 (−22.83, −12.56) | 1981–1984 | −36.63 (−64.07, 11.79) | |||
| 1984–1996 | 12.55 (6.49, 18.94) | |||||
| 1996–2011 | −9.63 (−14.46, −4.51) | |||||
| Mexico | 5 | 1959–1970 | 19.26 (9.51, 29.88) | 5 | 1959–1970 | 16.98 (8.81, 25.76) |
| 1970–1978 | −16.55 (−25.62, −6.37) | 1970–1978 | −10.99 (−19.62, −1.44) | |||
| 1978–1985 | 33.24 (18.61, 49.67) | 1978–1985 | 44.37 (30.41, 59.83) | |||
| 1985–1989 | −8.47 (−22.61, 8.25) | 1985–1989 | −11.77 (−24.29, 2.82) | |||
| 1989–1992 | −44.16 (−68.6, −0.67) | 1989–1992 | −47.91 (−70.46, −8.15) | |||
| 1992–2011 | −11.11 (−17.34, −4.41) | 1992–2011 | −8.81 (−14.16, −3.13) | |||
| Nicaragua | 2 | 1959–1992 | 0.56 (−1.01, 2.16) | 3 | 1959–1980 | 2.92 (−0.50, 6.45) |
| 1992–1996 | 22.26 (−5.75, 58.59) | 1980–1983 | −31.22 (−67.46, 45.4) | |||
| 1996–2011 | −28.46 (−34.53, −21.82) | 1983–1996 | 13.24 (8.25, 18.46) | |||
| 1996–2011 | −27.44 (−33.18, −21.22) | |||||
| Panama | 5 | 1959–1964 | −20.44 (−30.74, −8.61) | 5 | 1959–1964 | −25.91 (−36.26, −13.9) |
| 1964–1969 | 16.18 (−11.35, 52.27) | 1964–1969 | 32.49 (−0.14, 75.77) | |||
| 1969–1977 | −27.92 (−36.63, −18) | 1969–1972 | −66.83 (−85.3, −25.16) | |||
| 1977–2000 | 1.34 (−2.80, 5.66) | 1972–1996 | 0.25 (−3.36, 4.00) | |||
| 2000–2004 | 50.90 (−1.07, 130.17) | 1996–2004 | 36.32 (17.16, 58.61) | |||
| 2004–2011 | −36.56 (−46.03, −25.44) | 2004–2011 | −33.85 (−45.14, −20.24) | |||
| Paraguay | 2 | 1959–1967 | 55.07 (25.65, 91.38) | 2 | 1959–1967 | 26.82 (4.89, 53.34) |
| 1967–1971 | −55.73 (−74.52, −23.08) | 1967–1971 | −46.79 (−68.81, −9.22) | |||
| 1971–2011 | 0.73 (−5.36, 7.22) | 1971–2011 | 2.67 (−3.11, 8.79) | |||
| Peru | 2 | 1959–1991 | 5.59 (2.39, 8.88) | 5 | 1959–1963 | −46.2 (−68.22, −8.90) |
| 1991–1996 | 36.45 (16.80, 59.41) | 1963–1977 | 31.7 (22.26, 41.88) | |||
| 1996–2011 | −14.10 (−16.73, −11.38) | 1977–1981 | −2.58 (−31.25, 38.04) | |||
| 1981–1993 | 14.81 (10.71, 19.06) | |||||
| 1993–2000 | −26.09 (−29.03, −23.03) | |||||
| 2000–2010 | −2.97 (−6.03, 0.19) | |||||
| Suriname | 2 | 1959–1989 | 0.27 (−2.68, 3.31) | 2 | 1959–1977 | −3.29 (−8.80, 2.57) |
| 1989–2001 | 15.51 (6.04, 25.83) | 1977–2001 | 9.29 (6.53, 12.11) | |||
| 2001–2011 | −25.11 (−33.26, −15.97) | 2001–2011 | −14.22 (−22.14, −5.49) | |||
| Venezuela | 4 | 1959–1971 | 22.63 (12.43, 33.76) | 4 | 1959–1971 | 40.54 (27.16, 55.33) |
| 1971–1980 | −20.66 (−28.63, −11.8) | 1971–1980 | −18.23 (−27.21, −8.13) | |||
| 1980–1990 | 29.95 (20.90, 39.67) | 1980–1988 | 36.17 (18.07, 57.05) | |||
| 1990–1993 | −34.41 (−57.92, 2.22) | 1988–1993 | −10.96 (−23.12, 3.12) | |||
| 1993–2011 | 3.91 (2.12, 5.74) | 1993–2011 | 2.22 (0.21, 4.27) | |||
During 1960–1969, most countries had significantly increasing API (P value < 0.05), and for a majority of these years, most countries also had a significantly increasing SPR. Malaria incidence (SPR and API) continued to increase throughout the 1970–1979 period and peaked in the early 1980s, when only Guatemala had a significant reduction in API and none of the countries had a significant declining SPR (Supplemental Tables 1–4). By 1989, a reversal in the malaria trends was observed; five countries had significantly decreasing API, but it was not accompanied by significant reductions in the SPR. During 1990–1999, this declining malaria trend was further accentuated; by the end of that decade, 13 countries showed reductions in API, and the number of countries with significantly declining SPR increased from 2 to 7. Malaria continued to decline, and by the middle of 2000–2011, 12 and 16 countries had significantly decreasing SPR and API, respectively. Between 2011 and the previous joinpoint year, only Haiti and Venezuela had significant increases in API, whereas Venezuela and the Dominican Republic also had significant increases in SPR. The change from an increasing or non-significant trend for API to a significantly declining API or joinpoint year was seen in the 1990–1999 period for most countries in the Americas, but in Colombia, Ecuador, Panama, and Suriname, this change was observed in the 2000–2011 period. In some countries, the significant declining API has not been of constant magnitude; in Belize, the annual rate of reduction declined from 29.69% in the 1994–2000 period to 12.89% in the subsequent period. In Bolivia, there was a similar change in the rate of decline from 37.22% between 1998 and 2001 to 7.44% since 2001. The rapid API reduction rates were consequent of the fact that, for the 53-year period under review, both of these countries reported the highest API during 1990–1999, and the initial rapid decline was from those observed peaks. In contrast, the annual rate of decline increased in Brazil and Guatemala from 2.20% and 4.95% to 12.20% and 28.78%, respectively, with the joinpoint year being 2005 for Guatemala and 2006 for Brazil. A major reversal of malaria trend toward decline was seen in the 2000–2011 period; 13 countries had a significantly declining SPR in 2011, and 7 of them (Brazil, Costa Rica, Ecuador, French Guiana, Guatemala, Panama, and Suriname) changed from previously increasing or non-significant SPR during 2000–2011. Of note is the significantly declining SPR for over two decades in Mexico and El Salvador, both of which are presently in the pre-elimination phase.
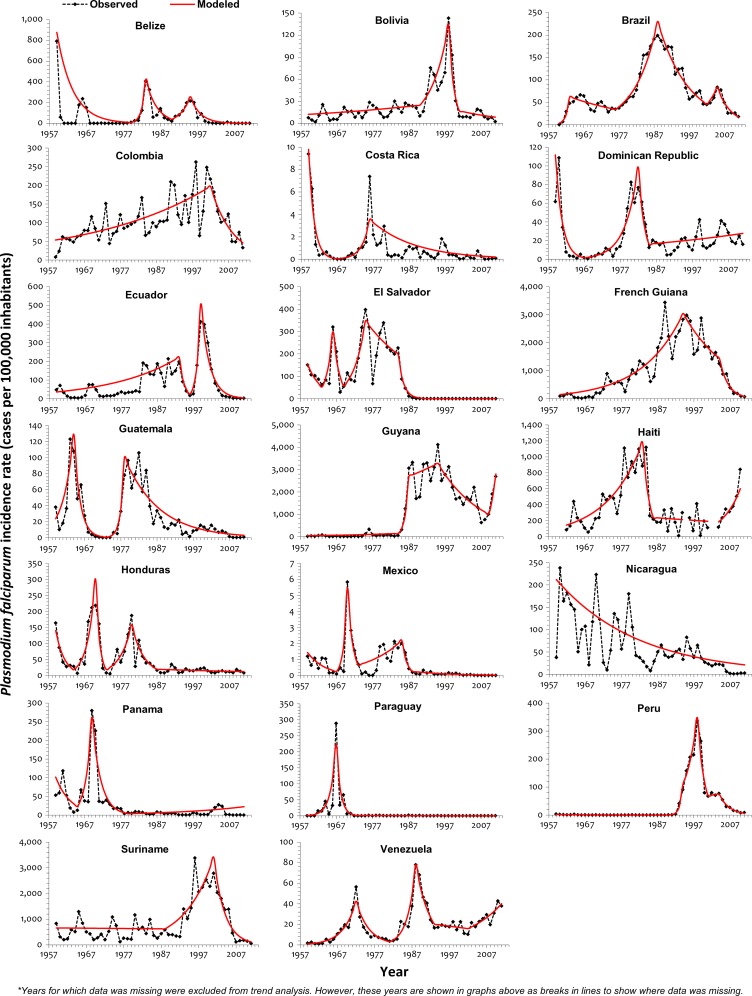
Species-specific trends.
Analysis of the P. falciparum incidence rate (PfIR) was not undertaken for Argentina and analysis of the P. vivax incidence rate (PvIR) was not undertaken for Haiti and Dominican Republic, because the number of malaria cases for the respective species was zero in those countries for most of the years. Trends in PvIR and PfIR were almost similar in Bolivia, Paraguay, and Venezuela (Figures 3 and 4 ). Joinpoint years for the two species-specific rates were not equal for any of the countries, signifying different species-specific trends for all countries (Table 3). There was no joinpoint in the PfIR for Nicaragua, but a significant reduction (P value < 0.05) was observed throughout the 53-year period studied. Similar to API and SPR trends, PvIR increased significantly in a number of countries during 1960–1985. Thereafter, a reversal was seen in subsequent years. PvIR reduced significantly in many countries in the latter half of 1990–1999 and almost all countries17 by the mid-2000–2011 period. By 1999, one-half of the countries had significantly declining PfIR, and this continued through 2000–2011. Increasing PfIR and PvIR have been observed in Guyana from 2008–2011. Argentina, El Salvador, Mexico, and Paraguay have not reported autochthonous P. falciparum cases in the past decade.
Figure 3.
Trend of PvIR (per 100,000 inhabitants) in the Americas, 1959–2011.
Figure 4.
Trend of PfIR (per 100,000 inhabitants) in the Americas, 1959–2011.
Table 3.
APC in PvIRs and PfIRs (per 100,000 inhabitants) in the Americas, 1959–2011
| Country | PvIR | PfIR | ||||
|---|---|---|---|---|---|---|
| Number of joinpoints | Year range | APC (%; 95% confidence interval) | Number of joinpoints | Year range | APC (%; 95% confidence interval) | |
| Argentina* | 3 | 1959–1962 | 48.27 (−3.63, 128.11) | |||
| 1962–1965 | −53.24 (−88.33, 87.33) | |||||
| 1965–1996 | 1.38 (−1.04, 3.86) | |||||
| 1996–2011 | −19.29 (−29.2, −8.00) | |||||
| Belize | 5 | 1959–1972 | −6.49 (−20.86, 10.49) | 4 | 1959–1979 | −20.72 (−27.41, −13.41) |
| 1972–1982 | 38.30 (21.53, 57.38) | 1979–1983 | 166.18 (11.90, 533.16) | |||
| 1982–1990 | −6.30 (−11.97, −0.25) | 1983–1990 | −28.45 (−41.49, −12.50) | |||
| 1990–1994 | 38.40 (17.06, 63.63) | 1990–1995 | 44.52 (5.65, 97.69) | |||
| 1994–1999 | −31.15 (−38.88, −22.45) | 1995–2011 | −30.70 (−41.79, −17.49) | |||
| 1999–2011 | −14.25 (−20.52, −7.49) | |||||
| Bolivia | 2 | 1959–1998 | 7.36 (6.15, 8.58) | 3 | 1959–1990 | 2.31 (−0.36, 5.06) |
| 1998–2001 | −31.03 (−56.57, 9.53) | 1990–1998 | 23.54 (11.52, 36.86) | |||
| 2001–2011 | −8.69 (−14.89, −2.03) | 1998–2001 | −49.70 (−70.98, −12.83) | |||
| 2001–2011 | −6.89 (−19.10, 7.16) | |||||
| Brazil | 4 | 1960–1963 | 58.56 (−22.50, 224.44) | 5 | 1960–1963 | 165.07 (−56.05, 1,498.76) |
| 1963–1970 | −17.49 (−29.42, −3.55) | 1963–1976 | −4.49 (−8.32, −0.49) | |||
| 1970–1989 | 11.66 (9.39, 13.98) | 1976–1988 | 17.21 (13.73, 20.80) | |||
| 1989–2006 | −0.18 (−1.38, 1.04) | 1988–2002 | −10.89 (−12.54, −9.20) | |||
| 2006–2011 | −11.06 (−17.98, −3.56) | 2002–2005 | 21.96 (−30.02, 112.57) | |||
| 2005–2011 | −23.18 (−30.20, −15.44) | |||||
| Colombia | 1 | 1959–1995 | 6.66 (5.47, 7.87) | 1 | 1959–2002 | 3.07 (2.19, 3.97) |
| 1995–2011 | −5.43 (−7.35, −3.48) | 2002–2011 | −14.93 (−21.08, −8.30) | |||
| Costa Rica | 4 | 1959–1967 | 9.92 (−1.70, 22.90) | 2 | 1959–1967 | −48.27 (−60.18, −32.79) |
| 1967–1970 | −64.33 (−86.86, −3.15) | 1967–1976 | 60.50 (7.44, 139.76) | |||
| 1970–1989 | 5.04 (−3.79, 14.67) | 1976–2011 | −7.87 (−10.26, −5.42) | |||
| 1989–1992 | 97.86 (−5.00, 312.07) | |||||
| 1992–2011 | −10.11 (−13.72, −6.34) | |||||
| Dominican Republic† | 3 | 1959–1967 | −41.15 (−55.42, −22.30) | |||
| 1967–1982 | 31.57 (18.90, 45.58) | |||||
| 1982–1985 | −45.09 (−77.19, 32.16) | |||||
| 1985–2011 | 2.06 (−0.36, 4.54) | |||||
| Ecuador | 5 | 1959–1969 | 28.75 (11.16, 49.11) | 3 | 1959–1993 | 5.48 (2.40, 8.63) |
| 1969–1973 | −54.00 (−74.64, −16.56) | 1993–1996 | −51.45 (−80.00, 17.82) | |||
| 1973–1984 | 31.51 (15.79, 49.37) | 1996–1999 | 170.85 (−4.70, 669.52) | |||
| 1984–1997 | −10.84 (−16.59, −4.69) | 1999–2011 | −33.39 (−42.77, −22.47) | |||
| 1997–2001 | 41.76 (2.36, 96.33) | |||||
| 2001–2011 | −33.35 (−43.61, −21.22) | |||||
| El Salvador | 2 | 1959–1966 | 23.34 (5.83, 43.76) | 5 | 1959–1963 | −23.54 (−43.78, 3.98) |
| 1966–1982 | 0.87 (−1.86, 3.68) | 1963–1966 | 78.10 (−40.59, 433.94) | |||
| 1982–2011 | −22.94 (−27.43, −18.17) | 1966–1969 | −42.34 (−83.90, 106.45) | |||
| 1969–1975 | 35.04 (15.90, 57.34) | |||||
| 1975–1984 | −6.08 (−11.57, −0.24) | |||||
| 1984–2011 | −49.49 (−61.39, −33.93) | |||||
| French Guiana | 5 | 1960–1986 | 9.29 (4.83, 13.95) | 2 | 1960–1994 | 9.27 (6.79, 11.80) |
| 1986–1990 | 82.59 (49.85, 122.49) | 1994–2004 | −7.25 (−11.66, −2.62) | |||
| 1990–1993 | −47.29 (−61.88, −27.11) | 2004–2011 | −33.84 (−44.92, −20.54) | |||
| 1993–2003 | −1.39 (−5.42, 2.81) | |||||
| 2003–2006 | 41.44 (−5.46, 111.60) | |||||
| 2006–2011 | −33.10 (−39.22, −26.37) | |||||
| Guatemala | 4 | 1959–1966 | 17.84 (−2.55, 42.50) | 3 | 1959–1964 | 40.28 (6.70, 84.43) |
| 1966–1975 | −15.12 (−26.77, −1.63) | 1964–1973 | −44.27 (−63.78, −14.24) | |||
| 1975–1978 | 124.65 (7.46, 369.63) | 1973–1978 | 172.21 (0.19, 639.63) | |||
| 1978–2005 | −4.75 (−5.97, −3.52) | 1978–2011 | −10.04 (−11.88, −8.15) | |||
| 2005–2011 | −28.38 (−40.59, −13.66) | |||||
| Guyana | 4 | 1959–1995 | 16.96 (14.42, 19.55) | 4 | 1959–1984 | 4.89 (−4.63, 15.35) |
| 1995–1999 | −18.52 (−36.77, 5.00) | 1984–1987 | 165.52 (−16.33, 742.54) | |||
| 1999–2005 | 8.14 (−3.68, 21.41) | 1987–1995 | 2.45 (−4.23, 9.60) | |||
| 2005–2008 | −34.79 (−71.43, 48.84) | 1995–2009 | −8.06 (−11.22, −4.79) | |||
| 2008–2011 | 23.72 (−16.31, 82.91) | 2009–2011 | 66.50 (−3.20, 186.39) | |||
| Haiti‡ | 3 | 1962–1983 | 10.50 (7.63, 13.45) | |||
| 1983–1986 | −41.13 (−82.66, 99.90) | |||||
| 1986–2004 | −1.46 (−6.41, 3.76) | |||||
| 2004–2011 | 21.75 (9.69, 35.13) | |||||
| Honduras | 1 | 1959–1996 | 1.52 (−0.08, 3.15) | 5 | 1959–1964 | −33.67 (−48.31, −14.87) |
| 1996–2011 | −17.61 (−22.62, −12.28) | 1964–1970 | 59.80 (24.75, 104.71) | |||
| 1970–1973 | −60.34 (−89.34, 47.55) | |||||
| 1973–1980 | 35.58 (14.36, 60.74) | |||||
| 1980–1987 | −25.87 (−37.06, −12.68) | |||||
| 1987–2011 | −1.94 (−4.56, 0.74) | |||||
| Mexico | 5 | 1959–1970 | 19.30 (9.50, 29.96) | 5 | 1959–1967 | −19.19 (−31.86, −4.17) |
| 1970–1978 | −16.41 (−25.51, −6.20) | 1967–1970 | 175.74 (−63.08, 1,959.58) | |||
| 1978–1985 | 33.31 (18.51, 49.96) | 1970–1973 | −50.62 (−77.09, 6.42) | |||
| 1985–1989 | −8.18 (−22.48, 8.75) | 1973–1985 | 10.55 (0.90, 21.13) | |||
| 1989–1992 | −44.45 (−69.06, −0.27) | 1985–1988 | −50.86 (−83.90, 50.00) | |||
| 1992–2011 | −11.12 (−17.22, −4.57) | 1988–2011 | −9.78 (−17.60, −1.23) | |||
| Nicaragua | 2 | 1959–1992 | 1.27 (−0.46, 3.02) | 0 | 1959–2011 | −4.33 (−5.57, −3.08) |
| 1992–1996 | 22.56 (−4.63, 57.51) | |||||
| 1996–2011 | −29.42 (−35.69, −22.53) | |||||
| Panama | 4 | 1959–1983 | −14.85 (−16.69, −12.96) | 3 | 1959–1965 | −21.45 (−43.60, 9.39) |
| 1983–1986 | 69.09 (−96.43, 7,909.03) | 1965–1969 | 81.97 (−2.21, 238.61) | |||
| 1986–1997 | −7.58 (−15.30, 0.84) | 1969–1979 | −33.98 (−42.17, −24.63) | |||
| 1997–2004 | 33.77 (18.25, 51.31) | 1979–2011 | 5.47 (1.10, 10.03) | |||
| 2004–2011 | −33.07 (−41.23, −23.77) | |||||
| Paraguay | 3 | 1959–1967 | 55.60 (31.33, 84.36) | 2 | 1959–1967 | 92.87 (57.41, 136.32) |
| 1967–1972 | −56.42 (−74.09, −26.70) | 1967–1973 | −57.43 (−69.27, −41.02) | |||
| 1972–1999 | 6.46 (−3.05, 16.90) | 1973–2011 | −5.60 (−11.53, 0.73) | |||
| 1999–2011 | −28.84 (−43.12, −10.98) | |||||
| Peru | 2 | 1959–1991 | 5.09 (2.07, 8.19) | 5 | 1959–1989 | −8.34 (−11.52, −5.06) |
| 1991–1995 | 37.77 (10.14, 72.32) | 1989–1994 | 264.76 (172.52, 388.23) | |||
| 1995–2011 | −11.84 (−14.32, −9.29) | 1994–1998 | 34.01 (27.09, 41.29) | |||
| 1998–2001 | −41.51 (−49.21, −32.64) | |||||
| 2001–2004 | 2.49 (−14.34, 22.63) | |||||
| 2004–2011 | −29.47 (−32.01, −26.84) | |||||
| Suriname | 3 | 1959–1988 | 12.48 (8.49, 16.62) | 2 | 1959–1988 | −0.19 (−3.33, 3.06) |
| 1988–1991 | −58.29 (−98.56, 1,110.36) | 1988–2001 | 13.94 (5.35, 23.24) | |||
| 1991–1998 | 75.14 (46.23, 109.76) | 2001–2011 | −28.84 (−39.98, −17.03) | |||
| 1998–2011 | −9.12 (−11.33, −6.86) | |||||
| Venezuela | 4 | 1959–1971 | 20.36 (8.29, 33.78) | 5 | 1959–1972 | 33.63 (21.99, 46.38) |
| 1971–1980 | −21.84 (−32.02, −10.13) | 1972–1981 | −25.25 (−32.71, −16.97) | |||
| 1980–1989 | 32.32 (17.93, 48.46) | 1981–1988 | 58.54 (38.76, 81.14) | |||
| 1989–1993 | −20.28 (−41.19, 8.06) | 1988–1993 | −23.54 (−32.98, −12.77) | |||
| 1993–2011 | 3.07 (0.75, 5.45) | 1993–2002 | −2.69 (−8.93, 3.99) | |||
| 2002–2011 | 10.80 (6.54, 15.23) | |||||
Data from Argentina were not analyzed for PfIR.
Data from Dominican Republic were not analyzed for PvIR.
Data from Haiti were not analyzed for PvIR.
Trends (AAPC) since 2000.
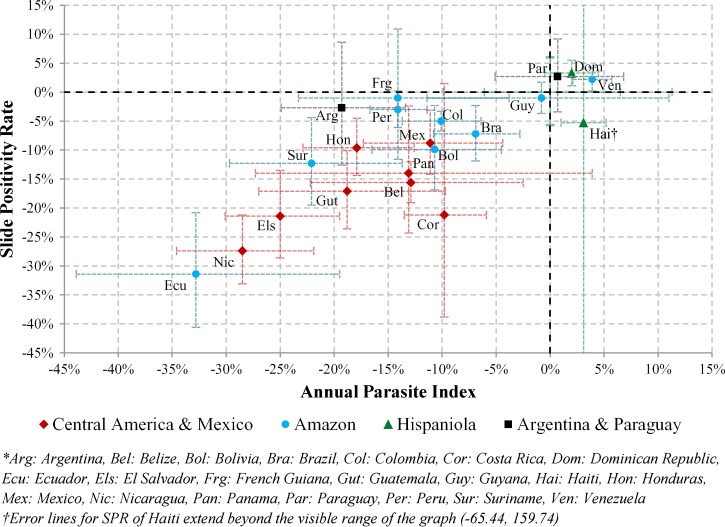
Significant decreases (P value < 0.05) in the AAPC of the API were observed in 16 of 21 endemic countries during the 2000–2011 period (Figure 5 ). In 11 of 16 countries, significant decreases in AAPC for the SPR were also observed. In the Mesoamerican subregion, there was a significant decline in malaria (Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, and Mexico), with reductions in the API of over 9% annually, and with the exception of Costa Rica, there were significant decreases in SPR, being higher than 8% annually (Table 4). This rapid and sustained decline in the whole subregion in the last decade has formed the basis for present regional malaria elimination efforts in these countries. Five of eight countries that share the Amazon Basin in South America, namely Bolivia, Brazil, Colombia, Ecuador, and Suriname, had significant reductions in AAPC for both API and SPR. In Ecuador, malaria declined at a very rapid pace: more than 30% annual decreases in both API and SPR. Suriname, El Salvador, and Nicaragua were other countries where malaria declined significantly: over 20% annual decline in API. Analyses for Paraguay revealed no significant trends for either the SPR or API, but after removing the epidemic years of the 1960s, significant decline in both the API and SPR was observed in recent years (2000–2011). However, API increased by over 3% annually in Haiti and Venezuela. Even in absolute numbers, the case burden has decreased since the last joinpoint for most countries in the American continents (Table 5).
Figure 5.
AAPC in API and SPR by subregion for 2000–2011.
Table 4.
AAPC in API and SPR by country, 2000–2011
| Country | AAPC | |
|---|---|---|
| API | SPR | |
| Argentina | −19.26 (−29.14, −8.00)* | −2.67 (−8.23, 3.22) |
| Belize | −12.89 (−22.18, −2.49)* | −15.56 (−19.02, −11.95)* |
| Bolivia | −10.66 (−16.42, −4.51)* | −9.91 (−16.93, −2.29)* |
| Brazil | −6.86 (−10.75, −2.80)* | −7.23 (−11.93, −2.29)* |
| Colombia | −10.08 (−13.63, −6.39)* | −5.02 (−6.68, −3.33)* |
| Costa Rica | −9.82 (−13.53, −5.94)* | −21.18 (−38.82, 1.53) |
| Dominican Republic | 2.00 (−0.49, 4.54) | 3.25 (1.09, 5.46)* |
| Ecuador | −32.81 (−43.93, −19.49)* | −31.41 (−40.56, −20.85)* |
| El Salvador | −24.98 (−30.08, −19.51)* | −21.42 (−28.58, −13.54)* |
| French Guiana | −14.14 (−23.30, −3.88)* | −1.00 (−11.60, 10.90)* |
| Guatemala | −18.79 (−27.01, −9.66)* | −17.09 (−23.58, −10.06)* |
| Guyana | −0.76 (−11.32, 11.06)* | −1.01 (−3.68, 1.72) |
| Haiti | 3.12 (1.02, 5.26)* | −5.268 (−6.44, 159.74) |
| Honduras | −17.86 (−22.83, −12.56)* | −9.63 (−14.46, −4.51)* |
| Mexico | −11.11 (−17.34, −4.41)* | −8.81 (−14.16, −3.13)* |
| Nicaragua | −28.46 (−34.53, −21.82)* | −27.44 (−33.18, −21.22)* |
| Panama | −13.07 (−27.28, 3.93) | −13.96 (−24.21, −2.33)* |
| Paraguay | 0.73 (−5.36, 7.22) | 2.67 (−3.11, 8.79) |
| Peru | −14.10 (−16.73, −11.38)* | −2.97 (−6.03, 0.19) |
| Suriname | −22.11 (−29.67, −13.73)* | −12.31 (−19.52, −4.46)* |
| Venezuela | 3.91 (2.12, 5.74)* | 2.22 (0.21, 4.27)* |
Significant at the 0.05 level.
Table 5.
Last joinpoint year (API) and number of malaria cases by country
| Country | Last joinpoint year | Number of malaria cases* | |
|---|---|---|---|
| Joinpoint year | 2011 | ||
| Argentina | 1996 | 2,048 | 18 |
| Belize | 2000 | 1,486 | 79 |
| Bolivia | 2001 | 15,765 | 7,143 |
| Brazil | 2006 | 549,469 | 267,045 |
| Colombia | 2001 | 231,233 | 64,436 |
| Costa Rica | 1992 | 6,951 | 17 |
| Dominican Republic | 1985 | 816 | 1,616 |
| Ecuador | 2001 | 108,903 | 1,233 |
| El Salvador | 1982 | 86,202 | 15 |
| French Guiana | 2009 | 3,462 | 1,209 |
| Guatemala | 2005 | 39,571 | 6,817 |
| Guyana | 2009 | 13,673 | 29,471 |
| Haiti | 2004 | 10,802 | 32,969 |
| Honduras | 1996 | 91,799 | 7,615 |
| Mexico | 1992 | 16,170 | 1,124 |
| Nicaragua | 1996 | 75,606 | 925 |
| Panama | 2004 | 5,095 | 354 |
| Paraguay | 1971 | 423 | 10 |
| Peru | 1996 | 211,561 | 22,878 |
| Suriname | 2001 | 16,003 | 795 |
| Venezuela | 1993 | 12,539 | 45,824 |
Total cases include cases imported from other countries.
Discussion
Trends presented here are based on reported cases, which are affected by robustness of surveillance systems and quality and coverage of diagnostic services. However, trends for most countries and periods in our study seem to generally reflect the historic incidence of disease. Several factors may be responsible for malaria increases in these years: some specific to countries and others specific to certain periods of time. In general, malaria increased during the first decade of the eradication efforts in the 1950s in the Americas, probably associated with improved case detection and reporting. Malaria spiked in the 1970s as vector resistance to insecticides, primarily dichloro diphenyl trichloroethane (DDT), became established in most Central American countries; between 1975 and 1978, malaria incidence increased by almost 11 times in Guatemala because of insecticide resistance.18 However, drug resistance was responsible for sporadic outbreaks in those years in South America; introduction of chloroquine-resistant P. falciparum from the Marowijne region of Suriname to the Upper Suriname river area led to a fivefold increase in cases in that area in 1974 and 1975.19 Hurricanes David and Federico in 1979 were reasons for increases in parts of Central America and the Caribbean countries.20
Disruptions in health services during political and social disturbances were responsible for increases in some countries; decrease and cessation of vector control activities by 1979 in Nicaragua because of the revolution led to an accompanying increase in malaria.21 Areas of this country affected by Contra attacks reported an increase in cases, whereas other unaffected areas reported decreases during 1983–1985, leading to only a slight change in malaria incidence overall. Change of agricultural practices was a principal reason for the increase in malaria in the 1990s in many Central American countries; switching from growing rice and cotton to African palm and banana was associated with an increase in breeding sites caused by deforestation and associated work-related migration of people, often from malaria-endemic areas.22 A 10-fold increase was reported in Costa Rica between 1989 and 1992 because of an earthquake in 1991, and as the banana plantation industry flourished in the Huetar Atlantic Region of the country, malaria transmission shifted from the Pacific to the Atlantic coast during 1991–1999.20,23 Most countries in South America, excluding Ecuador, had increased malaria incidence during 1993–1995, because indoor residual sprayings with DDT were decreased or abolished.24 Decentralization of health systems, integration of the vertical national malaria eradications programs into the system, displacement of health personnel dedicated to combat malaria, and reduced malaria-specific resources are among the factors associated with increases in malaria in most countries during different periods.25
Reduction in malaria started well in the 1990–1999 period and continued throughout 2000–2011. However, some countries had the highest malaria incidence rates of the five decades during the 1990–1999 period, including Belize, Bolivia, Costa Rica, Guyana, Nicaragua, Peru, and Suriname. Other countries also reported spikes in malaria incidence in the same period. Some of these were specific to the El Niño Southern Oscillation effect, especially the peak observed in 1996 in Colombia, Ecuador, and Peru. It has been previously shown that this environmental phenomenon has been associated with malaria epidemics in Colombia, Peru, Venezuela, and Guyana in the 1970s and 1980s.26–28 Malaria funding also declined regionally in the 1990s; between 1992 and 1998, funds available per person for malaria control declined from US$1.10 to US$0.40.29
The majority of the countries had simultaneous significant downtrends for both API and SPR during the 2000–2011 period, which can be considered a demonstration of a declining malaria incidence accompanied by consistently functional surveillance. This is related to increased malaria control and surveillance efforts in most endemic countries, 15 of which received external financial support through Global Fund for AIDS, Tuberculosis and Malaria (Global Fund) grants during that period. Policy changes and improvement in implementation of interventions based on evidence generated within the ambit of the Amazon Malaria Initiative (AMI) supported by the US Agency for International Development and with technical cooperation from PAHO, Centers for Disease Prevention and Control, Management Sciences for Health, US Pharmacopeia, Research Triangle Initiative, and Links Media have also led to this decrease.30
Venezuela, Dominican Republic, and Haiti show an increasing malaria incidence in recent years. In Venezuela, this is accompanied by an increasing proportion of cases caused by P. falciparum. Adjoining areas of Guyana have been presenting a similar picture in recent years associated with increased gold mining in the Amazon forest areas of these two countries. This phenomenon has also increased the population at risk of malaria and in most instances, in virgin rainforests with limited access to health clinics. This underlines the shared risk factors of mobile populations living at the high risk associated with gold mining in these two countries as well as Suriname, French Guiana, and adjoining areas of Brazil.31–33 Malaria has declined in all other countries sharing the Amazon Basin. Brazil and Colombia together report more than one-half of the malaria cases in the Americas and have shown efforts in identifying and successfully targeting at-risk populations.13,34–36 A project for malaria in the Andean region across international boundaries was successful in reduction of the disease and reinforced the need for such efforts.37 P. falciparum malaria declined in the countries sharing the Amazon Basin that all changed the first line of treatment to artemisinin-based combination therapies during 2000–2011 based on evidence generated by therapeutic efficacy studies within the Amazon Network for the Surveillance of Antimalarial Drug Resistance (RAVREDA) initiative supported by AMI. However, P. vivax malaria also declined at the same time, pointing to the probable impact of other interventions for malaria control or changes in socioeconomic and/or environmental factors that were at play. However, additional studies are required for analyzing these associations.
Haiti and Dominican Republic, sharing the island of Hispaniola, have exclusively P. falciparum transmission and present a challenge, because they are the only focus of continued malaria transmission in the Caribbean. A large proportion of cases in Dominican Republic has reportedly been imported from Haiti, leading to an increase in the total case incidence, although the number of autochthonous cases in the country has been declining. Natural disasters have been associated with increases in malaria incidence on the island; Hurricanes David and Federico led to increase in 1981–1982 and Hurricane George led to an increase in 1998 in Dominican Republic. Recently, the Haiti earthquake in 2010 led to a 67% increase compared with 2009. It is likely that this is an artefact, because the increase was mostly from the center department of the country, which was most affected by the earthquake, and the same persons may have been tested more than one time by different relief agencies, leading to duplication of data. Limited access to diagnosis and treatment and a weak surveillance system increase the uncertainty of observed malaria incidence trends in the country; for example, the SPR reported by the country varied from 97.4% in 1998 to 5.6% in 1999.38 Malaria cases imported from this country have been associated with outbreaks in other Caribbean countries; conditions for malaria transmission exist in many other island nations.39
Mexico, El Salvador, and Costa Rica, three countries in the pre-elimination phase, have had sustained reductions in malaria incidence for two or more recent decades. They do not report autochthonous P. falciparum cases, and malaria is limited to focal areas of these countries.40,41 In other Central American countries, namely Belize, Guatemala, Honduras, Nicaragua, and Panama, malaria has also declined significantly, and the disease is now focalized in pockets of the subregion. Although Honduras and Nicaragua have been reporting higher proportions of cases caused by P. falciparum in the last decade, this is considered a result of increased surveillance in areas with previously greater inaccessibility in the shared La Moskitia region between the two countries. All of the Central American countries are preparing to progress toward the pre-elimination and elimination phases. Political support for this goal was evidenced in the form of a recent resolution passed by the ministers of health of the Mesoamerican countries as well as the Dominican Republic and Haiti to eliminate malaria by 2020.42
Argentina and Paraguay, in the southern cone of South America, are also targeted for elimination; they are both presently in the pre-elimination phase with exclusively P. vivax transmission. Paraguay reported an outbreak of malaria in 1999, but thereafter, malaria decreased significantly, with only one autochthonous case reported in 2011 and none up to week 51 in 2012.43 Its efforts at decreasing malaria were recognized, and the national malaria control program of the country was awarded the Malaria Champion of the Americas in the year 2012.44
Overall, funding for malaria control from national sources kept increasing while financing from external sources declined between 1957 and 1986 with waning interest of donor agencies, largely mirroring the malaria problem as it grew during those years.22 In particular, in 1988 in Haiti, a major financial crisis led to closing of the National Malaria Eradication Program.38 However, funding for malaria control has been increasing again since 2000 from both national sources and external aid, particularly since 2002 through the Global Fund, and it has been accompanied with a decline in malaria in most countries. However, additional investigation is required to adeptly relate the malaria trend in the American continents to historic trends in malaria financing and coverage of other malaria control interventions. The new funding model of the Global Fund takes the countries' World Bank classification based on the gross domestic product (GDP) and malaria burden into account. Thus, many previously eligible countries in the Americas will have less likelihood of receiving external funding. Given the fact that 18 of the endemic countries have shown marked decreases in malaria burden and some of these have also intimated interest in eliminating the disease, it is imperative that national resources presently available continue to be destined to further malaria reduction. Also, additional resources should be made available to ensure functional surveillance systems in areas where elimination has been achieved to prevent reintroduction. Failure to adequately address funding shortages in years ahead could lead to increases in malaria, which has been previously seen.
In a 2011 article, White45 proposed that increased P. falciparum incidence could lead to an increase in P. vivax because of the activation of hypnozoites in areas where transmission of the two species occurs simultaneously. Such a temporal increase pattern was seen in Brazil and Venezuela during the 1985–1988 period and Ecuador during 1997–1999. However, such an association can be better ascertained with data collected at weekly or monthly intervals and at the level of localities or foci. Associations like these will require more detailed analysis to better establish this relationship, because the data presented here do not so permit.
Data for some years were missing in our study (< 1%), but the proportion of unavailable data points was small, and as such, the effect of missing data is considered to be minimal. Although Chaparro and Padillo46 used joinpoint regression analysis for malaria deaths in Colombia, this methodology has mostly been used for chronic diseases, such as cancer, which unlike malaria, are not prone to rapid changes in annual incidence rates.46–52 Thus, epidemic years could significantly affect the malaria trend, which was the case in Paraguay, where an otherwise non-significant trend in recent years changed to a significant decline on removing the epidemic year of 1969 and previous years. Furthermore, the innate variability in reporting of incidence data because of social, economic, and programmatic reasons also limits interpretation of trends. However, use of joinpoint regression in this study has provided satisfactory results. Decreased cases were reported in 1996 and 1997 in Guatemala owing to temporary weakening of information systems as a result of decentralization; however, this artefact did not affect the declining trend since the late 1970s that was evidenced by joinpoint methodology. However, large and abrupt variation in cases, which was seen in some countries in the 1960s and 1970s, resulted in non-significant trends, signifying possible inadequate quality of data. This suggests that joinpoint methodology is robust and may also be considered for other infectious diseases, like tuberculosis and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).
Although this study used country-level data, malaria incidence rates could both increase and decrease at the state or district level within a country in the same year or period. State- or district-level analyses would, thus, present a better understanding of underlying patterns of malaria incidence for use in decision-making at the subnational and local levels, especially when pursuing the goal of malaria elimination. API depends on the definition of the population at risk; the population of the whole country is not accurate for calculating API, because it underestimates the incidence. SPR is conservative, because it measures incidence of malaria in only suspected or tested cases, which are always fewer than the total population at risk. Although both the definition of a suspected case and the coverage of diagnosis impact SPR, it has been proven to be a reliable indicator for measurement of malaria incidence trends.53 Simultaneous analyses of both API and SPR provide more reliability in trends, because convergent trends in these two indicators signify actual decline in malaria incidence, whereas divergent trends could be caused by inadequate information or decreases in coverage of diagnosis. This is well-observed between 1986 and 2004 in Guatemala, where declining API would give an impression of decreasing malaria, although increasing SPR points to the problems of decreases in diagnosis and treatment coverage and associated surveillance quality. National surveillance systems and coverage of the national malaria programs have improved over the past 50 years in the endemic countries of the Americas.54,55 Countries of the Americas have moved from reporting of malaria cases as aggregate data to nominal case reporting, thereby decreasing the chances of duplication of data and improving the quality of data. Presently, all countries, except Haiti, have nominal reporting systems implemented completely or partially throughout the country. Laboratory-confirmed cases have been used in this study; improvement in the coverage and quality of diagnostic services over the years is, thus, a limiting factor in interpreting results. Furthermore, improvements in reporting completeness and timeliness and changes in surveillance systems, definitions, and health-seeking behavior also limit comparison of results over various decades. Recent annual incidence rates are more specific than those of the previous decades, which could lead to an under- or overestimation of trends. Use of annual incidence estimates adjusted for these improvements over the study period would have prevented this error; however, this is beyond the scope of this study. Factors affecting these historical trends and especially, those during the 2000–2011 period will be further analyzed and presented separately in a future manuscript.
The majority of the endemic countries on the American continents had significantly decreasing trends in malaria incidence in recent years. Although such significant decreases have been seen in previous years in several of these countries, in the past, a sustained decline spanning more than one decade and more so, in almost all of the countries at the same time has been uncommon. The recent achievements are considered a reflection of sustained efforts of country programs and increased funding for malaria and vector-borne disease control accompanied by political will and commitment at the national and global levels.2,14,56,57 The sustained focus on malaria through the establishment and monitoring of MDGs has been an important catalyst for this decline, and it gives credence to the suggestion that the post-MDG agenda should include goals for further decline and possible elimination of malaria. The current PAHO strategy and plan of action for malaria in 2011–2015 took the declining trend into account and included malaria elimination where feasible as a goal. It will transition into the post-2015 strategy and plan of action, which will be aligned with the global technical strategy for malaria being developed by the WHO. In that context, for the American continents, the current situation presents a unique opportunity to develop multicountry malaria elimination efforts as models for other parts of the world, which also highlight and underline the importance of sustained efforts for malaria control and its possible elimination in the Americas. However, newer challenges have emerged, including the recent report of a suspected decrease in artemisinin efficacy in Guyana and Suriname, which has necessitated immediate steps for the control of malaria in the Guiana Shield.58,59 Although the reported trends in the last decade can catalyze further efforts to eliminate the disease from the Americas, as the malaria burden decreases, the lessons of reintroduction in Jamaica in 2006 and the need for constant surveillance after elimination must not be forgotten.39
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the representatives of the national malaria programs of the ministries of health who have provided the data for this study over the years.
Disclaimer: K.H.C. had full access to all the data in the study and had the final responsibility for the decision to submit this article for publication. The funders had no role in study design, data collection, analysis and interpretation, writing of the manuscript, or decision to submit the manuscript. Views expressed in this article are those of the authors and in no manner reflect the official position of PAHO/WHO.
Footnotes
Financial support: The Amazon Malaria Initiative (AMI) funded by the US Government through its US International Agency for Development (USAID) in coordination with Pan American Health Organization/World Health Organization (PAHO/WHO) has supported efforts of the Amazon Network for the Surveillance of Antimalarial Drug Resistance (RAVREDA) of the malaria programs in the countries sharing the Amazon Basin since 2002 and in recent years, expanded to some countries in Central America.
Authors' addresses: Keith H. Carter, Prabhjot Singh, Rainier P. Escalada, Maria Paz Ade, Luis Gerardo Castellanos, and Marcos A. Espinal, Department of Communicable Diseases and Health Analysis, Pan American Health Organization/World Health Organization, Washington, DC, E-mails: carterke@paho.org, singhpra@paho.org, escaladr@paho.org, ademarap@paho.org, castellanosl@paho.org, and espinalm@paho.org. Oscar J. Mujica, Special Program for Sustainable Development and Health Equity, Pan American Health Organization/World Health Organization, Washington, DC, E-mail: mujicaos@paho.org.
References
- 1.Pan American Health Organization . Pro Salute Novi Mundi: A History of the Pan American Health Organization. Washington, DC: PAHO; 1992. [Google Scholar]
- 2.World Health Organization . World Malaria Report: 2012. Geneva: WHO Press; 2012. [Google Scholar]
- 3.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Global Malaria Control and Elimination: Report of a Technical Review. Geneva: World Health Organization; 2008. p. 54. [Google Scholar]
- 5.Najera JA, Gonzalez-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969) PLoS Med. 2011;8:e1000412. doi: 10.1371/journal.pmed.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002;15:564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidson C. Global malaria challenge: the Amsterdam summit. Southeast Asian J Trop Med Public Health. 1992;23:635–640. [PubMed] [Google Scholar]
- 8.Pan American Health Organization . CD42.R15—Roll Back Malaria in the Americas. The 42nd Directing Council. Washington, DC: PAHO; 2000. p. 2. [Google Scholar]
- 9.United Nations General Assembly 55/284–2001–2010: Decade to Roll Back Malaria in developing countries, particularly in Africa; Proceedings of the 55th Session of the General Assembly; New York, NY. 2001. September 28, 2001. [Google Scholar]
- 10.United Nations General Assembly 66/289—Consolidating gains and accelerating efforts to control and eliminate malaria in developing countries, particularly in Africa, by 2015; Proceedings of the 66th Session of the General Assembly; New York, NY. 2012. October 15, 2012. [Google Scholar]
- 11.Regional Malaria Program . Regional Strategic Plan for Malaria in the Americas, 2006–2010. Washington, DC: Pan American Health Organization; 2006. [Google Scholar]
- 12.Pan American Health Organization . CD;51/11—Strategy and Plan for Action for Malaria. 51st Directing Council. Washington, DC: Pan American Health Organization; 2011. [Google Scholar]
- 13.Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT. Malaria in Brazil: an overview. Malar J. 2010;9:115. doi: 10.1186/1475-2875-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 15.United Nations Department of Economic and Social Affairs Population Division . World Population Prospects: The 2010 Revision. CD-ROM Ed.; New York, NY: United Nations Department of Economic and Social Affairs Population Division; 2011. [Google Scholar]
- 16.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute . Joinpoint Regression Program. Calverton, MD: National Cancer Institute; 2012. Version 4.0. [Google Scholar]
- 18.Pan American Health Organization . XXVI Meeting of the Directing Council of the Pan American Health Organization and the XXXI Meeting of the Regional Committee of the World Health Organization. Washington, DC: Pan American Health Organization; 1979. Status of malaria programs in the Americas, XXVII report. [PubMed] [Google Scholar]
- 19.Rozendaal JA. Epidemiology and control of malaria in Suriname. Bull Pan Am Health Organ. 1991;25:336–355. [PubMed] [Google Scholar]
- 20.Pan American Health Organization Roll Back Malaria in Meso America; Proceedings of the Meeting in the Dominican Republic with the Participation of Central America Countries, Mexico, Haiti and Dominican Republic; San Pedro de Macoris, Dominican Republic. 2000. November 24, 2000. [Google Scholar]
- 21.Garfield R. Malaria control in Nicaragua: social and political influences on disease transmission and control activities. Lancet. 1999;354:414–418. doi: 10.1016/S0140-6736(99)02226-6. [DOI] [PubMed] [Google Scholar]
- 22.Najera J. Malaria Control: Achievements Problems & Strategies. Geneva: World Health Organization; 1999. p. 126. [Google Scholar]
- 23.Saenz R, Bissell RA, Paniagua F. Post-disaster malaria in Costa Rica. Prehosp Disaster Med. 1995;10:154–160. doi: 10.1017/s1049023x00041935. [DOI] [PubMed] [Google Scholar]
- 24.Roberts DR, Laughlin LL, Hsheih P, Legters LJ. DDT, global strategies, and a malaria control crisis in South America. Emerg Infect Dis. 1997;3:295. doi: 10.3201/eid0303.970305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan American Health Organization Roll Back Malaria initiative in the tropical forest area of South America; Proceedings of the Meeting in Colombia with the Participation of South American Countries; Cartagena, Colombia. 2000. August 25, 2000. [Google Scholar]
- 26.Bouma MJ, Dye C. Cycles of malaria associated with El Niño in Venezuela. JAMA. 1997;278:1772–1774. [PubMed] [Google Scholar]
- 27.Gagnon AS, Smoyer-Tomic KE, Bush AB. The El Niño southern oscillation and malaria epidemics in South America. Int J Biometeorol. 2002;46:81–89. doi: 10.1007/s00484-001-0119-6. [DOI] [PubMed] [Google Scholar]
- 28.Bouma M, Poveda G, Rojas W, Chavasse D, Quinones M, Cox J, Patz J. Predicting high-risk years for malaria in Colombia using parameters of El Niño Southern Oscillation. Trop Med Int Health. 1997;2:1122–1127. doi: 10.1046/j.1365-3156.1997.d01-210.x. [DOI] [PubMed] [Google Scholar]
- 29.Pan American Health Organization Report on the status of malaria in the Americas, 1999; Proceedings of the 41st Session of the Directing Council of the Pan American Health Organization and the 51st Session of the Regional Committee of the World Health Organization; San Juan, Puerto Rico. 1999. September 27-October 1, 1999. [Google Scholar]
- 30.World Health Organization . Global Report on Antimalarial Efficacy and Drug Resistance: 2000–2010. Geneva: World Health Organization; 2010. [Google Scholar]
- 31.Rodríguez-Morales AJ, Delgado L, Martínez N, Franco-Paredes C. Impact of imported malaria on the burden of disease in northeastern Venezuela. J Travel Med. 2006;13:15–20. doi: 10.1111/j.1708-8305.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- 32.Moreno J, Rubio-Palis Y, Páez E, Pérez E, Sánchez V. Abundance, biting behaviour and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med Vet Entomol. 2007;21:339–349. doi: 10.1111/j.1365-2915.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 33.Barbieri AF, Sawyer DO. Heterogeneity of malaria prevalence in alluvial gold mining areas in Northern Mato Grosso State, Brazil. Cad Saude Publica. 2007;23:2878–2886. doi: 10.1590/s0102-311x2007001200009. [DOI] [PubMed] [Google Scholar]
- 34.Da Silva NS, da Silva-Nunes M, Malafronte RS, Menezes MJ, D'Arcadia RR, Komatsu NT, Scopel KK, Braga ÉM, Cavasini CE, Cordeiro JA. Epidemiology and control of frontier malaria in Brazil: lessons from community-based studies in rural Amazonia. Trans R Soc Trop Med Hyg. 2010;104:343–350. doi: 10.1016/j.trstmh.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira MU, da Silva-Nunes M. Evidence-based public health and prospects for malaria control in Brazil. J Infect Dev Ctries. 2010;4:533–545. doi: 10.3855/jidc.760. [DOI] [PubMed] [Google Scholar]
- 36.Mateus JC, Carrasquilla G. Predictors of local malaria outbreaks: An approach to the development of an early warning system in Colombia. Mem Inst Oswaldo Cruz. 2011;106:107–113. doi: 10.1590/s0074-02762011000900014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fundación para la Investigación y Desarrollo de la Salud y la Seguridad Social . El Impacto de las Reformas en Salud en Colombia, Ecuador, Peru y Venezuela en la situation de Malaria. Control de la Malaria en Zonas Fronterizas de los Países Andinos: Un enfoque comunitario—PAMAFRO. Bogota, Columbia: Organismo Andino de Salud/Convenio Hipólito Unanue; 2007. p. 122. [Google Scholar]
- 38.Anonymous Epidemiological stratification of malaria in the region of the Americas. Epidemiol Bull. 1991;12:1–16. [PubMed] [Google Scholar]
- 39.Webster-Kerr K, Peter Figueroa J, Weir PL, Lewis-Bell K, Baker E, Horner-Bryce J, Lewis-Fuller E, Bullock DuCasse M, Carter KH, Campbell-Forrester S. Success in controlling a major outbreak of malaria because of Plasmodium falciparum in Jamaica. Trop Med Int Health. 2011;16:298–306. doi: 10.1111/j.1365-3156.2010.02700.x. [DOI] [PubMed] [Google Scholar]
- 40.Hernández-Avila JE, Rodríguez MH, Betanzos-Reyes AF, Danis-Lozano R, Méndez-Galván JF, Velázquez-Monroy OJ, Tapia-Conyer R. Determinant factors for malaria transmission on the coast of Oaxaca State, the main residual transmission focus in Mexico. Salud Publica Mex. 2006;48:405–417. doi: 10.1590/s0036-36342006000500007. [DOI] [PubMed] [Google Scholar]
- 41.Ávila-Agüero ML. Epidemiología de la malaria en Costa Rica. Acta Med Costarric. 2008;50:72–74. [Google Scholar]
- 42.Consejo de Ministros de Salud de Centroamérica y República Dominicana (COMISCA) Declaración- Hacia la eliminación de la malaria en Mesoamérica y la Isla de la Española en el 2020. San José, Costa Rica: XXXVIII Reunión Ordinaria del COMISCA; 2013. [Google Scholar]
- 43.Gestion de Datos Epidemiologicos D Boletin Informativo Semanal del SENEPA. Sem Epidemiol. 2012;51 http://www.mspbs.gov.py/senepa/paludismo/ Available at. Accessed April 24, 2013. [Google Scholar]
- 44.Pan American Health Organization . PAHO Honors 2012 Malaria Champions of the Americas. 2012. http://new.paho.org/hq/index.php?option=com_content&view=article&id=7429&Itemid=39639&lang=en Available at. Accessed March 23, 2013. [Google Scholar]
- 45.White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaparro P, Padilla J. Mortalidad por paludismo en Colombia, 1979–2008. Biomedica. 2012;32:95–105. doi: 10.1590/S0120-41572012000500011. [DOI] [PubMed] [Google Scholar]
- 47.Bosetti C, Bertuccio P, Levi F, Lucchini F, Negri E, La Vecchia C. Cancer mortality in the European Union, 1970–2003, with a joinpoint analysis. Ann Oncol. 2008;19:631–640. doi: 10.1093/annonc/mdm597. [DOI] [PubMed] [Google Scholar]
- 48.Cayuela A, Rodriguez-Dominguez S, Lopez-Campos JL, Otero Candelera R, Rodriguez Matutes C. Joinpoint regression analysis of lung cancer mortality, Andalusia 1975–2000. Ann Oncol. 2004;15:793–796. doi: 10.1093/annonc/mdh170. [DOI] [PubMed] [Google Scholar]
- 49.Chen HS, Portier K, Ghosh K, Naishadham D, Kim HJ, Zhu L, Pickle LW, Krapcho M, Scoppa S, Jemal A, Feuer EJ. Predicting US- and state-level cancer counts for the current calendar year. Part I: evaluation of temporal projection methods for mortality. Cancer. 2012;118:1091–1099. doi: 10.1002/cncr.27404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez E, Gonzalez JR, Borras JM, Moreno V, Sanchez V, Peris M. Recent decline in cancer mortality in Catalonia (Spain). A joinpoint regression analysis. Eur J Cancer. 2001;37:2222–2228. doi: 10.1016/s0959-8049(01)00279-9. [DOI] [PubMed] [Google Scholar]
- 51.Jung KW, Shin HR, Kong HJ, Park S, Won YJ, Choi KS, Park EC. Long-term trends in cancer mortality in Korea (1983–2007): a joinpoint regression analysis. Asian Pac J Cancer Prev. 2010;11:1451–1457. [PubMed] [Google Scholar]
- 52.Kisic Tepavcevic D, Matejic B, Gazibara T, Pekmezovic T. Trends and patterns of ovarian cancer mortality in Belgrade, Serbia: a joinpoint regression analysis. Int J Gynecol Cancer. 2011;21:1018–1023. doi: 10.1097/IGC.0b013e31821dc8d1. [DOI] [PubMed] [Google Scholar]
- 53.Jensen TP, Bukirwa H, Njama-Meya D, Francis D, Kamya MR, Rosenthal PJ, Dorsey G. Use of the slide positivity rate to estimate changes in malaria incidence in a cohort of Ugandan children. Malar J. 2009;8:213. doi: 10.1186/1475-2875-8-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider D, Evering-Watley M, Walke H, Bloland P. Training the global public health workforce through applied epidemiology training programs: CDC's experience, 1951–2011. Public Health Rev. 2011;33:190–203. [Google Scholar]
- 55.Lescano AG, Salmon-Mulanovich G, Pedroni E, Blazes DL. Epidemiology. Outbreak investigation and response training. Science. 2007;318:574–575. doi: 10.1126/science.1146837. [DOI] [PubMed] [Google Scholar]
- 56.Korenromp EL, Hosseini M, Newman RD, Cibulskis RE. Progress towards malaria control targets in relation to national malaria programme funding. Malar J. 2013;12:18. doi: 10.1186/1475-2875-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Regional Malaria Program . Report on the Situation of Malaria in the Americas 2008. Washington, DC: Pan American Health Organization; 2010. [Google Scholar]
- 58.Pan American Health Organization . PAHO/WHO to Support Guyana and Suriname in Studies of Resistance to Artemisinin-Based Combination Therapy (ACT) 2013. http://www.paho.org/hq/index.php?option=com_content&view=article&id=8636%3Apahowho-to-support-guyana-and-suriname-in-studies-of-resistance-to-artemisinin-based-combination-therapy-act&catid=1443%3Anews-front-page-items&Itemid=1926&lang=en Available at. Accessed August 29, 2013. [Google Scholar]
- 59.WHO Malaria Policy Advisory Committee and Secretariat Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of March 2013 meeting. Malar J. 2013;12:213. doi: 10.1186/1475-2875-12-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.