Abstract
Infection with a dengue virus (DENV) serotype induces cross-reactive, weakly neutralizing antibodies to different dengue serotypes. It has been postulated that cross-reactive antibodies form a virus–antibody immune complex and enhance DENV infection of Fc gamma receptor (FcγR)-bearing cells. We determined whether infectious DENV–antibody immune complex is formed in vivo in marmosets after passive transfer of DENV-specific monoclonal antibody (mAb) and DENV inoculation and whether infectious DENV–antibody immune complex is detectable using FcγR-expressing cells. Marmosets showed that DENV–antibody immune complex was exclusively infectious to FcγR-expressing cells on days 2, 4, and 7 after passive transfer of each of the mAbs (mAb 4G2 and mAb 6B6C) and DENV inoculation. Although DENV–antibody immune complex was detected, contribution of the passively transferred antibody to overall viremia levels was limited in this study. The results indicate that DENV cross-reactive antibodies form DENV–antibody immune complex in vivo, which is infectious to FcγR-bearing cells but not FcγR-negative cells.
Introduction
Dengue virus (DENV) is estimated to cause more than 300 million infections annually.1 Infection with any of the four DENV serotypes (DENV-1 to −4) can cause an array of symptoms ranging from asymptomatic to undifferentiated fever, dengue fever, and severe life-threatening dengue hemorrhagic fever.2 Infection with one DENV serotype confers lifelong protection against infection with the same serotype and short-lived protective immunity against other serotypes. Severe dengue cases often occur in sequentially infected patients with a heterologous DENV serotype or a serotype that differs from that of primary infection.3–5 Antibody (Ab) -dependent enhancement (ADE) of DENV infection has been proposed to be the underlying mechanism of severe DENV infection.6 In ADE, non-neutralizing antibodies that cross-react with a heterologous serotype form immune complexes with DENV and enhance infection through FcγR-bearing cells, leading to higher virus replication.7,8
DENV–Ab immune complex has been associated with severe dengue.9,10 Because infectious virus–Ab immune complex is associated with higher viremia titers during secondary DENV infection, understanding of their role during DENV infection is important for elucidating the pathogenesis of severe dengue.11 In vitro studies have shown ADE activity to heterotypic serotype in undiluted serum samples from human dengue patients and non-human primates.12–14 In infants, maternal antibodies are associated with disease enhancement, resulting in the onset of severe illness and the development of severe dengue.12 A mouse model of maternal ADE antibodies showed higher viremia correlates with increased disease severity.15 ADE has also been shown in mouse and non-human primate models, in which passive transfer of a cross-reactive monoclonal Ab (mAb) and sera from dengue patients resulted in higher viremia levels.16–18 However, the formation of infectious virus–immune complex in these primate models has not been shown.
In this study, marmosets were used to elucidate the interaction of DENV with cross-reactive, non-neutralizing antibodies and the formation of infectious immune complex in vivo. We previously reported the use of marmosets as an animal model of DENV infection. The marmoset model consistently developed high levels of viremia on primary and secondary DENV infection.19,20 Marmosets sequentially infected with DENV have also been shown to show infectious virus–Ab immune complex in plasma samples.20 In this study, marmosets passively transferred with two types of infection-enhancement antibodies and then inoculated with DENV-2 were used to determine virus–immune complex formation in vivo.
Materials and Methods
Primates.
In total, 10 marmosets were used in the study. Marmosets were purchased from Clea Japan Inc. (Tokyo, Japan) and caged singly at 27°C ± 2°C in 50% ± 10% humidity with a 12-hour light–dark cycle (lighting from 7:00 am to 7:00 pm) at the National Institute of Infectious Diseases of Japan. Animals were fed with a standard marmoset diet (Clea New World Monkey Diet, CMS-1M; CLEA Japan Inc., Tokyo, Japan) supplemented with fruit. Water was given ad libitum.19,20 All animal studies were conducted in accordance with the guidelines for animal experiments at the National Institute of Infectious Diseases after obtaining study approval from the Animal Welfare and Animal Care Committee of the National Institute of Infectious Diseases of Japan (approval numbers 512005 and 613005).
DENV strains and propagation.
DENV-2 DHF0663 strain (Genbank accession number AB189122) was used for the experiment.19,20 The DENV-2 strain was used within four passages on cell culture. Culture supernatant from infected baby hamster kidney (BHK) cells was centrifuged at 800 × g for 5 minutes to remove cell debris and stored at −80°C until use.
Antibodies.
To examine the formation of infectious virus–Ab immune complex on passive transfer of ADE Ab, two mouse mAbs with ADE activity in vitro to the DENV-2 DHF0663 strain (mAb 4G2 and mAb 6B6C) were used.21 The Ab mAb 4G2 enhanced DENV-2 infection (fold enhancement range = 2.4–10.8) at dilutions between 0.26 and 260 μg/mL Ab using FcγR-expressing cells. No neutralizing activity was detected at mAb 4G2 Ab concentrations using BHK cells. The mAb 6B6C enhanced DENV-2 infection (fold enhancement range = 2.0–6.1) at 0.59–590 μg/mL Ab using FcγR-expressing cells.22,23 Neutralizing activity was detected at mAb 6B6C Ab concentrations of 59–5,900 μg/mL using BHK cells.22
Inoculation of marmosets with DENV.
Marmosets were randomly assigned to three groups: group A (N = 3), group B (N = 3), and control (N = 4). Group A marmosets (A1–A3) were intravenously administered with 1 mL 26 μg/mL (0.09 mg/kg) mAb 4G2, group B marmosets (B1–B3) were intravenously administered with 1 mL 590 μg/mL (2.0 mg/kg) mAb 6B6C, and group C marmosets (C1–C3) were intravenously administered with 1 mL saline (normal saline; Otsuka Pharmaceutical Co., Tokyo, Japan). The mean weight of marmosets at the day 0 was 288 ± 30 g (group A), 297 ± 66 g (group B), and 276 ± 19 g (group C). At 20 minutes after intravenous administration, all 10 marmosets were subcutaneously inoculated with 1 × 105 plaque forming units (PFU)/dose DENV-2 DHF0663 strain in the upper back.19,20 The 50% plaque reduction neutralizing test (PRNT50) of mAb 6B6C at a concentration of 590 μg/mL was 1:80 using BHK cells and 1:20 using FcγR-expressing BHK cells. No neutralizing activity was detected in mAb 4G2 at a concentration of 26 μg/mL using BHK and FcγR-expressing BHK cells.22 Inoculation with DENV and blood drawing were performed under anesthesia with 5 mg/kg ketamine hydrochloride. Blood samples were centrifuged at 200 × g for 10 minutes for plasma collection. Blood samples were collected before Ab and saline administration and 20 minutes after Ab and saline administration (before virus inoculation). Day 0 was defined as the day of virus inoculation.
Quantification of viremia by quantitative reverse transcriptase polymerase chain reaction and plaque assay.
Levels of DENV genome in plasma samples (High Pure Viral RNA Kit; Roche Diagnostics GmbH, Mannheim, Germany) were determined by quantitative TaqMan real-time reverse transcriptase polymerase chain reaction (RT-PCR).24 Viral genome levels determined by RT-PCR were expressed as log10 genome copies per milliliter. To determine viremia levels (infectious particles) in plasma samples, the samples were serially diluted 10-fold from 1:101 to 1:106 with Eagle's minimum essential medium (EMEM; Sigma) supplemented with 10% fetal bovine serum (FBS).11 Fifty microliters diluted serum samples were inoculated onto BHK and FcγR-expressing BHK cell monolayers in 12-well plates. The plates were incubated for 1 hour at 37°C in 5% CO2. After virus absorption, the cells were overlaid with maintenance medium containing 1% methylcellulose (Wako Pure Chemical Industry, Osaka, Japan). The plates were incubated at 37°C in 5% CO2 until visible plaques could be observed (5–7 days of incubation). The cells were fixed with formaldehyde, stained with methylene blue, and washed with water. Assays were conducted in duplicate. Viral titers were expressed as PFU per millimeter using the following formula: (number of plaques per well) × (dilution)/(inoculum volume).11
Serological studies.
DENV-specific immunoglobulin M (IgM) Ab was detected by Dengue Fever IgM Capture Enzyme-Linked Immunosorbent Assay (ELISA; Focus), and DENV-specific IgG Ab was determined using Dengue IgG Indirect ELISA (PanBio) in accordance with the manufacturer's instructions.19,20 Concentrations of mouse IgG antibodies in marmoset plasma samples were determined in accordance with the manufacturer's instructions (Mouse IgG ELISA Quantitation Set; Bethyl, TX). The positive/negative (P/N) ratio was calculated by the following formula: optical density (OD) of the test sample/OD of a negative sample. Plasma samples collected from three DENV-naïve marmosets were used as the negative samples. P/N ratios of < 2 and ≥ 2 were considered to be negative and positive, respectively. All ELISA assays were conducted in duplicate.20
Neutralizing Ab titers were determined by plaque reduction neutralization to the inoculated DENV-2 DHF 0663 strain.19,25,26 Heat-inactivated plasma samples were serially diluted twofold from 1:5 to 1:160 with EMEM supplemented with 2% FBS. Virus–Ab mixture was prepared by mixing 25 μL DENV-2 (DHF 0663 strain) at titers of 2,000 PFU/mL with 25 μL serially diluted plasma samples to make up a final plasma dilution of 1:10 to 1:320. Controls were prepared by mixing 25 μL DENV-2 at titers of 2,000 PFU/mL with 25 μL EMEM supplemented with 2% FBS. Virus–plasma sample mixture was incubated at 37°C for 1 hour. Fifty microliters virus–plasma sample mixture was inoculated onto BHK and FcγR-expressing BHK cell monolayers in 12-well plates. After 5 days of inoculation, cells were fixed and stained, and plaques were counted. Neutralizing assay was not tested in some samples because of insufficient volume. Tests were conducted in duplicate. Neutralization titer was expressed as the maximum dilution of plasma sample that yielded a > 50% plaque reduction in the virus inoculum compared with control.25
Statistics.
Results were expressed as the mean value of each group. Non-parametric Kruskal–Wallis and Mann–Whitney tests were used to compare values between the marmoset groups and determine statistical significance. Microsoft Excel (Microsoft Corporation, Redmond, WA) was used for raw data processing, and GraphPad Prism (GraphPad Software Inc., La Jolla, CA) was used for statistical analysis. A P value of < 0.05 was considered to be a statistically significant difference.
Results
Detection of infectious DENV–Ab immune complex using FcγR-expressing cells in marmosets passively transferred with mAbs.
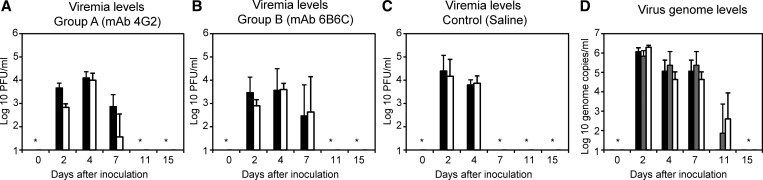
Plasma samples were collected from marmosets passively transferred with mAb 4G2 and then inoculated with DENV-2. The plasma samples were analyzed for viremia levels using BHK and FcγR-expressing BHK cells (Figure 1 and Table 1). Viremia was detected in all three marmosets (A1–A3) on days 2, 4, and 7 (Figure 1A and Table 1). Viremia levels were 4–15 times higher using FcγR-expressing cells than using BHK cells on day 2 in marmosets A1–A3. On day 7, viremia was detected using FcγR-expressing BHK cells but not BHK cells in plasma samples from marmosets A2 and A3 (Table 1). In two of three marmosets (B2 and B3) passively transferred with mAb 6B6C, viremia levels were higher on days 2 and 4 using FcγR-expressing BHK cells than BHK cells (Figure 1B and Table 1). In the control group (marmosets C1–C4), viremia was detected on days 2 and 4, and viremia levels were similar using BHK and FcγR-expressing BHK cells (Figure 1C ). The results suggested the presence of a DENV–Ab immune complex that is infectious to FcγR-expressing cells but not FcγR-negative cells and confirmed that FcγR-expressing BHK cells are useful for detecting infectious DENV–Ab complexes developed in vivo.11
Figure 1.
Viremia kinetics in Ab-treated and untreated marmosets after DENV inoculation. (A–C) Viremia levels determined using FcγR-expressing BHK cells (black bars) and FcγR-negative BHK cells (white bars) in plasma samples from marmosets administered (A) mAb 4G2, (B) mAb 6B6C, or (C) saline. (D) Viremia levels as determined by RT-PCR. Black bars indicate marmosets in the mAb 4G2 Ab-treated group (group A), gray bars indicate marmosets in the mAb 6B6C Ab-treated group (group B), and white bars indicate marmosets in the control (saline) group. *Values below the detection level.
Table 1.
Dengue viremia levels in plasma samples from 10 marmosets after passive transfer of Ab and inoculation with DENV-2
| Animal identification | Administered Ab/saline | Inoculated virus dose (PFU/dose) | Infectious virus titer of BHK/FcγR-BHK cells (log10 PFU/mL; VN/VO ratio) days after inoculation | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 7 | 11 | 15 | |||
| Group A | ||||||||
| A1 | mAb 4G2 | 1 × 105 DENV-2 | −/− (ND) | 2.7/3.9 (15.8)* | 4.3/4.4 (1.3) | 2.7/2.3 (0.4) | −/− (ND) | −/− (ND) |
| A2 | mAb 4G2 | 1 × 105 DENV-2 | −/− (ND) | 2.8/3.5 (5.0)* | 4.0/3.9 (0.8) | −/3.0 (> 100)* | −/− (ND) | −/− (ND) |
| A3 | mAb 4G2 | 1 × 105 DENV-2 | −/− (ND) | 3.0/3.6 (4.0)* | 3.7/4.0 (2.0)* | −/3.3 (> 100)* | −/− (ND) | −/− (ND) |
| Group B | ||||||||
| B1 | mAb 6B6C | 1 × 105 DENV-2 | −/− (ND) | 2.6/2.7 (1.3) | 3.3/2.5 (0.2) | 2.9/2.8 (0.8) | −/− (ND) | −/− (ND) |
| B2 | mAb 6B6C | 1 × 105 DENV-2 | −/− (ND) | 3.1/3.8 (5.0)* | 3.8/4.2 (2.5)* | 4.0/3.6 (0.4) | −/− (ND) | −/− (ND) |
| B3 | mAb 6B6C | 1 × 105 DENV-2 | −/− (ND) | 3.0/3.9 (7.9)* | 3.7/4.0 (2.0)* | −/− (ND) | −/− (ND) | −/− (ND) |
| Control | ||||||||
| C1 | Saline | 1 × 105 DENV-2 | −/− (ND) | 3.2/3.3 (1.3) | 4.0/4.0 (1.0) | −/− (ND) | −/− (ND) | −/− (ND) |
| C2 | Saline | 1 × 105 DENV-2 | −/− (ND) | 3.4/3.3 (0.8) | 4.3/4.3 (1.0) | −/− (ND) | −/− (ND) | −/− (ND) |
| C3 | Saline | 1 × 105 DENV-2 | −/− (ND) | 4.6/4.5 (0.8) | 3.7/3.9 (1.6) | −/− (ND) | −/− (ND) | −/− (ND) |
| C4 | Saline | 1 × 105 DENV-2 | −/− (ND) | 4.5/4.4 (0.8) | 3.6/3.8 (1.6) | −/− (ND) | −/− (ND) | −/− (ND) |
VN/VO ratio indicates the ratio of viremia titer determined by FcγR-expressing cells (VN) to viremia titer determined by BHK cells (VO), and – indicates viremia below detection levels (< 2.0 log10 PFU/mL). ND = not determined.
VN/VO ratio ≥ 2.0.
Detection of mouse IgG Ab in plasma samples from marmosets after administration of mAbs.
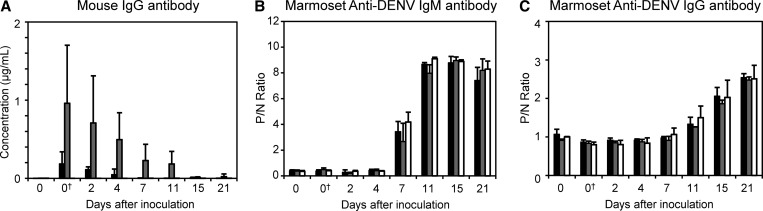
Concentration of mouse IgG Ab was assessed in marmosets using anti-mouse IgG ELISA. As expected, before mAb administration, all marmosets were negative for mouse antibodies (Figure 2A and Table 2). All six marmosets (A1–A3 and B1–B3) were positive for mouse Ab: up to day 15 for mAb 4G2 and day 21 for mAb 6B6C.
Figure 2.
Anti-DENV and mouse IgG Ab levels in Ab-treated and untreated marmosets after DENV inoculation. (A) Mouse IgG Ab levels after passive transfer of mouse mAbs and DENV challenge in marmosets. (B) Levels of marmoset anti-DENV IgM Abs and (C) levels of marmoset anti-DENV IgG Abs. Black bars indicate marmosets in the mAb 4G2 Ab-treated group (group A), gray bars indicate marmosets in the mAb 6B6C Ab-treated group (group B), and white bars indicate marmosets in the control (saline) group. P/N ratio ≥ 2.0 was considered as Ab-positive. †Values after mouse mAb treatment.
Table 2.
Concentration of mouse IgG Ab in plasma samples from marmosets before and after passive transfer of the Ab
| Animal identification | Concentration of mouse IgG Ab (ng/mL) days after inoculation | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0* | 2 | 4 | 7 | 11 | 15 | 21 | |
| Group A (mAb 4G2) | ||||||||
| A1 | − | 258 | 118 | − | 3 | − | 15 | − |
| A2 | − | 289 | 70 | 9 | − | − | − | − |
| A3 | − | 5 | 141 | 130 | 5 | − | − | − |
| Group B (mAb 6B6C) | ||||||||
| B1 | − | 1,789 | 177 | 419 | 512 | 299 | 6 | − |
| B2 | − | 730 | 1,365 | 192 | 410 | ND | − | − |
| B3 | − | 357 | 579 | 871 | 269 | 250 | 20 | 61 |
| Control (saline) | ||||||||
| C1 | − | − | − | − | − | − | − | − |
| C2 | − | − | − | − | − | − | − | − |
| C3 | − | − | − | − | − | − | − | − |
| C4 | − | − | − | − | − | − | − | − |
Mouse IgG concentrations below detection levels (< 2.0 ng/mL) are indicated by –. ND = not determined.
Values after Ab or saline administration.
Absence of marmoset anti-DENV IgM and IgG on days 0, 2, and 4 after DENV inoculation.
Ab response after inoculation with DENV was analyzed using IgM and IgG ELISA. DENV-specific marmoset IgM was first detected on day 7 after virus inoculation in control marmosets and those administered with mAb 4G2 or mAb 6B6C (Figure 2B and Table 3). The IgM Ab response in the mAb-administered groups was similar to that in the control group and primary DENV infection.19,20
Table 3.
IgM Ab responses in marmosets on passive transfer of mAb and inoculation with DENV-2
| Animal identification | Days after inoculation | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0* | 2 | 4 | 7 | 11 | 15 | 21 | |
| Group A (mAb 4G2) | ||||||||
| A1 | 0.4 | 0.4 | 0.5 | 0.5 | 2.9† | 8.8† | 9.3† | 8.5† |
| A2 | 0.3 | 0.3 | 0.2 | 0.3 | 4.3† | 8.6† | 8.4† | 6.5† |
| A3 | 0.4 | 0.5 | 0.2 | 0.4 | 3.1† | 8.6† | 8.6† | 7.2† |
| Group B (mAb 6B6C) | ||||||||
| B1 | 0.3 | 0.4 | 0.3 | 0.3 | 1.1 | 7.5† | 8.9† | 7.4† |
| B2 | 0.4 | 0.5 | 0.2 | 0.5 | 3.1† | ND | 9.3† | 9.1† |
| B3 | 0.5 | 0.6 | 0.2 | 0.4 | 3.8† | 8.4† | 8.8† | 8.1† |
| Control (saline) | ||||||||
| C1 | 0.3 | 0.5 | 0.3 | 0.3 | 4.5† | 9.0† | 9.0† | 8.2† |
| C2 | 0.3 | 0.4 | 0.3 | 0.4 | 4.8† | 9.2† | 9.0† | 9.0† |
| C3 | 0.3 | 0.3 | 0.3 | 0.3 | 4.7† | 9.0† | 8.9† | 8.0† |
| C4 | 0.4 | 0.5 | 0.5 | 0.4 | 3.1† | 9.1† | 8.8† | 7.8† |
ND = not determined.
Values after Ab or saline administration.
Positive detection of DENV IgM (P/N ≥ 2.0).
Anti-DENV IgG was first detected on day 11 in marmoset C1 and day 15 in all marmosets administered with mAbs (Figure 2C and Table 4). The IgG Ab response patterns in groups A and B were similar to those in the control group and marmosets with primary DENV infection.19,20 The results indicate that DENV-specific marmoset IgG was absent on days 2, 4, and 7 in all of the DENV-inoculated marmosets. These results, along with those shown in Tables 1 and 2, indicate that infectious DENV–Ab immune complex detected on days 2, 4, and 7 was formed by administrated mouse mAb and DENV.
Table 4.
IgG Ab responses in marmosets on passive transfer of mAb and inoculation with DENV-2
| Animal identification | Days after inoculation | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0* | 2 | 4 | 7 | 11 | 15 | 21 | |
| Group A (mAb 4G2) | ||||||||
| A1 | 0.9 | 0.8 | 0.9 | 0.9 | 0.9 | 1.2 | 2.1† | 2.6† |
| A2 | 1.2 | 0.9 | 1.0 | 0.9 | 1.0 | 1.2 | 1.8 | 2.4† |
| A3 | 1.1 | 0.9 | 0.9 | 0.9 | 1.0 | 1.5 | 2.3† | 2.6† |
| Group B (mAb 6B6C) | ||||||||
| B1 | 0.9 | 0.8 | 0.9 | 0.9 | 1.0 | 1.3 | 2.0† | 2.5† |
| B2 | 0.9 | 0.8 | 0.8 | 0.8 | 0.9 | ND | 1.8 | 2.6† |
| B3 | 1.0 | 0.9 | 0.9 | 0.9 | 0.9 | 1.3 | 1.8 | 2.4† |
| Control (saline) | ||||||||
| C1 | 1.0 | 1.0 | 1.0 | 1.0 | 1.2 | 2.1 | 2.9† | 3.1† |
| C2 | 1.0 | 0.9 | 0.9 | 1.0 | 1.3 | 1.7 | 2.2† | 2.8† |
| C3 | 1.0 | 0.8 | 0.8 | 0.8 | 0.9 | 1.4 | 1.8 | 2.1† |
| C4 | 1.0 | 0.7 | 0.7 | 0.8 | 1.0 | 1.4 | 2.1† | 2.6† |
ND = not determined.
Values after Ab or saline administration.
Positive detection of DENV IgM (P/N ≥ 2.0).
Neutralizing Ab in marmosets after Ab administration and DENV inoculation.
Levels of neutralizing activity to the inoculated serotype (DENV-2) were assessed using BHK cells and FcγR-expressing BHK cells (Table 5). Neutralizing activity was detected in plasma samples from marmosets B1 and B2 on days 0, 2, and 4 but not in those from group A antibodies or the control group. DENV-specific marmoset IgM and IgG were not detected on days 0, 2, and 4 (Tables 3 and 4).Thus, the results suggest that the neutralizing activity, which was detected in B1 and B2 from days 0 to 4, occurred because of the administration of mAb 6B6C. Neutralizing activity was detected from day 7 in the marmosets of groups A and B and the control group, possibly reflecting marmoset DENV-specific Ab responses.
Table 5.
Neutralizing Ab titers to inoculated DENV-2 (DHF0663 strain) in marmosets using BHK and FcγR-expressing BHK cells
| Animal identification | Days after inoculation (PRNT50 titers by BHK, FcγR-expressing BHK cells) | ||||||
|---|---|---|---|---|---|---|---|
| 0* | 2 | 4 | 7 | 11 | 15 | 21 | |
| Group A (mAb 4G2) | |||||||
| A1 | < 10, < 10 | < 10, < 10 | < 10, < 10 | 10, 10 | 10, 10 | 10, 10 | 20, 10 |
| A2 | < 10, < 10 | < 10, < 10 | < 10, < 10 | 10, < 10 | 10, 20 | 10, 10 | 10, ND |
| A3 | < 10, < 10 | < 10, < 10 | < 10, < 10 | 10, < 10 | 20, 20 | 10, 10 | 10, 10 |
| Group B (mAb 6B6C) | |||||||
| B1 | 10, < 10 | 10, < 10 | 10, < 10 | 10, < 10 | 10, 10 | ND, 10 | 40, 10 |
| B2 | 10, < 10 | 10, < 10 | < 10, < 10 | < 10, < 10 | ND, ND | 10, 10 | 40, 10 |
| B3 | < 10, < 10 | < 10, < 10 | 10, < 10 | 10, < 10 | 10, 20 | 20, 10 | 10, ND |
| Control (saline) | |||||||
| C1 | < 10, < 10 | < 10, < 10 | < 10, < 10 | < 10, < 10 | 20, 20 | 10, 10 | 20, 10 |
| C2 | < 10, < 10 | < 10, < 10 | < 10, < 10 | 10, 10 | ND, ND | 10, 10 | 160, 10 |
| C3 | < 10, < 10 | < 10, < 10 | < 10, < 10 | 10, < 10 | ND, ND | 10, 10 | 40, 20 |
| C4 | < 10, < 10 | < 10, < 10 | < 10, < 10 | 10, < 10 | ND, ND | 10, 10 | 80, 40 |
Results are shown as reciprocal of the highest plasma dilution (end-point titer) that results in < 50% input of plaque count and were expressed as PRNT50 values. ND = not determined because of low sample volumes.
Values after Ab or saline administration.
Limited effect of administration of a DENV infection-enhancing mAb in marmosets.
Mean peak viremia in all three groups was comparable using BHK and FcγR-expressing BHK cells (PBHK = 0.08, PFcγR-BHK = 0.26; Kruskal–Wallis test). Mean peak viremia between marmosets (A1–A3) that were passively transferred with mAb 4G2 (BHK = 4.0 log10 PFU/mL, FcγR-expressing BHK cells = 4.1 log10 PFU/mL) and control marmosets (C1–C4) administered with saline and then inoculated with DENV-2 was comparable (BHK = 4.4 log10 PFU/mL, FcγR-expressing BHK cells = 4.3 log10 PFU/mL, PBHK = 0.23, PFcγR-BHK = 0.46; Mann–Whitney test). The mean peak viremia for marmosets passively transferred with mAb 6B6C was 3.6 log10 PFU/mL using BHK cells and 3.6 log10 PFU/mL using FcγR-expressing BHK cells (PBHK = 0.09 and PFcγR-BHK = 0.14, respectively, compared with control; Mann–Whitney test). The levels of DENV-2 RNA among the three groups on days 2–7 were comparable (P = 0.06; Kruskal–Wallis test) (Figure 1D and Table 6). IgM Ab responses increased rapidly from day 7, and levels of DENV-specific IgM in the three groups were comparable (Pday7 = 0.09; Kruskal–Wallis test) (Table 3). Marmoset IgG Ab response was detected on days 15 and 21, and the levels of specific IgG in the three groups were also comparable (Pday15,21 = 0.48; Kruskal–Wallis test) (Table 4). The results suggest that the effects of DENV–mAb immune complex on the levels of viremia and IgM and IgG immune responses were limited in this study.
Table 6.
Levels of dengue viral RNA in plasma samples from marmosets treated with mouse Ab or saline and inoculated with DENV-2
| Animal identification | Administered Ab/saline | Inoculated virus dose (PFU/dose) | Dengue viral RNA copy numbers (log10 copies/mL) days after inoculation | |||||
|---|---|---|---|---|---|---|---|---|
| 0* | 2 | 4 | 7 | 11 | 15 | |||
| Group A | ||||||||
| A1 | mAb 4G2 | 1 × 105 DENV-2 | − | 6.0 | 6.9 | 5.7 | − | − |
| A2 | mAb 4G2 | 1 × 105 DENV-2 | − | 6.3 | 6.5 | 4.6 | − | − |
| A3 | mAb 4G2 | 1 × 105 DENV-2 | − | 5.9 | 6.4 | 4.9 | − | − |
| Group B | ||||||||
| B1 | mAb 6B6C | 1 × 105 DENV-2 | − | 5.5 | 5.9 | 5.5 | − | − |
| B2 | mAb 6B6C | 1 × 105 DENV-2 | − | 6.0 | 6.4 | 6.0 | − | − |
| B3 | mAb 6B6C | 1 × 105 DENV-2 | − | 6.0 | 6.4 | 4.6 | 3.6 | − |
| Control | ||||||||
| C1 | Saline | 1 × 105 DENV-2 | − | 6.2 | 6.7 | 5.4 | 4.0 | − |
| C2 | Saline | 1 × 105 DENV-2 | − | 6.4 | 6.9 | 4.5 | − | − |
| C3 | Saline | 1 × 105 DENV-2 | − | 6.3 | 6.6 | 4.7 | 3.6 | − |
| C4 | Saline | 1 × 105 DENV-2 | − | 6.2 | 6.7 | 4.7 | 3.2 | − |
Viral RNA below detection levels is indicated by –.
Levels of viral genome were quantitated using plasma samples from marmosets after passive transfer of mouse mAb but before DENV inoculation.
Discussion
DENV–immune complex formation in vivo contributes to viral replication, and the presence of the immune complex triggers an array of effector cells and pathways through FcγR, ultimately leading to severe dengue disease.27–31 In the absence of an anamnestic immune response, passive Ab transfer, including maternal Abs, is speculated to be more effective at enhancing DENV infection than infection-acquired immunity.12,32 However, the presence of DENV–immune complex formation in vivo has not been fully defined. In this study, DENV infection-enhancing mAbs were passively transferred to marmosets to determine the formation of DENV–Ab immune complex in vivo and the effects of these antibodies on DENV infection. Ab was administered at concentrations that showed ADE in vitro. In plasma samples from marmosets passively transferred with infection-enhancing mAb, we showed the formation of infectious DENV–Ab immune complex before the induction of DENV-specific IgM and IgG antibodies. The results suggest that the DENV–Ab immune complex develops on passive administration of infection-enhancing Abs.
There have been other attempts to show ADE of DENV infection in non-human primate models using sequential infection with heterologous serotypes. Primates with sequential heterotypic DENV-2 infection (mean peak viremia = 2.8 × 103 PFU/0.5 mL) have been shown to have higher titers of viremia compared with primates with primary DENV-2 infection (mean peak viremia = 2.4 × 102 PFU/0.5 mL; P < 0.001).17 In marmosets, infectious virus–Ab complex has been detected using FcγR-expressing cells during sequential secondary heterologous infection.20 These findings concerning secondary infection are concurrent with the results of this study, which shows the formation of DENV–Ab immune complex in vivo with passively acquired DENV cross-reactive infection-enhancing Abs. The results of this study are also concurrent with a study on secondary infection in human dengue patients,11 in which viremia levels were found to be higher using FcγR-expressing cells than BHK cells.
In other non-human primate models of passive Ab transfer, peak viremia range was higher in primates passively immunized with chimpanzee–human chimeric Ab (passive transfer group viremia range = 0.58–2.76 log10 focus forming units (FFU)/mL; control group viremia = 0.40 log10 FFU/mL)16 and human sera from dengue patients (passive transfer group viremia range = 2.7–4.2 log10 PFU/mL; control group peak viremia range = 2.0–2.9 log10 PFU/mL).33 In this study, levels of viremia were comparable between marmosets passively transferred with infection-enhancement Ab and non-treated marmosets, although the peak viremia titer in both infection-enhancement Ab-treated and non-treated marmosets (peak viremia range = 3.3–4.6 log10 PFU/mL) in this study was higher than those of macaques treated with infection-enhancing chimeric Ab.16 Additional studies using Abs with infection-enhancing activities and Abs originating from primates are needed to clarify the factors contributing to overall higher viremia titers in the presence of virus–Ab immune complex in vivo. Because Callithrix jacchus marmosets possess Ig receptors, including FcγRs and leukocyte immunoglobin-like receptors (LILRs),34 the marmoset model is potentially useful in ADE and therapeutic Ab studies.35,36 Our results confirm that DENV cross-reactive antibodies form virus–Ab immune complexes in vivo and that these immune complexes are infectious only to FcγR-bearing cells. Our results also concur with those of prior studies, which show that infectious virus–Ab immune complexes occur in the presence of DENV cross-reactive infection-enhancing Abs before DENV infection or with transfer of maternal Ab. Overall, our results suggest the feasibility of the marmoset model for elucidating the role of DENV–Ab immune complex in vivo. Together with our earlier studies on primary and sequential DENV infection in marmosets, our results also suggest that the marmoset model could prove useful for studies on the immune response after passive Ab transfer and as a model for therapeutic Ab studies.
ACKNOWLEDGMENTS
The authors thank Prof. Duane J. Gubler (Duke University–National University of Singapore) for the generous gift of the 6B6C monoclonal antibody-producing hybridoma and Dr. Akira Igarashi (Nagasaki University) for assistance in obtaining the hybridoma. The authors thank Dr. Jeffrey V. Ravetch (Rockefeller University) for generously providing the FcγRIIA complementary DNA and Dr. Susheela Tridandapani (Ohio State University College of Medicine) for assistance in obtaining the FcγRIIA complementary DNA used in this work. We also thank Dr. Eiji Konishi (Research Institute for Microbial Diseases [BIKEN], Osaka University) for providing us with valuable advice on dengue virus infection-enhancing activity in animal models. We also thank Ms. Makiko Ikeda (Department of Virology 1, National Institute of Infectious Diseases) for her excellent technical support.
Footnotes
Financial support: This work was supported by Research on Emerging and Re-Emerging Infectious Diseases Grants H23-shinkou-ippan-010 and H26-shinkou-jitsuyouka-007 from the Ministry of Health, Labour and Welfare, Japan and Kiban B Grant-in-Aid for Scientific Research 25293112 and Young Scientists B Grant-in-Aid for Scientific Research 26870872 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Authors' addresses: Meng Ling Moi, Chang-Kweng Lim, Masayuki Saijo, and Tomohiko Takasaki, Department of Virology 1, National Institute of Infectious Diseases, Tokyo, Japan, E-mails: sherry@nih.go.jp, ck@nih.go.jp, msaijo@nih.go.jp, and takasaki@nih.go.jp. Yasushi Ami and Yuriko Suzaki, Division of Experimental Animal Research, National Institute of Infectious Diseases, Musashimurayama, Tokyo, Japan, E-mails: yami@nih.go.jp and ysuzaki@nih.go.jp. Kenji Shirai, Kazutaka Kitaura, and Ryuji Suzuki, Department of Rheumatology and Clinical Immunology, Clinical Research Center for Allergy and Rheumatology, Sagamihara National Hospital, National Hospital Organization, Kanagawa, Japan, E-mails: k.shirai0727@gmail.com, kitaura@nih.go.jp, and r-suzuki@sagamihara-hosp.gr.jp. Yuka Saito, Department of Virology 1, National Institute of Infectious Diseases, Tokyo, Japan, and College of Bioresource Sciences, Nihon University, Fujisawa, Kanagawa, Japan, E-mail: saito-y@nih.go.jp. Ichiro Kurane, National Institute of Infectious Diseases, Tokyo, Japan, E-mail: kurane@nih.go.jp.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 3.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 4.Guzman MG, Kouri GP, Bravo J, Calunga M, Soler M, Vazquez S, Venereo C. Dengue haemorrhagic fever in Cuba. I. Serological confirmation of clinical diagnosis. Trans R Soc Trop Med Hyg. 1984;78:235–238. doi: 10.1016/0035-9203(84)90285-2. [DOI] [PubMed] [Google Scholar]
- 5.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158:1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 6.Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 7.Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontny U, Kurane I, Ennis FA. Gamma interferon augments Fc gamma receptor-mediated dengue virus infection of human monocytic cells. J Virol. 1988;62:3928–3933. doi: 10.1128/jvi.62.11.3928-3933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruangjirachuporn W, Boonpucknavig S, Nimmanitya S. Circulating immune complexes in serum from patients with dengue haemorrhagic fever. Clin Exp Immunol. 1979;36:46–53. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WK, Chen HL, Yang CF, Hsieh SC, Juan CC, Chang SM, Yu CC, Lin LH, Huang JH, King CC. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis. 2006;43:1023–1030. doi: 10.1086/507635. [DOI] [PubMed] [Google Scholar]
- 11.Moi ML, Lim CK, Kotaki A, Takasaki T, Kurane I. Detection of higher levels of dengue viremia using FcγR-expressing BHK-21 cells than FcγR-negative cells in secondary infection but not in primary infection. J Infect Dis. 2011;203:1405–1414. doi: 10.1093/infdis/jir053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–419. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 13.Ito M, Mukai RZ, Takasaki T, Kotaki A, Kurane I. Antibody-dependent enhancement of dengue virus infection in vitro by undiluted sera from monkeys infected with heterotypic dengue virus. Arch Virol. 2010;155:1617–1624. doi: 10.1007/s00705-010-0741-x. [DOI] [PubMed] [Google Scholar]
- 14.Moi ML, Takasaki T, Saijo M, Kurane I. Dengue virus infection-enhancing activity of undiluted sera obtained from patients with secondary dengue virus infection. Trans R Soc Trop Med Hyg. 2013;107:51–58. doi: 10.1093/trstmh/trs007. [DOI] [PubMed] [Google Scholar]
- 15.Ng JK, Zhang SL, Tan HC, Yan B, Maria Martinez Gomez J, Tan WY, Lam JH, Tan GK, Ooi EE, Alonso S. First experimental in vivo model of enhanced dengue disease severity through maternally acquired heterotypic dengue antibodies. PLoS Pathog. 2014;10:e1004031. doi: 10.1371/journal.ppat.1004031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci USA. 2007;104:9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J Infect Dis. 1973;128:15–22. doi: 10.1093/infdis/128.1.15. [DOI] [PubMed] [Google Scholar]
- 18.Balsitis SJ, Williams KL, Lachica R, Flores D, Kyle JL, Mehlhop E, Johnson S, Diamond MS, Beatty PR, Harris E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010;6:e1000790. doi: 10.1371/journal.ppat.1000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omatsu T, Moi ML, Hirayama T, Takasaki T, Nakamura S, Tajima S, Ito M, Yoshida T, Saito A, Katakai Y, Akari H, Kurane I. Common marmoset (Callithrix jacchus) as a primate model of dengue virus infection: development of high levels of viraemia and demonstration of protective immunity. J Gen Virol. 2011;92:2272–2280. doi: 10.1099/vir.0.031229-0. [DOI] [PubMed] [Google Scholar]
- 20.Moi ML, Takasaki T, Omatsu T, Nakamura S, Katakai Y, Ami Y, Suzaki Y, Saijo M, Akari H, Kurane I. Demonstration of marmosets (Callithrix jacchus) as a non-human primate model for secondary dengue virus infection: high levels of viraemia and serotype cross-reactive antibody responses consistent with secondary infection of humans. J Gen Virol. 2014;95:591–600. doi: 10.1099/vir.0.060384-0. [DOI] [PubMed] [Google Scholar]
- 21.Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982;31:830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- 22.Moi ML, Takasaki T, Saijo M, Kurane I. Determination of antibody concentration as the main parameter in a dengue virus antibody-dependent enhancement assay using FcγR-expressing BHK cells. Arch Virol. 2014;159:103–116. doi: 10.1007/s00705-013-1787-3. [DOI] [PubMed] [Google Scholar]
- 23.Moi ML, Lim CK, Kotaki A, Takasaki T, Kurane I. Development of an antibody-dependent enhancement assay for dengue virus using stable BHK-21 cell lines expressing Fc gammaRIIA. J Virol Methods. 2010;163:205–209. doi: 10.1016/j.jviromet.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Ito M, Takasaki T, Yamada K, Nerome R, Tajima S, Kurane I. Development and evaluation of fluorogenic TaqMan reverse transcriptase PCR assays for detection of dengue virus types 1 to 4. J Clin Microbiol. 2004;42:5935–5937. doi: 10.1128/JCM.42.12.5935-5937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moi ML, Lim CK, Chua KB, Takasaki T, Kurane I. Dengue virus infection-enhancing activity in serum samples with neutralizing activity as determined by using FcγR-expressing cells. PLoS Negl Trop Dis. 2012;6:e1536. doi: 10.1371/journal.pntd.0001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito M, Katakai Y, Ono F, Akari H, Mukai RZ, Takasaki T, Kotaki A, Kurane I. Serotype-specific and cross-reactive neutralizing antibody responses in cynomolgus monkeys after infection with multiple dengue virus serotypes. Arch Virol. 2011;156:1073–1077. doi: 10.1007/s00705-011-0959-2. [DOI] [PubMed] [Google Scholar]
- 27.Boonnak K, Slike BM, Donofrio GC, Marovich MA. Human FcγRII cytoplasmic domains differentially influence antibody-mediated dengue virus infection. J Immunol. 2013;190:5659–5665. doi: 10.4049/jimmunol.1203052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KR, Ong EZ, Tan HC, Zhang SL, Zhang Q, Tang KF, Kaliaperumal N, Lim AP, Hibberd ML, Chan SH, Connolly JE, Krishnan MN, Lok SM, Hanson BJ, Lin CN, Ooi EE. Leukocyte immunoglobulin-like receptor B1 is critical for antibody-dependent dengue. Proc Natl Acad Sci USA. 2014;111:2722–2727. doi: 10.1073/pnas.1317454111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis. 2010;10:712–722. doi: 10.1016/S1473-3099(10)70166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ubol S, Halstead SB. How innate immune mechanisms contribute to antibody-enhanced viral infections. Clin Vaccine Immunol. 2010;17:1829–1835. doi: 10.1128/CVI.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ubol S, Phuklia W, Kalayanarooj S, Modhiran N. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. J Infect Dis. 2010;201:923–935. doi: 10.1086/651018. [DOI] [PubMed] [Google Scholar]
- 32.Halstead SB. Pathogenesis: Risk factors prior to infection. In: Halstead SB, Pasvol G, Hoffman SL, editors. Dengue (Tropical Medicine: Science and Practice) London, United Kingdom: Imperial College Press; 2009. pp. 219–256. [Google Scholar]
- 33.Halstead SB. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979;140:527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- 34.The Marmoset Genome Sequencing and Analysis Consortium The common marmoset genome provides insight into primate biology and evolution. Nat Genet. 2014;46:850–857. doi: 10.1038/ng.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan KR, Ong EZ, Tan HC, Zhang SL, Zhang Q, Tang KF, Kaliaperumal N, Lim AP, Hibberd ML, Chan SH, Connolly JE, Krishnan MN, Lok SM, Hanson BJ, Lin CN, Ooi EE. Leukocyte immunoglobulin-like receptor B1 is critical for antibody-dependent dengue. Proc Natl Acad Sci USA. 2014;111:2722–2777. doi: 10.1073/pnas.1317454111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nimmerjahn F, Lux A. LILR-B1 blocks activating FcγR signaling to allow antibody dependent enhancement of dengue virus infection. Proc Natl Acad Sci USA. 2014;111:2404–2405. doi: 10.1073/pnas.1324286111. [DOI] [PMC free article] [PubMed] [Google Scholar]