Abstract
Hepatitis E virus (HEV) is a common cause of acute viral hepatitis in developing countries; however, its contribution to acute jaundice syndrome is not well-described. A large outbreak of hepatitis E occurred in northern Uganda from 2007 to 2009. In response to this outbreak, acute jaundice syndrome surveillance was established in 10 district healthcare facilities to determine the proportion of cases attributable to hepatitis E. Of 347 acute jaundice syndrome cases reported, the majority (42%) had hepatitis E followed by hepatitis B (14%), malaria (10%), hepatitis C (5%), and other/unknown (29%). Of hepatitis E cases, 72% occurred in Kaboong district, and 68% of these cases occurred between May and August of 2011. Residence in Kaabong district was independently associated with hepatitis E (adjusted odds ratio = 13; 95% confidence interval = 7–24). The findings from this surveillance show that an outbreak and sporadic transmission of hepatitis E occur in northern Uganda.
Introduction
Hepatitis E virus (HEV) infection is a major public health problem, causing large outbreaks and sporadic acute hepatitis in developing countries. In 2005, an estimated 20.1 million HEV infections occurred, resulting in 3.4 million acute cases and 56,000 deaths.1,2 HEV infection is particularly severe during pregnancy; pregnant women are at risk to develop fulminant liver failure and obstetric complications, with a mortality rate as high as 10–25%.3 During outbreaks, HEV is transmitted by drinking fecally contaminated water, and direct person-to-person contact may contribute to sustained transmission after a common source is removed.4
The contribution of hepatitis E to acute viral hepatitis (AVH) is not well-understood. Few studies have shown that hepatitis E is the leading cause of AVH in developing countries.5,6 However, in many countries, routine etiologic AVH surveillance and laboratory capacity to do so do not exist.7 Thus, the burden of AVH and the proportion attributable to hepatitis E are not well-known. Owing to the lack of capacity for AVH surveillance, acute jaundice syndrome (AJS) surveillance is used for viral hepatitis in humanitarian emergencies and resource-limited settings.
In recent years, outbreaks of hepatitis E involving several thousand cases have been observed in a number of countries, including countries where it has not been reported in the past.5 In October of 2007, the largest hepatitis E epidemic among displaced persons in Africa and the first epidemic in Uganda occurred in Kitgum district, northern Uganda.8 In response to this outbreak, AJS surveillance was established to determine the etiology of AJS and the proportion of cases attributable to hepatitis E and detect outbreaks in neighboring districts of the 2007 outbreak. This report summarizes findings from this surveillance system.
Methods
Ten healthcare facilities, including Kitgum Health Center located in northern Uganda, participated in AJS surveillance. Healthcare workers in these facilities were trained to identify cases of jaundice. A case of AJS was defined as a person presenting with jaundice of less than 1 month duration and any of the following symptoms: nausea, vomiting, abdominal pain, loss of appetite, fever, itching, joint pain, dark urine, malaise, and headache. A standard form was used to collect data, including age, gender, residence (rural versus urban), disease outcome (including hospitalization and death), and potential risk factors (including drinking water source, treatment of drinking water, handwashing, soap use, latrine use, and contact with a person with jaundice within 2 months before symptom onset). Blood samples were collected by phlebotomy, processed, and shipped to the Uganda Virus Research Institute (UVRI) for hepatitis serology and HEV nucleic acid testing. Blood film was tested for malaria on site.
Laboratory criteria for each type of hepatitis were (1) hepatitis A, immunoglobulin M (IgM) antihepatitis A virus-positive; (2) acute hepatitis B, IgM anti-hepatitis B core antibody (HBc)–positive and hepatitis B surface antigen (HBsAg)-positive; (3) hepatitis C, antihepatitis C virus-positive and IgM antihepatitis A virus-negative, HBsAg-negative, and IgM anti-HEV negative; and (4) hepatitis E, IgM anti-HEV–positive or HEV RNA-positive.
Statistical analyses
Data were entered into the Epi Info database, and analysis was conducted using SAS, version 9.2.9 Descriptive statistics were used to summarize demographic variables and the proportion of cases attributable to hepatitis E. Logistic regression analysis was used to determine the association between demographics, sanitation and hygiene conditions, and hepatitis E.
Results
From February of 2010 to March of 2012, 347 cases of AJS were reported. The median age of cases was 24 years old (range = 1–81 years old), 56% were male, 83% resided in rural areas, 21% were hospitalized, and 2% died. Of 347 AJS cases, 144 (42%) had hepatitis E (Table 1). Of 144 hepatitis E cases, 135 were positive for IgM anti-HEV, 107 of which were also positive for IgG anti-HEV. Of 110 IgM anti-HEV–positive cases that were tested for HEV RNA, 64 (58%) had detectable HEV RNA. Of 122 IgM anti-HEV–negative AJS cases, 9 (7.3%) had detectable HEV RNA by reverse transcription polymerase chain reaction (RT-PCR). The remaining 115 cases were not tested for HEV RNA. Of 144 hepatitis E cases, 44% were female, 86% were in the age group 15–49 years old, 72% were from Kaabong district, 17% were hospitalized, and 1% died. The two deaths in hepatitis E cases were among a 32-year-old non-pregnant female and a 24-year-old male.
Table 1.
Selected demographic and clinical characteristics of patients with AJS by etiology of jaundice in northern Uganda from 2010 to 2012 (N = 347)
| Characteristic | n (%) | |||||
|---|---|---|---|---|---|---|
| Hepatitis B | Hepatitis C | Hepatitis E | Malaria | Other*/ unknown† | Total‡ | |
| Overall | 50 (14) | 17 (5) | 144 (42) | 35 (10) | 101 (29) | 347 (100)‡ |
| Sex | ||||||
| Female | 16 (32) | 10 (59) | 63 (44) | 18 (51) | 47 (47) | 154 (44) |
| Male | 34 (68) | 7 (41) | 81 (56) | 17 (49) | 54 (53) | 193 (56) |
| Age group | ||||||
| ≤ 10 | 10 (20) | 3 (18) | 5 (3) | 9 (26) | 20 (20) | 50 (14) |
| 11–20 | 14 (28) | 3 (18) | 36 (25) | 5 (14) | 22 (23) | 80 (23) |
| 21–30 | 13 (26) | 3 (18) | 60 (42) | 14 (40) | 31 (32) | 121 (35) |
| 31–40 | 7 (14) | 3 (18) | 32 (22) | 3 (9) | 12 (12) | 57 (16) |
| 40+ | 6 (12) | 5 (29) | 11 (18) | 4 (11) | 13 (13) | 39 (11) |
| District of healthcare facility | ||||||
| Abim | 4 (8) | 3 (18) | 10 (6) | 16 (46) | 23 (22) | 56 (16) |
| Amuru | 1 (2) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 2 (1) |
| Dokolo | 10 (20) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 12 (3) |
| Gulu | 2 (4) | 1 (6) | 1 (1) | 1 (3) | 0 (0) | 5 (1) |
| Kaabong | 11 (22) | 3 (18) | 104 (72) | 6 (17) | 14 (14) | 138 (40) |
| Kitgum | 8 (16) | 2 (12) | 10 (7) | 1 (3) | 18 (18) | 39 (11) |
| Kotido | 10 (20) | 5 (29) | 13 (9) | 7 (20) | 36 (36) | 71 (20) |
| Lira | 3 (6) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 5 (1) |
| Mbarara | 0 (0) | 2 (12) | 3 (2) | 2 (6) | 7 (7) | 14 (4) |
| Padler | 1 (2) | 1 (6) | 0 (0) | 2 (6) | 1 (1) | 5 (1) |
| Place of residence | ||||||
| Rural | 42 (88) | 14 (82) | 124 (87) | 28 (80) | 78 (77) | 286 (83) |
| Urban | 5 (10) | 0 (0) | 17 (12) | 7 (20) | 21 (21) | 50 (15) |
| Camp | 1 (2) | 2 (12) | 2 (1) | 0 (0) | 0 (0) | 5 (1) |
| Other | 0 (0) | 1 (6) | 1 (1) | 0 (0) | 1 (1) | 3 (1) |
| Clinical profile | ||||||
| Inpatient | 7 (14) | 4 (24) | 24 (17) | 13 (37) | 23 (23) | 71 (21) |
| Outpatient | 32 (65) | 12 (71) | 114 (79) | 22 (63) | 69 (69) | 249 (72) |
| Unknown | 10 (20) | 1 (6) | 6 (4) | 0 (0) | 8 (8) | 25 (7) |
| Clinical outcome | ||||||
| Alive | 33 (67) | 13 (76) | 127 (89) | 32 (94) | 89 (89) | 294 (86) |
| Dead | 1 (2) | 1 (6) | 2 (1) | 1 (3) | 1 (1) | 6 (2) |
| Unknown | 15 (31) | 3 (18) | 14 (10) | 1 (3) | 10 (110) | 43 (13) |
Other causes of AJS include hepatitis A (seven cases) and yellow fever (seven cases).
Unknown causes of AJS (92 cases).
Percentages might not add up to 100% because of missing data or rounding.
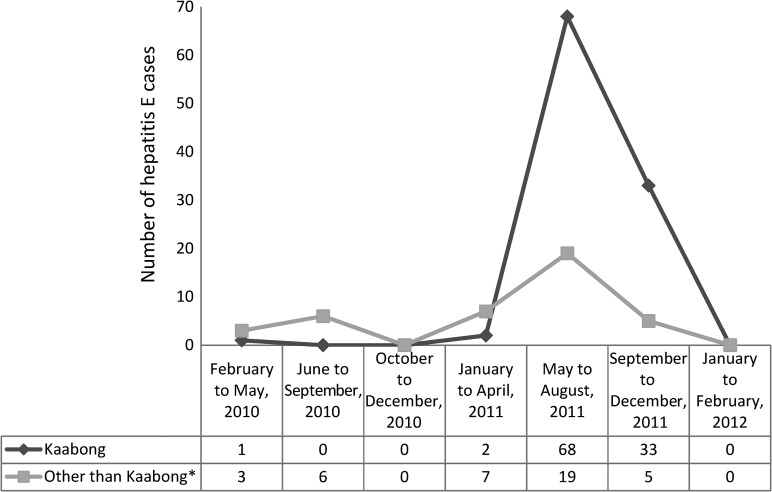
Almost all (101 of 104) of the cases from Kaabong were reported in 2011, of which 68% were reported during the months from May to August of 2011 and the remaining 29% occurred during the months from September to December (Figure 1).
Figure 1.
Number of hepatitis E cases by months of symptom onset and district in northern Uganda from 2010 to 2012. *Other than Kaabong includes Abim, Amuru, Dokolo, Gulu, Kitgum, Kotido, Lira, Mbarara, and Padler.
Of seven pregnant women with hepatitis E, three women were in the third trimester of their pregnancy, and one woman was hospitalized. Other AJS causes included hepatitis B (14%), hepatitis C (5%), malaria (10%), and other/unknown (29%; includes hepatitis A [2%] and yellow fever [0.7%]) (Table 1). In univariate analyses, age, place of residence, and contact with a jaundiced person were associated with hepatitis E. Residence in Kaabong district was associated with increased odds of hepatitis E (Table 2).
Table 2.
Univariate and multivariate analyses of factors associated with hepatitis E compared with other causes of AJS in northern Uganda from 2010 to 2012 (N = 144)
| Characteristic | Hepatitis E n (%) | Univariate OR (95% CI) | Multivariate OR (95% CI) |
|---|---|---|---|
| Sex | |||
| Female | 63 (44) | Reference | NS |
| Male | 81 (56) | 1.05 (0.68–1.61) | |
| Age group (years) | |||
| ≤ 10 | 5 (4) | 0.12 (0.05–0.34)* | 0.20 (0.05–0.80)† |
| 11–20 | 36 (25) | 0.83 (0.47–1.47) | 0.92 (0.45–1.86) |
| 21–30 | 60 (42) | Reference | Reference |
| 31–40 | 32 (22) | 1.30 (0.69–2.45) | 1.60 (0.73–3.48) |
| 40+ | 11 (7) | 0.40 (0.18–0.87)† | 0.45 (0.18–1.13)† |
| Pregnant | |||
| Yes | 7 (15) | 0.95 (0.35–2.61) | NS |
| No | 40 (85) | Reference | |
| District of residence | |||
| Kaabong | 104 (72) | 12.92 (7.70–21.70)* | 13.04 (7.02–24.23)* |
| Other than Kaabong | 40 (28) | Reference | |
| Source of drinking water | |||
| Safe water‡ | 114 (88) | Reference | NS |
| Surface | 30 (12) | 0.89 (0.53–1.50) | |
| Boiled and treated | |||
| Yes | 29 (21) | Reference | NS |
| No | 111 (79) | 0.90 (0.51–1.564) | |
| Used latrine | |||
| Yes | 89 (64) | Reference | NS |
| No | 51 (36) | 1.00 (0.63–1.58) | |
| Contact with jaundiced person | |||
| Yes | 54 (39) | 1.78 (1.10–2.86)† | NS |
| No | 83 (61) | Reference | |
| Total | 144 (42) | ||
P < 0.0001.
P < 0.05.
Safe water included piped and protected well.
CI = confidence interval; NS = not significant; OR = odds ratio.
Discussion
AJS surveillance showed that hepatitis E was the most common cause of AJS among patients seeking medical care in northern Uganda. Although hepatitis E infection has been recognized as a major cause of jaundice in developing countries,10,11 data from this surveillance system clearly showed that a combination of sporadic and outbreak hepatitis E is the leading cause of AJS in northern Uganda. In this study, hepatitis E was independently associated with age and residence in Kaabong district. The temporal clustering of cases from Kaabong represents an outbreak of hepatitis E from May to August of 2011. The characteristics of hepatitis E cases by age group and gender are not different from the commonly observed epidemiologic characteristics of hepatitis E as reported previously.10,12,13 A high proportion of AJS cases was hospitalized, suggesting that people with severe illness were seeking care at these health facilities.
Unlike reports from India14,15 and Pakistan,16 when we exclude cases from Kaboong district (where an outbreak occurred in 2011), we found that hepatitis E is not the most common cause of jaundice in sporadic hepatitis cases in the surveillance sites in northern Uganda. The etiologic identification also showed that other hepatitides, including hepatitis B and hepatitis C, were relatively common causes of jaundice. Not surprisingly, hepatitis A was the least common of the causes of AJS.17
There are few limitations of our study. The sites were conveniently selected to be contiguous to the outbreak epicenter in northern Uganda. Patients with mild disease without jaundice would not have been detected. Infected infants and young children usually do not have jaundice18 and therefore, would not be detected by AJS surveillance. Thus, jaundice surveillance might be incomplete but can be a useful index of hepatitis E incidence in an endemic population. We could not ascertain whether antihepatitis C virus-positive cases were currently infected, because we did not have the capacity to determine hepatitis C virus RNA. In some hepatitis B cases, we were unable to determine whether these were acute or chronic infections because of the lack of IgM anti-HBc tests during some of the surveillance period.
Jaundice surveillance is useful in resource-limited settings. If supplemented by etiologic confirmation, this system is effective in identifying cases of hepatitis E, detecting outbreaks, and providing essential data for prevention and control. The lessons learned from AJS surveillance in northern Uganda could be used to improve population-level surveillance and inform comprehensive hepatitis prevention and control strategies and policy, including vaccination.
ACKNOWLEDGMENTS
The authors thank our many helpful partners: the Ugandan Ministry of Health, the Ugandan Virus Research Institute, the Centers for Disease Control and Prevention–Uganda, and the staff in all participating health institutions.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Gemechu B. Gerbi, Roxanne Williams, Stephen Liu, Jan Drobeniuc, Saleem Kamili, Fujie Xu, Scott D. Holmberg, and Eyasu H. Teshale, Division of Viral Hepatitis, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: ggerbi@msm.edu, rewilli8@yahoo.com, ice5@cdc.gov, jqd6@cdc.gov, sek6@cdc.gov, fax1@cdc.gov, sdh1@cdc.gov, and eht4@cdc.gov. Barnabas Bakamutumaho, Uganda Virus Research Institute–Centers for Disease Control and Prevention Influenza Surveillance Project, World Health Organization Influenza Collaborating Center, Entebbe, Uganda, E-mail: bbarnabas2001@yahoo.com. Robert Downing, Centers for Disease Control and Prevention–Uganda, Kampala, Uganda, E-mail: rqd6@ug.cdc.gov.
References
- 1.Rein DBSG, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthmann JP, Klovstad H, Boccia D, Hamid N, Pinoges L, Nizou JY, Tatay M, Diaz F, Moren A, Grais RF, Ciglenecki I, Nicand E, Guerin PJ. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: the role of water treatment methods. Clin Infect Dis. 2006;42:1685–1691. doi: 10.1086/504321. [DOI] [PubMed] [Google Scholar]
- 4.Teshale EH, Grytdal SP, Howard C, Barry V, Kamili S, Drobeniuc J, Hill VR, Okware S, Hu DJ, Holmberg SD. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in northern Uganda. Clin Infect Dis. 2010;50:1006–1010. doi: 10.1086/651077. [DOI] [PubMed] [Google Scholar]
- 5.Isaacson M, Frean J, He JK, Seriwatana J, Innis BL. An outbreak of hepatitis E in northern Namibia, 1983. Am J Trop Med Hyg. 2000;62:619–625. doi: 10.4269/ajtmh.2000.62.619. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen GC, Segev DL, Thuluvath PJ. Nationwide increase in hospitalizations and hepatitis C among inpatients with cirrhosis and sequelae of portal hypertension. Clin Gastroenterol Hepatol. 2007;5:1092–1099. doi: 10.1016/j.cgh.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 7.WHO WHO Recommended Surveillance Standard of Acute Viral Hepatitis. http://www.who.int/immunization_monitoring/diseases/hepatitis_surveillance/en/ Available at. Accessed March 17, 2013.
- 8.The International Federation The International Federation’s Disaster Relief Emergency Fund Operation Final Report. 2011. http://www.ifrc.org/docs/appeals/08/MDRUG009fr.pdf Available at. Accessed March 15, 2013.
- 9.SAS Institute Inc. Cary, NC: [Google Scholar]
- 10.Buisson YGM, Nicand E, Cheval P, van Cuyck-Gandre H, Innis B, Rehel P, Coursaget P, Teyssou R, Tsarev S. Identification of a novel hepatitis E virus in Nigeria. J Gen Virol. 2000;81:903–909. doi: 10.1099/0022-1317-81-4-903. [DOI] [PubMed] [Google Scholar]
- 11.Bradley DW. Enterically-transmitted non-A, non-B hepatitis. Br Med Bull. 1990;46:442–461. doi: 10.1093/oxfordjournals.bmb.a072409. [DOI] [PubMed] [Google Scholar]
- 12.Isaacson MFJ, He J, Seriwatana J, Innis BL. An outbreak of hepatitis E in northern Namibia, 1983. Am J Trop Med Hyg. 2000;62:619–625. doi: 10.4269/ajtmh.2000.62.619. [DOI] [PubMed] [Google Scholar]
- 13.Saeddi MI, Mahmood K, Amanullah, Ziauddin M, Ilyas N, Zarif M. Frequency and clinical course of hepatitis E in tertiary care hospitals. J Coll Physicians Surg Pak. 2004;14:527–529. [PubMed] [Google Scholar]
- 14.Chadha MS, Walimbe AM, Chobe LP, Arankalle VA. Comparison of etiology of sporadic acute and fulminant viral hepatitis in hospitalized patients in Pune, India during 1978–81 and 1994–97. Indian J Gastroenterol. 2003;22:11–15. [PubMed] [Google Scholar]
- 15.Das K, Agarwal A, Andrew R, Frosner GG, Kar P. Role of hepatitis E and other hepatotropic virus in aetiology of sporadic acute viral hepatitis: a hospital based study from urban Delhi. Eur J Epidemiol. 2000;16:937–940. doi: 10.1023/a:1011072015127. [DOI] [PubMed] [Google Scholar]
- 16.Khan A, Tanaka Y, Kurbanov F, Elkady A, Abbas Z, Azam Z, Subhan A, Raza S, Hamid S, Jafri W, Shih J, Xia N, Takahashi K, Mishiro S, Mizokami M. Investigating an outbreak of acute viral hepatitis caused by hepatitis E virus variants in Karachi, South Pakistan. J Med Virol. 2011;83:622–629. doi: 10.1002/jmv.22036. [DOI] [PubMed] [Google Scholar]
- 17.Manmohan Gupta. Retrospective hospital based study of infective causes of jaundice in Tamil Nadu, India. Calcutta Med J. 2011;9:e4. [Google Scholar]
- 18.Teshale EH, Grytdal SP, Howard C, Barry V, Kamili S, Drobeniuc J, Hill VR, Okware S, Hu DJ, Holmberg SD. Evidence of person-to-person transmission of hepatitis e virus during a large outbreak in northern Uganda. Clin Infect Dis. 2010;50:1006–1010. doi: 10.1086/651077. [DOI] [PubMed] [Google Scholar]