Abstract
Few studies have focused on Rift Valley fever virus (RVFV) transmission in less arid, transitional landscapes surrounding known high-risk regions. The objective of this study was to identify evidence of RVFV exposure in Bodhei Village in a forested area at the edge of the RVFV-epidemic Garissa region. In a household cluster-based survey conducted between epidemics in early 2006, 211 participants were enrolled. Overall seroprevalence for anti-RVFV was high (18%) and comparable with rates in the more arid, dense brush regions farther north. Seroprevalence of adults was 28%, whereas that of children was significantly lower (3%; P < 0.001); the youngest positive child was age 3 years. Males were more likely to be seropositive than females (25% versus 11%; P < 0.01), and animal husbandry activities (birthing, sheltering, and butchering) were strongly associated with seropositivity. The results confirm that significant RVFV transmission occurs outside of recognized high-risk areas and independent of known epidemic periods.
Introduction
Rift Valley fever (RVF) is a zoonotic disease endemic to Kenya and other countries of Africa. The two predominant modes of RVF virus (RVFV) transmission to humans are through mosquito bites and contact with infected livestock.1,2 In humans, RVFV-related disease usually presents with headache, fever, and malaise but can progress to much more severe manifestations that include retinitis, encephalitis, or hemorrhagic fever.2,3 The overall fatality rate is estimated at 0.5–1%. However, among patients whose clinical illness is sufficiently severe to bring them to the attention of medical personnel, mortality has been reported to be as high as 29%, which was seen during the Kenya 2006–2007 outbreak.4 RVFV is a mosquito-borne virus that, because of its significant morbidity and mortality in humans and livestock, is considered to be a bioterror threat.5
RVFV was first identified in the 1930s in Kenya after isolation from a sheep in Rift Valley Province.6 RVFV is now present throughout Africa and has caused outbreaks off the east coast of Africa in Madagascar as well as in Yemen and Saudi Arabia.7 Although in semiarid areas, blooms in mosquito numbers (caused by excess rainfall) play an important role in epidemic/enzootic transmission of RVFV to both humans and animals, much is still not known about the drivers of disease occurrence in other areas of Kenya.8–11 For this study, we hypothesized that patterns of RVFV exposure and transmission in a more settled forested region (Bodhei) would prove different from those among seminomadic populations of more arid areas farther north who had been included in our previous surveys.12
Materials and Methods
Location.
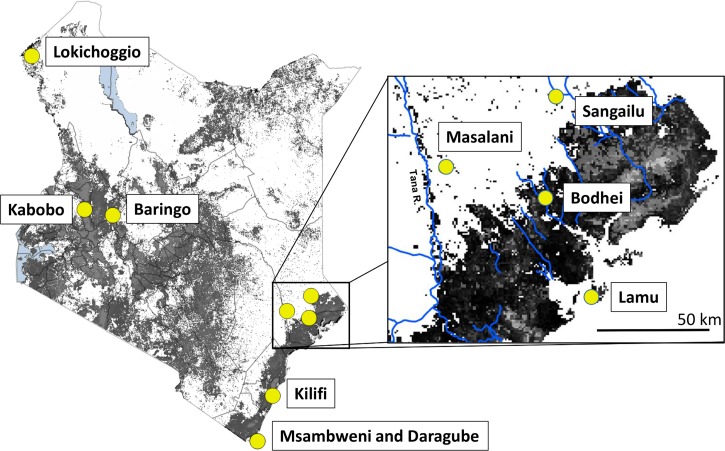
This cross-sectional, cluster-randomized survey was conducted in September of 2006 during an interepidemic period in Bodhei Village (latitude: 1.8271° S, longitude: 40.684° E), Lamu County, Kenya (55 km NNW of Lamu Town on the Garissa-Lamu County border) (Figure 1 ). This is an area of mixed grasslands and open canopy forests near the Dodori National Reserve, and the village serves as a market town, police post, and base for the Kenya Wildlife Service. Local agriculture includes small-hold cropping and livestock production of cattle, sheep, and goats. Climate and other environmental details are presented in Table 1.
Figure 1.
Map of Kenya with inset of the Bodhei study area. Tree cover15 is indicated as gray shading on each map. Shaded circles with labels indicate the town of Lamu and the Kenyan locations in Table 1 previously evaluated for RVFV transmission.10,12,13
Table 1.
Environmental characteristics15 of RVFV transmission communities in Kenya
| Location | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Northeast coast border (Bodhei) | Northeast | Coast | Rift Valley | ||||||
| Masalani | Sangailu | Daragube | Msambweni | Kilifi | Lokichoggio | Baringo | Kabobo | ||
| Community interepidemic prevalence of anti-RVFV IgG ELISA (%) | 18 | 13* | 15† | 3‡ | 2† | n/a§ | 19‡ | n/a§ | 1‡ |
| Agroecological zone | Coastal lowland semiarid | Pastoralist | Pastoralist | Coastal lowland semihumid | Coastal lowland semihumid | Coastal lowland semihumid | Pastoralist | Lower midland semiarid | Lower highland subhumid |
| Land use/land cover | Woodland and dense bush | Dense bush | Dense bush | Dense agriculture and bush | Dense agriculture and bush | Dense agriculture and bush | Sparse bush | Dense bush and swamp | Dense agriculture |
| Annual rainfall (mm) | 699 | 514 | 460 | 1,298 | 1,298 | 1,008 | 335 | 659 | 1,056 |
| Absolute maximum temperature (°C) | 32.7 | 34.6 | 33.8 | 33.2 | 33.2 | 32.6 | 32.6 | 32.6 | 26.6 |
| Absolute minimum temperature (°C) | 21.6 | 20.5 | 20.9 | 20.0 | 20.0 | 21.2 | 20.7 | 14.3 | 9.2 |
| Soil type10,15 | Planosol | Solonetz | Solonetz | Variable Arenasol, Ferrasol, Planosol | Variable Arenasol, Ferrasol, Planosol | Variable Arenasol, Ferrasol, Planosol | Variable Luvisol, Solonchak, Fluvisol | Variable Solonchak, Cambisol, Xerosol | Nitisol |
| Soil drainage | Extremely slow | Well-drained | Well-drained | Slow to well-drained | Slow to well-drained | Extremely slow, to slow to well-drained | Extremely slow to rapid | Extremely slow to well-drained | Slow |
| Tsetse presence | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| Altitude | 29 | 46 | 71 | 21 | 15 | 14 | 644 | 1,711 | 2,011 |
| Cattle density per division | 2,760 | Unknown | Unknown | 47,050 | 47,050 | 74,375 | Unknown | 60,446 | 68,796 |
| Camel density | 3 | 5 | 5 | 0 | 0 | 2 | 4 | 3 | 0 |
Population.
All long-term residents of Bodhei Village over the age of 1 year old were eligible for inclusion. After a community household census, a target 250 residents were selected for enrollment by randomized cluster sampling of households, with probability of selection proportional to household size. Of these, 219 residents were enrolled, and 211 residents completed all phases of the basic survey. In addition, 81 older children and adult participants completed vision screening and retinal examination by the project ophthalmologist (F.K.).
Study procedures.
After informational meetings with local residents, community leaders, and local healthcare officers, written informed consent was obtained from selected subjects or their parents and assent was obtained from children over 7 years old before participation following a protocol approved by the Kenya Medical Research Institute Ethical Review Committee. Subjects completed a standard demographic, exposure, and symptom questionnaire12 administered in the local language by a health worker and then received a limited physical examination. Vision testing for each eye was performed using standard Snellen wall charts. Blood for serological testing was collected at the local dispensary, and serum was separated, refrigerated, and transported to the Division of Vector-Borne and Neglected Tropical Diseases (DVBNTD) in Nairobi for anti-RVFV testing.
Laboratory procedures.
The primary outcome of the study was prior RVFV exposure measured as seropositivity of anti-RVFV immunoglobulin G (IgG) detected by enzyme-linked immunosorbent assay (ELISA) of subject serum. Specimens were screened for the presence of anti-RVF IgG by ELISA using lysates of Vero cells infected with the MP-12 strain (vaccine strain) of RVFV as the test antigen and lysates of mock-infected cells as the internal control antigen. This ELISA was established and validated in previous studies by LaBeaud and others13 and Niklasson and others.14 Serum samples diluted 1:100 were read at 405 nm, and those scoring an optical density (OD) value greater than the mean + 2 SDs of the values for negative control sera were deemed positive.
Climate, landscape, and livestock data.
Climate data and geologic and geographic data, including soil types, tree cover, land use, and livestock density patterns, were obtained from the geographic information system (GIS) site of the International Livestock Research Institute (ILRI; Nairobi, Kenya).10,15
Statistical analysis.
Initial exploratory analysis to identify the personal and environmental factors associated with anti-RVFV seropositivity was performed using χ2 testing for categorical data and Student's t testing for continuous data. For these multiple comparisons, the Bonferroni method was used to adjust cutoffs for P value significance. Next, multiply-adjusted odds ratios for seropositivity were estimated using logit-distributed generalized linear estimating equation (GEE) models that accounted for intraclass correlation at the household level. For these multivariable models, backward selection of demographic, exposure, and symptom variables was used, and significance for inclusion was set at the 0.05 level. SPSS version 21 software (IBM, Armonk, NY) was used for all statistical analysis.
Results
Of 219 residents enrolled from 86 randomly selected households, 211 residents completed surveys and full physical examinations, were evaluated for anti-RVFV seropositivity by ELISA testing, and were included for analysis in the study. Among the study group, 89 (42%) residents were children (ages 1–15 years old), and 130 (58%) residents were adults (details in Table 2). The male to female ratio for participants was 0.97, 121 (57%) residents practiced nomadic pastoralist herding, 180 (85%) residents sheltered livestock, and almost all (208; 97%) residents reported having been displaced by floods during or after the 1997 El Niño-related excess rainfall events (Table 2).
Table 2.
Demographic features and frequency of exposures of the population surveyed in Bodhei Village in September of 2006
| Characteristic | Anti-RVFV seropositive N (%) | Anti-RVFV seronegative N (%) | Total N (%) |
|---|---|---|---|
| Study cohort | 38 (18) | 173 (82) | 211 (100) |
| Sex | |||
| Male | 26 (25)* | 78 (75) | 104 |
| Female | 12 (11) | 95 (89) | 107 |
| Age class | |||
| Children (1–15 years) | 3 (3.4)† | 85 (97) | 88 |
| Adults (> 15 years) | 35 (28) | 88 (72) | 123 |
| Nomadic | |||
| Yes | 25 (21) | 96 (79) | 121 |
| No | 13 (14) | 77 (86) | 90 |
| Displaced by floods | |||
| Yes | 38 (18) | 170 (82) | 208 |
| No | 0 (0) | 3 (100) | 3 |
| Sheltered livestock | |||
| Yes | 36 (20) | 144 (80) | 180 |
| No | 2 (7) | 29 (93) | 31 |
| Killed animals | |||
| Yes | 17 (24) | 55 (76) | 72 |
| No | 21 (15) | 118 (85) | 139 |
| Butchered animals | |||
| Yes | 28 (35)† | 52 (65) | 80 |
| No | 10 (8) | 121 (92) | 131 |
| Skinned animals | |||
| Yes | 32 (32)† | 67 (68) | 99 |
| No | 6 (5) | 106 (95) | 112 |
| Milked animals | |||
| Yes | 31 (25)† | 92 (75) | 123 |
| No | 7 (8) | 81 (92) | 88 |
| Drank raw milk | |||
| Yes | 37 (20) | 153 (80) | 190 |
| No | 1 (5) | 20 (95) | 21 |
| Birthed livestock | |||
| Yes | 28 (32)† | 60 (68) | 88 |
| No | 10 (8) | 113 (92) | 123 |
| Abortus contact | |||
| Yes | 26 (34)† | 50 (66) | 76 |
| No | 12 (9) | 123 (91) | 135 |
Significant difference between sexes (P < 0.01) by χ2 test.
Significant difference between age or exposure subgroups (P < 0.001) by χ2 test.
Overall, 38 of 211 (18%) participants were anti-RVFV antibody seropositive by ELISA testing. This level of community seropositivity was high and comparable with rates found in previous serosurveys of communities in more arid regions of Garissa County and Turkana Province (Table 1).12,13
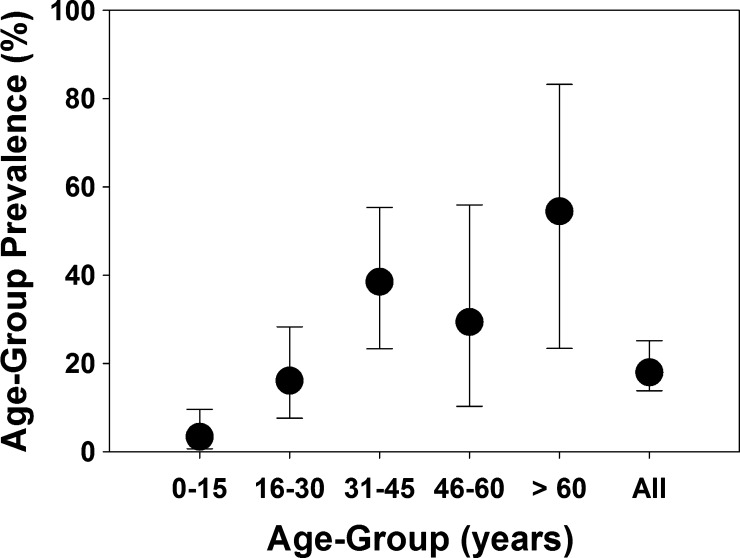
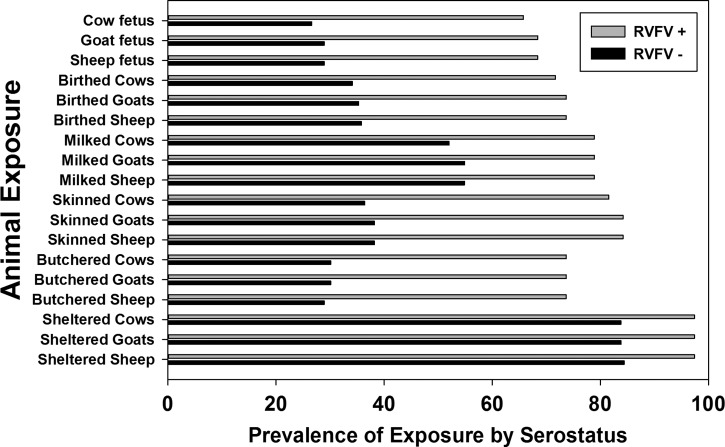
In this Bodhei sample, there were significant differences in seropositivity rates between males (25%) and females (11%) and between adults (28%) and children (3%) (Table 2). The youngest seropositive subject was 3 years old, and the oldest seropositive subject was 76 years old. Across different age groups, seroprevalence increased significantly above 15 years of age and again above 30 years of age (Figure 2 ). Personal exposures significantly associated with seropositivity were (1) history of milking an animal; (2) butchering, skinning, or birthing cattle, sheep, or goats, and (3) disposing of livestock abortus (Figure 3 and Table 2). Reported mosquito net usage was quite high (208 of 211; 97%) along with use of fire to repel mosquitoes (209 of 211), and these factors were not statistically associated with serostatus. Among reported symptoms, a history of painful eyes, poor vision, or a history of scleral injection (red eyes) was positively associated with RVFV seropositivity, but other symptoms were not (Table 3).
Figure 2.
Black circles indicate the prevalence of anti-RVFV seropositivity for the different Bodhei age groups indicated on the horizontal axis. Error bars show the 95% confidence intervals for seroprevalence. Significant differences were found between the 1–15 years old age group and the other older subjects (χ2 = 20.1, 1 degree of freedom, P < 0.001) and between the 16–30 years old and the > 30 years old age groups (χ2 = 6.7, 1 degree of freedom, P < 0.01).
Figure 3.
Differences detected between anti-RVFV seropositive versus seronegative subjects in their reported specific animal exposures. Significance was P < 0.001 for butchering, skinning, milking, birthing, or abortus disposal activities related to sheep, goats, or cattle. P values for animal sheltering were < 0.03.
Table 3.
Symptom prevalence among the population surveyed in Bodhei Village in September of 2006
| Symptom reported | Anti-RVFV seropositive N (%) | Anti-RVFV seronegative N (%) | Total N |
|---|---|---|---|
| Malaise | |||
| Yes | 27 (16) | 146 (84) | 173 |
| No | 11 (29) | 27 (71) | 38 |
| Myalgia | |||
| Yes | 28 (20) | 109 (80) | 137 |
| No | 10 (14) | 64 (86) | 74 |
| Backache | |||
| Yes | 30 (20) | 120 (80) | 150 |
| No | 8 (13) | 53 (87) | 61 |
| Eye pain | |||
| Yes | 23 (24)* | 72 (76) | 95 |
| No | 15 (13) | 101 (87) | 116 |
| Red eyes | |||
| Yes | 25 (25)* | 76 (75) | 101 |
| No | 13 (12) | 97 (88) | 110 |
| Photophobia | |||
| Yes | 13 (19) | 57 (81) | 70 |
| No | 25 (18) | 116 (82) | 141 |
| Pre-syncope, syncope, or coma | |||
| Yes | 16 (22) | 57 (78) | 73 |
| No | 22 (16) | 116 (84) | 138 |
| Poor vision | |||
| Yes | 18 (26)* | 52 (74) | 70 |
| No | 20 (14) | 121 (86) | 141 |
Significant difference between symptom subgroups (P < 0.05) by χ2 test.
For 81 subjects who had full ophthalmologic examinations, a reduced vision score by formal eye chart testing (more than six of nine vision in either eye) was significantly associated with seropositivity: 11 (46%) of 24 subjects with reduced acuity were seropositive versus 8 of 57 (14%) seropositive among those with normal vision (P = 0.002). Conversely, for this association, 58% of seropositives had poor vision in one or both eyes compared with 21% of seronegatives.
Children were less likely than adults to give a history of performing activities associated with higher risk of RVFV aerosol exposure, such as butchering and birthing.12,16,17 Similarly, animal exposures differed according to gendered roles—males had overall more live animal contact and butchering exposure, whereas females had more exposure by cooking.
To adjust for possible age, sex, or other confounding in the sample selection, we performed multivariable logistic modeling of the odds of individual seropositivity as shown in Table 4. Our multivariable GEE model confirmed the independent associations of age > 15 years old, animal exposure by skinning or birthing of livestock, and history of poor vision or past episode of pre-syncope, syncope, or coma as significant independent predictors of anti-RVFV seropositivity in the Bodhei community.
Table 4.
Unadjusted and multiply-adjusted odds ratios for anti-RVFV seropositivity in Bodhei Village, Kenya: Results of GEE logistic modeling
| Variable | Unadjusted | Multiply-adjusted* | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% Confidence interval | P value | Adjusted odds ratio | 95% Confidence interval | P value | |
| Adult > 15 years old | 11.3* | 3.3, 38 | < 0.001 | 4.4† | 1.3, 15 | 0.018 |
| Male | 2.6* | 1.2, 5.7 | 0.009 | |||
| Animal exposures | ||||||
| Sheltered animals | 3.6 | 0.8, 16 | 0.07 | |||
| Killed animals | 1.7 | 0.8, 3.6 | 0.13 | |||
| Butchered animals | 6.5* | 3.0, 14 | < 0.001 | |||
| Skinned animals | 8.4* | 3.3, 21 | < 0.001 | 4.1† | 1.6, 10.5 | 0.004 |
| Milked animals | 3.9* | 1.6, 9.3 | 0.001 | |||
| Drank raw milk | 4.8 | 0.6, 37 | 0.1 | |||
| Birthed livestock | 5.3* | 2.4, 11.6 | < 0.001 | 2.5† | 1.01, 6.3 | 0.048 |
| Abortus contact | 5.3* | 2.5, 11.4 | < 0.001 | |||
| Symptoms | ||||||
| Malaise | 0.45 | 0.2, 1.02 | 0.053 | |||
| Myalgia | 1.6 | 0.75, 3.6 | 0.21 | |||
| Backache | 1.7 | 0.7, 3.9 | 0.24 | |||
| Eye pain | 2.2* | 1.05, 4.4 | 0.034 | |||
| Red eyes | 2.5* | 1.2, 5.1 | 0.015 | |||
| Photophobia | 1.06 | 0.5, 2.2 | 0.88 | |||
| Pre-syncope, syncope, or coma | 1.5 | 0.72, 3.0 | 0.28 | 3.6† | 1.5, 8.4 | 0.003 |
| Poor vision | 2.1* | 1.02, 4.3 | 0.04 | 2.6† | 1.1, 6.2 | 0.026 |
Significant associations.
Variables remaining in the best fit parsimonious multivariable GEE logistic model accounting for intrahousehold correlation of serostatus outcomes.
Discussion
This survey documents a high level of exposure to RVFV in a coastal forest area of Lamu Province. Evidence of past exposure was detected in terms of anti-RVFV antibody among long-term residents of the Bodhei area. Measured seroprevalence in this location was as high as in other previously surveyed high-risk locations of Garissa and Turkana Counties (Table 1) and well above the 1–3% seroprevalence seen in low-risk areas of the coastal plain (Msambweni) and the central highlands (Kabobo) of Kenya.12,13 Risk of seropositivity was not uniform—adults > 15 years old, especially those who worked with livestock, had significantly higher rates of seropositivity. Among animal contact activities, skinning, butchering, and assisting at delivery were most strongly linked to past RVFV exposure. As previously noted in our other surveys, symptoms of poor vision and objective visual impairment were significantly associated with evidence of past RVFV infection.16 Among our Bodhei study population, a history of sensorial changes (pre-syncope, syncope, or coma) proved to be a significant correlate of anti-RVFV seropositivity among subjects without a history of livestock skinning or birthing exposure, perhaps suggesting a difference in disease manifestations depending on route of exposure. Visual disturbances, retinitis,12,18 and long-term neurological complications19 remain less well-known complications of RVFV infection.
Our study confirms that RVFV transmission is not limited to semiarid regions, although more arid pastoralist areas experienced some of the most prominent epizootics and epidemics in 1997 and 19984,17 and again, in 2006 and 2007.2,4,10 For our study, it was hypothesized that, because rains foster local tsetse and yield better pasturage in the north Garissa region, seasonal cattle migration away from the Bodhei forested region would have resulted in less exposure to RVFV during the rainy periods of transmission. However, Bodhei is a transit point for the animals being taken from the interior Ijara District to Lamu for marketing. Although seasonal movement of cattle may play a role, the serology evidence indicates that Bodhei residents working with livestock still have significant exposure to infection. The age discrepancy in seroprevalence (over and under the age of 15 years old), as noted by others,17 suggests that occupational exposure to RVFV through aerosolized transmission rather than mosquito vector-borne transmission is the predominant source of RVFV exposure in Bodhei and elsewhere in Kenya.2
Transmission in non-arid areas has been previously documented in Kenya and other endemic countries of Africa. Within Kenya, significant human and animal RVF outbreaks have occurred in the Lake Naivasha, Lake Baringo, and Kilifi areas.10,20,21 Independent of elevation and average annual rainfall, local soil structure causing poor drainage combined with periods of excess rainfall and abundant local domestic ungulate populations seem to foster augmentation of mosquito numbers along with RVFV amplification in livestock.8–10 For Bodhei, poor drainage combined with extensive local animal herd exposure were likely to have been the most influential factors in the high local rates of transmission, whether epidemic (1997–1998 and 2006–2007) or interepidemic, which are evidenced in this survey by the seropositivity found among very young Bodhei children (age = 3 years old).
There are limitations to this study. The sample size, although sufficient to identify an area of high seroprevalence, was likely underpowered for identification of some infection-associated exposure factors and potentially risk-reducing preventive measures. Within the study location, the residents' experience was universal (fully homogeneous) in terms of flood exposure, mosquito exposure, and use of mosquito prevention, thus limiting our ability to assess the impact of these factors. Diagnosis of our subjects was by direct ELISA for anti-RVFV IgG. Although this screening tool is effective for surveillance, its sensitivity and specificity are less than those for the plaque reduction neutralization assay,12,14 meaning that a fraction of our subjects were misclassified in terms of RVFV exposure. Finally, the survey was performed approximately 8 years after the last known RVF epidemic, and subject recall bias was likely to have affected the reported symptom histories.
In summary, our results suggest that significant RVFV transmission often occurs outside of the recognized high-risk RVFV areas of northeast Kenya and at times, independent of known epidemic events.12,21,22 Effective surveillance and prevention (livestock vaccination) will require inclusion of border areas, such as the transitional coastal forests of Kenya, as risk zones during future periods of El Niño/Southern Oscillation (ENSO) excess rainfall in the Horn of Africa.23 Improved warning systems through more detailed mapping combined with early intervention should aid in preventing recurrence of the severe RVF outbreaks of the past.24
ACKNOWLEDGMENTS
The authors thank the population of Bodhei Village for their hospitality and willing participation in this survey project.
Footnotes
Financial support: This work was supported by the Kenya Ministry of Public Health and Sanitation and for laboratory testing, funding from US National Institutes of Health Grants U01AI45473-S1, 1KL2RR024990, and T32AI52067 and the Robert E. Shope Fellowship in Infectious Diseases Award.
Authors' addresses: Samuel Muiruri, Vector Borne Diseases Control Unit, Ministry of Health, Nairobi, Kenya, E-mail: smuiruri37@yahoo.com. Ephantus W. Kabiru, School of Public Health, Kenyatta University, Nairobi, Kenya, E-mail: ewkabiru@yahoo.com. Eric M. Muchiri, Meru University of Science and Technology, Meru, Kenya, E-mail: ericmmuchiri@gmail.com. Hassan Hussein, Office of Director of Health, Garissa County, Kenya, E-mail: hhnuriye@yahoo.com. Frederick Kagondu, Department of Ophthalmology, Thika Level 5 Hospital, Thika, Kenya, E-mail: fkagondu@yahoo.com. A. Desirée LaBeaud, Stanford University School of Medicine, Palo Alto, CA, E-mail: dlabeaud@stanford.edu. Charles H. King, Center for Global Health and Diseases, Case Western Reserve University, Cleveland, OH, E-mail: chk@cwru.edu.
References
- 1.Ksiazek TG, Jouan A, Meegan JM, Le Guenno B, Wilson ML, Peters CJ, Digoutte JP, Guillaud M, Merzoug NO, Touray EM. Rift Valley fever among domestic animals in the recent West African outbreak. Res Virol. 1989;140:67–77. doi: 10.1016/s0923-2516(89)80086-x. [DOI] [PubMed] [Google Scholar]
- 2.Anyangu AS, Gould LH, Sharif SK, Nguku PM, Omolo J, Mutonga D, Rao CY, Lederman E, Schnabel DC, Paweska JT, Katz M, Hightower A, Njenga MK, Feikin DR, Breiman RF. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am J Trop Med Hyg. 2010;83((2 Suppl)):14–21. doi: 10.4269/ajtmh.2010.09-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahlon SS, Peters CJ, Leduc J, Muchiri EM, Muiruri S, Njenga MK, Breiman RF, Clinton White A, Jr, King CH. Severe Rift Valley fever may present with a characteristic clinical syndrome. Am J Trop Med Hyg. 2010;82:371–375. doi: 10.4269/ajtmh.2010.09-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC Rift Valley fever outbreak–Kenya, November 2006–January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:73–76. [PubMed] [Google Scholar]
- 5.Hartley DM, Rinderknecht JL, Nipp TL, Clarke NP, Snowder GD. National Center for Foreign Animal and Zoonotic Disease Defense Advisory Group on Rift Valley Fever Potential effects of Rift Valley fever in the United States. Emerg Infect Dis. 2011;17:e1. doi: 10.3201/eid1708.101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or Rift Valley fever: an undescribed virus disease of sheep, cattle and man from East Africa. J Pathol Bacteriol. 1931;34:545–579. [Google Scholar]
- 7.Clements AC, Pfeiffer DU, Martin V, Otte MJ. A Rift Valley fever atlas for Africa. Prev Vet Med. 2007;82:72–82. doi: 10.1016/j.prevetmed.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Davies FG, Linthicum KJ, James AD. Rainfall and epizootic Rift Valley fever. Bull World Health Organ. 1985;63:941–943. [PMC free article] [PubMed] [Google Scholar]
- 9.Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285:397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- 10.Hightower A, Kinkade C, Nguku PM, Anyangu A, Mutonga D, Omolo J, Njenga MK, Feikin DR, Schnabel D, Ombok M, Breiman RF. Relationship of climate, geography, and geology to the incidence of Rift Valley fever in Kenya during the 2006–2007 outbreak. Am J Trop Med Hyg. 2012;86:373–380. doi: 10.4269/ajtmh.2012.11-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munyua P, Murithi RM, Wainwright S, Githinji J, Hightower A, Mutonga D, Macharia J, Ithondeka PM, Musaa J, Breiman RF, Bloland P, Njenga MK. Rift Valley fever outbreak in livestock in Kenya, 2006–2007. Am J Trop Med Hyg. 2010;83((2 Suppl)):58–64. doi: 10.4269/ajtmh.2010.09-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaBeaud AD, Muchiri EM, Ndzovu M, Mwanje MT, Muiruri S, Peters CJ, King CH. Interepidemic Rift Valley fever virus seropositivity, northeastern Kenya. Emerg Infect Dis. 2008;14:1240–1246. doi: 10.3201/eid1408.080082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaBeaud AD, Ochiai Y, Peters CJ, Muchiri EM, King CH. Spectrum of Rift Valley fever virus transmission in Kenya: insights from three distinct regions. Am J Trop Med Hyg. 2007;76:795–800. [PMC free article] [PubMed] [Google Scholar]
- 14.Niklasson B, Grandien M, Peters CJ, Gargan TP. Detection of Rift Valley fever virus antigen by enzyme-linked immunosorbent assay. J Clin Microbiol. 1983;17:1026–1031. doi: 10.1128/jcm.17.6.1026-1031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruska A, Kariuki P. International Livestock Research Institute GIS Database. 2007. http://www.ilri.org/gis Available at. Accessed June 20, 2014.
- 16.LaBeaud AD, Muiruri S, Sutherland LJ, Dahir S, Gildengorin G, Morrill J, Muchiri EM, Peters CJ, King CH. Postepidemic analysis of Rift Valley fever virus transmission in northeastern Kenya: a village cohort study. PLoS Negl Trop Dis. 2011;5:e1265. doi: 10.1371/journal.pntd.0001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods CW, Karpati AM, Grein T, McCarthy N, Gaturuku P, Muchiri E, Dunster L, Henderson A, Khan AS, Swanepoel R, Bonmarin I, Martin L, Mann P, Smoak BL, Ryan M, Ksiazek TG, Arthur RR, Ndikuyeze A, Agata NN, Peters CJ, WHO Hemorrhagic Fever Task Force An outbreak of Rift Valley fever in Northeastern Kenya, 1997–98. Emerg Infect Dis. 2002;8:138–144. doi: 10.3201/eid0802.010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hazmi A, Al-Rajhi AA, Abboud EB, Ayoola EA, Al-Hazmi M, Saadi R, Ahmed N. Ocular complications of Rift Valley fever outbreak in Saudi Arabia. Ophthalmology. 2005;112:313–318. doi: 10.1016/j.ophtha.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Riou O, Philippe B, Jouan A, Coulibaly I, Mondo M, Digoutte JP. Neurologic and neurosensory forms of Rift Valley fever in Mauritania. Bull Soc Pathol Exot. 1989;82:605–610. [PubMed] [Google Scholar]
- 20.Davies FG. The historical and recent impact of Rift Valley fever in Africa. Am J Trop Med Hyg. 2010;83((2 Suppl)):73–74. doi: 10.4269/ajtmh.2010.83s2a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murithi RM, Munyua P, Ithondeka PM, Macharia JM, Hightower A, Luman ET, Breiman RF, Njenga MK. Rift Valley fever in Kenya: history of epizootics and identification of vulnerable districts. Epidemiol Infect. 2011;139:372–380. doi: 10.1017/S0950268810001020. [DOI] [PubMed] [Google Scholar]
- 22.LaBeaud AD, Cross PC, Getz WM, Glinka A, King CH. Rift Valley fever virus infection in African buffalo (Syncerus caffer) herds in rural South Africa: evidence of interepidemic transmission. Am J Trop Med Hyg. 2011;84:641–646. doi: 10.4269/ajtmh.2011.10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anyamba A, Chretien JP, Small J, Tucker CJ, Formenty PB, Richardson JH, Britch SC, Schnabel DC, Erickson RL, Linthicum KJ. Prediction of a Rift Valley fever outbreak. Proc Natl Acad Sci USA. 2009;106:955–959. doi: 10.1073/pnas.0806490106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consultative Group for RVF Decision Support Decision-support tool for prevention and control of Rift Valley fever epizootics in the greater Horn of Africa. Am J Trop Med Hyg. 2010;83((Suppl 2)):75–85. doi: 10.4269/ajtmh.2010.83s2a03. [DOI] [PMC free article] [PubMed] [Google Scholar]