Abstract
In this study, we characterize the ability of the previously described Infoscitex tent (IST) to capture mosquitoes in comparison to either the Centers for Disease Control Light Trap hung next to individuals under a bed net (LTC) or to human landing catches (HLC). In Senegal, the IST caught 6.14 times the number of Anopheles gambiae sensu lato (s.l.), and 8.78 times the Culex group V mosquitoes as LTC. In one of two locations in Burkina Faso, the IST caught An. gambiae at a rate not significantly different than HLC. Of importance, 9.1–36.1% of HLC caught An. gambiae were blood fed, mostly with fresh blood, suggesting they fed upon the collector, whereas only 0.5–5.0% from the IST had partial or old blood. The IST also caught outdoor biting species in proportions comparable to HLC. The results show this tent provides a safer and effective alternative to the skill-dependent, risky, and laborious HLC method.
Introduction
The current “gold-standard” approach for the collection of anthropophilic vectors is the human landing catch (HLC) technique. This approach involves the use of human participants as bait to attract arthropod vectors, which can be collected as they attempt to bite. It has been well documented that this technique, although effective and the benchmark for calculation of a variety of entomological measures, is laborious, skill-dependent, difficult to manage, and puts participants at direct risk of vector-borne disease.1–5 As a result of these concerns, development of alternatives to HLC for the capture of host-seeking vectors is important. Currently used alternative approaches include the Centers for Disease Control Light Trap,3 baited odor boxes,6 vector-electrocuting grids,4 and a variety of human-baited tent trap designs including the Mbita, Ifakara B and C, and Furvela tent traps.2,5,7 These techniques have found varying degrees of success and applicability, though unreliability in biting estimation,2,3 failure to distinguish between endophagic/exophagic populations,4 and possible exposure to vectors2 have all been reported. Furthermore, most of the previously described tent traps have been passive rather than active designs, and did not make use of modern camping tent materials for increased durability, collector comfort, and ease of use and setup.
Previously, we described the design, laboratory testing, and initial field testing of the Infoscitex Tent (IST), which features an active, battery-powered trapping design and is constructed with modern camping tent materials.1 The IST tent meets the initial critical requirement of essentially zero exposure of the person in the tent to biting vectors, while still attracting and collecting vectors. It successfully sampled An. gambiae s.l. and Culex group V (CGV) but these initial comparative trials only investigated the crepuscular biting period in which the primary African malaria vector, An. gambiae, rarely feeds.8 Here, we directly evaluated the IST tent trap in overnight sampling against either light trap catches (LTC) or HLC to analyze its safety and to compare the sampling efficiency of all three approaches in three regions of West Africa that are hyper or holoendemic for mosquito-borne diseases.
Materials and Methods
Operation, trapping, locations, and sampling design.
The design and operation of the IST tent trap has been previously described.1 For all experiments, a human collector rested or slept in the custom designed camping tent for the duration of trapping each night, with the doors zipped and the airflow panels on all four sides opened to make a 5 cm gap of exposed micro-mesh fabric. All tests were performed outside, and the tent was always deployed with its rainfly attached to protect the collector from rainfall that would often occur during the nights of testing. The IST tent is pictured in Figure 1 deployed in Liberia during testing, with arrows marking mosquito entry gaps through the rainfly. A battery-operated fan attached to the inside tent ceiling was activated through the control box in the tent by the collector at the start of each night's sampling period. The fan speed on the control box was set at 9V in all sampling. The fan blows air onto the resting collector and forces the collector's odors and volatiles out through the open micro-mesh panels, creating odor plumes emanating from all four sides of the tent. This same fan creates negative suction to capture vectors that navigate up the odor plumes to the micro-mesh panel. Suction ports positioned immediately above each open mesh panel aspirate the host-seeking vectors into a trap, which is accessible to the collector inside the tent. We tested the tent from July to September 2012 in southeastern Senegal, from June 7 to 30, 2013 in northeastern Liberia, and from August to October 2013 in southwestern Burkina Faso. In all of these tests, the IST tent was directly compared with LTC or HLC. These trapping sessions were during portions of the rainy season in each location, when mosquito numbers and malaria transmission are high.9
Figure 1.
Infoscitex Tent in “location 1” of Bolahun, Liberia. Three openings for mosquito entry on this side are marked.
In Senegal, sampling was performed overnight (10:00 PM–6:00 AM) over 16 nights in the semi-urban town of Kedougou, which has been described previously.1 Testing was performed outdoors with a rotation of two collectors that would sleep underneath either a deltamethrin-treated Permanet 2.0 (Vestergaard Frandsen SA, Denmark) with a CDC Light trap hung near their feet, or sleeping in the IST that was placed > 10 m away. A treated net was used to prevent the sleeping human from being bitten if they inadvertently moved in the night and rested an exposed body part against the net; it was also shown in another study that the use of insecticide-coated nets had no effect on the ability of the CDC Light trap to catch An. gambiae s.l. compared with untreated nets.10
In Liberia, we compared the IST tent trap to HLC in a 2 × 2 rotational design using four local collectors over 8 nights in the town of Bolahun. This area of Liberia is a tropical rainforest, with an average yearly rainfall of 2346.6 mm (Climatic Research Unit of University of East Anglia, Norwich, UK).11 The area is holoendemic for malaria, with the Bolahun Health Care Center reporting year-round cases of malaria (diagnosed by Plasmodium falciparum Histidine-Rich Protein-2-specific rapid diagnostic test) with peak transmission during rains from May to October (Jallah H, personal communication). Testing was performed outdoors, with two IST tents deployed and HLC stations placed roughly 20 m away. The collector performing the HLC was next to a canopy under which he could work if it rained. Two individuals would sleep in the IST tents and two individuals would perform HLC overnight (10:00 PM–6:00 AM). Over this period, HLC collectors would sample 45 minutes with their legs exposed to biting vectors, which they would collect either by mouth aspirator (model 412; John W. Hock Company, Gainesville, FL) or 15 mL conical tube (Thermo-Fisher Scientific, Lafayette, CO) and then place in a paper container. The final 15 minutes of every hour the HLC collector was given a break in which they could rest. As a result of sampling for only 45 minutes of each hour with the HLC, the collection numbers with the tent were multiplied by 0.75 so sampling periods were equivalent. Each collector sampled in each of the four locations (Tent no. 1, Tent no. 2, HLC no. 1, and HLC no. 2) twice.
In Burkina Faso, sampling was performed outdoors in the rural villages of Bougouriba and Diarkadougou. These are Sudan savannah zones that are hyperendemic for malaria transmission, with peak rainfall in July and August, and a yearly average rainfall of 829.29 mm (Climatic Research Unit of University of East Anglia).11,12 These villages are separated by ∼10 km, the Nabere forest, and the Bougouriba River that borders the fishing village of Bougouriba. Eight collections were completed in Bougouriba, and nine collections were completed in Diarkadougou using local collectors. Each night of collection involved an individual collecting by HLC (as described previously) or with the IST tent for the first half of the night (9:00 PM–1:30 AM), and then switching techniques for the second half of the night (1:30 AM–6:00 AM) for a total of 9 hours of collection. Collectors also rotated which technique they began with, and in which village they sampled throughout the duration of testing. This approach allowed for sampling to be continuous throughout the night for HLC, while not forcing the collector to attempt the laborious HLC process for 9 hours, consecutively, and allowed for slightly earlier sampling than Liberia to account for any earlier feeding. This also allowed us to avoid the correction factors used in Liberia, which may artificially inflate or deflate numbers. Additional aspiration sampling of indoor-resting blood-fed mosquitoes was performed in both villages on the morning after overnight trapping using InsectaZooka field aspirators (BioQuip Products, Rancho Dominguez, CA).
Mosquito identification and processing.
After collection, mosquitoes were frozen until dead or killed with chloroform, and then identified to species or lowest taxa group using published keys.13–15 Mosquito DNA was extracted from the head and thorax using the 96-well format DNeasy Blood & Tissue Kit following manufacturer instructions for insect tissue (Qiagen Sciences Inc., Germantown, MD). Anopheles gambiae s.l. complex members were analyzed with multiplex polymerase chain reaction (PCR) that distinguishes between An. gambiae sensu stricto (s.s.), Anopheles arabiensis, Anopheles quadriannulatus, and Anopheles merus/melas.16 Plasmodium sporozoites were detected from extracted DNA using a quantitative PCR protocol that distinguishes between Plasmodium falciparum and Plasmodium ovale/vivax/malariae.17
Statistical analysis.
Catch numbers for each method were compared on a nightly basis using a Wilcoxon matched-pairs Signed-ranks test with Graphpad Prism Version 5 (GraphPad Software, La Jolla, CA). This test was used as it is appropriate for normal and non-normal data, which allows for comparisons between sets to use the same statistical test. Correlations were performed using Pearson correlations on log(x+1) transformed data because of the largely linear relationship between methods and to assure normality for all sites presented.3 The majority of data sets had normal distributions (D'Agostino-Pearson omnibus normality test), with the exception of LTC in Kedougou, likely as a result of there being five zero-catch nights. Differences in P. falciparum infection rates and An. gambiae s.l. complex species identities between methods were analyzed with 2 × 2 contingency tables and two-tailed Fisher's exact tests also using Graphpad Prism.
Ethical considerations.
Sampling using human subjects was approved by the Institutional Review Board at Colorado State University (protocol 11-2874H), and by human subjects research reviews in each country (Senegal, no. 132/12_/DSK; Liberia, EC/LIBR/012/033; Burkina Faso, 28-2013/CE-CM) in compliance with the Helsinki Declaration. Informed consent was obtained from all adult, paid mosquito collectors. Antimalarial prophylaxis drug regimens (either atovaquone-proguanil or doxycycline) were made available to all collectors, and diagnosis and treatment of any malaria infections, for the duration of testing. No collectors became ill over the course of the collections.
Results
Capture efficiency of IST compared with other methods.
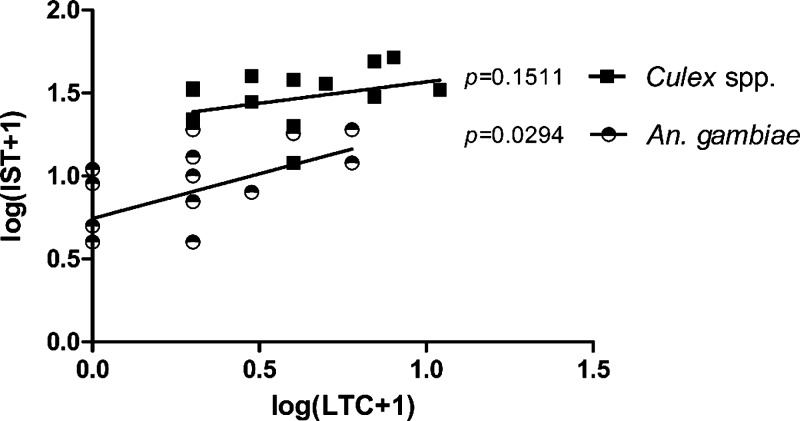
In Senegal, the IST tent trap outperformed the LTC across 16 nights for CGV and An. gambiae s.l. (Tables 1 and 2). Overnight mean catch numbers for CGV and An. gambiae using LTC were 3.38 and 1.38, respectively. Mean catch numbers per night for the tent were 29.63 and 8.44 for CGV and An. gambiae, respectively, or 8.78 times the LTC catch rates for Culex and 6.14 times the rate for Anopheles. The difference in capture numbers were highly significant for both species (Wilcoxon signed-rank test, P = 0.0005). Collection numbers between methods correlated between the LTC and IST tent for An. gambiae s.l. (Pearson r = 0.6451, P = 0.0070), but not for Culex spp. (Pearson r = 0.3761, P = 0.1511) (Figure 2).
Table 1.
Anopheles gambiae s.l. catch numbers per night and species composition of the Infoscitex tent compared with reference methods
| Location | Method | Trap nights | Mean no. An. gambiae s.l. caught (95% CI) | Total | % Of Ref. method | P value* | Proportion An. gambiae s.s (no. tested) | P value† |
|---|---|---|---|---|---|---|---|---|
| Kedougou, Senegal | IST | 16 | 8.44 (5.51–11.37) | 135 | 613.67% | 0.0005 | 1.000 (69) | 1.000 |
| LTC | 16 | 1.38 (0.51–2.24) | 22 | − | 1.000 (16) | |||
| Bolahun, Liberia | IST‡ | 14 | 11.30 (7.32–15.29) | 211 | 38.03% | 0.0203 | 1.000 (86) | 1.000 |
| HLC | 14 | 29.71 (17.23–42.20) | 416 | − | 1.000 (140) | |||
| Bougouriba, Burkina Faso | IST | 8 | 52.38 (21.23–83.52) | 419 | 52.91% | 0.0391 | 0.965 (141) | 0.070 |
| HLC | 8 | 99.00 (56.00–142.0) | 792 | − | 1.000 (109) | |||
| Diarkadougou, Burkina Faso | IST | 9 | 57.56 (15.53–99.58) | 518 | 71.56% | 0.1289 | 1.000 (142) | 0.228 |
| HLC | 9 | 80.44 (30.89–130.0) | 724 | − | 0.985 (130) |
P values are calculated by Wilcoxon signed-rank test comparing nightly catch numbers for each method.
P values for proportion An. gambiae s.s. calculated by two-tailed Fisher's exact. “−” = not applicable as it is the reference method.
Mean IST catch values from Liberia were reduced by 0.25× to compare with the 45-minute HLC sampling duration for each hour of the testing.
IST = Infoscitex tent; LTC = light trap catches; HLC = human landing catches.
Table 2.
Nightly catch numbers of all caught species over all locations/methods
| Location | Method | Anopheles gambiae s.l. | Average/night [percentage of species in total catch] (n # total) | Aedes spp. | Mansonia uniformis | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| An. nili | An. funestus | An. coustani | An. pharoensis | Other Anopheles | Culex spp. | ||||||
| Kedougou, Senegal | IST | 8.44 [22.1%] (135) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 29.63 [77.6%] (474) | 0.13 [0.3%] (2) | 0.00 [0%] (0) | 611 |
| LTC | 1.38 [26.5%] (22) | 0.00 [0%] (0) | 0.063 [1.2%] (1) | 0.00 [0%] (0) | 0.00 [0] (0) | 0.00 [0%] (0) | 3.38 [65.1%] (54) | 0.31 [6.0%] (5) | 0.00 [0%] (0) | 83 | |
| Bolahun, Liberia | IST | 15.07 [100%] (211) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 211 |
| HLC | 29.71 [98.8%] (416) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.00 [0%] (0) | 0.21 [0.7%] (3) | 0.00 [0] (0) | 0.14 [0.5%] (2) | 421 | |
| Bougouriba, Burkina Faso | IST | 52.38 [67.9%] (419) | 15.88 [20.6%] (127) | 1.50 [1.9%] (12) | 2.00 [2.6%] (16) | 1.88 [2.4%] (15) | 0.25 [0.3%] (2) | 2.63 [3.4%] (21) | 0.63 [0.8%] (5) | 0.00 [0%] (0) | 617 |
| HLC | 99.00 [60.7%] (792) | 48.50 [29.7%] (388) | 11.00 [6.7%] (88) | 1.13 [0.7%] (9) | 0.25 [0.2%] (2) | 0.25 [0.2%] (2) | 1.00 [0.6%] (8) | 2.00 [1.2%] (16) | 0.00 [0%] (0) | 1,305 | |
| Asp | N/A [98.1%] (790) | N/A [0.2%] (2) | N/A [0.4%] (4) | N/A [0%] (0) | N/A [0%] (0) | N/A [0.1%] (1) | N/A [1.0%] (8) | N/A [0%] (0) | N/A [0%] (0) | 805 | |
| Diarkadougou, Burkina Faso | IST | 57.56 [87.9%] (518) | 1.11 [1.7%] (10) | 4.78 [7.3%] (43) | 0.22 [0.3%] (2) | 0.00 [0%] (0) | 0.67 [1.0%] (6) | 1.00 [1.5%] (9) | 0.11 [0.2%] (1) | 0.00 [0%] (0) | 589 |
| HLC | 80.44 [89.6%] (724) | 1.89 [2.1%] (17) | 4.33 [4.8%] (39) | 0.56 [0.6%] (5) | 0.00 [0%] (0) | 0.33 [0.4%] (3) | 1.89 [2.1%] (17) | 0.33 [0.4%] (3) | 0.00 [0%] (0) | 808 | |
| Asp | N/A [95.3%] (988) | N/A [0.4%] (4) | N/A [4.1%] (43) | N/A [0%] (0) | N/A [0%] (0) | N/A [0.2%] (2) | N/A [0%] (0) | N/A [0%] (0) | N/A [0%] (0) | 1, 037 | |
Figure 2.
Correlation of log (x + 1) transformed nightly catch numbers between the Infoscitex Tent and Light Trap Catch in Kedougou, Senegal.
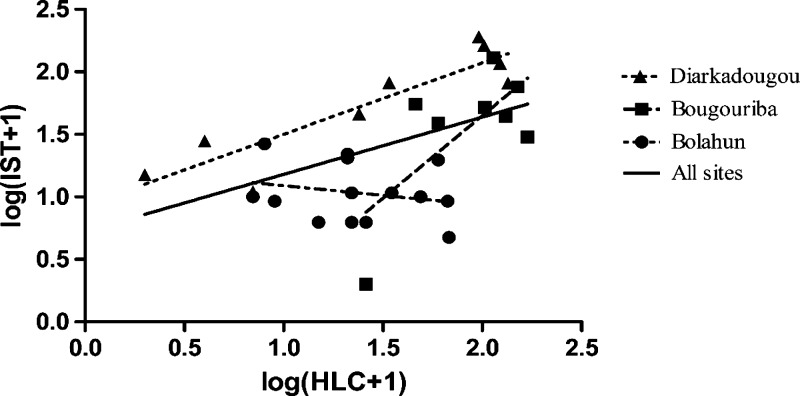
In Liberia, there were 14 successful pair-matched nights of sampling, with one night having battery failures in both tents at ∼12:30 am caused by user error. Though the trap was restarted with new batteries, it is unknown how many mosquitoes were lost during this period, thus we removed these IST/HLC pair data from analysis. In this sampling, the dominant mosquito caught was An. gambiae s.l. (N = 627, 99.2% of all caught-species). Mean nightly catch numbers for each method were 11.30 and 29.71 for IST and HLC, respectively. With IST tent data adjusted for the 45 minutes sampling, the difference in median catch number between methods differed significantly (Wilcoxon signed-rank test, P = 0.0203). The IST tent trap caught no other species in Liberia, although the HLC caught a minimal number of Culex spp. (N = 3) and Mansonia uniformis (n = 1) (Table 2). Methods failed to correlate in this location (Pearson r = −0.2215, P = 0.4466) (Figure 3). Additionally, collection numbers by HLC increased 1.8 or 4.1 mosquitoes per night of sampling on average (5.8% and 14.9%, respectively) depending on location (Supplemental Figure 1, all nights included). In the same time period, IST efficiency increased by 0.29 or decreased by 0.56 mosquitoes per night of sampling on average (3.0% and −4.6%, respectively) over the two collection locations.
Figure 3.
Correlation of log (x + 1) transformed nightly catch numbers between the Infoscitex Tent and Human Landing Catch in one location in Liberia, and two locations in Burkina Faso. Dashed lines are linear regressions for each site individually, and the solid line is the regression from all sites combined.
Testing in Burkina Faso again analyzed the HLC against the IST tent. In this location, mosquito density and diversity was higher than in the other sites of this trial (Table 2). Anopheles gambiae s.l. collection numbers for the tent were 52.38/night and 57.56/night in Bougouriba and Diarkadougou, respectively, and 99.00/night and 80.44/night for the HLC in these respective locations (Table 1). The difference in collection numbers between methods was significant in Bougouriba (Wilcoxon signed-rank test, P = 0.0391), but not Diarkadougou (Wilcoxon signed-rank test, P = 0.1289). Additionally, in Bougouriba Anopheles nili were sampled more often by both HLC and the IST tent (48.50/night and 15.88/night, respectively) than in all other locations (Table 2). The sampling efficiency difference between approaches was comparable (Wilcoxon signed-rank test, P = 0.0547). The methods were comparable in Bougouriba (Pearson r = 0.6918, P = 0.0573) and correlated significantly in Diarkadougou (Pearson r = 0.8951, P = 0.0011) (Figure 3). The increase in HLC sampling efficiency relative to the tent seen in Liberia was not seen in Burkina Faso, with equivalent increasing regression slopes in Bougouriba (HLC:9.4, IST:5.2), and an inverse relationship in Diarkadougou (HLC: −0.6, IST: 5.2) (Supplemental Figure 1).
An. gambiae s.l. species discrimination, Plasmodium infection rates, and proportions with blood meals.
In Senegal, a proportion of captured An. gambiae s.l. were analyzed for species composition from each method, and of the 85 successfully amplified samples from the two methods, all were An. gambiae s.s. (Table 1). Of those collected, 5.2% of An. gambiae caught by the IST tent and 9.1% of the LTC-caught had an appreciable blood meal, and these differences were not statistically significant (Table 3). As a result of low relative catch numbers, Plasmodium infection status was not analyzed at this location.
Table 3.
Anopheles gambiae s.l. Plasmodium falciparum infection status, monthly entomological inoculation rate, and bloodedness based on collection method*
| Location | Method | Trap nights | Proportion Pf sporozoite+ | P value | 30-day EIR | Proportion with blood meal (no. analyzed) | P value |
|---|---|---|---|---|---|---|---|
| Kedougou, Senegal | IST | 16 | − | − | − | 0.052 (135) | 0.3662 |
| LTC | 16 | − | − | − | 0.091 (22) | ||
| Bolahun, Liberia | IST | 14 | 0.036 | 0.678 | 16.219 | 0.005 (236) | < 0.0001 |
| HLC | 14 | 0.047 | 41.563 | 0.214 (416) | |||
| Bougouriba, Burkina Faso | IST | 8 | 0.024 | 0.334 | 37.714 | 0.007 (282) | < 0.0001 |
| HLC | 8 | 0.051 | 151.017 | 0.361 (288) | |||
| Diarkadougou, Burkina Faso | IST | 9 | 0.020 | 1.000 | 35.241 | 0.012 (258) | < 0.0001 |
| HLC | 9 | 0.015 | 37.126 | 0.261 (230) |
“−” = not tested due to low catch numbers; Pf = Plasmodium falciparum.
Mosquitoes captured in Bolahun, Liberia were also analyzed by molecular sibling species discrimination (Table 1). Of the 226 successfully amplified specimens (86 IST, 140 HLC) all were found to be An. gambiae s.s. Because of a limited presence of cattle and the early season sampling, it is unsurprising that An. arabiensis were not captured by either method at this location. Testing for Plasmodium sporozoites was performed on 82.3% of all HLC (n = 386) and 94.5% of all IST tent (n = 223) An. gambiae s.l. samples. As sporozoite infection rates are low, the data were pooled for analysis. From this, no statistically significant difference was found in Plasmodium infection status of mosquitoes captured between the two methods (3.6% and 4.7% sporozoite positive for IST and HLC respectively, two-tailed Fisher's exact test, P = 0.6782). The dominant Plasmodium species was falciparum, with only one mosquito testing positive for either Plasmodium ovale/vivax/malariae (Data not shown, determined by quantitative reverse transcription-PCR).17
In both locations in Burkina Faso, proportions of An. gambiae s.s. and An. arabiensis were not significantly different with sampling by either method (Table 1). Unlike the other locations, An. arabiensis were collected with both methods, though in the tested sub-sample this species was still a small fraction of those caught (3.5% of tent caught An. gambiae s.l. in Bougouriba and 1.6% of An. gambiae s.l. in Diarkadougou) (Table 1, “Proportion An. gambiae s.s.”). No significant differences in Plasmodium sporozoite infection status was observed from An. gambiae s.l. collected in either location (2.4% positive of IST caught in Bougouriba; 5.1% of HLC caught, p = 0.334; and 2.0% of IST in Diarkadougou, 1.5% HLC, P = 1.000) (Table 3). Plasmodium falciparum was again the dominant species of parasite, with only one IST-caught Anopheles funestus testing positive for P. ovale/vivax/malariae.
Mosquitoes from the IST were significantly less likely to be blood fed than those caught by HLC in all locations (P < 0.0001, two-tailed Fisher's exact) (Table 3). Furthermore, many of the blood meals from HLC-captured mosquitoes were fresh (the blood meal was red), but this was less often true of the fewer blood meals observed in IST-captured mosquitoes. There was no statistical difference in the proportion of mosquitoes containing blood meals of those caught between LTC and the IST tent.
Discussion
In this work the efficiency and safety of a novel mosquito-trapping human-baited tent was examined against the “gold standard” trapping methods of CDC Light Traps and HLC in areas of West Africa with high endemicity for mosquito-borne disease. Overall, catch numbers for the IST tent trap were significantly higher than LTC in the one location tested (Kedougou, Senegal). This ∼6–9× increase in mosquito catch numbers (depending on species) is promising as the tent is easy to use, has comparable safety and comfort to sleeping under a bed net, and it can be more easily set up outside as it does not need additional shelter (e.g., a tarpaulin fixed over the bed and bed net). Additionally, the tent avoids any biases present in HLC, such as skill of collector. The battery powered system in the tent would also be conducive to inclusion of regular or UV light sources, similar to those in the LTC, if vectors in the area were highly phototaxic. Though the inclusion of light sources may also increase the amount of non-host-seeking insects that can also be highly phototaxic. This is seen commonly in CDC Light Traps and can significantly slow vector processing.
The IST tent trap's catch numbers were not significantly different than HLC in 1 of 2 locations in Burkina Faso (Table 1). However, the mean number of An. gambiae s.l. captured per night was lower for the IST tent compared with HLC over the three sampling locations where these comparisons were performed (IST = 35.33 [20.49–50.17] An. gambiae s.l./night, HLC = 62.32 [42.63–82.01]/night), and in Bolahun, Liberia and Bougouriba, Burkina Faso the mean catch numbers were significantly different (Table 1). There are at least two reasons for the reduced sampling efficiency of the tent compared with HLC. First, the lack of visual and/or thermal cues from an individual in the tent could limit the mosquito's fine host-seeking ability after it has followed the odor plume to the open mesh panels, as has been suggested with the IST tent in crepuscular sampling of Aedes spp. and in other trapping methods for An. gambiae.1,18 Second, it is possible that some mosquitoes or mosquito species are more able to escape from the suction at the ports positioned above the open-mesh panels. This possibility might be easily remedied by increasing the fan speed by the control box. Nevertheless, the reduced trapping efficiency of the IST tent could be fixed by having a collector sleep in the tent on sequential days, which would also alleviate temporal collecting bias (changes in daily catch numbers caused by weather and other uncontrollable environmental and biological factors). Sequential sampling is not practical with HLC as it is difficult for collectors to be awake and focused many nights in a row.
One drawback to increased sampling is the necessity of maintaining charged batteries. Testing overnight in Burkina Faso lasted 9 hours as opposed to the 8-hour testing period in Liberia. Consequently, there were several battery failures in Burkina Faso in the last hour of sampling as this extended time drained the 12-volt, 14 amperage batteries that were available. Having larger batteries with higher amperage would likely have eliminated this problem. Previous descriptions of tent traps with passive designs highlighted the advantage of requiring no batteries in simplifying the tents' use and lowering their cost. In contrast, the active design of the IST mandates a power system that will increase its cost and the batteries need to be regularly charged after use. With cell phones and solar charging systems now often found in even the most remote areas, this latter critique may be less of a concern. Other than cost, the main trade-offs of a passive versus an active design may then be 1) the comfort of the collector in each respective tent, 2) trapping efficiency when an odor plume passively diffuses from the tent versus when an odor plume is forced from the tent, and 3) the ability to trap exophagic mosquitoes. The forced air of the IST tent's fan blowing down on the user increases the comfort for the collector in the tent throughout the night; it remains to be seen if the resulting active odor plume is more successful for trapping mosquitoes than that from passive designs. Future experiments should be conducted to directly compare these two designs.
To understand how the IST samples mosquitoes with exophagic or endophagic tendencies, it may be useful to examine the proportions of Anopheles species caught relative to their biting location preference as described by the literature.18 Although each field site has unique mosquito populations, in general An. gambiae s.s. have been observed biting indoors and outdoors in approximately equal proportions,19 whereas An. arabiensis, An. funestus, An. nili, and An. coustani have been observed to bite outdoors more often than bite indoors.4,10,18,20,21 Indoor resting aspiration collections in Bougouriba and Diarkadougou, respectively, conducted the morning immediately after overnight IST and HLC testing resulted in 95.3, 98.1% An. gambiae s.l. (98.5% of which are An. gambiae s.s.); but only 0.4, 4.1% An. funestus; 0.2, 0.4% An. nili; and 0% An. coustani (Table 2). Compared with both HLC and IST tent collections the night prior, significantly lower proportions of predominantly outdoor biting species were collected (Table 2). Although some of these data could be explained by these secondary vector species having bitten indoors and then immediately exiting the house, they nevertheless highlight the importance of collecting outdoor biting mosquitoes. Overall, the data show that the IST tent, although slightly less efficient than HLC, samples outdoor biting mosquito populations in approximately equal proportions to HLC, and the reduced efficiency could be addressed by sequential sampling. This is important because exophagic vectors are thought to be increasingly maintaining Plasmodium transmission in the face of wide-scale implementation of indoor vector control measures such as indoor-residual spraying, insecticide-treated bed nets and use of indoor spatial repellents.22,23
Finally, the Plasmodium sporozoite infection status was examined between Anopheles vectors captured between methods. No differences in Bolahun, Liberia nor in either location of Burkina Faso were observed (Table 3). Catch numbers in Kedougou, Senegal were too low for meaningful testing. These data were used to calculate the entomological inoculation rate (EIR), or the number of bites/person/day, expanded over the month of testing, and multiplied by the percentage of mosquitoes infected. The monthly EIR was high, and ranged from 16 to 151 infectious bites/person/month depending on the collection method and location (Table 3). At least three P. falciparum sporozoite positive mosquitoes caught by HLC contained blood in their abdomens. It is unknown if this blood was taken from the collector performing HLC, but as the overall percentage of blood fed An. gambiae s.l. was significantly higher in HLC approaches (27.1%) than tent (0.7%), it is likely that the blood was taken during HLC. Additionally, it was frequently noted that blooded mosquitoes from HLC often had fresh blood meals and many were blood fed to near repletion, which makes it unlikely that they were actively host seeking after having bitten some other person in the village. These data clearly show the risk to HLC collectors, and it has been previously shown that Brugia nematodes, Plasmodium protozoa, and arboviruses can be transmitted through probing alone.24–27 The HLC is also a skill-dependent approach (Table 2 and Supplementary Figure 1). In Liberia, the trained collectors were novices, whereas in Burkina Faso, the collectors had performed this method many times previously on other projects. Reflecting this, the sampling efficiency of HLC in Liberia increased over successive nights, but the efficiency did not correspondingly increase with the tent. This is compared with the efficiencies of the two methods in Burkina Faso, which held relatively constant to each other. On the other hand, the Liberian collectors seemed slightly more diligent at preventing mosquito engorgement from their exposed legs. Compared with HLC, the IST tent affords a standardized approach that is not skill-dependent, has minimal risk, and removes the over-sampling bias associated with HLC (performing an unnatural behavior of sitting outdoors overnight, exposing their legs purposely to biting vectors).
In light of the dangers presented from Human Landing Catch, alternative methods of modern sampling are needed. In our testing, we found that the Infoscitex Tent was much safer than the Human Landing Catch. This approach more closely mimics realistic overnight behavior of individuals in an area, having them sleep throughout the night, rather than intentionally expose themselves to mosquito biting. Additionally, it provides a standardized platform that removes the skill component present in HLC whereby previous experience, or a willingness to allow mosquitoes to probe fully, can increase catch numbers. The primary disadvantages of the tent are likely to be cost relative to HLC and LTC (although it has not yet been marketed), and electric infrastructure must be present in the sampling area to adequately charge batteries for frequent sampling. However, long-term costs may be minimized if it is decided that ethical considerations could be minimized and drug prophylaxis would not be needed, and there may be additional cost reductions associated with training fewer collectors. Ultimately, the IST tent approach presented here catches fewer numbers of mosquitoes compared with HLC, but allows for a more robust sampling design, may more accurately reflect true disease risk, and limits disease risk to human volunteers.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nemo Equipment, Inc. for their assistance in designing the tent. We thank Massamba Sylla for his assistance in facilitating this research in Southeastern Senegal. We thank the people and collectors of Kedougou, Bolahun, Bougouriba, and Diarkadougou without whom this work would not have been possible. We also thank Henry Jallah at the Bolahun Health Care Center for his administration of prophylaxis, RDT kits, and his helpful correspondence about disease in the area. The thank the staff of the Liberian Institute for Biomedical Research and the Institut de Recherche en Sciences de la Santé for their assistance throughout. We thank Jason Richardson, Edgar Rowton, and Erica Lindroth for helpful discussions and guidance throughout this project. Finally, we acknowledge Desmond Foley and Richard Wilkerson for their development of the initial idea, and on the design and testing of the tent trap.
Footnotes
Financial support: Financial support for this project was through a Small Business Innovation Research Grant W81XWH-10-C-0214 from the U.S. Department of Defense, and research in Senegal, Liberia, and Burkina Faso was partially funded by grant 1R01AI094349-01A1 from the U.S. National Institutes of Health.
Authors' addresses: Benjamin J. Krajacich, Haoues Alout, Nathan D. Grubaugh, Jacob I. Meyers, Doug E. Brackney, Timothy A. Burton, Jonathan A. Seaman, and Brian D. Foy, Colorado State University, Fort Collins, CO, E-mails: benjamin.krajacich@colostate.edu, haoues.alout@colostate.edu, nathan.grubaugh@colostate.edu, Jacob.I.Meyers@gmail.com, Doug.Brackney@colostate.edu, timaburton@gmail.com, jonathan.seaman@rams.colostate.edu, and brian.foy@colostate.edu. Jeremiah R. Slade, Robert F. Mulligan, and Brendan LaBrecque, Infoscitex Corporation, Littleton, MA, E-mails: jslade@infoscitex.com, rfm3@email.com, and labrecque.b@gmail.com. Lawrence S. Fakoli III and Fatorma K. Bolay, Liberian Institute for Biomedical Research (LIBR), Charlesville, Margibi County, Liberia, E-mails: lawfako2008@yahoo.com and director.libr@gmail.com. Joseph W. Diclaro II, Vector Biology Research Program, Naval Medical Research Unit No. 3 Cairo Egypt, E-mail: joseph.diclaro@med.navy.mil. Roch K. Dabiré, Institut de Recherche en Sciences de la Santé (IRSS), Bobo Dioulasso, Burkina Faso, E-mail: dabire_roch@hotmail.com.
References
- 1.Krajacich BJ, Slade JR, Mulligan RF, LaBrecque B, Kobylinski KC, Gray M, Kuklinski WS, Burton TA, Seaman JA, Sylla M, Foy BD. Design and testing of a novel, protective human-baited tent trap for the collection of anthropophilic disease vectors. J Med Entomol. 2014;51:253–263. doi: 10.1603/me13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Govella NJ, Chaki PP, Geissbuhler Y, Kannady K, Okumu F, Charlwood JD, Anderson RA, Killeen GF. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J. 2009;8:157. doi: 10.1186/1475-2875-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overgaard HJ, Saebø S, Reddy MR, Reddy VP, Abaga S, Matias A, Slotman MA. Light traps fail to estimate reliable malaria mosquito biting rates on Bioko Island, Equatorial Guinea. Malar J. 2012;11:56. doi: 10.1186/1475-2875-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majambere S, Massue DJ, Mlacha Y, Govella NJ, Magesa SM, Killeen GF. Advantages and limitations of commercially available electrocuting grids for studying mosquito behavior. Parasit Vectors. 2013;6:53. doi: 10.1186/1756-3305-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathenge EM, Omweri GO, Irungu LW, Ndegwa PN, Walczak E, Smith TA, Killeen GF, Knols BG. Comparative field evaluation of the Mbita trap, the Centers for Disease Control light trap, and the human landing catch for sampling of malaria vectors in western Kenya. Am J Trop Med Hyg. 2004;70:33–37. [PubMed] [Google Scholar]
- 6.Njiru BN, Mukabana WR, Takken W, Knols BG. Trapping of the malaria vector Anopheles gambiae with odor-baited MM-X traps in semi-field conditions in western Kenya. Malar J. 2006;5:39. doi: 10.1186/1475-2875-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govella NJ, Moore JD, Killeen GF. An exposure-free tool for monitoring adult malaria mosquito populations. Am J Trop Med Hyg. 2010;83:596–600. doi: 10.4269/ajtmh.2010.09-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontenille D, Lochouarn L, Diatta M, Sokhna C, Dia I, Diagne N, Lemasson J-J, Ba K, Tall A, Rogier C, Trape J-F. Four years' entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and A. arabiensis. Trans R Soc Trop Med Hyg. 1997;91:647–652. doi: 10.1016/s0035-9203(97)90506-x. [DOI] [PubMed] [Google Scholar]
- 9.Monlun E, Zeller H, Le Guenno B, Traoré-Lamizana M, Hervy JP, Adam F, Ferrara L, Fontenille D, Sylla R, Mondo M. Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal. Bull Soc Pathol Exot. 1993;86:21–28. [PubMed] [Google Scholar]
- 10.Fornadel CM, Norris LC, Norris DE. Centers for Disease Control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am J Trop Med Hyg. 2010;83:838–842. doi: 10.4269/ajtmh.2010.10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Climatic Research Unit U of EA Climate Change Knowledge Portal 2.0. 2014. http://sdwebx.worldbank.org/climateportal/index.cfm?page=country_historical_climate&ThisRegion=Africa&ThisCCode=LBR Available at. Accessed May 5, 2014.
- 12.Dabiré KR, Diabaté A, Namountougou M, Toé KH, Ouari A, Kengne P, Bass C, Baldet T. Distribution of pyrethroid and DDT resistance and the L1014F kdr mutation in Anopheles gambiae s.l. from Burkina Faso (West Africa) Trans R Soc Trop Med Hyg. 2009;103:1113–1120. doi: 10.1016/j.trstmh.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Pecor J. Adult Female Identification Key to Culex Species of Africa (AFRICOM), with Emphasis on Medically Important Species. 2012. http://www.wrbu.org/keys/AF_CX_A/Culex_Afrotropical_AFRICOM_A.html Available at. Accessed March 10, 2014.
- 14.Diagne N, Fontenille D, Konate L, Faye O, Lamizana MT, Legros F, Molez JF, Trape JF. Anopheles of Senegal. An annotated and illustrated list. Bull Soc Pathol Exot. 1993;87:267–277. [PubMed] [Google Scholar]
- 15.Rueda LM. Adult Identification Key to the Genus Anopheles in Africa (AFRICOM), with Emphasis on Medically Important Mosquitoes. 2014. http://www.wrbu.org/keys/AF_AN_A/Anopheles_Afro_AFRICOM_A.html Available at. Accessed March 18, 2014.
- 16.Wilkins EE, Howell PI, Benedict MQ. IMP PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Savanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar J. 2006;5:125. doi: 10.1186/1475-2875-5-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bass C, Nikou D, Blagborough AM, Vontas J, Sinden RE, Williamson MS, Field LM. PCR-based detection of Plasmodium in Anopheles mosquitoes: a comparison of a new high-throughput assay with existing methods. Malar J. 2008;7:177. doi: 10.1186/1475-2875-7-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dia I, Diallo D, Duchemin J, Ba Y, Konate L, Costantini C, Diallo M. Comparisons of human-landing catches and odor-baited entry traps for sampling malaria vectors in Senegal. J Med Entomol. 2005;42:104–109. doi: 10.1093/jmedent/42.2.104. [DOI] [PubMed] [Google Scholar]
- 19.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, Slotman MA. Outdoor host seeking behavior of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirados I, Costantini C, Gibson G, Torr SJ. Blood-feeding behavior of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006;20:425–437. doi: 10.1111/j.1365-2915.2006.652.x. [DOI] [PubMed] [Google Scholar]
- 21.Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, Ofulla AV, Atieli FK, Ondijo SO, Genga IO, Odada PK, Situbi PA, Oloo JA. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 22.Govella NJ, Ferguson H. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol. 2012;3:199. doi: 10.3389/fphys.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewert A, Ho BC. The fate of Brugia pahangi larvae immediately after feeding by infective vector mosquitoes. Trans R Soc Trop Med Hyg. 1967;61:659–662. doi: 10.1016/0035-9203(67)90129-0. [DOI] [PubMed] [Google Scholar]
- 25.Ho BC, Ewert A. Experimental transmission of filarial larvae in relation to feeding behavior of the mosquito vectors. Trans R Soc Trop Med Hyg. 1967;61:663–666. doi: 10.1016/0035-9203(67)90130-7. [DOI] [PubMed] [Google Scholar]
- 26.Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007;3:1262–1270. doi: 10.1371/journal.ppat.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medica D, Sinnis P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect Immun. 2005;73:4363–4369. doi: 10.1128/IAI.73.7.4363-4369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.